This post hoc analysis of the open-label cohort study Safety Events in Vismodegib reports the outcomes of vismodegib treatment in participants with periocular locally advanced basal cell carcinoma.
Key Points
Question
What were the outcomes of vismodegib treatment for study participants with periocular locally advanced basal cell carcinoma?
Findings
The post hoc analysis of an open-label cohort study of 244 participants revealed that 22 (9.0%) died, 70 (28.7%) achieved complete response, and 94 (38.5%) achieved partial response. Two hundred thirty-two participants (95.1%) had more than 1 adverse event, and vismodegib was discontinued owing to an adverse event in 58 (23.8%).
Meaning
These data may be helpful when considering vismodegib treatment for patients with periocular locally advanced basal cell carcinoma.
Abstract
Importance
The outcomes of vismodegib treatment in a relatively large cohort of study participants with periocular locally advanced basal cell carcinoma (POLA-BCC) may guide physicians when considering this treatment.
Objective
To report the outcomes of vismodegib treatment in patients with POLA-BCC in the Safety Events in Vismodegib (STEVIE) study.
Design, Setting, and Participants
This post hoc subgroup analysis from the STEVIE single-arm, multicenter, open-label cohort study screened all 1215 participants for ocular or periocular involvement and identified 244 participants with POLA-BCC or metastatic BCC. Data for the first STEVIE trial were collected from 167 treatment locations in 36 countries from June 30, 2011, to June 14, 2017. This post hoc analysis was performed from April 1 to August 31, 2019.
Main Outcomes and Measures
Response to treatment and adverse events.
Results
Ocular or periocular involvement was found in 244 of 1215 STEVIE participants (20.1%), who constituted the analytic sample. The median age of the study participants was 72.0 (interquartile range [IQR], 60.0-82.0]) years, and they included 143 men (58.6%). Locally advanced BCC was diagnosed in 238 of the 244 participants (97.5%) and metastatic BCC, in 6 (2.5%). The median duration of exposure to vismodegib was 40.0 (IQR, 20.0-78.0) weeks, specifically 39.7 (IQR, 19.9-76.0) weeks for POLA-BCC and 92.4 (IQR, 53.2-163.0) weeks for metastatic BCC. Sixty-nine participants (28.3%) sustained serious adverse events (alopecia, muscle spasms, dysgeusia, weight loss, decreased appetite, asthenia, ageusia, nausea, fatigue, and diarrhea). Two hundred thirty-two study participants (95.1%) sustained more than 1 adverse effect. The overall mean (SD) number of drug-related adverse effects per study participant by first adverse event, regardless of the severity, was 5.48 (3.84). Discontinuation of vismodegib treatment owing to an adverse event was recorded in 58 participants (23.8%). During the study, 22 participants (9.0%) died, 70 (28.7%) achieved complete response, and 94 (38.5%) achieved partial response.
Conclusions and Relevance
Vismodegib was well tolerated by the study participants with POLA-BCC. The safety of vismodegib treatment according to the STEVIE trial findings is consistent with that reported in previous studies. These data may be helpful when considering vismodegib for patients with POLA-BCC.
