Abstract
Recent studies have shown that MDR could be induced by the high stemness of cancer cells. In a previous study, we found bufalin could reverse MDR and inhibit cancer cell stemness in colorectal cancer, but the relationship between them was unclear. Here we identified overexpressing CD133 increases levels of Akt/nuclear factor‐κB signaling mediators and MDR1, while increasing cell chemoresistance. Furthermore, bufalin reverses colorectal cancer MDR by regulating cancer cell stemness through the CD133/nuclear factor‐κB/MDR1 pathway in vitro and in vivo. Taken together, our results suggest that bufalin could be developed as a novel 2‐pronged drug that targets CD133 and MDR1 to eradicate MDR cells and could ultimately be combined with conventional chemotherapeutic agents to improve treatment outcomes for patients with colorectal cancer.
Keywords: bufalin, CD133, colorectal cancer, MDR1, multidrug resistance
Overexpressing CD133 increases levels of Akt/NF‐κB signaling mediators and MDR1. Bufalin reverses colorectal cancer multidrug resistance by regulating cancer cell stemness via the CD133/NF‐κB/MDR1 pathway.
Abbreviations
- ADR
adriamycin
- BU
bufalin
- CRC
colorectal cancer
- CSC
cancer stem cell
- CTX
cyclophosphamide
- DOX
doxorubicin
- IHC
immunohistochemistry
- MMC
mitomycin C
- NF‐κB,
nuclear factor‐κB
- OE,
overexpressing
- qPCR,
quantitative PCR
- VCR
vincristine
1. INTRODUCTION
Colorectal carcinoma is one of the most common malignant tumors in the world, with the third highest incidence among cancers. 1 The incidence of CRC has continued to rise in many countries in recent years. Although chemotherapy is an effective treatment method for CRC, MDR is the main cause of chemotherapy failure and disease progression. 2 , 3 Cancer stem cells play important roles in MDR according to the viability of self‐renewal and infinite proliferation. 4 , 5 , 6 Therefore, it is important to discover new agents that are capable of specifically targeting MDR cells and CSCs, which is difficult but essential for reversing MDR and curing CRC.
To date, some agents derived from nature have been identified to overcome MDR by inhibiting ABC transporters and/or targeting CSCs. 7 Bufalin has shown strong antitumor activity against various cancers. 8 , 9 , 10 , 11 The anticancer mechanisms of BU can be summarized as follows: inhibition of proliferation, promotion of apoptosis, inhibition of angiogenesis, inhibition of metastasis, reversal of drug resistance, and induction of autophagy. 12 , 13 , 14 Recent studies suggested that BU could inhibit the differentiation, proliferation, and drug resistance of cancers by inhibiting stemness in osteosarcoma, and improve the sensitivity of human glioma stem‐like cells to temozolamide. 15 , 16 , 17
Our previous studies showed that BU could effectively reverse MDR in colon cancer by inhibiting the expression of the MDR1/P‐gp protein. 18 In addition, BU can reverse acquired cisplatin resistance by inhibiting the expression of CD133. 19 It was reported that CD133+ colon CSCs often show excessive activation of the PI3K/Akt pathway; 20 PI3K/Akt activates NF‐κB, which induces MDR1/P‐gp expression through binding to its promoter. 21 , 22 , 23 Therefore, we hypothesized that BU can reverse MDR1/P‐gp‐mediated MDR by the inhibition of stemness through the AKT/NF‐κB axis in CRC.
The purpose of our work was to investigate the potential molecular mechanism of the BU‐mediated reversal of MDR in CRC, including the relationship between CD133 and MDR1/P‐gp, and the role of the AKT/NF‐κB pathway in this relationship.
2. METHODS
2.1. Cell lines and reagents
The human colon cancer cell lines LoVo and HCT8 were obtained from the Cell Bank of the Chinese Academy of Sciences. Doxorubicin‐selected LoVo/ADR and HCT8/ADR cell lines were purchased from Shanghai Yan Sheng Industrial Co. All cell lines were used for the reversal study and were cultured in RPMI‐1640 or F12K containing 10% FBS at 37°C in a humidified atmosphere of 5% CO2. All the DOX‐selected cells were seeded in medium containing 1 μmol/L DOX to maintain the drug resistance phenotype. LoVoCD133+ and HCT8CD133+ cells were established by infecting the LoVo and HCT8 cell lines with a lentivirus containing an empty plasmid or full‐length CD133 and were cultured in medium containing 10 μg/mL puromycin.
Bufalin, DOX, MMC, VCR, and CTX were obtained from Sigma‐Aldrich Chemical Co. The AKT overexpression plasmid, the NF‐κB/p65 overexpression plasmid, and the MDR1 promoter plasmid were purchased from Addgene.
2.2. Cell viability and apoptosis assays
Colon cancer cells were plated in 96‐well plates and treated with various chemotherapeutic agents for the indicated times. After 48 hours, cell viability was assessed using a CCK‐8 assay (Dojindo Molecular Technologies). For apoptosis, an annexin V‐FITC apoptosis detection kit (Invitrogen) was used according to the manufacturer’s instructions.
2.3. DOX uptake and retention
To visualize the uptake of DOX, the cells incubated with DOX (1 μg/mL). Nuclei were stained with DAPI (Invitrogen). The cells were visualized with a Zeiss 510 confocal laser‐scanning microscope using a 63 N (NA1.32) objective.
To investigate the cellular DOX retention level, DOX concentrations in the cell lysates were measured using a Wallac Victor2 TM1420 multilabel counter (Perkin Elmer). Cellular DOX uptake is expressed as nanomoles per milligram of protein. The protein concentrations were determined with a BCA Protein Assay Kit (Thermo Fisher Scientific).
2.4. Immunofluorescence
Cells cultivated on glass cover slides were fixed in 4% paraformaldehyde/PBS for 10 minutes, permeabilized in 0.2% Triton X‐100 for 20 minutes, and blocked in 100% FBS for 1 hour. The fixed cells were incubated with a primary Ab specific for CD133, NF‐κB/p65, or P‐gp (Cell Signaling Technology) and a secondary Ab. Images were obtained by using a confocal laser‐scanning microscope (LSM 510; Carl Zeiss).
2.5. Quantitative RT‐PCR
Total RNA was extracted with TRIzol (Invitrogen), and reverse transcription was carried out using the PrimeScript RT‐PCR Kit (TaKaRa Biotechnology) based on the instructions of the manufacturer. Quantitative RT‐PCR was undertaken by using the PrimeScript RT‐PCR Kit according to the procedures of specification. Sequences of the PCR primers were as follows: MDR1, 5′‐AACGGAAGCCAGAACATTCC‐3′ and 5′‐AGGCTTCCTGTGGCAAAGAG‐3′; CD133, 5′‐TTCTATGCTGTGTCCTGGGGC‐3′ and 5′‐TTGTTGGTGCAAGCTCTTCAAGGT‐3′; and GAPDH, 5′‐CTCCATCCTGGCCTCGCTGT‐3′ and 5′‐GCTGTCACCTTCACCGTTCC‐3′.
2.6. Luciferase activity assay
The cells (2 × 104) were cotransfected with 500 ng plasmid containing the MDR1 promoter (Plasmid #37627; Addgene) with or without BU treatment. A luciferase activity assay was carried out 48 hours after transfection with the dual‐luciferase reporter assay system (Promega).
2.7. Western blot analysis
Proteins were resolved using SDS/PAGE and subjected to immunoblot analysis with specific Abs (Cell Signaling Technology). All Abs were used at a 1 mg/mL working concentration. The membranes were further probed with HRP‐conjugated rabbit anti‐mouse IgG (Santa Cruz Biotechnology). Quantification of protein bands was undertaken using ImageJ software (Wayne Rasband, National Institutes of Health).
2.8. In vivo xenograft model
LoVoCD133+ or LoVo/ADR colon cancer cells were injected into the flanks of male athymic nude mice (4‐5 weeks old). Two weeks after injection, DOX (0.5 mg/kg) or bufalin (0.1 mg/kg) was given by i.p. injection 5 days per week for 4 weeks. Tumor volumes were measured at the beginning of the treatment and every 4 days until the mice were killed. The estimated tumor volumes (V) were calculated by the formula V = W2 × L × 0.5, where W represents the largest tumor diameter in centimeters and L represents the next largest tumor diameter. Tumors were dissected out and weighed. Harvested tumors were weighed and immediately fixed in formalin for IHC.
All proposals were approved and supervised by the institutional animal care and use committee of Putuo Hospital, Shanghai University of Traditional Chinese Medicine, China. All animal studies were carried out in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals.
2.9. Immunohistochemistry
Tissues were fixed in 10% formalin, embedded in paraffin, and sectioned (5‐mm thickness). For IHC of TUNEL, CD133 and P‐gp was carried out. For quantifications, positive expression cells of 30 random images (400×) per experimental group were captured with a microscope (Leica).
2.10. Statistical analysis
Each experimental value was expressed as the mean ± SD. Statistical analysis was carried out using a t test to evaluate the significance of differences between cell line groups, with significance accepted at *P < .05 and **P < .01. All data points represent the mean value of triplicate measurements. Statistical analysis of tissue samples was undertaken using the Spearman’s rank statistical test and the Mann‐Whitney test to evaluate the significance of differences between groups.
3. RESULTS
3.1. Bufalin reverses MDR in ADR‐resistant CRC cells
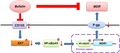
We treated ADR‐resistant CRC cells with BUF (20 nmol/L). The results showed that BU could significantly increase the sensitivity of ADR‐resistant cells to DOX, MMC, VCR, and CTX and remarkably reduce the corresponding IC50 values (Figure 1A,B). The DOX uptake experiment showed that BU significantly enhanced the concentration of intracellular DOX in ADR‐resistant cells (Figure 1C,D). The detection of CD133 by immunofluorescence was decreased in ADR‐resistant cells (Figure 1E) after BU treatment. Moreover, BU significantly decreased CD133 and MDR1/P‐gp expression in those cells (Figure 1F). Taken together, these results suggest that BU could reverse MDR in ADR‐resistant cells by inhibiting both CD133 and P‐gp.
Figure 1.
Bufalin reverses MDR in adriamycin (ADR)‐resistant colorectal cancer cells. A, B, Bufalin (BU) significantly enhanced the sensitivity of LoVo/ADR and HCT8/ADR cells to doxorubicin (DOX), mitomycin C (MMC), vincristine (VCR), and cyclophosphamide (CTX) and significantly reduced the corresponding IC50 values based on a CCK‐8 assay. C, D, BU significantly enhanced the intracellular concentration of DOX in LoVo/ADR and HCT8/ADR cells. E, BU significantly decreased CD133 expression in LoVo/ADR and HCT8/ADR cells by immunofluorescence. F, BU significantly decreased CD133 and P‐gp expression in LoVo/ADR and HCT8/ADR cells by western blotting. *P < .05, **P < .01. Each bar represents the mean ± SD of 3 independent experiments
3.2. Multidrug resistance correlates with CSC properties
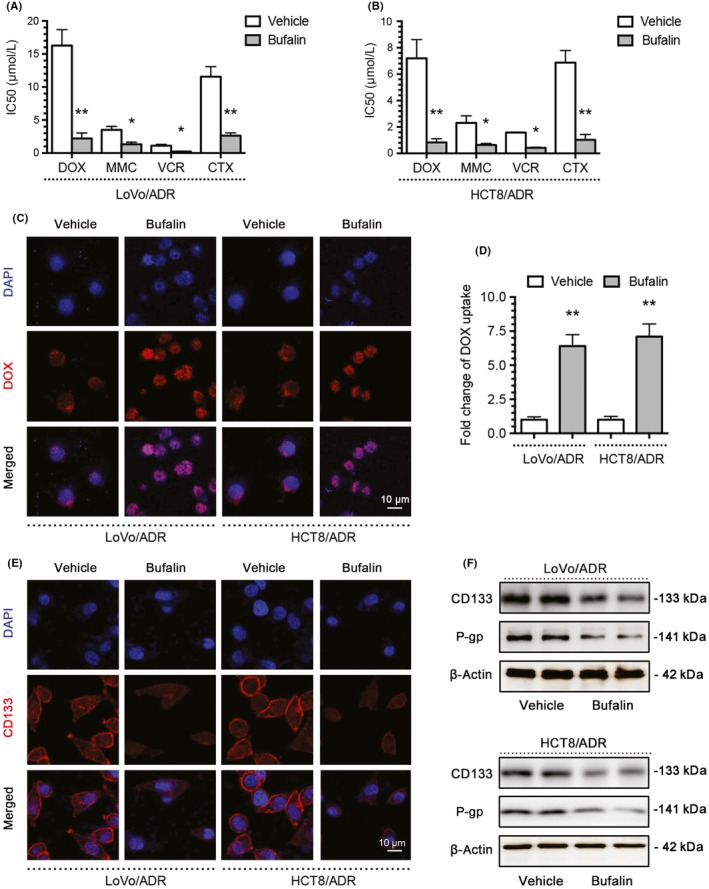
We generated CD133‐OE LoVoCD133+ and HCT8CD133+ CRC cells by a lentivirus vector system. The high levels of CD133 in LoVoCD133+ and HCT8CD133+ cells were also confirmed by flow cytometry, immunofluorescence, qPCR, and western blotting (Figure 2A,B,G,H). Compared to their respective control cells, LoVoCD133+ and HCT8CD133+ cells displayed significant resistance to chemotherapeutic agents (DOX, MMC, VCR, and CTX) (Figure 2C,D) and markedly inhibited DOX uptake (Figure 2E,F). Notability, MDR1/P‐gp mRNA and protein levels were clearly increased in CD133‐OE cells (Figure 2G,H). The above‐mentioned studies indicated that CD133‐OE cells possess MDR and features similar to CSCs; in other words, MDR is related to CSC characteristics.
Figure 2.
Multidrug resistance correlates with cancer stem cell properties. A, B, CD133 expression in LoVo and HCT8 colorectal cancer cells and CD133‐overexpressing LoVoCD133+ and HCT8CD133+ cells was determined by flow cytometry and immunofluorescence (blue, DAPI; red, CD133). C, D, IC50 values of doxorubicin (DOX), mitomycin C (MMC), vincristine (VCR), and cyclophosphamide (CTX) in LoVo, HCT8, LoVoCD133+, and HCT8CD133+ cells were determined with a CCK‐8 assay. E, Intracellular distribution of DOX (red) in LoVo, HCT8, LoVoCD133+, and HCT8CD133+ cells 6 h after a 1‐h incubation with 1 μg/mL DOX. F, Quantitative DOX profiles over 6 h. G, H, CD133 and MDR1/P‐gp expression in LoVo, HCT8, LoVoCD133+, and HCT8CD133+ cells was determined by quantitative PCR and western blotting. *P < .05, **P < .01. Each bar represents the mean ± SD of 3 independent experiments
3.3. Bufalin reverses CD133‐associated MDR
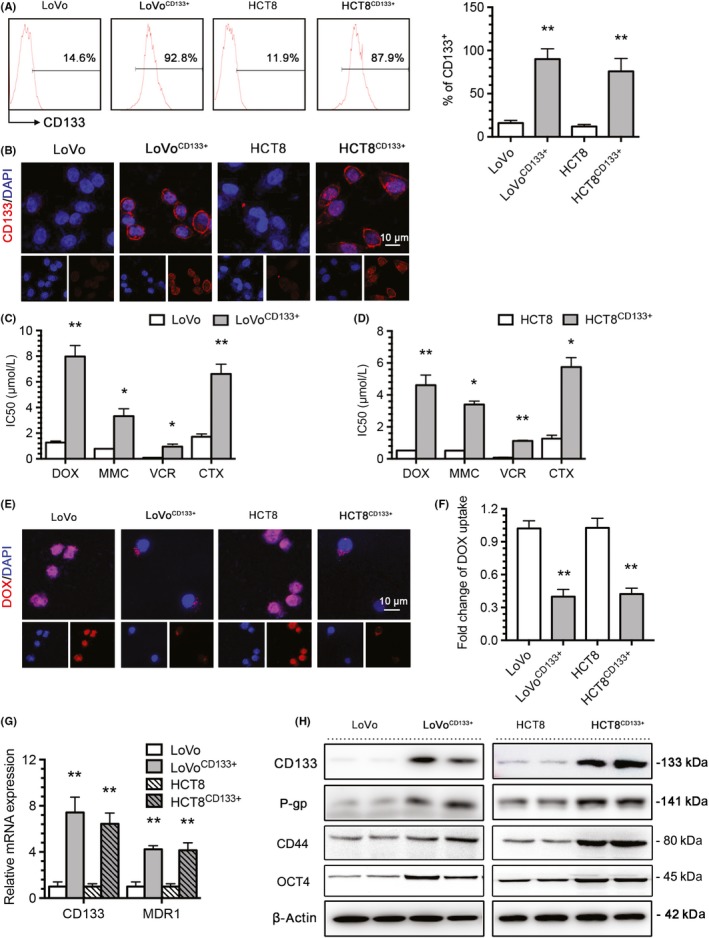
To determine the relationship between the stemness protein CD133 and the MDR protein MDR1/P‐gp in the BU‐mediated reversal of MDR in CRC, we treated CD133‐OE cells with BU. The results showed that BU could significantly increase the sensitivity of CD133 cells to DOX, MMC, VCR, and CTX and remarkably reduce the corresponding IC50 values (Figure 3A,B). One of the important indicators of tumor cell sensitivity to chemotherapy is induced apoptosis; thus, we detected the apoptotic capacity of LoVoCD133+ and HCT8CD133+ cells by flow cytometry. After 48 hours of treatment, the BU group displayed greater sensitivity to DOX (Figure 3C). The DOX uptake experiment showed that BU significantly enhanced the concentration of intracellular DOX in CD133‐OE cells (Figure 3D,E). The detection of CD133 by immunofluorescence was decreased in both CD133‐OE cell lines (Figure 3F) after BU treatment. Moreover, BU significantly decreased CD133 and MDR1/P‐gp expression in those cells (Figure 3G). Taken together, these results suggest that BU could reverse CD133‐associated MDR in CRC.
Figure 3.
Bufalin (BU) reverses CD133‐associated MDR. A, B, BU significantly enhanced the sensitivity of LoVoCD133+ and HCT8CD133+ cells to doxorubicin (DOX), mitomycin C (MMC), vincristine (VCR), and cyclophosphamide (CTX) and significantly reduced the corresponding IC50 values based on a CCK‐8 assay. C, BU significantly enhanced the apoptosis level of LoVoCD133+ and HCT8CD133+ cells in response to DOX, as indicated by an annexin V‐PI assay. D, E, BU significantly enhanced the intracellular concentration of DOX. F, BU significantly decreased CD133 expression in LoVoCD133+ and HCT8CD133+ cells by immunofluorescence. G, BU significantly decreased CD133 and P‐gp expression in LoVoCD133+ and HCT8CD133+ cells by western blotting. *P < .05, **P < .01. Each bar represents the mean ± SD of 3 independent experiments
3.4. Bufalin regulates MDR1/P‐gp expression through CD133
Protein kinase B signal transduction is related to MDR1 expression. In addition, CD133 activates the Akt pathway in CD133+ glioma stem cells. Therefore, we hypothesized that CD133 regulates MDR1 through the Akt‐NF‐κB axis, whereas BU regulates the expression of MDR1/P‐gp through the Akt‐NF‐κB axis. To test this hypothesis, we treated LoVoCD133+, HCT8CD133+, LoVo/ADR, and HCT8/ADR cells with BU. The qPCR results showed that MDR1 mRNA expression was inhibited by BU (Figure 4A,B), and MDR1 gene promoter activity was downregulated in a luciferase reporter assay (Figure 4C,D). Moreover, the western blot results showed that CD133, p‐Akt, p‐NF‐κB/p65, and P‐gp levels were all decreased in whole cells after BU treatment, and total NF‐κB/p65 and p‐NF‐κB/p65 levels were decreased in the nucleus (Figure 4E,F). Immunofluorescence showed that NF‐κB localized to the cytoplasm after BU or CD133 KD plasmid treatment (Figure 4G,H). The above studies showed that BU could decrease MDR1/P‐gp expression by inhibiting CD133 expression, AKT phosphorylation, NF‐κB/p65 nuclear translocation, and MDR1 translation in CD133‐OE cells and ADR‐resistant cells.
Figure 4.
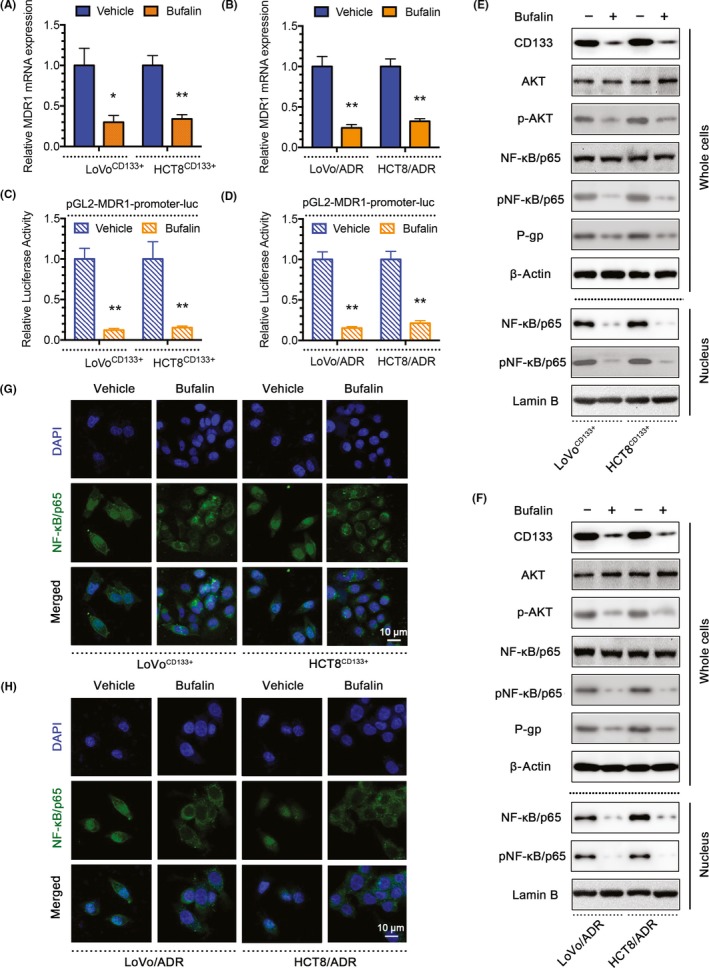
Bufalin (BU) regulates MDR1/P‐gp expression through CD133. A, B, Quantitative PCR showing MDR1 mRNA expression in LoVoCD133+, HCT8CD133+, LoVo/ADR, and HCT8/ADR cells treated with BU. C, D, Luciferase reporter assay showing MDR1 gene promoter activity in LoVoCD133+, HCT8CD133+, LoVo/ADR, and HCT8/ADR cells treated with BU. E, F, Western blots showing protein profiles of CD133, total Akt, p‐Akt, total nuclear factor (NF)‐κB/p65 (whole cells or nucleus), p‐NF‐κB/p65 (whole cells or nucleus), and P‐gp in LoVoCD133+, HCT8CD133+, LoVo/ADR, and HCT8/ADR cells treated with BU. G, H, Immunofluorescence showing the localization of NF‐κB/p65 in LoVoCD133+ and HCT8CD133+ cells treated with BU. *P < .05, **P < .01. Each bar represents the mean ± SD of 3 independent experiments
3.5. Protein kinase B‐NF‐κB signaling pathway is the crucial mechanism by which BU regulates MDR1/P‐gp expression through CD133
To investigate whether the Akt‐NF‐κB signaling pathway is the crucial mechanism by which BU regulated MDR1/P‐gp expression through CD133, we undertook a rescue experiment with the Akt OE plasmid or the NF‐κB/p65 OE plasmid. The protein and mRNA level of MDR1/P‐gp induced by BU were restored by Akt or NF‐κB/p65 (Figure 5A‐C). The same factors restored MDR1 gene promoter activity, as indicated by luciferase reporter assay (Figure 5D). Moreover, Akt or NF‐κB/p65 OE significantly decreased DOX uptake (Figure 5E,F). Finally, the CCK‐8 assay results showed that the Akt OE plasmid or the NF‐κB/p65 OE plasmid could reverse the enhanced sensitivity of LoVoCD133+ and LoVo/ADR cells after treatment with BU (Figure 5G). These results suggest that BU regulates the expression of MDR1/P‐gp through CD133, and the Akt‐NF‐κB signaling pathway plays an important role in this process.
Figure 5.
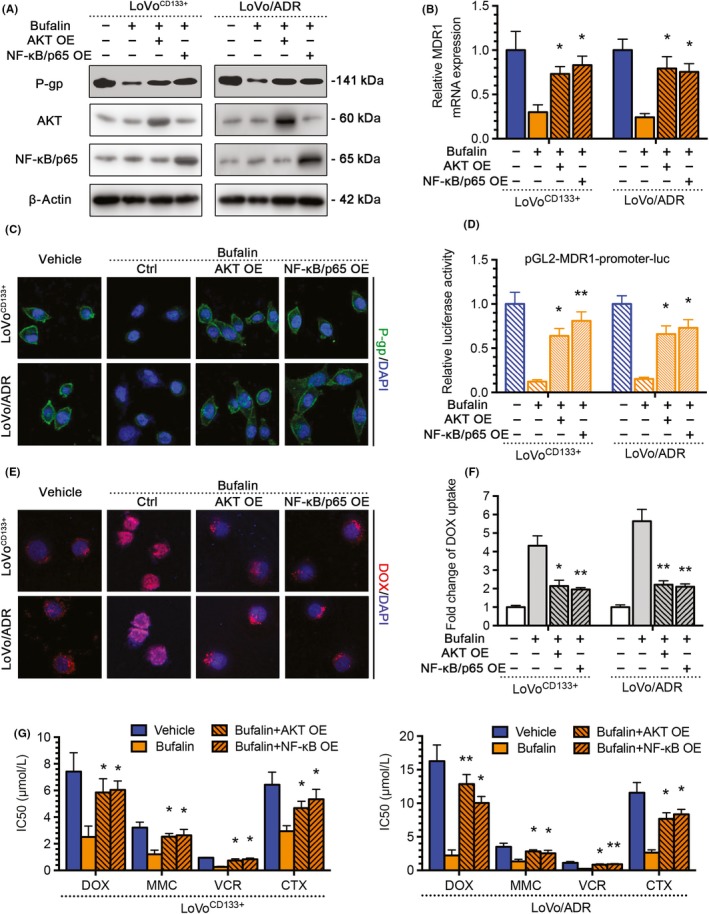
Akt‐nuclear factor (NF)‐κB signaling pathway is the crucial mechanism by which bufalin (BU) regulates MDR1/P‐gp expression through CD133. The AKT overexpressing (OE) plasmid or the NF‐κB/p65 OE plasmid could reverse the effects of BU or the CD133 KD plasmid in LoVoCD133+ and LoVo/ADR cells. A, Reversal of reduced P‐gp expression in western blots. B, Reversal of reduced MDR1 mRNA expression by quantitative PCR. C, Reversal of reduced P‐gp expression by immunofluorescence. D, Reversal of reduced MDR1 gene promoter activity by a luciferase reporter assay. E, F, Reversal of enhanced intracellular doxorubicin (DOX) concentrations. G, Reversal of enhanced sensitivity in CCK‐8 assay. *P < .05, **P < .01. Each bar represents the mean ± SD of 3 independent experiments
3.6. Bufalin increases antitumor effects of DOX in vivo
We observed that BU could reverse CD133‐associated MDR in CRC cells in vitro. We used in vivo experiments to verify this finding. We established a xenograft model of colon tumors by s.c. inoculation of 1 × 106 ADR‐resistant LoVo/ADR cells or 1 × 106 CD133‐OE LoVoCD133+ cells into nude mice. After 2 weeks, the mice were randomly divided into 4 groups (control group, BU group, DOX group, and BU + DOX group). After 4 weeks of treatment, tumor growth and therapeutic sensitivity were monitored until the mice were killed.
After measuring the tumor weight, we plotted a growth curve of the transplanted tumors. The results showed that BU significantly increased the sensitivity of LoVo/ADR cells and LoVoCD133+ cells to DOX (Figure 6A,B), and the tumor size and tumor weight showed similar results, as expected (Figure 6C). As shown in Figure S1, none of the test subjects lost body weight or died in any of the 4 groups at the doses tested, suggesting minimal toxicities. Moreover, the qPCR results showed that MDR1 mRNA expression was inhibited by BU, as observed in vitro (Figure 6D). To assess whether BU sensitized the tumors to DOX and induced tumor growth regression through the CD133/P‐gp pathway in vivo, representative samples from harvested tumor tissues were analyzed by IHC for active CD133 or P‐gp, as described previously. Consistent with our in vitro observations, a discernible decline was observed in tumor tissues after BU treatment, and BU significantly increased the apoptotic effect of DOX in a TUNEL assay (Figure 6E,F). The positive staining rates of CD133, P‐gp, and TUNEL based on IHC are shown (Figure S2). These data indicated that BU could enhance the antitumor effect of chemotherapy agents through CD133/MDR1 in vivo.
Figure 6.
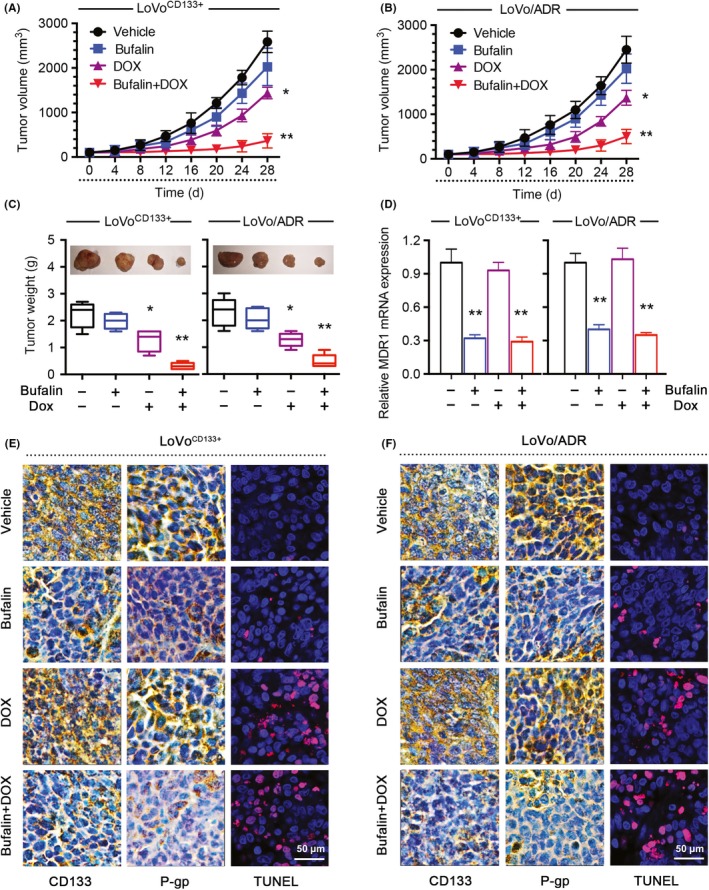
Bufalin (BU) increased the antitumor effects of doxorubicin (DOX) in vivo. BU increased the effectiveness of DOX in the inhibition of tumor growth in vivo. A, B, Xenograft tumor growth curves. C, Photographs of tumors and tumor weights. D, BU decreased MDR1 mRNA expression in vivo. E, F, BU significantly increased apoptosis in response to DOX, as indicated by TUNEL, and reduced CD133 and P‐gp expression levels in vivo. Images are representative of multiple fields of tumor sections from each group. Percentage of cells with positive TUNEL, CD133, and P‐gp staining for quantifications, positive expression cells of 30 random images (400×) per experimental group were captured with a microscope (Leica). *P < .05, **P < .01. Each bar represents the mean ± SD of 3 independent experiments
3.7. Discussion
Multidrug resistance of tumor cells contributes to severe clinical restrictions in CRC. An additional limitation is the fact that many available drugs target the quickly dividing cells and not the CSCs. 24 Bufalin was shown to have reversed of MDR in various tumors, such as leukemia cells, 25 hepatocellular carcinoma, 26 myeloma, 27 and colorectal cancer. 18 However, the precise underlying mechanisms remain unknown, and more efforts should be directed toward clarifying its effects on CRCs.
Prior studies have shown that BU can inhibit CSCs or stemness in different cancer cells. 15 Meng et al reported that BU suppressed stemness through the Hedgehog signaling pathway in gemcitabine‐resistant pancreatic cancer cells. 28 Chang et al found that BU was capable of inhibiting the proliferation and differentiation of CSCs in human osteosarcoma cells. 16 CD133 has been identified as a cell surface marker for CRC. 29 , 30 Evidence suggests that CD133 regulates tumor biology including drug resistance. El‐Khattouti et al found that CD133+ melanoma cells showed increased drug resistance to paclitaxel compared with CD133− cells. 31 Another study found that CD133 was capable of regulating MDR of glioma cells. 32
In our previous study, we first identified the ability of BU to reverse acquired cisplatin resistance by inhibiting stemness in CRC. 19 Therefore, we postulated that the mechanism by which BU inhibits MDR1/P‐gp expression might be related to the stemness of CRC. In this study, our results revealed that BU could decrease both CD133 and MDR1/P‐gp expression and increase chemosensitivity to DOX in CD133+ cells.
Furthermore, we examined the potential interaction of CD133 and MDR1/P‐gp, which contributed to BU‐mediated MDR reversal in CRC. The current work found that BU‐mediated inhibition of MDR1/P‐gp was regulated by CD133 through PI3K‐Akt‐NF‐κB signaling. In addition, silencing CD133 reversed chemoresistance in DOX‐resistant cells and increased AKT‐ and NF‐κB‐induced drug resistance, confirming that these molecules are relevant for chemotherapy and play roles in this process. In vivo, BU significantly increased the effect of DOX against LoVo/ADR and LoVoCD133+ colorectal cancer cell xenografts in nude mice, decreasing the expression of MDR1/P‐gp and CD133.
In summary, we identified that BU reverses CRC MDR by regulating cancer cell stemness through the CD133/NF‐κB/MDR1 pathway in vitro and in vivo. Therefore, BU could be developed as a novel 2‐pronged drug that targets CD133 and MDR1/P‐gp to eradicate CD133+ drug‐resistant cells and could ultimately be combined with conventional chemotherapeutic agents to improve treatment outcomes for patients with CRC.
CONFLICT OF INTEREST
There are no conflicts of interest.
Supporting information
FigureS1‐S2
ACKNOWLEDGMENTS
This project was sponsored by the National Nature Science Foundation of China (81873137, 81603502, 81703885), the Shanghai Rising‐Star Program (17QA1403400), and The Budget project of Shanghai University of Traditional Chinese Medicine (2019LK039). This project was also sponsored by “The twelfth five year” key subject of traditional Chinese medicine of State Administration of traditional Chinese medicine and the Action Plan of Shanghai Municipality for Further Accelerating the Development of Traditional Chinese Medicine [ZY(2018‐2020)‐RCPY‐2016].
Zhan Y, Qiu Y, Wang H, et al. Bufalin reverses multidrug resistance by regulating stemness through the CD133/nuclear factor‐κB/MDR1 pathway in colorectal cancer. Cancer Sci. 2020;111:1619–1630. 10.1111/cas.14345
Yueping Zhan, Yanyan Qiu, and Haijing Wang contributed equally.
Contributor Information
Zeting Yuan, Email: yuan340202@163.com.
Ke Xu, Email: kexu2577@shutcm.edu.cn.
Peihao Yin, Email: yinpeihao@shutcm.edu.cn.
REFERENCES
- 1. Van der Jeught K, Xu HC, Li YJ, Lu XB, Ji G. Drug resistance and new therapies in colorectal cancer. World J Gastroenterol. 2018;24:3834‐3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baguley BC. Multiple drug resistance mechanisms in cancer. Mol Biotechnol. 2020;46:308‐316. [DOI] [PubMed] [Google Scholar]
- 3. Lage H. An overview of cancer multidrug resistance: a still unsolved problem. Cell Mol Life Sci. 2008;65:3145‐3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Palomeras S, Ruiz‐Martinez S, Puig T. Targeting breast cancer stem cells to overcome treatment resistance. Molecules. 2018;23(9):2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fesler A, Guo S, Liu H, Ju J, Wu N. Overcoming chemoresistance in cancer stem cells with the help of microRNAs in colorectal cancer. Epigenomics. 2017;9(6):793‐796. [DOI] [PubMed] [Google Scholar]
- 6. Guo P, Wang J, Gao W, et al. Salvianolic acid B reverses multidrug resistance in nude mice bearing human colon cancer stem cells. Mol Med Rep. 2018;18:1323‐1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang Q, Feng Y, Kennedy D. Multidrug‐resistant cancer cells and cancer stem cells hijack cellular systems to circumvent systemic therapies, can natural products reverse this? Cell Mol Life Sci. 2017;74:777‐801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yin P‐H, Liu X, Qiu Y‐Y, et al. Anti‐tumor activity and apoptosis‐regulation mechanisms of bufalin in various cancers: new hope for cancer patients. Asian Pac J Cancer Prev. 2012;13:5339‐5343. [DOI] [PubMed] [Google Scholar]
- 9. Liu F, Tong D, Li H, et al. Bufalin enhances antitumor effect of paclitaxel on cervical tumorigenesis via inhibiting the integrin α2/β5/FAK signaling pathway. Oncotarget. 2016;7:8896‐8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang Z, Yang Y, Wu W. Bufalin attenuates the stage and metastatic potential of hepatocellular carcinoma in nude mice. J Transl Med. 2014;12(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yuan Z, Shi X, Qiu Y, et al. Reversal of P‐gp‐mediated multidrug resistance in colon cancer by cinobufagin. Oncol Rep. 2017;37:1815‐1825. [DOI] [PubMed] [Google Scholar]
- 12. Feng Y, Chen Y, Meng Y, et al. Bufalin suppresses migration and invasion of hepatocellular carcinoma cells elicited by poly (I:C) therapy. Oncoimmunology. 2018;7:e1426434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Y, Tian X, Liu X, Gong P. Bufalin inhibits human breast cancer tumorigenesis by inducing cell death through the ROS‐mediated RIP1/RIP3/PARP‐1 pathways. Carcinogenesis. 2018;39:700‐707. [DOI] [PubMed] [Google Scholar]
- 14. Zhai B, Hu F, Yan H, et al. Bufalin reverses resistance to sorafenib by inhibiting Akt activation in hepatocellular carcinoma: the role of endoplasmic reticulum stress. PLoS ONE. 2015;10:e0138485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chang Y, Zhao Y, Gu W, et al. Bufalin inhibits the differentiation and proliferation of cancer stem cells derived from primary osteosarcoma cells through Mir‐148a. Cell Physiol Biochem. 2015;36:1186‐1196. [DOI] [PubMed] [Google Scholar]
- 16. Chang YW, Zhao YF, Zhan HS, Wei XE, Liu TJ, Zheng B. Bufalin inhibits the differentiation and proliferation of human osteosarcoma cell line hMG63‐derived cancer stem cells. Tumour Biol. 2014;35:1075‐1082. [DOI] [PubMed] [Google Scholar]
- 17. Liu J, Zhang Y, Sun S, et al. Bufalin induces apoptosis and improves the sensitivity of human glioma stem‐like cells to temozolamide. Oncol Res. 2019;27:475‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yuan Z, Shi X, Yuan Y, et al. Bufalin reverses ABCB1‐mediated drug resistance in colorectal cancer. Oncotarget. 2017;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sun J, Xu K, Qiu Y, et al. Bufalin reverses acquired drug resistance by inhibiting stemness in colorectal cancer cells. Oncol Rep. 2017;38:1420‐1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang J, Wang W, Cai H, et al. MACC1 facilitates chemoresistance and cancer stem celllike properties of colon cancer cells through the PI3K/AKT signaling pathway. Mol Med Rep. 2017;16:8747‐8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang H, Jia XH, Chen JR, Wang JY, Li YJ. Osthole shows the potential to overcome P‐glycoproteinmediated multidrug resistance in human myelogenous leukemia K562/ADM cells by inhibiting the PI3K/Akt signaling pathway. Oncol Rep. 2016;35:3659‐3668. [DOI] [PubMed] [Google Scholar]
- 22. Wang H, Jia XH, Chen JR, et al. HOXB4 knockdown reverses multidrug resistance of human myelogenous leukemia K562/ADM cells by downregulating P‐gp, MRP1 and BCRP expression via PI3K/Akt signaling pathway. Int J Oncol. 2016;49:2529‐2537. [DOI] [PubMed] [Google Scholar]
- 23. Zhang LL, Mu GG, Ding QS, et al. Phosphatase and Tensin Homolog (PTEN) represses colon cancer progression through inhibiting paxillin transcription via PI3K/AKT/NF‐kappaB pathway. J Biol Chem. 2015;290:15018‐15029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adorno‐Cruz V, Kibria G, Liu X, et al. Cancer stem cells: targeting the roots of cancer, seeds of metastasis, and sources of therapy resistance. Cancer Res. 2015;75:924‐929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Efferth T, Davey M, Olbrich A, Rücker G, Gebhart E, Davey R. Activity of drugs from traditional Chinese medicine toward sensitive and MDR1‐ or MRP1‐overexpressing multidrug‐resistant human CCRF‐CEM leukemia cells. Blood Cells Mol Dis. 2002;28:160‐168. [DOI] [PubMed] [Google Scholar]
- 26. Gu W, Liu L, Fang F, Huang F, Cheng B, Li B. Reversal effect of bufalin on multidrug resistance in human hepatocellular carcinoma BEL‐7402/5‐FU cells. Oncol Rep. 2014;31:216‐222. [DOI] [PubMed] [Google Scholar]
- 27. Fujii E, Inada Y, Kakoki M, et al. Bufalin induces DNA damage response under hypoxic condition in myeloma cells. Oncol Lett. 2018;15:6443‐6449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang H, Ning Z, Li Y, Zhu X, Meng Z. (2016) Bufalin suppresses cancer stem‐like cells in gemcitabine‐resistant pancreatic cancer cells via Hedgehog signaling. Mol Med Rep. 2016;14:1907‐1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen S, Song X, Chen Z, et al. CD133 expression and the prognosis of colorectal cancer: a systematic review and meta‐analysis. PLoS ONE. 2013;8:e56380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang R, Mo D, Wu J, Ai H, Lu Y. CD133 expression correlates with clinicopathologic features and poor prognosis of colorectal cancer patients: an updated meta‐analysis of 37 studies. Medicine. 2018;97:e10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. El‐Khattouti A, Selimovic D, Haïkel Y, Megahed M, Gomez C, Hassan M. Identification and analysis of CD133(+) melanoma stem‐like cells conferring resistance to taxol: an insight into the mechanisms of their resistance and response. Cancer Lett. 2014;343:123‐133. [DOI] [PubMed] [Google Scholar]
- 32. Xi G, Hayes E, Lewis R, et al. CD133 and DNA‐PK regulate MDR1 via the PI3K‐ or Akt‐NF‐κB pathway in multidrug‐resistant glioblastoma cells in vitro. Oncogene. 2016;35:241‐250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FigureS1‐S2


