This meta-analysis of 13 studies comprising 1095 patients provides associations of patient-related factors with cochlear implant speech recognition outcomes.
Key Points
Question
What are the associations between patient-related factors and cochlear implant (CI) speech recognition outcomes?
Findings
In this meta-analysis of 13 studies comprising 1095 patients, CI speech recognition outcomes were found to be negligibly associated with age at implantation, duration of hearing loss, preimplant pure-tone average, and preimplant word recognition.
Meaning
Patient-related factors often thought to influence CI speech recognition ability offer limited assistance in clinical decision-making in cochlear implantation, which presents an opportunity for additional research to identify patient-related and other factors that predict CI outcomes.
Abstract
Importance
Multiple studies have evaluated associations between post–cochlear implant (CI) speech recognition outcomes and patient-related factors. Current literature often appears equivocal or contradictory, so little is known about the factors that contribute to successful speech recognition outcomes with CIs.
Objective
To use a meta-analysis to pool data from the extant literature and provide an objective summary of existing evidence on associations of patient-related factors and CI speech recognition outcomes.
Data Sources
A literature search was performed using PubMed, Scopus, and CINAHL databases in January 2019 using the following search terms: cochlear implant or cochlear implants or cochlear implantation and speech recognition or word recognition or sentence recognition. Studies of postlingually deafened adult CI recipients that reported word or sentence recognition scores were included.
Study Selection
Inclusion criteria were postlingual adult CI recipients 18 years or older with word or sentence recognition scores at minimum 6-month postimplantation. Studies that included patients undergoing revision or reimplantation surgery were excluded.
Data Extraction and Synthesis
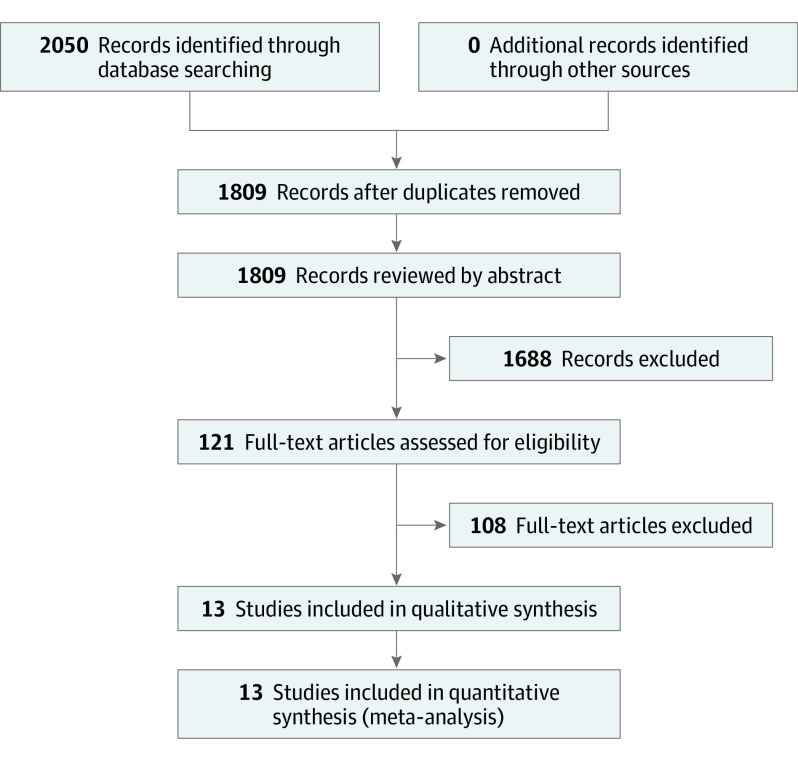
Following the Preferred Reporting Items for Systemic Reviews and Meta-analyses (PRISMA) guidelines, 1809 unique articles underwent review by abstract, and 121 articles underwent full-text review, resulting in 13 articles of 1095 patients for a meta-analysis of correlations. Random-effects model was used when the heterogeneity test yielded a low P value (P < .05).
Main Outcomes and Measures
The planned primary outcome was the pooled correlation values between postimplant speech recognition scores and patient-related factors.
Results
Of the 1095 patients included from the 13 studies, the mean age at implantation ranged from 51.2 to 63.7 years and the mean duration of hearing loss ranged from 9.5 to 31.8 years; for the 825 patients for whom sex was reported, 421 (51.0%) were women. A weak negative correlation was observed between age at implantation and postimplant sentence recognition in quiet (r = −0.31 [95% CI, −0.41 to −0.20]). Other correlations between patient-related factors and postimplant word or sentence recognition were statistically significant, but all correlations were absent to negligible (r = 0.02-0.27).
