This single-arm, multicenter phase 2 trial investigates the efficacy and safety of low-dose erlotinib in elderly or frail patients with EGFR mutation–positive non–small cell lung cancer.
Key Points
Question
How safe and effective is low-dose erlotinib for elderly or frail patients with epidermal growth factor receptor mutation–positive non–small cell lung cancer?
Findings
In this single-arm, multicenter phase 2 trial of 80 patients with epidermal growth factor receptor mutation–positive non–small cell lung cancer, results showed that the objective response rate of low-dose erlotinib was 60.0%, while the adverse events observed were mild, with few patients exhibiting those of grade 3 or higher.
Meaning
Low-dose erlotinib may be a treatment option for elderly or frail patients with non–small cell lung cancer.
Abstract
Importance
Although the efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for EGFR gene mutation–positive non–small cell lung cancer is well established, optimal dosing remains to be established, especially in elderly or frail patients.
Objective
To investigate the efficacy and safety of low-dose erlotinib in elderly or frail patients with EGFR mutation–positive non–small cell lung cancer.
Design, Setting, and Participants
Single-arm phase 2 trial with the Southwest Oncology Group (SWOG) 2-stage design that enrolled frail patients from 21 Japanese institutions after meeting the inclusion criteria. Chemotherapy-naive patients with EGFR-activating mutation–positive non–small cell lung cancer who were considered frail based on age, the Charlson Comorbidity Index, and Eastern Cooperative Oncology Group performance status were eligible for the study.
Interventions
Patients were initially administered 50 mg/d erlotinib for 4 weeks, which was modified based on response or adverse events. Dose increase was permitted for patients with stable disease after 4 weeks.
Main Outcomes and Measures
The primary end point was the independent review committee–confirmed objective response rate (ORR) at the dose of 50 mg/d. The study also evaluated the pharmacokinetics of low-dose erlotinib and influence of ABCB1 gene polymorphisms.
Results
Eighty patients were enrolled, with a median (range) age of 80 (49-90) years; 54 (68%) were men. An independent review committee confirmed a significant ORR of 60.0% (90% CI, 50.2%-69.2%). The disease control rate was 90.0% (90% CI, 82.7%-94.9%), median progression-free survival was 9.3 months (95% CI, 7.2-11.4 months), and median overall survival was 26.2 months (95% CI, 21.9-30.4 months). Mild adverse events were observed in some participants, with few patients exhibiting grade 3 or greater adverse events. Low-dose erlotinib treatment was temporarily suspended for 10 patients owing to adverse events. Five of 80 patients (6%) had their erlotinib dose reduced to 25 mg because of oral mucositis, paronychia, erythema multiforme, diarrhea, and anorexia. Two patients discontinued treatment because of adverse events (cutaneous ulcer and bone infection, and oral mucositis, respectively). There were no cases of interstitial lung disease or treatment-related deaths. The median (range) erlotinib plasma concentration was measured at 685 (153-1950) ng/mL. Seventy-three patients discontinued study treatment owing to disease progression (n = 60), death (n = 3), AEs (n = 4), and patient requests (n = 6). No clear association was observed between the pharmacokinetics of low-dose erlotinib and the treatment outcome.
Conclusions and Relevance
Low-dose erlotinib appears to be safe and effective in elderly or frail patients with EGFR mutation–positive non–small cell lung cancer and can be a valid treatment option.
Trial Registration
UMIN-CTR Identifier: UMIN000015949
Introduction
Recent advances in molecular-targeted drugs tailored for gene alterations have greatly improved treatment efficacy for non–small cell lung cancer (NSCLC). First-, second-, and third-generation epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) have exhibited efficacy against epidermal growth factor receptor (EGFR) mutation–positive NSCLC, contributing to prolonged patient survival.1,2,3
Most patients enrolled in clinical trials are nonelderly with good performance status (PS), whereas patients with high-risk conditions, such as significant comorbidities, are usually excluded.1,2,3 Previous trials of elderly patients with EGFR mutation–positive NSCLC, including the NEJ0014 and NEJ0035 trials for gefitinib, erlotinib,6 and afatinib,7 have reported favorable results.
Recently, frailty has been shown to play a vital role in determining treatment adaptation and outcome.8 Predictors of frailty are related to complex factors, such as age, comorbidities, and the cancer itself.8 Not all frail patients are elderly, and vice versa, but frailty is substantially correlated with age.9 With the increasing number of elderly patients with cancer, many of whom also have significant comorbidities,10,11 there is a considerable value in investigating whether EGFR-TKIs are effective for the frail population. Furthermore, it is difficult to identify the appropriate dose of molecular-targeted drugs. In fact, several studies have reported that low-dose and standard-dose molecular-targeted drugs were equally effective.12,13
The clinical efficacies of erlotinib and gefitinib are considered equivalent.14 However, the recommended dose of gefitinib, 250 mg/d, is one-third of the maximum tolerated dose (MTD), whereas the approved, recommended dose of erlotinib is set at an MTD of 150 mg/d. If gefitinib 250 mg, which is pharmacologically equivalent to one-third of erlotinib 150 mg, is as effective as erlotinib 150 mg, erlotinib in one-third of the standard MTD dose could maintain sufficient concentration and would also be as effective. A lower dose will lead to reduction of adverse events (AEs) and costs.
Previously, we reported the activity and reduced toxicity of low-dose erlotinib (50 mg/d) in patients with prior systemic treatment for EGFR mutation–positive NSCLC.15 Based on the rationales, we hypothesized that low-dose erlotinib would be safe and effective in elderly or frail patients with EGFR mutation–positive NSCLC. We thus conducted this multicenter phase 2 clinical trial to investigate the efficacy and safety of low-dose erlotinib in the population.
In an additional exploratory investigation, we performed a pharmacokinetics study of erlotinib and its association with ABCB1 gene polymorphisms. Erlotinib is a substrate for ABC transporters, such as ABCB1 (P-glycoprotein) and ABCB2, which play an important role in the pharmacokinetics of several xenobiotics.16 Thus, ABCB1 gene polymorphisms might be associated erlotinib-induced AEs. Our investigation could contribute to a customized dosing strategy in elderly/frail population.
Methods
Patients Selection
This single-arm phase 2 trial with the Southwest Oncology Group (SWOG) 2-stage design enrolled frail patients from 21 Japanese institutions after meeting the inclusion criteria. This study was compliant with the ethical principles of the Declaration of Helsinki. The trial protocol was approved by the institutional review boards of all the participating institutions. All patients provided written informed consent before enrollment and were not compensated for participation. The trial was registered in the UMIN Clinical Trials Registry (UMIN000015949). Patients were selected based on the Charlson Comorbidity Index (CCI), which is widely used to score patients’ physical condition and organ dysfunction. It is a simple tool to assess patient vulnerability.17 We used the age-adjusted CCI (a-CCI) to classify the enrolled elderly/frail patients into 3 cohorts: cohort 1, aged 81 years or older with any a-CCI and PS; cohort 2, aged 75 to 80 years with a-CCI of 6 points or higher and/or PS of 1 or higher; and cohort 3, aged 20 to 74 years with a-CCI of 6 points or higher and/or PS of 2 or greater.
Other eligible criteria included age of 20 years or older; histologically or cytologically diagnosed with EGFR mutation–positive NSCLC; advanced or relapsing diseases without prior systemic treatment; measurable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST); and at least minimally preserved organ function. In anticipation of enrolling patients with complications, we set highly relaxed criteria for organ function. (See the trial protocol in Supplement 1 and eMethods in Supplement 2).
Study Design
Patients received daily administration of low-dose erlotinib (50 mg) for 4 weeks and were evaluated with radiologic imaging. Patients with a complete response (CR) or partial response (PR) continued to receive low-dose erlotinib until progressive disease (PD) or unacceptable AEs, including interstitial lung disease of any grade. Patients with stable disease (SD) received increased erlotinib dosage at the discretion of the attending physicians. There were no posttherapy regulations on for those patients, including erlotinib continuation.
AEs were graded and categorized according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). Dose modifications and discontinuation criteria on AEs are specified in the trial protocol (Supplement 1).
Tumor size reduction was evaluated using RECIST, version 1.1.18 The best tumor responses to the protocol therapy were confirmed by an independent review committee (IRC) comprising 2 radiologists. Follow-up evaluation and monitoring were conducted as prespecified in the trial protocol (Supplement 1). Exploratory study on the pharmacokinetics of erlotinib and the association with ABCB1 gene polymorphisms was performed, as described in the eResults in Supplement 2.
Statistical Analysis
The primary end point was IRC-confirmed objective response rate (ORR) of 50 mg/d erlotinib based on RECIST, version 1.1. The secondary end points were disease control rate, ORR including patients who received increased erlotinib dose after the first 4 weeks, progression-free survival (PFS), overall survival (OS), and patient safety throughout the treatment. Proportions and 90% CIs were calculated for ORR. Definition and analysis of the secondary end points were described in the eMethods (Supplement 2). Patients were observed for at least 18 months after enrollment. Efficacy and safety analyses were conducted on the full analysis set population, which was defined as all enrolled patients excluding those who were found ineligible after enrollment or who did not receive any study treatment.
Multivariate analyses were performed to find the association between clinical factors and patient outcome. Baseline variables, such as age, sex, advanced vs recurrent disease, PS, smoking status, EGFR mutation type, and CCI (not age adjusted), were entered into a logistic regression model for association with clinical response to treatment (CR/PR vs SD/PD); those were also entered into Cox proportional hazards regression models for association with PFS and OS. P values of .05 or less were deemed significant. Data analysis took place from November 2018 to January 2019. Statistical analyses were performed using BellCurve for Excel, version 3.20 (Social Survey Research Information Co, Ltd).
Results
Patient Characteristics
We enrolled 80 patients from 21 Japanese institutions from December 2014 through April 2017. Participants had a median (range) age of 80 (49-90) years; 54 (68%) were men. All patients were eligible and received treatment. Therefore, the full analysis set was identical with the intention-to-treat population. Based on the study hypothesis, if 48 of 80 patients responded, low-dose erlotinib would be considered effective. Prior to evaluation at 4 weeks of treatment, 2 patients died owing to reasons unrelated to treatment (namely, acute myocardial infarction and cerebral aneurysm rupture), and they were included in the efficacy and safety analyses. Patient characteristics are summarized in Table 1. Twenty-five patients (31%) showed PS of 2 or higher, and 37 (46%) patients belonged to cohort 1 (those aged 81 years and older).
Table 1. Patient Characteristics.
| Characteristic | No. (%) | |
|---|---|---|
| All (N = 80) | Erlotinib PC (n = 48) | |
| Age, median (range), y | 80 (49-90) | 80 (54-90) |
| Sex | ||
| Female | 26 (33) | 31 (65) |
| Male | 54 (68) | 17 (35) |
| Pathology | ||
| Adenocarcinoma | 76 (95) | 46 (96) |
| Other | 4 (5) | 2 (4) |
| Stage | ||
| IIIA | 2 (3) | 1 (2) |
| IIIB | 2 (3) | 1 (2) |
| IV | 51 (64) | 30 (63) |
| Recurrence | 25 (31) | 16 (33) |
| ECOG performance status | ||
| 0 | 9 (11) | 5 (10) |
| 1 | 46 (58) | 30 (63) |
| 2 | 15 (19) | 7 (15) |
| 3 | 7 (9) | 3 (6) |
| 4 | 3 (4) | 3 (6) |
| Smoking history | ||
| Yes | 26 (33) | 13 (27) |
| No | 54 (68) | 35 (73) |
| EGFR mutation | ||
| Exon 19 | 42 (53) | 23 (48) |
| Exon 21 | 38 (48) | 25 (52) |
| Cohorta | ||
| 1 | 37 (46) | 22 (46) |
| 2 | 28 (35) | 19 (40) |
| 3 | 15 (19) | 7 (15) |
Abbreviations: EGFR, epidermal growth factor receptor; PC, plasma concentration.
The enrolled patients were grouped as follows: cohort 1, aged ≥81 years; cohort 2, aged 75-80 years and age-adjusted Charlson Comorbidity Index (a-CCI) ≥ 6 points or PS ≥ 1; and cohort 3, aged 20-74 years and a-CCI ≥ 6 points or PS ≥ 2.
Efficacy
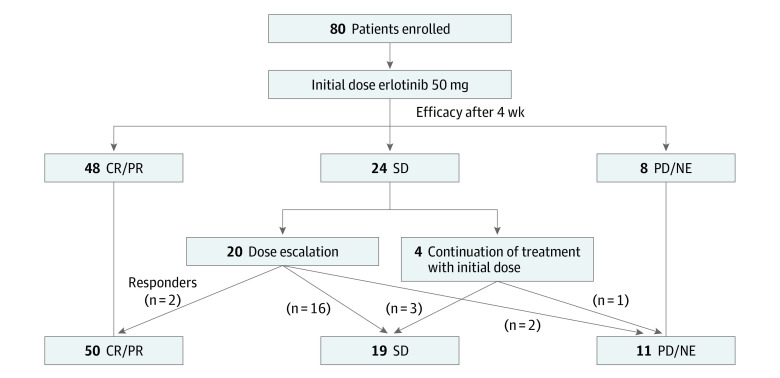
Efficacy evaluation was performed at each institution, and patients who were determined to be responders were confirmed by the IRC (Table 2). The ORR was 60.0% (90% CI, 50.2%-69.2%), and the study met its predefined criteria of efficacy. The disease control rate was 90.0% (90% CI, 82.7%-94.9%). Figure 1 shows the dose increase implementation for nonresponders at 4 weeks of treatment. The overall ORR and disease control rate for the study period were 62.5% (90% CI, 52.7%-71.6%) and 86.3% (90% CI, 78.3%-92.1%), respectively. The ORRs for the 3 cohorts were 57% (cohort 1, 21 of 37), 71% (cohort 2, 20 of 28), and 47% (cohort 3, 7 of 15), without statistically significant differences (P = .27 [Fisher exact test]). At the median follow-up period of 28.7 months, the median PFS was 9.3 months (95% CI, 7.2-11.4 months) (Figure 2A), and the median OS was 26.2 months (95% CI, 21.9-30.4 months) (Figure 2B). The PFS and OS of each cohort are shown in Figures 2C and 2D, respectively.
Table 2. Tumor Response.
| Response | No. (%) | ||
|---|---|---|---|
| All (N = 80) | PC (n = 48), low-dose erlotinib [50 mg] | ||
| Low-dose erlotinib (50 mg) | All erlotinib dosesa | ||
| Response | |||
| Complete | 1 (1)b | 1 (1) | 1 (2) |
| Partial | 47 (59)b | 49 (61) | 30 (63) |
| Disease | |||
| Stable | 24 (30) | 19 (24) | 11 (23) |
| Progressive | 5 (6) | 8 (10) | 3 (6) |
| Not evaluable | 3 (4) | 3 (4) | 3 (6) |
| Rate, No. (%) [90% CI] | |||
| Overall response | 48 (60) [50.2-69.2]b | 50 (62.5) [52.7-71.6] | 31 (64.6) [51.7-76.0] |
| Disease control | 72 (90) [82.7-94.9] | 69 (86.3) [78.3-92.1] | 42 (87.5) [76.8-94.4] |
Abbreviation: PC, plasma concentration.
A dose increase was implemented for the 20 nonresponders following 4 weeks of treatment (100 mg, n = 17; 150 mg, n = 3).
Confirmed by the independent review committee.
Figure 1. Flow Diagram for Efficacy of the Study.
CR indicates complete response; NE, not evaluated; PD, progressive disease; PR, partial response; SD, stable disease.
Figure 2. Progression-Free Survival (PFS) and Overall Survival .
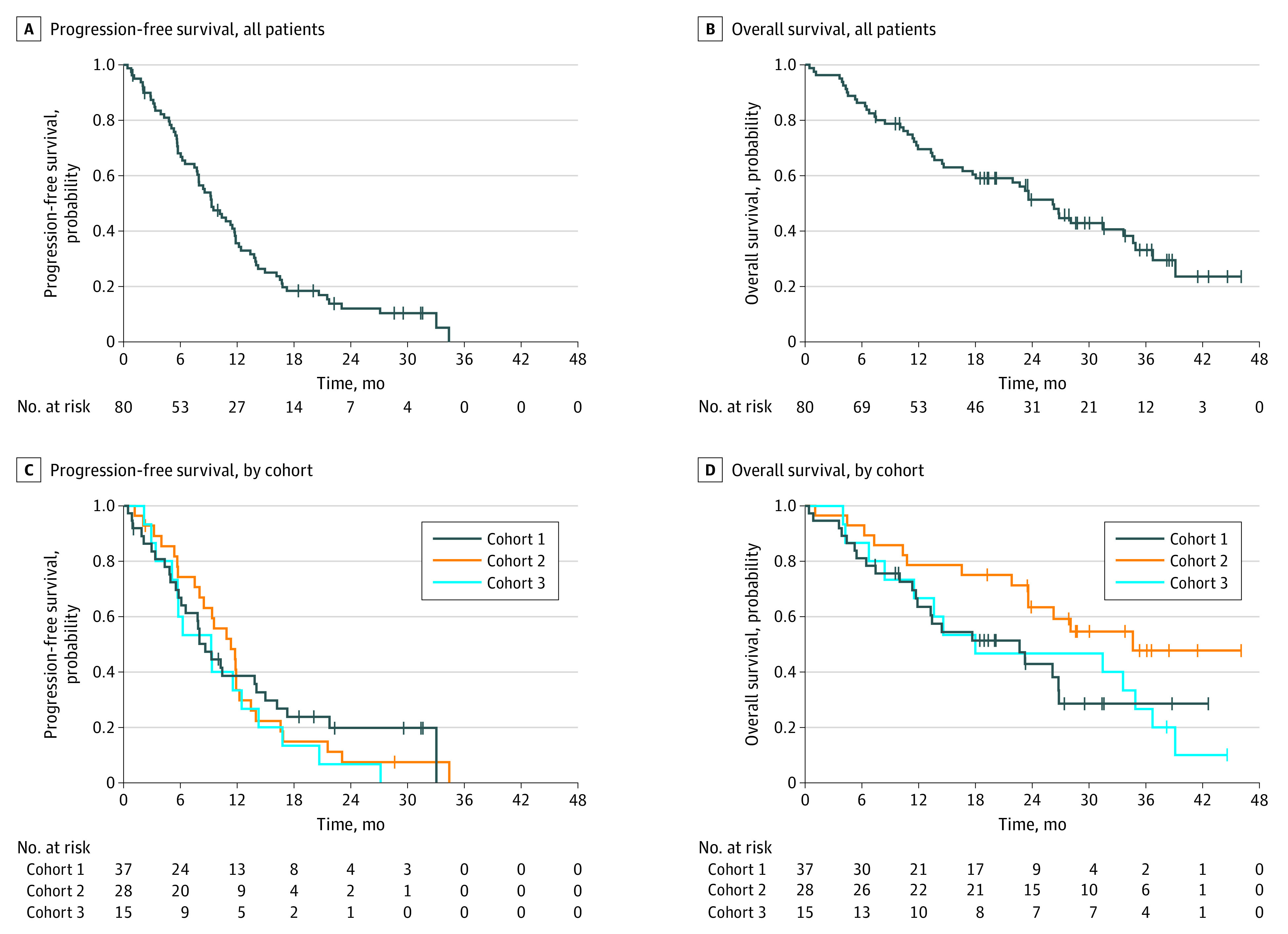
For all patients, median PFS, 9.3 months (95% CI, 7.2-11.4 months) (A); median OS, 26.2 months (95% CI, 21.9-30.4 months) (B). Tick marks indicate censoring. PFS (C) and OS (D) according to the cohorts, defined by age, performance status, and age-adjusted Charlson Comorbidity Index as follows: cohort 1, aged ≥81 years; cohort 2, aged 75-80 years and age-adjusted Charlson Comorbidity Index (a-CCI) ≥ 6 points or PS ≥ 1; and cohort 3, aged 20-74 years and a-CCI ≥ 6 points or PS ≥ 2. Median PFS and OS were as follows: cohort 1, 8.6 months (95% CI, 5.8-14.0 months) and 22.7 months (95% CI, 11.7-26.8 months); cohort 2, 11.3 months (95% CI, 7.5-12.2 months) and 34.6 months (95% CI, 23.6-37.5 months); cohort 3, 9.20 months (95% CI, 3.4-12.4 months) and 18.0 months (95% CI, 6.7-34.9 months).
Seventy-three patients discontinued study treatment owing to disease progression (n = 60), death (n = 3), AEs (n = 4), and patient requests (n = 6). Seven patients are still receiving low-dose erlotinib treatment. Erlotinib administration was continued in 30 patients beyond PD, while 20 patients were switched to other drugs at PD (other EGFR-TKIs, n = 12; cytotoxic drugs, n = 6; immune checkpoint inhibitors, n = 2). For 19 patients, the subsequent treatment included osimertinib.
Multivariate analyses showed that PS of 2 or worse was significantly associated with shorter PFS (hazard ratio [HR], 1.98; 95% CI, 1.18-8.32; P = .009) and shorter OS (HR, 1.83; 95% CI 1.02-3.28; P = .04), whereas CCI (without age adjustment) of 2 or more (vs 0 or 1) was significantly associated with shorter PFS (HR, 1.98; 95% CI, 1.16-3.40; P = .01). Patients 80 years or older were more likely to achieve CR/PR (odds ratio, 1.88; 95% CI, 1.02-3.44; P = .04). No other significant factors were identified.
Safety
AEs were evaluated in all enrolled patients (Table 3). Low-dose erlotinib treatment was temporarily suspended for 10 patients owing to AEs. Five of 80 patients (6%) had their erlotinib dose reduced to 25 mg because of oral mucositis, paronychia, erythema multiforme, diarrhea, and anorexia. Two patients discontinued treatment because of AEs (cutaneous ulcer and bone infection, and oral mucositis, respectively). There were no reports of interstitial lung disease or treatment-related death. The median (range) period of 50 mg erlotinib administration was 6.0 (0.4-34.4) months.
Table 3. Adverse Events During Erlotinib Treatment.
| Adverse event | No. of patients | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Erlotinib 50 mg/d (n = 80) | Overall treatment time (n = 80) | |||||||||
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 3-4, % | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 3-4, % | |
| Acneiform rash | 46 | 8 | 0 | 0 | 0 | 44 | 14 | 2 | 0 | 3 |
| Hypoalbuminemia | 37 | 15 | 1 | 0 | 1 | 39 | 17 | 2 | 0 | 3 |
| Anemia | 29 | 10 | 3 | 0 | 4 | 32 | 11 | 5 | 0 | 6 |
| Alkaline phosphatase increase | 26 | 8 | 4 | 0 | 5 | 28 | 8 | 4 | 0 | 5 |
| Dry skin | 28 | 8 | 0 | NA | 0 | 34 | 11 | 1 | NA | 1 |
| AST increase | 29 | 0 | 0 | 1 | 1 | 30 | 1 | 0 | 2 | 3 |
| Diarrhea | 23 | 2 | 2 | 0 | 3 | 29 | 5 | 2 | 0 | 3 |
| Creatinine increase | 21 | 5 | 1 | 0 | 1 | 24 | 6 | 1 | 0 | 1 |
| ALT increase | 23 | 0 | 0 | 1 | 1 | 30 | 0 | 1 | 1 | 3 |
| Paronychia | 17 | 5 | 0 | NA | 0 | 17 | 12 | 1 | NA | 1 |
| Malaise | 14 | 9 | 0 | NA | 0 | 18 | 10 | 0 | NA | 0 |
| Blood bilirubin increase | 20 | 0 | 0 | 0 | 0 | 30 | 1 | 0 | 0 | 0 |
| Fatigue | 10 | 5 | 1 | NA | 1 | 14 | 6 | 1 | NA | 1 |
| Oral mucositis | 12 | 3 | 0 | 0 | 0 | 17 | 2 | 2 | 0 | 3 |
| Dyspnea | 9 | 2 | 1 | NA | 1 | 9 | 4 | 1 | NA | 1 |
| Neutrophil count decrease | 5 | 4 | 1 | 0 | 1 | 5 | 5 | 1 | 0 | 1 |
| Erythema multiforme | 5 | 0 | 1 | 0 | 1 | 6 | 1 | 1 | 0 | 1 |
| Vomiting | 3 | 0 | 1 | 0 | 1 | 6 | 1 | 1 | 0 | 1 |
| Dizziness | 1 | 1 | 1 | NA | 1 | 1 | 1 | 1 | NA | 1 |
| Lung infection | NA | 1 | 1 | 0 | 1 | NA | 1 | 1 | 0 | 1 |
| Skin ulceration | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 |
| Bone infection | NA | 0 | 1 | 0 | 1 | NA | 0 | 1 | 0 | 1 |
| Vasovagal reaction | NA | NA | 1 | 0 | 1 | NA | NA | 1 | 0 | 1 |
| Myocardial infarction | NA | 0 | 1 | 0 | 1 | NA | 0 | 1 | 0 | 1 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; NA, not applicable.
Because the study enrolled frail patients, AEs such as fatigue and malaise were common. Grade 4 increased aspartate aminotransferase and alanine aminotransferase levels were observed in 1 patient, which was ascribed to cardiac tamponade resulting from exacerbation of the primary disease. Fourteen patients experienced grade 3 or greater AEs owing to low-dose erlotinib; this incidence was higher than that in our previous trial,15 probably reflecting the poorer-risk status of the study population. In fact, most of the laboratory abnormalities, such as anemia and elevation of alkaline phosphatase, or general symptoms, such as fatigue and dyspnea, resulted from baseline abnormalities. Diarrhea or skin AEs were mild. These AEs were generally manageable.
Pharmacokinetic Analysis
Plasma erlotinib concentration could be measured in 48 patients (Table 1). The ORR for low-dose erlotinib in the 48 patients was 64.6%, with a median (range) trough of 685 (153-1950) ng/mL (median [range] during administration period, day 13 [6-27]), which surpassed the reported effective level (500 ng/mL).19 Responses occurred in 9 of 15 patients (60%) who failed to achieve this level.
Plasma erlotinib concentrations were not significantly associated with response: the median trough (ng/mL) of the CR + PR, SD, and PD cases was 687 (171-1950), 752 (244-1390), and 590 (195-632) (P = .43) (Kruskal-Wallis test) (eFigure 1A in Supplement 2). Although there was no difference in plasma erlotinib concentration between the cohorts (P = .23, Mann-Whitney test), plasma erlotinib concentration tended to be associated with worsening PS (P = .13, Mann-Whitney test) (eFigures 1B and 1C in Supplement 2). The severity of acneiform rash tended to be associated with higher erlotinib plasma concentration (Spearman’s rank correlation coefficient [ρ] = 0.273; P = .06), but no association was observed between erlotinib plasma concentration and diarrhea (ρ = 0.038; P = .80) (eFigure 2 in Supplement 2). ABCB1 gene polymorphism analysis is described in the eAppendix and eFigure 3 in Supplement 2.
Discussion
To our knowledge, this is the first prospective study assessing low-dose erlotinib in elderly or frail patients with EGFR mutation–positive NSCLC. We verified the clinical efficacy of low-dose erlotinib in these patients.
Comorbidities and reduced physiologic function make challenging obstacles in the treatment of frail patients. Reduced intracellular water content and reduced metabolism affect pharmacodynamics and pharmacokinetics, which could have relevant influences on tolerability and adherence.
The results of our pharmacokinetics analysis showed that the median trough plasma concentration of erlotinib exceeded the effective level even after administration at a low dose. No association was found between trough erlotinib concentration and diarrhea, whereas rash tended to be associated with erlotinib concentration. Furthermore, plasma concentrations of low-dose erlotinib in frail patients were higher than those in the phase 1 trial19 and tended to be higher in patients with PS of 3 or 4. These pharmacokinetic changes in the population could result in AEs owing to relatively excessive drug doses.
Because low-dose erlotinib could achieve effective plasma concentration and clinical efficacy, the conventional dosing strategy of target-based drugs based on MTD may not be optimal. In fact, there are several reports of trials of the strategy to use lower-dose drugs to reduce toxicity, improve tolerability, and decrease costs while maintaining efficacy. These include abiraterone in prostate cancer, dasatinib in chronic myeloid leukemia, and regorafenib in colorectal cancer, all with favorable results.13,20,21 With the increased number of elderly and frail patients with cancer, more patients would receive benefit from this value-based treatment to enhance risk-benefit and cost-benefit ratios. In fact, our low-dose erlotinib therapy could substantially reduce the treatment cost.
Limitations
Our trial had several limitations. First, the frail patients were heterogeneous. Our patient selection using PS and a-CCI may include elderly but active patients. Second, we analyzed the pharmacokinetics of low-dose erlotinib at only 1 point. Pharmacokinetic analysis after dose increases may be necessary to evaluate the efficacy of low-dose erlotinib.
Third, the recently published results of the FLAURA randomized clinical trial showed that osimertinib was superior to first-generation TKIs, including erlotinib.3 Therefore, our results have now only limited influence on the standard of care. However, because the FLAURA trial enrolled only good-risk patients, the results cannot be automatically applied to real-world frail patient groups. More large-scale studies are needed.
Conclusions
In conclusion, low-dose erlotinib was associated with efficacy and safety in frail patients with EGFR mutation–positive lung cancer. More research on the dosing strategy of target-based drugs is warranted, especially in frail patients in the real-world setting.
Trial Protocol
eMethods.
eResults.
eReferences.
eFigure 1. (A) Erlotinib Plasma Concentration (PC) According to Clinical Response. (B) PC between Cohorts. (C) PC for Each Performance Status (PS).
eFigure 2. Plasma Concentration According to Diarrhea and Rash.
eFigure 3. Plasma Concentration According to ABCB1 Polymorphism.
References
- 1.Maemondo M, Inoue A, Kobayashi K, et al. ; North-East Japan Study Group . Gefitinib or chemotherapy for non–small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380-2388. doi: 10.1056/NEJMoa0909530 [DOI] [PubMed] [Google Scholar]
- 2.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327-3334. doi: 10.1200/JCO.2012.44.2806 [DOI] [PubMed] [Google Scholar]
- 3.Soria JC, Ohe Y, Vansteenkiste J, et al. ; FLAURA Investigators . Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378(2):113-125. doi: 10.56/NEJMoa1713137 [DOI] [PubMed] [Google Scholar]
- 4.Inoue A, Kobayashi K, Usui K, et al. ; North East Japan Gefitinib Study Group . First-line gefitinib for patients with advanced non–small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J Clin Oncol. 2009;27(9):1394-1400. doi: 10.1200/JCO.2008.18.7658 [DOI] [PubMed] [Google Scholar]
- 5.Maemondo M, Minegishi Y, Inoue A, et al. First-line gefitinib in patients aged 75 or older with advanced non–small cell lung cancer harboring epidermal growth factor receptor mutations: NEJ 003 study. J Thorac Oncol. 2012;7(9):1417-1422. doi: 10.1097/JTO.0b013e318260de8b [DOI] [PubMed] [Google Scholar]
- 6.Inoue Y, Inui N, Asada K, et al. Phase II study of erlotinib in elderly patients with non–small cell lung cancer harboring epidermal growth factor receptor mutations. Cancer Chemother Pharmacol. 2015;76(1):155-161. doi: 10.1007/s00280-015-2784-x [DOI] [PubMed] [Google Scholar]
- 7.Imai H, Kaira K, Suzuki K, et al. A phase II study of afatinib treatment for elderly patients with previously untreated advanced non–small-cell lung cancer harboring EGFR mutations. Lung Cancer. 2018;126:41-47. doi: 10.1016/j.lungcan.2018.10.014 [DOI] [PubMed] [Google Scholar]
- 8.Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326-2347. doi: 10.1200/JCO.2018.78.8687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Handforth C, Clegg A, Young C, et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol. 2015;26(6):1091-1101. doi: 10.1093/annonc/mdu540 [DOI] [PubMed] [Google Scholar]
- 10.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27(17):2758-2765. doi: 10.1200/JCO.2008.20.8983 [DOI] [PubMed] [Google Scholar]
- 11.Vestal RE. Aging and pharmacology. Cancer. 1997;80(7):1302-1310. doi: [DOI] [PubMed] [Google Scholar]
- 12.Reiss KA, Yu S, Mamtani R, et al. Starting dose of sorafenib for the treatment of hepatocellular carcinoma: a retrospective, multi-institutional study. J Clin Oncol. 2017;35(31):3575-3581. doi: 10.1200/JCO.2017.73.8245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naqvi K, Jabbour E, Skinner J, et al. Early results of lower dose dasatinib (50 mg daily) as frontline therapy for newly diagnosed chronic-phase chronic myeloid leukemia. Cancer. 2018;124(13):2740-2747. doi: 10.1002/cncr.31357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urata Y, Katakami N, Morita S, et al. Randomized phase III study comparing gefitinib with erlotinib in patients with previously treated advanced lung adenocarcinoma: WJOG 5108L. J Clin Oncol. 2016;34(27):3248-3257. doi: 10.1200/JCO.2015.63.4154 [DOI] [PubMed] [Google Scholar]
- 15.Yamada K, Aono H, Hosomi Y, et al. A prospective, multicentre phase II trial of low-dose erlotinib in non–small cell lung cancer patients with EGFR mutations pretreated with chemotherapy: Thoracic Oncology Research Group 0911. Eur J Cancer. 2015;51(14):1904-1910. doi: 10.1016/j.ejca.2015.06.120 [DOI] [PubMed] [Google Scholar]
- 16.Hamada A, Sasaki J, Saeki S, et al. Association of ABCB1 polymorphisms with erlotinib pharmacokinetics and toxicity in Japanese patients with non–small-cell lung cancer. Pharmacogenomics. 2012;13(5):615-624. doi: 10.2217/pgs.11.176 [DOI] [PubMed] [Google Scholar]
- 17.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245-1251. doi: 10.1016/0895-4356(94)90129-5 [DOI] [PubMed] [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 19.Hidalgo M, Siu LL, Nemunaitis J, et al. Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol. 2001;19(13):3267-3279. doi: 10.1200/JCO.2001.19.13.3267 [DOI] [PubMed] [Google Scholar]
- 20.Szmulewitz RZ, Peer CJ, Ibraheem A, et al. Prospective international randomized phase II study of low-dose abiraterone with food versus standard dose abiraterone in castration-resistant prostate cancer. J Clin Oncol. 2018;36(14):1389-1395. doi: 10.1200/JCO.2017.76.4381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bekaii-Saab TS, Ou FS, Ahn DH, et al. Regorafenib dose-optimisation in patients with refractory metastatic colorectal cancer (ReDOS): a randomised, multicentre, open-label, phase 2 study. Lancet Oncol. 2019;20(8):1070-1082. doi: 10.1016/S1470-2045(19)30272-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eMethods.
eResults.
eReferences.
eFigure 1. (A) Erlotinib Plasma Concentration (PC) According to Clinical Response. (B) PC between Cohorts. (C) PC for Each Performance Status (PS).
eFigure 2. Plasma Concentration According to Diarrhea and Rash.
eFigure 3. Plasma Concentration According to ABCB1 Polymorphism.