Introduction
Basal cell carcinoma (BCC) is the most common cancer worldwide and more common than all other cancers combined.1 About 3.3 million individuals in the United States are diagnosed each year with nonmelanoma skin cancer, and approximately 80% of the lesions are BCC tumors.2 Periocular BCC is diagnosed in 4.4% to 18.0% of all BCCs, accounting for approximately 90% of malignant periocular tumors.3,4 Although most periocular BCCs are curable by surgery, the disease progresses in a minority of patients to a more extensive locally advanced BCC for which surgery and radiotherapy are inappropriate because a cure is unlikely and/or because surgery might result in severe ocular morbidity. Treatment of periocular locally advanced BCC (POLA-BCC) is especially complex because the need to remove periocular or orbital tissues (exenteration or full-thickness eyelid resection) often results in severe disfigurement and poor visual outcome or blindness, leading to a dramatic reduction in functionality and quality of life.5,6
The key molecular driver in the development of BCC is abnormal hedgehog pathway signaling, found in more than 90% of BCCs.7,8 The hedgehog signal transduction pathway is one of the key regulators of cell proliferation and differentiation during embryogenesis, and it plays a major role in ensuring proper embryonic development. In adults, the hedgehog pathway is normally inactive except in stem cells, skin cells, and hair follicles. Studies have shown that aberrant uncontrolled activation of the hedgehog pathway is maintained in 95% of sporadic BCCs as well as several other malignant neoplasms. Activation of the hedgehog pathway eventually leads to the promotion of cell division and tumorigenesis.9
Vismodegib is a first-in-class inhibitor of the hedgehog signaling pathway, approved by the US Food and Drug Administration and the European Medicines Agency for the treatment of adults with metastatic BCC or locally advanced BCC that is inappropriate for surgery or radiotherapy.10,11,12,13,14 The seminal ERIVANCE BCC study demonstrated an overall response rate (ORR) for vismodegib of 60.3%, as assessed by the investigator, in study participants with locally advanced BCC, with a durable response and a consistent efficacy and safety profile.15,16 The Safety Events in Vismodegib trial (STEVIE), a single-arm, multicenter, open-label study, confirmed the safety and efficacy of vismodegib on a much larger patient population in a setting representative of clinical practice.17,18 A total of 1119 study participants with locally advanced BCC treated with vismodegib demonstrated a response rate (investigator-assessed) of 68.5% (33.4% of them with complete response) and a safety profile similar to that of the ERIVANCE study (23.8% with grade ≥3 treatment-emergent adverse events).
Evidence on the efficacy and safety of vismodegib in the management of POLA-BCC is limited to case reports (first reported in 2013) and a few small case series that showed a wide variability of response to treatment (30%-67% of complete clinical response).19,20,21,22,23,24 We now report the outcomes of vismodegib treatment for POLA-BCC in a large population (n = 244) of study participants with POLA-BCC from the STEVIE trial who were followed up for 12 months or longer, making this the largest series, to our knowledge, to be reported. The STEVIE protocol included a subgroup analysis for head tumors, but it was not specific for ocular tumors, which is a substantially different and complicated medical field. The significance of this post hoc analysis is relevant mostly for ophthalmologists because it represents a crucial part of the oculoplastic clinic.
Methods
Study Design and Study Participants
STEVIE is a single-arm, multicenter, open-label study involving 167 treatment locations in 36 countries. The study design and methods were described in detail elsewhere.17,18 The first STEVIE trial was initiated on July 1, 2011. First results were published on May 13, 2015, and the final results were published on June 14, 2017. Our post hoc analysis was initiated April 1 and completed August 31, 2019. Because this is a post hoc analysis without recruitment of new study participants, no approval by our institutional review board was required according to our institutional protocol. Participant consent was also waived by our institutional review board owing to the fact that this is a post hoc analysis without recruitment of new patients. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
The original STEVIE study design did not include ocular tumor involvement as a defined tumor site nor any other specific consideration. Data mining techniques were used to construct an ophthalmic database of the STEVIE study. Ophthalmic involvement was identified by scanning the researcher’s tumor-free text description field for the following specific key words: “eye,” “orbit,” “ophthalmic,” “eyelid,” “canthus,” and “lacrimal.” Due to the nature of the free text description and its variation, each relevant description was assessed individually for the detection of duplication (ie, ≥2 tumors per patient) and then combined. After reviewing all ocular tumor descriptions, a list of study participants with ocular or periocular involvement was constructed, and each ocular tumor was given a study tumor identification number. All relevant tumor descriptions were evaluated by an oculoplastic specialist (I.Y.) for validation. Further assessment according to the Response Evaluation Criteria in Solid Tumors (RECIST) of target lesions (those with the longest diameter and suitability for accurate repeated measurements) vs nontarget lesion (all others) was performed.25 Only study participants with ocular or periocular target lesions were evaluated for demographic features, and statistical comparisons were made among the study participants with locally advanced BCC and those with metastatic BCC.
The best overall response for each patient was identified and extracted for a specific assessment. The overall response was compared between the study participants with and without POLA disease using the χ2 test. An additional analysis identified all adverse events in study participants with ocular and periocular involvement by matching study participants with ocular and periocular involvement in an adverse event data set.