Conclusions and Relevance
Given that most associations were weak, negligible, or absent, patient-related factors often thought to affect CI speech recognition ability offer limited assistance in clinical decision-making in cochlear implantation. Additional research is needed to identify patient-related and other factors that predict CI outcomes, including speech recognition and other important variables related to success with CIs.
Introduction
Cochlear implantation has become the standard of care for the millions of adults with severe-to-profound sensorineural hearing loss. Despite advances in cochlear implant (CI) technologies, speech recognition outcomes remain highly variable.1,2 Reasons for this variability are uncertain but likely involve several device-specific and patient-specific factors. Some of the device-specific variables that have been studied include signal processing strategies,3,4,5,6,7 intracochlear electrode location,8,9,10,11,12,13,14,15 and operative approach.16,17,18,19 However, intrinsic patient-specific demographic and audiologic factors, including age at implantation, duration of hearing loss, preimplant audiologic measurements, and hearing aid (HA) use prior to implantation, likely affect CI outcomes independent from these device-related variables.
The success of cochlear implantation is currently evaluated primarily by open-set speech recognition scores,20 which most often include word recognition in quiet, sentence recognition in quiet, and sentence recognition in various levels of background noise. Although many patient-specific factors are thought to be associated with these outcomes, the strength of the associations of these factors with CI speech-recognition outcomes has been equivocal. For example, Kim et al21 and Francis et al22 both showed that older age at implantation was associated with poorer postimplant speech recognition abilities as compared with younger CI users, whereas Park et al23 and Guerra-Jimenez et al24 did not report this association. Similar inconsistencies exist for the majority of patient-specific factors that have been studied and reported.
To date, most published reports examining the effect of patient-specific factors on CI outcomes have small sample sizes and are from single institutions, so results may not be broadly representative of CI users and can generate contradictory findings. To address this limitation, the present study uses meta-analyses of correlation to pool data across studies with the goal of identifying patient-specific factors that predict CI speech recognition outcomes. Meta-analyses provide the means to combine data from smaller studies to increase statistical power and precision to detect associations and provide an objective summary of existing evidence.
The current meta-analysis examines the association between patient-specific factors and speech recognition outcomes in adult CI users. A better understanding of the strength of associations between patient characteristics and postimplant outcomes is important to inform CI candidates and provide appropriate advice regarding likely postoperative success. Poor postimplant performance and low perceived benefit coupled with inappropriate expectations have been associated with elective nonuse,25 which makes careful candidate selection essential to justify the cost of intervention. In addition, it is important to identify and target patient characteristics that are potentially modifiable in order to improve CI outcomes.
Methods
Literature Search
A literature search was performed following the Preferred Reporting Items for Systemic Reviews and Meta-analyses (PRISMA) guidelines.26 Two reviewers (E.E.Z. and C.L.) independently searched the PubMed, Scopus, and CINAHL databases in January 2019 for studies of postlingually deafened adult CI recipients that reported word or sentence recognition scores. The following search terms were used: cochlear implant or cochlear implants or cochlear implantation and speech recognition or word recognition or sentence recognition. These searches resulted in 1809 unique articles that were reviewed by title and abstract for inclusion and exclusion criteria. Inclusion criteria were as follows: postlingual adult CI recipients with word or sentence recognition scores at minimum 6-month postimplantation. Case reports, letters to the editor, conference proceedings, and full-text articles not available in English were excluded. Studies that included patients younger than 18 years, revision or reimplantation surgery, and prelingually deafened patients were excluded. A minimum of 3 pure-tone thresholds at frequencies of 500, 1000, or 2000 Hz were required for inclusion in analysis of preimplant pure-tone average (PTA). Studies that included patients with a single condition (eg, Meniere’s disease27) were excluded. No exclusions were made based on time range or publication date.
After review of 1809 unique abstracts, 121 articles underwent full-text review for inclusion. After full-text review, 13 articles were included in the meta-analysis (Table 1).1,21,22,24,28,29,30,31,32,33,34,35,36 Disagreements among reviewers were mediated by the senior author (T.R.M.). Articles were reviewed to ensure no overlapping study samples were included. In studies with bilateral CI recipients, data were included if implanted ears were reported separately. Figure 1 displays the study selection process.
Table 1. Studies Included in the Meta-analysis of Correlationsa.
| Source | Patient-related factors | Postoperative speech recognition task | Follow-up, mo |
|---|---|---|---|
| Beyea et al,28 2016 | Age at CI, DoHL | Sentences in quiet: HINT, AzBio | ≥12 |
| Derinsu et al,29 2018 | Age at CI, DoHL, WR | Words in quiet: phonetically balanced monosyllable word lists | ≥12 |
| Fabie et al,30 2018 | PTA, WR | Words in quiet: CNC | ≥6 |
| Sentences in quiet: AzBio in quiet | |||
| Sentences in noise: AzBio in noise | |||
| Francis et al,22 2005 | Age at CI, DoHL, PTA | Words in quiet: CNC | 6 |
| Guerra-Jimenez et al,24 2016 | Age at CI, DoHL | Words in quiet: NS | ≥12 |
| Holden et al,1 2013 | Age at CI, DoHL | Words in quiet: CNC | ≥24 |
| Holden et al,31 2016 | Age at CI, DoHL, PTA | Words in quiet: CNC | ≥6 |
| Sentences in quiet: AzBio in quiet | |||
| Sentences in noise: AzBio in noise | |||
| Kamakura et al,32 2016 | Age at CI | Words in quiet: CNC, NU-6 | >12 |
| Kim et al,21 2018 | Age at CI, DoHL | Words in quiet: NS | 24 |
| Li et al,33 2007 | Age at CI | Words in quiet: NU-6 or CNC | ≥12 |
| Medina et al,34 2017 | DoHL | Words in quiet: DWR | 6 |
| Sentences in quiet: NS | |||
| Plant et al,35 2016 | DoHL | Words in quiet: CNC | 12 |
| Rubinstein et al,36 1999 | DoHL | Words in quiet: NU-6 or CNC | 9 |
Abbreviations: CI, cochlear implant; CNC, consonant-nucleus-consonant test; DoHL, duration of hearing loss; DWR, disyllabic word recognition; HINT, Hearing in Noise Test; NS, not specified; NU-6, Northwestern University Auditory Test Number 6; PTA, preimplant earphone pure-tone average; WR, preimplant-aided word recognition.
Studies listed satisfied inclusion criteria for meta-analysis of correlations, factors analyzed, speech recognition measure used, and follow-up time in months.
Figure 1. PRISMA Diagram.
Literature review process using the Preferred Reporting Items for Systemic Reviews and Meta-analysis (PRISMA) guidelines.
Data Extraction
Articles were selected for the meta-analysis of correlations based on a priori inclusion and exclusion criteria as previously described. Data were reviewed by 2 independent reviewers (E.E.Z. and C.L.) who recorded the author, sample size, patient demographics, year of publication, and correlation values.
In studies with multiple time points longer than 6 months, the 6-month postimplant data were included to ensure consistency. In studies that reported results for more than 1 test within the same category of speech recognition measurements (eg, AzBio and HINT [Hearing in Noise Test] for sentence recognition in quiet) correlation and number of patients were both listed as separate samples. Data reported in graphical plots were extracted if numerical values were available. Authors were contacted for more complete data when necessary for inclusion into the study. Level of evidence for each selected article was determined to be level 4, as determined by the Oxford Centre for Evidence-Based Medicine.37
Biostatistics
A meta-analysis of correlations was performed using MedCalc, version 18.10.2 (MedCalc Software). The resulting forest plot shows the correlation coefficients (with 95% CIs) found in the different studies included in the meta-analysis, as well as the overall effect with 95% CIs. Under the fixed-effects model, it is assumed that all studies come from a common population and that the correlation coefficient is not statistically significantly different among the different trials. This assumption is tested by the heterogeneity test. If this test yields a low P value (P < .05), then the fixed-effects model may be invalid. In this case, the random-effects model might be more appropriate, in which both the random variation within the studies and the variation between the different studies is incorporated.38 Each study was weighted according to the number of patients included. MedCalc uses the Hedges-Olkin method for calculating the weighted summary correlation coefficient under the fixed effects model, using a Fisher z transformation of the correlation coefficients.39 Under the random-effects model, the heterogeneity statistic is incorporated to calculate the summary correlation coefficient.40
For this analysis, the null hypothesis was that there was no association between preimplant patient-specific factors (eg, age at implantation, duration of hearing loss, preimplant PTA) and postimplant word and sentence recognition scores. The following criteria were used for subjective assessment of correlation values (r): 0 to 0.3, negligible; 0.3 to 0.5, low; 0.5 to 0.7, medium; 0.7 to 0.9, high; 0.9 to 1.0, very high.40,41 Potential publication bias was evaluated by visual inspection of the funnel plot and Egger regression test, which examines the asymmetry of the funnel plot42 (see eFigure in the Supplement). In a funnel plot, treatment effect is plotted on the horizontal axis, and MedCalc plots the standard error (SE) on the vertical axis.43 The vertical line represents the summary derived using fixed-effect meta-analysis. Two diagonal lines represent (pseudo) 95% CIs (effect ±1.96 SE) around the summary effect for each SE on the vertical axis. These show the expected distribution of studies in the absence of heterogeneity of selection bias. In the absence of heterogeneity, 95% of the studies should lie within the funnel defined by these diagonal lines. The need for institutional review board approval was waived by the Medical University of South Carolina, and all data analyzed in this study were deidentifed before data access, handling, or analysis.
Results
Studies that met criteria for inclusion in the meta-analysis of correlations are summarized in Table 1. Table 2 summarizes the patient characteristics of studies included in the meta-analysis of correlations, and Table 3 summarizes the patient-related factors and correlations. Thirteen studies were included with a total of 1095 patients. Sex was reported for 825 of these patients: 405 (49.1%) were men and 421 (51.0%) were women. The mean age at implantation ranged from 51.2 to 63.7 years. The mean duration of hearing loss ranged from 9.5 to 31.8 years. Nine studies were included in the meta-analysis of correlations for age at implantation, 10 for duration of hearing loss, 3 for preimplant PTA, and 2 for preimplant word recognition. Reasons for exclusion beyond the a priori criteria were incomplete statistics, overlapping study samples, insufficient follow-up time, use of phonemes as sole postoperative speech recognition outcome, combined reporting of word recognition and sentence recognition as a single correlation value, and grouping data for patients older and younger than 18 years. Egger regression test suggested an association between the sample size of these studies and their effect sizes, which indicates a high likelihood of publication bias for the analysis of duration of hearing loss and post-CI word recognition (I2 = 79.9%; P < .001). The funnel plot of the meta-analysis of duration of hearing loss and word recognition in quiet is available as eFigure in the Supplement. There was no indication of publication bias for any other meta-analyses of correlation performed.
Table 2. Patients Included in the Meta-analysis of Correlationsa.
| Patient-related factor | Studies | No. of patients | Sex, No. (%) | Mean range | |
|---|---|---|---|---|---|
| Male | Female | ||||
| Age at implantation | 9 | 518 | 152 (46) | 179 (54) | 51.2-72 y |
| Duration of hearing loss | 10 | 738 | 205 (44) | 264 (56) | 9.5-31.8 y |
| Preimplant | |||||
| Earphone PTA | 3 | 478 | 201 (55) | 166 (45) | 87.6-96.3 dB |
| Aided word recognition | 2 | 404 | 182 (55) | 146 (45) | 7.2%-8.6% |
| All studies | 13 | 1095 | 405 (49) | 421 (51) | NA |
Abbreviations: NA, not applicable; PTA, pure-tone average.
More than 1 factor for each article could be included in a meta-analysis, so all studies are not totals. Subject sex was not reported for all subjects.
Table 3. Meta-analysis of Correlationsa.
| Characteristic | r (95% CI) | I2, % | P value |
|---|---|---|---|
| Age at implantation | |||
| Word recognition in quiet | −0.27 (−0.35 to −0.19) | 0.0 | .58 |
| Sentence recognition | |||
| In quiet | −0.31 (−0.41 to −0.20) | 0.0 | .87 |
| In noise | NA | NA | NA |
| Duration of hearing loss | |||
| Word recognition in quiet | −0.25 (−0.41 to −0.07) | 79.9 | <.001b |
| Sentence recognition | |||
| In quiet | 0.02 (−0.08 to 0.12) | 7.7 | .37 |
| In noise | NA | NA | NA |
| Preimplant earphone PTA | |||
| Word recognition in quiet | −0.16 (−0.26 to −0.06) | 0.0 | .76 |
| Sentence recognition | |||
| In quiet | −0.15 (−0.25 to −0.05) | 0.0 | .67 |
| In noise | −0.19 (−0.34 to −0.04) | 10.5 | .33 |
| Preimplant word recognition | |||
| Word recognition in quiet | 0.22 (0.13 to 0.32) | 18.5 | .27 |
| Sentence recognition | |||
| In quiet | NA | NA | NA |
| In noise | NA | NA | NA |
Abbreviations: NA, not applicable; PTA, pure-tone average.
Pooled correlation values and heterogeneity statistics (I2 and P value) for meta-analysis of correlations.
Indicates significance for heterogeneity. Random effect is used.
Age at Implantation and Postoperative Speech Recognition Outcomes
Results from 9 studies showed age at implantation had a negligible negative correlation with postoperative word recognition (r = −0.27 [95% CI, −0.35 to −0.19]) and a low negative correlation with postoperative sentence recognition in quiet (r = −0.31 [95% CI, −0.41 to −0.20]), as shown in Figure 2A and B.22,28,31 Association of age at implantation with postoperative sentence recognition in noise was not analyzed because only 1 study31 met inclusion criteria for review. Results showed no significant heterogeneity for word recognition in quiet (I2 = 0%; P = .58) or sentence recognition in quiet (I2 = 0%; P = .87).
Figure 2. Forest Plots of Meta-analysis of Correlations by Patient-Related Factors.
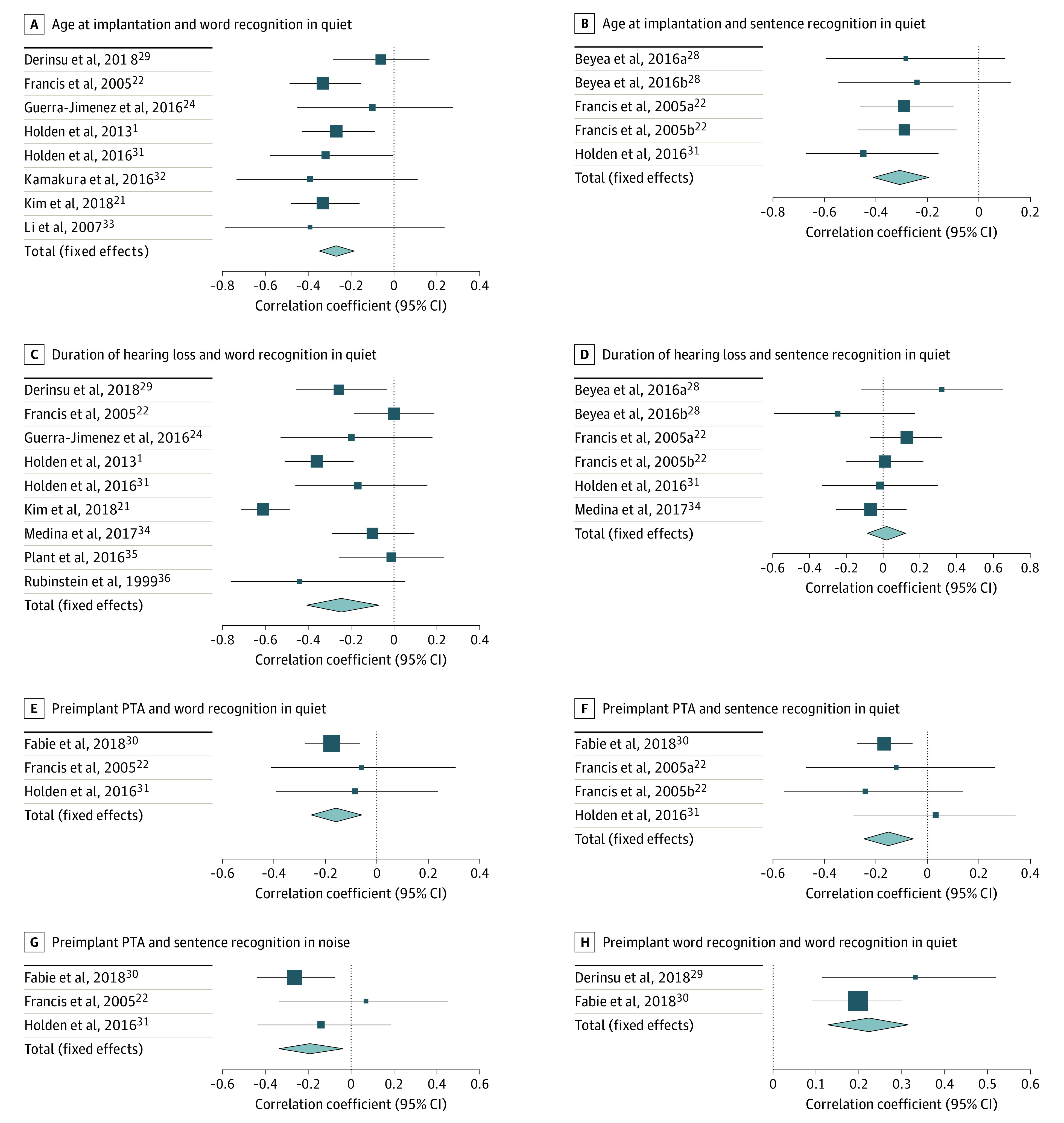
Studies with more than 1 task for the same speech recognition measurement are listed as separate samples. Beyea 2016a28 tested patients with HINT (Hearing in Noise Test), and Beyea 2016b28 tested patients with AzBio. Francis 2015a22 tested patients with CID sentences, and Francis 2015b22 tested subjects with HINT.
Abbreviation: PTA, pure-tone average.
Duration of Hearing Loss and Postoperative Speech Recognition Outcomes
Ten studies of duration of hearing loss were included in the analysis (Figure 2C and D).1,21,22,26,28,29,31,34,35,36 A negligible negative correlation was observed between duration of hearing loss and postoperative word recognition scores (r = −0.25 [95% CI, −0.41 to −0.07]). Notably, postoperative sentence recognition in quiet showed no correlation with duration of hearing loss (r = 0.02 [95% CI, −0.08 to 0.12]). Due to only 1 study31 reporting association of duration of hearing loss and sentence recognition in noise, no analysis was performed. Word recognition results showed significant heterogeneity (I2 = 79.9%; P < .001), while sentence recognition in quiet did not (I2 = 0%; P = .48).
Preimplant PTA and Postoperative Speech Recognition Outcomes
Three studies had sufficient data for analysis of preimplant earphone PTA. Out of the 3 studies, 2 studies22,30 used 3-frequency averages of 500, 1000, and 2000 Hz thresholds, and 1 study31 used 4-frequency averages of 250, 500, 1000, and 2000 Hz to calculate PTA. Given the lack of frequency-specific data, we were not able to standardize how PTA was calculated for the meta-analyses. All postoperative speech recognition tests showed negligible negative correlations with preimplant PTA (word recognition in quiet, r = −0.16 [95% CI, −0.26 to −0.06]; sentence recognition in quiet, r = −0.15 [95% CI, −0.25 to −0.05]; sentence recognition in noise, r = −0.19 [95% CI, −0.34 to −0.04]) (Figure 2E, F, and G).22,30,31 These results indicate that poorer preoperative hearing thresholds (as measured by higher preimplantation PTA) are associated with statistically significant, but negligibly lower, postimplant speech recognition scores. No significant heterogeneity was observed for word recognition (I2 = 0%; P = .76), sentence recognition in quiet (I2 = 0%; P = .67), and sentence recognition in noise (I2 = 10.5%; P = .33).
Preoperative Aided and Postoperative Word Recognition Ability
We then sought to evaluate the association between preoperative and postoperative speech recognition ability. A negligible positive correlation was observed between preimplant aided word recognition and postoperative word recognition in quiet (r = 0.22 [95% CI, 0.13-0.31]) with low heterogeneity (I2 = 18.5%; P = .27) in data pooled from 2 studies. A forest plot of correlation of preimplant hearing and postoperative speech recognition is shown in Figure 2H.29,30 Only 1 study compared preoperative and postoperative sentence recognition (in noise or quiet) and met inclusion/exclusion criteria; analysis could not be performed.
Discussion
The original intent of this study was to perform a metaregression of commonly reported patient-specific variables to assess the independent influence of each while controlling for confounders. However, only 3 studies that fulfilled the inclusion criteria contained the necessary data. Most studies that included correlation values did not report preimplant and postimplant audiologic data. As a result, we were only able to perform univariate analyses. In doing so, the preimplant patient-specific factors available for analysis were found to have absent to low correlations with postoperative speech recognition outcomes. While these results showed statistical significance in most of the factors analyzed, the clinical significance of these associations is likely minimal. To illustrate, the correlation between age at implantation and sentence recognition is one of the strongest noted in the present study (r = −0.31). However, the coefficient of determination for this value means that age accounts for only 9.6% of the variance in sentence recognition ability. This degree of correlation is fairly consistent for all investigated factors—all account for fewer than 10% of variance in postimplant speech recognition scores. This suggests that these patient-specific factors that are routinely reported in the literature offer limited assistance in clinical decision-making in cochlear implantation. Moreover, there is evidence that the influence of patient factors on speech recognition outcomes has decreased over time. Blamey et al44,45 found a lower association between speech recognition ability and age at implantation and onset of hearing loss in more recent data when comparing 2 large cohorts separated by 17 years. Reasons for the differences may be related to changes in CI candidacy or implant technology but are not completely understood at the present time. Owing to the limited number of available studies, we were unable to analyze these associations based on date of publication.
Care should be taken in interpreting data from analyses where high heterogeneity (I2) exists, such as the correlation between duration of hearing loss and post-CI word recognition (I2 = 79.9%). This suggests that there may be features of the included studies that introduce variability in outcomes. For example, multiple word-recognition and sentence-recognition tests were used in the included studies. In addition, the different means by which duration of hearing loss was defined and quantified likely introduced additional variability in the analyses. Many authors relied on self-report, and many failed to report their criteria at all.
In addition, duration of severe profound hearing loss is likely more influential for patient outcomes than onset of self-reported symptomatic hearing loss, but these are seldom differentiated in the literature. Holden et al1 and Derinsu et al29 measured the association between word recognition scores and duration of hearing loss defined as the duration of severe-to-profound hearing loss. The reported correlation values (−0.380 and −0.256, respectively) were within the confidence interval of the pooled correlation for word recognition and duration of hearing loss for all studies included in the current meta-analysis. To determine how duration of hearing loss should best be quantified to serve as a predictor of adult CI user outcomes, a study design is needed that includes a large cohort of CI recipients with severe-to-profound hearing loss who have had serial audiologic testing for an extended period of time prior to implantation, or a prospective, longitudinal study design that includes serial audiologic testing. However, given the relatively rare progression of hearing loss to the severe/profound range, the number of patients needed for these studies may be prohibitive. Similar inconsistencies are unfortunately found in many of the factors studied in this analysis, which likely introduce variability in the studies leading to a poor understanding of the patient-specific factors that may influence CI outcomes.
To that end, in our literature search, we identified several factors studied for CI outcomes that were too scarce to analyze in the current study but warrant discussion. One such factor was duration of preimplant HA use. Unfortunately reporting on the influence of HA use is inconsistent and variable, with some studies reporting significant positive correlations with postimplant word recognition,21 while several others found no or even negative significant associations.1,46,47 In theory, preimplant HA use may provide auditory stimulation to the affected ear, which has been suggested as a possible protective factor for maintaining central auditory neuroplasticity47; however, only 1 study met our inclusion criteria,21 and this topic was unable to be addressed in the current meta-analysis of correlations. Although we were able to analyze preimplant PTA and preimplant word recognition ability, the analysis included only 3 and 2 studies, respectively, which made interpretation difficult.
Overall, we found that the literature assessing the effects of patient-specific factors on CI speech recognition outcomes is limited to only a few factors, but evidence is growing that other factors may contribute. For example, working memory and cognition have been more recently cited as potential indicators for CI performance. Moberly et al48 found positive correlations between working memory capacity and visual working memory capacity with preimplant AzBio sentences in quiet and postimplant AzBio sentences in noise, respectively.49 In addition, Heydebrand et al50 found verbal learning measures to be the best predictor of postimplant CNC (consonant-nucleus-consonant) 6-month performance. The numerous constructs that contribute to cognitive ability and the many available tests used to measure these constructs make it difficult at this time to pool these results in a quantitative analysis. Therefore, we were not able to perform a meta-analysis of correlations for this topic.
Limitations
The present study is limited by the availability and quality of published data. As in any meta-analysis, the possibility of a publication bias exists. For example, some studies might have provided only significant correlation values, which excluded nonsignificant factors from these analyses and influenced our pooled results. We attempted to contact the authors of all studies that did not report a nonsignificant correlation value; however, additional data were not always available. In addition, the reporting of follow-up interval in this literature is limited. CI candidates often reach peak performance between 6 and 12 months postimplantation.1 Several of the included studies provided a standard follow-up time for all patients; however, many studies lacked consistent follow-up or failed to report these data. It is possible that the patient-specific factors analyzed might have a greater effect on speech recognition scores after 6 months, but available data from this time frame are very limited. Finally, the standardization of statistical reporting in journals and following recently published guidelines for reporting CI outcomes51 would greatly advance the ability to synthesize available evidence for future meta-analyses.
Conclusions
The current meta-analysis of correlations demonstrated statistically significant low to negligible associations between patient-specific factors and postimplant speech recognition. These correlations provide limited guidance in counseling patients regarding expectations and should not be the sole deciding factor for patients considering CIs. Further studies are needed to determine associations with postimplant sentence recognition in noise because this is rarely reported. Complete and standardized data reporting and exploration of additional patient factors in future studies may identify clinically significant predictors that can guide clinical practice.
eFigure. Duration of hearing loss and word recognition in quiet
References
- 1.Holden LK, Finley CC, Firszt JB, et al. Factors affecting open-set word recognition in adults with cochlear implants. Ear Hear. 2013;34(3):342-360. doi: 10.1097/AUD.0b013e3182741aa7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moberly AC, Lowenstein JH, Nittrouer S. Word recognition variability with cochlear implants: “perceptual attention” versus “auditory sensitivity”. Ear Hear. 2016;37(1):14-26. doi: 10.1097/AUD.0000000000000204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Firszt JB, Holden LK, Reeder RM, Skinner MW. Speech recognition in cochlear implant recipients: comparison of standard HiRes and HiRes 120 sound processing. Otol Neurotol. 2009;30(2):146-152. doi: 10.1097/MAO.0b013e3181924ff8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koch DB, Osberger MJ, Segel P, Kessler D. HiResolution and conventional sound processing in the HiResolution bionic ear: using appropriate outcome measures to assess speech recognition ability. Audiol Neurootol. 2004;9(4):214-223. doi: 10.1159/000078391 [DOI] [PubMed] [Google Scholar]
- 5.Skinner MW, Holden LK, Whitford LA, Plant KL, Psarros C, Holden TA. Speech recognition with the nucleus 24 SPEAK, ACE, and CIS speech coding strategies in newly implanted adults. Ear Hear. 2002;23(3):207-223. doi: 10.1097/00003446-200206000-00005 [DOI] [PubMed] [Google Scholar]
- 6.Holden LK, Skinner MW, Holden TA. Speech recognition with the MPEAK and SPEAK speech-coding strategies of the Nucleus cochlear implant. Otolaryngol Head Neck Surg. 1997;116(2):163-167. doi: 10.1016/S0194-5998(97)70319-X [DOI] [PubMed] [Google Scholar]
- 7.Cohen NL, Waltzman SB. Influence of processing strategies on cochlear implant performance. Ann Otol Rhinol Laryngol Suppl. 1995;165:9-14. [PubMed] [Google Scholar]
- 8.Finley CC, Holden TA, Holden LK, et al. Role of electrode placement as a contributor to variability in cochlear implant outcomes. Otol Neurotol. 2008;29(7):920-928. doi: 10.1097/MAO.0b013e318184f492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakravorti S, Noble JH, Gifford RH, et al. Further evidence of the relationship between cochlear implant electrode positioning and hearing outcomes. Otol Neurotol. 2019;40(5):617-624. doi: 10.1097/MAO.0000000000002204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee J, Nadol JB Jr, Eddington DK. Depth of electrode insertion and postoperative performance in humans with cochlear implants: a histopathologic study. Audiol Neurootol. 2010;15(5):323-331. doi: 10.1159/000289571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skinner MW, Ketten DR, Holden LK, et al. CT-derived estimation of cochlear morphology and electrode array position in relation to word recognition in Nucleus-22 recipients. J Assoc Res Otolaryngol. 2002;3(3):332-350. doi: 10.1007/s101620020013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friesen LM, Shannon RV, Slattery WH III. Effects of electrode location on speech recognition with the Nucleus-22 cochlear implant. J Am Acad Audiol. 2000;11(8):418-428. [PubMed] [Google Scholar]
- 13.Fu QJ, Shannon RV. Effects of electrode location and spacing on phoneme recognition with the Nucleus-22 cochlear implant. Ear Hear. 1999;20(4):321-331. doi: 10.1097/00003446-199908000-00005 [DOI] [PubMed] [Google Scholar]
- 14.Wanna GB, Noble JH, McRackan TR, et al. Assessment of electrode placement and audiological outcomes in bilateral cochlear implantation. Otol Neurotol. 2011;32(3):428-432. doi: 10.1097/MAO.0b013e3182096dc2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodges AV, Villasuso E, Balkany T, et al. Hearing results with deep insertion of cochlear implant electrodes. Am J Otol. 1999;20(1):53-55. [PubMed] [Google Scholar]
- 16.O’Connell BP, Cakir A, Hunter JB, et al. Electrode location and angular insertion depth are predictors of audiologic outcomes in cochlear implantation. Otol Neurotol. 2016;37(8):1016-1023. doi: 10.1097/MAO.0000000000001125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Basta D, Todt I, Ernst A. Audiological outcome of the pull-back technique in cochlear implantees. Laryngoscope. 2010;120(7):1391-1396. doi: 10.1002/lary.20942 [DOI] [PubMed] [Google Scholar]
- 18.Wanna GB, Noble JH, Carlson ML, et al. Impact of electrode design and surgical approach on scalar location and cochlear implant outcomes. Laryngoscope. 2014;124(suppl 6):S1-S7. doi: 10.1002/lary.24728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen NL, Waltzman SB. Partial insertion of the nucleus multichannel cochlear implant: technique and results. Am J Otol. 1993;14(4):357-361. [PubMed] [Google Scholar]
- 20.Luxford WM; Ad Hoc Subcommittee of the Committee on Hearing and Equilibrium of the American Academy of Otolaryngology-Head and Neck Surgery . Minimum speech test battery for postlingually deafened adult cochlear implant patients. Otolaryngol Head Neck Surg. 2001;124(2):125-126. doi: 10.1067/mhn.2001.113035 [DOI] [PubMed] [Google Scholar]
- 21.Kim H, Kang WS, Park HJ, et al. Cochlear implantation in postlingually deaf adults is time-sensitive towards positive outcome: prediction using advanced machine learning techniques. Sci Rep. 2018;8(1):18004. doi: 10.1038/s41598-018-36404-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Francis HW, Yeagle JD, Bowditch S, Niparko JK. Cochlear implant outcome is not influenced by the choice of ear. Ear Hear. 2005;26(4)(suppl):7S-16S. doi: 10.1097/00003446-200508001-00003 [DOI] [PubMed] [Google Scholar]
- 23.Park E, Shipp DB, Chen JM, Nedzelski JM, Lin VY. Postlingually deaf adults of all ages derive equal benefits from unilateral multichannel cochlear implant. J Am Acad Audiol. 2011;22(10):637-643. doi: 10.3766/jaaa.22.10.2 [DOI] [PubMed] [Google Scholar]
- 24.Guerra-Jimenez G, Ramos De Miguel Á, Falcón González JC, Borkoski Barreiro SA, Pérez Plasencia D, Ramos Macías Á. Cochlear implant evaluation: prognosis estimation by data mining system. J Int Adv Otol. 2016;12(1):1-7. doi: 10.5152/iao.2016.510 [DOI] [PubMed] [Google Scholar]
- 25.Summerfield AQ, Marshall DH. Non-use of cochlear implants by post-lingually deafened adults. Cochlear Implants Int. 2000;1(1):18-38. doi: 10.1002/cii.26 [DOI] [PubMed] [Google Scholar]
- 26.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lustig LR, Yeagle J, