Abstract
This case series describes subclinical development of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies in some patients and health care workers in a pediatric dialysis unit after contact with a seropositive patient.
Dialysis units are at especially high risk of infectious disease transmission, and concern exists about spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Dialysis units in Wuhan, China, have reported high coronavirus disease 2019 (COVID-19) prevalence, due in part to unique exposure challenges that limit social distancing efforts, including open bay formats and rotating/multiple nursing assignments.1,2 This study describes SARS-CoV-2 seroconversion in patients and health care workers in a pediatric dialysis unit.
Methods
Serial SARS-CoV-2 antibody levels were measured in patients, nurses, physicians, and staff in a freestanding outpatient 5-bed/3–isolation room pediatric hemodialysis unit at Riley Hospital for Children, Indianapolis, Indiana. Hemodialysis occurs during 2 shifts on Monday, Wednesday, and Friday. All patients had temperature and symptoms of COVID-19 screened before entry. Patients wore surgical masks at all times, as did health care workers, who also had temperatures checked before and after shifts.
One week before this study began (day 0; March 25, 2020), a single patient presented with fever and generalized symptoms. A reverse transcriptase–polymerase chain reaction (PCR) test result for SARS-CoV-2 on a nasopharyngeal swab was positive, and results on subsequent swabs remained positive on days 7 and 14 until day 19 (April 11, 2020). The patient was dialyzed in an isolation room on day 0 and thereafter. Serum IgM and IgG levels were measured on sera from whole blood samples from all study participants on days 7, 14, and 21 (April 1, 2020, to April 15, 2020) using SARS-CoV-2 enzyme-linked immunosorbent assays (ELISAs) (#KA5826, Abnova). Confirmatory ELISAs were performed at Mount Sinai Medical Center. Manufacturer’s instructions were followed for ELISAs and confirmatory tests as previously published.3 We determined the threshold for a positive ELISA result at 0.14, a value greater than the mean plus 3 times the standard deviation of negative control, consistent with standard methodology and with serum values of PCR-confirmed positive control patients.4 Participants were considered to have seroconverted if positive for IgM or IgG. The ELISA sensitivity and specificity were not provided by the manufacturer.
All participants (or legal representatives) provided written or verbal consent to participate. Human subjects approval was obtained through the Indiana University institutional review board.
Results
Thirteen patients, 9 dialysis nurses, 2 nurse practitioners, 4 staff, and 10 physicians participated in the study. All participant characteristics and results are presented in the Table. Between day 0 and day 7, 2 health care workers had negative PCR test results despite upper respiratory tract symptoms and fevers. One of these health care workers subsequently seroconverted on day 21 despite 3 negative PCR results. No other study participants had nasopharyngeal testing or symptomatology consistent with COVID-19 before day 7.
Table. Characteristics and Cumulative SARS-CoV-2 Seroconversion for Patients Receiving Dialysis and Health Care Workers.
| Characteristic | No. (%) | |
|---|---|---|
| Patients (n = 13) | Health care workers (n = 25)a | |
| Age, median (range), y | 13 (2-16) | 40.5 (25-61) |
| Male sex | 9 (69) | 3 (12) |
| Serostatus by week 3 | ||
| IgM+ | 2 (15) | 7 (28) |
| IgG+ | 3 (23) | 4 (16) |
| IgM+ or IgG+ | 3 (23) | 11 (44) |
| COVID-19–like symptoms | 1 (8) | 2 (8) |
| Positive PCR (symptomatic)b | 1 (100) | 0 |
| Asymptomatic IgM positive | 1 (8) | 4 (16) |
| Positive PCR (asymptomatic)c | 0 | 1 (25) |
Abbreviations: COVID-19, coronavirus disease 2019; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Health care workers include 9 dialysis nurses, 2 nurse practitioners, 4 staff, and 10 physicians.
PCR testing was performed on patients or health care workers with COVID-19–like symptoms (n = 3).
PCR testing was performed on asymptomatic patients or health care workers with IgM and no IgG (n = 5).
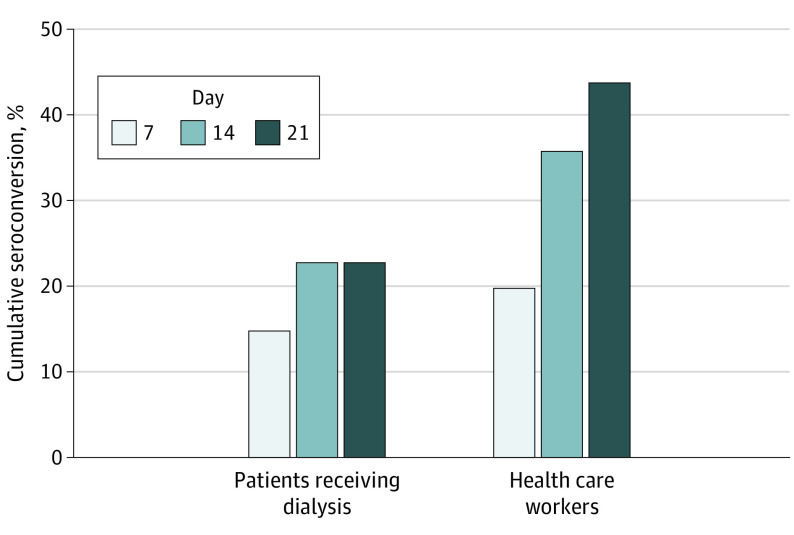
By day 21, 11 of 25 health care workers (44%) and 3 of 13 patients (23%) had positive SARS-CoV-2 antibodies (Figure). No participants developed symptoms between days 7 and 21. No health care workers who directly cared for the PCR-positive patient seroconverted.
Figure. Cumulative Seroconversion (Development of SARS-CoV-2 IgM or IgG Antibodies) Rates by Week of Study in Patients Receiving Dialysis and Health Care Workers.
Individuals were considered seropositive based on the study day in which they were first found to be seropositive for IgM, IgG, or both. SARS-CoV-2 indicates severe acute respiratory syndrome coronavirus 2.
Two of 11 health care workers who cared for 2 patients with subclinical seroconversion developed SARS-CoV-2 antibodies. Both health care workers remained asymptomatic, but one had a positive result on a nasopharyngeal PCR test obtained because of IgM seroconversion.
Discussion
This study found a high prevalence of subclinical seroconversion in individuals interacting in a pediatric dialysis unit. To our knowledge, no other studies of seroconversion in health care settings exist. The 1 symptomatic, PCR-positive patient may have been the source of spread, but other health care environment or community transmission cannot be ruled out. The prevalence of subclinical seroconversion in the health care workers suggests that more health care workers may be antibody-positive than would otherwise be expected. Information on seroprevalence can allow strategically staffing the care of SARS-CoV-2–positive or patients suspected to be positive with seroconverted nurses and physicians. This study has limitations including a small sample size, short follow-up, lack of large-scale sensitivity/specificity of ELISA, lag of antibody positivity from PCR positivity, and the setting of a single pediatric dialysis unit.
Replication in additional sites is needed to define the broad applicability of these findings, as is longer-term follow up to determine the persistence of the antibody response to SARS-CoV-2.
Section Editor: Jody W. Zylke, MD, Deputy Editor.
References
- 1.Su K, Ma Y, Wang Y, et al. How we mitigate and contain COVID-19 outbreak in hemodialysis center (HD): lessons and experiences. Infect Control Hosp Epidemiol. Published online April 23, 2020. doi: 10.1017/ice.2020.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kliger AS, Silberzweig J. Mitigating risk of COVID-19 in dialysis facilities. Clin J Am Soc Nephrol. 2020;15(5):707-709. doi: 10.2215/CJN.03340320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stadlbauer D, Amanat F, Chromikova V, et al. SARS-CoV-2 seroconversion in humans: a detailed protocol for a serological assay, antigen production, and test setup. Curr Protoc Microbiol. 2020;57(1):e100. doi: 10.1002/cpmc.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo L, Ren L, Yang S, et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clin Infect Dis. Published online March 21, 2020. doi: 10.1093/cid/ciaa310 [DOI] [PMC free article] [PubMed] [Google Scholar]