The primary end point of the STEVIE study was safety. Assessments included treatment-emergent adverse events (defined as occurring from the first administration to 30 days after the last administration of the study drug [inclusive] and assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0),26 physical examination, vital signs, Eastern Cooperative Oncology Group performance status, and laboratory parameters. Secondary end points included investigator-assessed objective response according to RECIST, version 1.1,25 duration of response, time from date of earliest response to date of progressive disease or death, time to response (time from date of first treatment to date of first documentation of confirmed complete response or partial response), progression-free survival (time from date of first treatment to date of progression or death), and overall survival (time from date of first treatment to date of death). It is important to state that complete response is a clinical term and does not necessarily mean that the patient has a histologic cure. Study participants with a complete response may still harbor significant subclinical disease.
Statistical Analysis
Study participants with histologically confirmed, measurable BCC who received at least 1 dose of the study drug were included in all analyses. Patient demographics and clinical data were evaluated using descriptive statistics. Overall response rates were compared using χ2 tests or Fisher exact test as appropriate. Survival analysis was achieved by Kaplan-Meier test. All P values were calculated as 2 sided, and P < .05 indicated statistical significance. Statistical analysis was performed using R, version 3.4.2 (R Core Team [2017]) Multiple comparisons were adjusted according to the Bonferroni correction.
Results
Patient Demographics and Characteristics
From June 30, 2011, to June 14, 2017, 1232 study participants were enrolled in the STEVIE trial. At study completion, 1215 study participants had received at least 1 dose of the study drug. Ocular or periocular involvement was identified in 287 study participants, and further screening selected 244 study participants (20.1% of those who received at least 1 dose) with ocular or periocular involvement for analysis (101 women [41.4%] and 143 men [58.6%]; median age 72.0 [interquartile range (IQR), 60.0-82.0] years) (eFigure 1 in the Supplement). Participants had a high level of comorbidities (233 [95.5%]) at baseline, and 64 (26.2%) had undergone radiotherapy. Of those 244 study participants, 238 (97.5%) had locally advanced BCC and 6 (2.5%) had metastatic BCC (Table 1). Most study participants had at least 1 additional nonocular BCC, of which 212 (86.9%) were target lesions and 66 (27.7%) were nontarget lesions. Nineteen participants (7.8%) had another periocular nontarget lesion. The STEVIE study reported an incidence of cutaneous squamous cell carcinoma of 4.20%. In our post hoc subgroup analysis, the incidence of squamous cell carcinoma was 2.5% (6 participants). This analysis is limited by the lack of histological data and a control arm.
Table 1. Baseline Characteristics and Demographics of Study Participants Given the Study Drug.
| Variable | Participant BCC groupa | |
|---|---|---|
| Locally advanced (n = 238) | Metastatic (n = 6) | |
| Sex (%) | ||
| Female | 97 (40.8) | 4 (66.7) |
| Male | 141 (59.2) | 2 (33.3) |
| Age, median (IQR), y | 72.0 (60.0-82.0) | 74.5 (63.0-78.5) |
| Time from initial diagnosis, median (IQR), y | 7.9 (2.5-17.3) | 12.0 (8.6-13.4) |
| Histologically confirmed | ||
| No | 2 (0.8) | 0 |
| Yes | 236 (99.2) | 6 (100) |
| Operability of locally advanced diseaseb | ||
| Inoperable | 91 (38.2) | 0 |
| Surgery medically contraindicated | 147 (61.8) | 0 |
| Surgery unlikely to be curative | 60 (25.2) | 0 |
| Anticipated substantial morbidity and/or deformity from surgery | 98 (41.2) | 0 |
| Administration of radiotherapy to ≥1 lesion | ||
| No | 179 (75.2) | 0 |
| Yes | 58 (24.4) | 6 (100) |
| Reason radiotherapy not administered | ||
| Contraindicated | 78 (32.8) | 0 |
| Inappropriate | 101 (42.4) | 0 |
| Measurable disease | 238 (100) | 6 (100) |
| Gorlin syndrome | ||
| No | 203 (85.3) | 6 (100) |
| Yes | 34 (14.3) | 0 |
Abbreviations: BCC, basal cell carcinoma; IQR, interquartile range.
Unless otherwise indicated, data are expressed as number (percentage) of participants. Percentages may not total 100 owing to missing data.
More than 1 reason was allowed.
Adverse Events
The median duration of exposure to vismodegib was 40.0 (IQR, 20.0-78.0) weeks, specifically, 39.7 (IQR, 19.9-76.0) weeks for locally advanced BCC and 92.4 (IQR, 53.2-163.0) weeks for metastatic BCC. Almost all the study participants (232 [95.1%]) had more than 1 adverse effect of the medication. The overall mean (SD) number of drug-related adverse effects per study participant by first adverse event, regardless of the severity, was 5.48 (3.84). The vismodegib therapy was discontinued owing to an adverse event(s) in 58 participants (23.8%), and most of these adverse events were of mild or moderate severity (43 [17.6%]). The first onset of any adverse event regardless of grade was at a mean of 20.2 (IQR, 8.1-47.0) weeks. Mean time to an adverse event changed when the group was divided according to the 5 adverse event grades of mild (17.6 [IQR, 7.3-43.8] weeks), moderate (26.3 [IQR, 12.0-57.0] weeks), severe (24.1 [IQR, 10.2-65.3] weeks), life threatening (24.1 [IQR, 8.6-36.0] weeks), and death (37.3 [IQR, 15.6-48.1] weeks). The Kaplan-Meier curve (eFigure 2 in the Supplement) demonstrates a trend regarding the time of onset of an adverse event, with study participants on a drug holiday (ie, temporary discontinuation of therapy for ≤8 weeks) typically having an earlier onset of adverse events.
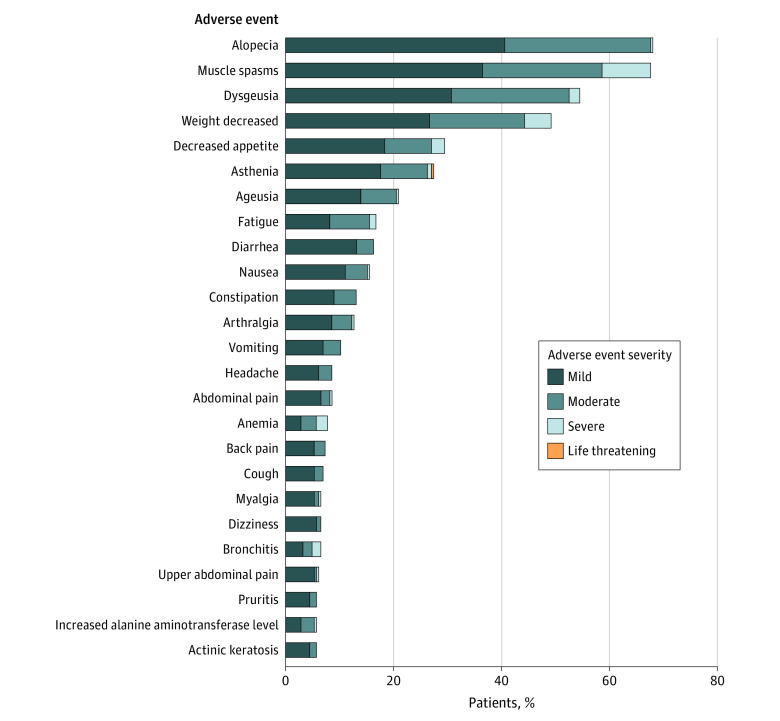
The 10 most common adverse events were alopecia (166 [68.0%]), muscle spasms (165 [67.6%]), dysgeusia (133 [54.5%]), weight loss (120 [49.2%]), decreased appetite (72 [29.5%]), asthenia (67 [27.5%]), ageusia (51 [20.9%]), nausea (38 [15.6%]), fatigue (41 [16.8%], and diarrhea (40 [16.4%]) (Figure and eFigure 3 in the Supplement). Serious adverse events were recorded for 69 of 244 participants (28.3%). During the study period, 22 participants (9.0%) died, including 21 with locally advanced BCC and 1 with metastatic BCC.
Figure. Demonstration of the Top 25 Adverse Events Due to Vismodegib.
The colors indicate the severity of the adverse events, ranging from mild to death.
The 3 main causes of death were an adverse event in 14 participants (5.7%), disease progression in 5 (2.0%), and miscellaneous in 3 (1.2%). Only 2 of the 14 deaths (0.8%) that were registered as resulting from an adverse event were considered related to treatment (myocardial infarction), and the rest were considered to be unrelated.
Efficacy
The median follow-up was 39 (IQR, 23.46-56.28) weeks. Seventy of the 244 study participants (28.7%) had achieved complete response and 94 (38.5%) had achieved partial response, reaching an ORR of 67.2% (complete plus partial responses). Study participants with Gorlin syndrome (n = 34) achieved a better ORR (27 [79.4%]; P = .10). None of the 6 study participants with metastatic BCC achieved a complete response, although 4 of them had a partial response. The number of treatment cycles was obtained for each response. The lowest median number of cycles was 8.50 (IQR, 5.00-18.25), which was recorded for stable disease, and the highest median number of cycles was 17.00 (IQR, 7.00-26.50), which was recorded for progressive disease (Table 2).
Table 2. Best Overall Treatment Response.
| Best overall responsea | Participant BCC group | No. of treatment cycles, median (IQR) | |
|---|---|---|---|
| Locally advanced | Metastatic | ||
| Complete response | 70 | 0 | 13.00 (9.00-23.50) |
| Partial response | 92 | 2 | 10.00 (5.00-18.00) |
| Not evaluable | 6 | 1 | 8.50 (7.25-9.75) |
| Stable disease | 57 | 3 | 8.50 (5.00-18.25) |
| Progressive disease | 5 | 0 | 17.00 (7.00-26.50) |
| Not available | 8 | 0 | 3.00 (2.00-10.00) |
Abbreviations: BCC, basal cell carcinoma; IQR, interquartile range.
Definitions are from the RECIST guideline (version 1.1). Complete response indicates disappearance of all target lesions. Any pathological lymph nodes (whether target or nontarget) must have reduction in short axis to less than 10 mm. Partial response indicates at least a 30% decrease in the sum of diameters of target lesions, taking as reference the baseline sum diameters. Stable disease indicates neither sufficient shrinkage to qualify for partial response nor sufficient increase to qualify for progressive disease, taking as reference the smallest sum diameters during the study. Progressive disease indicates at least a 20% increase in the sum of diameters of target lesions, taking as reference the smallest sum on study must also demonstrate an absolute increase of at least 5 mm.
Discussion
The 244 study participants with POLA-BCC from the STEVIE study provided important data on the possible effects of long-term vismodegib treatment in patients with advanced disease. Previous data were limited to case reports and small case series, with the largest one involving only 21 patients.19,20,21,22,23 The large group described herein establishes a solid base of evidence for future analysis and comparison.
The ORR rate of 67.2% (28.7% with complete response and 38.5% partial response) in our analysis is similar to the results of the seminal ERIVANCE studies and those of the STEVIE study (BCC in all body sites).16,18 Responses rates were comparable between the participants with and without POLA disease in the STEVIE study (P = .46). Several small studies have showed a better ORR for POLA study participants (70%-100% compared with our 67.2% ORR).20,21,22,23 These data are not in agreement with the high success rates published in ophthalmology journals. This difference could be explained by a small sample size (≤21 study participants) compared with the larger sample size of the current study (244 study participants), patient disease characteristics, or differences in treatment protocol: although the STEVIE study followed a strict protocol, some of the smaller studies allowed dose adjustments and/or more drug holidays to improve tolerability and duration of treatment.13 The difference in results demonstrates the importance of relying on data from large prospective studies.
The demographic characteristics of the STEVIE population with POLA-BCC are similar to those of other large studies on locally advanced BCC in all body sites and to the population of study participants who had undergone exenteration before the era of sonic hedgehog inhibitors.3,5,6 The study participants were elderly (median age, 72.0 years) with a tendency toward male sex (58.6%) and a high level of comorbidities (95.5%) at baseline. In addition, 64 of our study participants (26.2%) had undergone radiotherapy for BCC. Our cohort of 244 patients with POLA-BCC most probably accurately represents the population with advanced-stage BCC seen in oculoplastic oncologic clinics.
There is still no uniform definition of the term POLA-BCC accepted by ophthalmologists and nonophthalmologists alike, and the exact definition awaits multidisciplinary team guidelines and adjustment to the American Joint Committee on Cancer 8th edition eye cancer staging system. The fact that 20.1% of all cases of locally advanced BCC in the STEVIE study were POLA-BCC emphasizes the importance of including an ophthalmologist in such a multidisciplinary team.
The outcomes of vismodegib treatment in our POLA-BCC group are consistent with the safety profile described in earlier large analyses of whole-body locally advanced BCC from the ERIVANCE and STEVIE studies and in other studies on vismodegib in study participants with advanced BCC.8,15 Almost all of our study participants (95.1%) had more than 1 adverse event. The overall mean (SD) number of drug-related adverse effects per study participant by first adverse event, regardless of the severity, was 5.48 (3.84). Common adverse events included alopecia, muscle spasms, dysgeusia, weight loss, decreased appetite, asthenia, ageusia, nausea, fatigue, and diarrhea. Vismodegib treatment was withdrawn owing to an adverse event in 58 study participants (23.8%), and most of these adverse events (43 [17.6%]) were of mild or moderate severity. The mean time to adverse events, regardless of their grade, was 20.2 weeks (mild, 17.6 weeks; moderate, 26.3 weeks; severe, 24.1 weeks; life threating, 24.1 weeks; and death, 37.3 weeks).
The use of vismodegib as a neoadjuvant treatment is currently being investigated as a new option for eye-sparing surgery, and several small case series have already been published with variable degrees of success.19,21,23 Optimization between time to adverse events and time to best response is needed to determine the most appropriate time for surgical intervention. The mean time to first moderate or severe adverse event in the current study was 24.1 weeks. Strategies for improving tolerability and delaying the onset of adverse effects, including breaks between treatment cycles and an intermittent treatment protocol, have been explored and may further improve the utility of vismodegib as a neoadjuvant treatment.13,20,22 Data regarding time to best response need further analysis and, when they become available, may help plan the optimal time for surgical intervention after neoadjuvant vismodegib.
Sixty-nine of the 244 study participants (28.3%) sustained serious adverse events, and 22 (9.0%) died during the study period. Of the 14 deaths registered as being due to an adverse event, only 2 (0.8%) were deemed treatment related. This drug-related death rate is very low compared with the natural history of these study participants before the era of sonic hedgehog inhibitors, even with exenteration and radiation.3,5,27 Death due to disease progression (despite treatment) recorded in the present study far exceeded that of death due to drug-related causes.
Limitations
Some limitations to this study bear mention. The main limitations are the known possible biases of a post hoc subgroup analysis.28 We attempted to reduce the number of possible biases by several means. The hypothesis assessment of the subgroup was in line with the original study aim, and this lessens the probability of discovering statistical associations that suggest other causes and effects. Second, to reduce statistical multiplicity, we conducted our statistical analysis only on a specific preplanned subgroup and did not analyze any other group. Third, most known biases of a subgroup analysis apply to randomized clinical trials and less to our original study design, which was an open-label, single-arm study. Finally, the STEVIE study was not designed as an ocular study. Our study database does not include full ocular examinations, and the primary follow-up staff did not include an ophthalmologist, nor did it focus on preservation of vision. The primary end point of this study was the outcome of vismodegib treatment for POLA-BCC tumors based on data from the STEVIE trial. The possibility remains that the optimal treatment for patients with POLA-BCC may include different treatment durations and dose adjustments as well as a combination of adjuvant or neoadjuvant treatments, and those alternatives should be tested in prospective clinical trials.
Conclusions
This exploratory analysis of the periocular cases of the STEVIE trial is, to our knowledge, the largest series of patients with ophthalmic disease and advanced BCC ever reported and provides important data on the possible effects and adverse events of long-term vismodegib treatment. Selecting the patients with ocular disease from the STEVIE study participants with BCC in other sites revealed that vismodegib is tolerable in patients with POLA-BCC, with an associated adverse event profile consistent with that reported in previous studies. Despite a lower treatment response that was documented in the present study compared with previous small ophthalmic studies, it still provides a promising prognosis for patients with ocular and periocular BCC and may be helpful when considering vismodegib for patients with POLA-BCC.
eFigure 1. Trial Profile-Patient Selection Flowchart
eFigure 2. Kaplan-Meier Curves for Time to Adverse Event by Response Group in Accordance With Extended Treatment Breaks
eFigure 3. Demonstration of the Upper 10% of Vismodegib Adverse Events
References
- 1.Rubin AI, Chen EH, Ratner D. Basal-cell carcinoma. N Engl J Med. 2005;353(21):2262-2269. doi: 10.1056/NEJMra044151 [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Key Statistics for Basal and Squamous Cell Skin Cancers Updated January 8, 2020. Accessed January 8, 2020. https://www.cancer.org/cancer/basal-and-squamous-cell-skin-cancer/about/key-statistics.html
- 3.Shi Y, Jia R, Fan X. Ocular basal cell carcinoma: a brief literature review of clinical diagnosis and treatment. Onco Targets Ther. 2017;10:2483-2489. doi: 10.2147/OTT.S130371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu A, Sun MT, Huilgol SC, Madge S, Selva D. Histological subtypes of periocular basal cell carcinoma. Clin Exp Ophthalmol. 2014;42(7):603-607. doi: 10.1111/ceo.12298 [DOI] [PubMed] [Google Scholar]
- 5.Hoffman GR, Jefferson ND, Reid CBA, Eisenberg RL. Orbital exenteration to manage infiltrative sinonasal, orbital adnexal, and cutaneous malignancies provides acceptable survival outcomes: an institutional review, literature review, and meta-analysis. J Oral Maxillofac Surg. 2016;74(3):631-643. doi: 10.1016/j.joms.2015.09.019 [DOI] [PubMed] [Google Scholar]
- 6.Aryasit O, Preechawai P, Hirunpat C, Horatanaruang O, Singha P. Factors related to survival outcomes following orbital exenteration: a retrospective, comparative, case series. BMC Ophthalmol. 2018;18(1):186. doi: 10.1186/s12886-018-0850-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epstein EH. Basal cell carcinomas: attack of the hedgehog. Nat Rev Cancer. 2008;8(10):743-754. doi: 10.1038/nrc2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LoRusso PM, Rudin CM, Reddy JC, et al. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res. 2011;17(8):2502-2511. doi: 10.1158/1078-0432.CCR-10-2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sternfeld A, Rosenwasser-Weiss S, Ben-Yehuda G, et al. Gene-related response of basal cell carcinoma to biologic treatment with vismodegib. Sci Rep. 2020;10(1):1244. doi: 10.1038/s41598-020-58117-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US Food and Drug Administration. 2012. Notifications. Published February 13, 2018. Accessed July 10, 2019. https://www.fda.gov/drugs/resources-information-approved-drugs/2012-notifications
- 11.Unsworth SP, Heisel CJ, Kahana A. A new paradigm in the treatment of advanced periocular basal cell carcinoma? Am J Ophthalmol. 2019;206:215-216. doi: 10.1016/j.ajo.2019.06.027 [DOI] [PubMed] [Google Scholar]
- 12.Dréno B, Kunstfeld R, Hauschild A, et al. Two intermittent vismodegib dosing regimens in patients with multiple basal-cell carcinomas (MIKIE): a randomised, regimen-controlled, double-blind, phase 2 trial. Lancet Oncol. 2017;18(3):404-412. doi: 10.1016/S1470-2045(17)30072-4 [DOI] [PubMed] [Google Scholar]
- 13.Becker LR, Aakhus AE, Reich HC, Lee PK. A novel alternate dosing of vismodegib for treatment of patients with advanced basal cell carcinomas. JAMA Dermatol. 2017;153(4):321-322. doi: 10.1001/jamadermatol.2016.5058 [DOI] [PubMed] [Google Scholar]
- 14.Mortier L, Bertrand N, Basset-Seguin N, et al. Vismodegib in neoadjuvant treatment of locally advanced basal cell carcinoma: first results of a multicenter, open-label, phase 2 trial (VISMONEO study). J Clin Oncol. 2018;36(15 suppl):9509. doi: 10.1200/JCO.2018.36.15_suppl.9509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366(23):2171-2179. doi: 10.1056/NEJMoa1113713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sekulic A, Migden MR, Lewis K, et al. ; ERIVANCE BCC investigators . Pivotal ERIVANCE basal cell carcinoma (BCC) study: 12-month update of efficacy and safety of vismodegib in advanced BCC. J Am Acad Dermatol. 2015;72(6):1021-1026.e8. doi: 10.1016/j.jaad.2015.03.021 [DOI] [PubMed] [Google Scholar]
- 17.Basset-Seguin N, Hauschild A, Grob J-J, et al. Vismodegib in patients with advanced basal cell carcinoma (STEVIE): a pre-planned interim analysis of an international, open-label trial. Lancet Oncol. 2015;16(6):729-736. doi: 10.1016/S1470-2045(15)70198-1 [DOI] [PubMed] [Google Scholar]
- 18.Basset-Séguin N, Hauschild A, Kunstfeld R, et al. Vismodegib in patients with advanced basal cell carcinoma: primary analysis of STEVIE, an international, open-label trial. Eur J Cancer. 2017;86:334-348. doi: 10.1016/j.ejca.2017.08.022 [DOI] [PubMed] [Google Scholar]
- 19.Kahana A, Worden FP, Elner VM. Vismodegib as eye-sparing adjuvant treatment for orbital basal cell carcinoma. JAMA Ophthalmol. 2013;131(10):1364-1366. doi: 10.1001/jamaophthalmol.2013.4430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demirci H, Worden F, Nelson CC, Elner VM, Kahana A. Efficacy of vismodegib (erivedge) for basal cell carcinoma involving the orbit and periocular area. Ophthalmic Plast Reconstr Surg. 2015;31(6):463-466. doi: 10.1097/IOP.0000000000000388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sagiv O, Nagarajan P, Ferrarotto R, et al. Ocular preservation with neoadjuvant vismodegib in patients with locally advanced periocular basal cell carcinoma. Br J Ophthalmol. 2019;103(6):775-780. doi: 10.1136/bjophthalmol-2018-312277 [DOI] [PubMed] [Google Scholar]
- 22.González AR, Etchichury D, Gil ME, del Aguila R. Neoadjuvant vismodegib and Mohs micrographic surgery for locally advanced periocular basal cell carcinoma. Ophthal Plast Reconstr Surg. 2019;35(1):56-61. doi: 10.1097/IOP.0000000000001166 [DOI] [PubMed] [Google Scholar]
- 23.Eiger-Moscovich M, Reich E, Tauber G, et al. Efficacy of vismodegib for the treatment of orbital and advanced periocular basal cell carcinoma. Am J Ophthalmol. 2019;207:62-70. doi: 10.1016/j.ajo.2019.04.013 [DOI] [PubMed] [Google Scholar]
- 24.Yin VT, Pfeiffer ML, Esmaeli B. Targeted therapy for orbital and periocular basal cell carcinoma and squamous cell carcinoma. Ophthalmic Plast Reconstr Surg. 2013;29(2):87-92. doi: 10.1097/IOP.0b013e3182831bf3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz LH, Litière S, de Vries E, et al. RECIST 1.1-update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132-137. doi: 10.1016/j.ejca.2016.03.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0. Published May 28, 2009. Accessed August 15, 2019. https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
- 27.Rasmussen MLR, Ekholm O, Prause JU, Toft PB. Quality of life of eye amputated patients. Acta Ophthalmol. 2012;90(5):435-440. doi: 10.1111/j.1755-3768.2010.02092.x [DOI] [PubMed] [Google Scholar]
- 28.Delgado-Rodríguez M, Llorca J. Bias. J Epidemiol Community Health. 2004;58(8):635-641. doi: 10.1136/jech.2003.008466 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Trial Profile-Patient Selection Flowchart
eFigure 2. Kaplan-Meier Curves for Time to Adverse Event by Response Group in Accordance With Extended Treatment Breaks
eFigure 3. Demonstration of the Upper 10% of Vismodegib Adverse Events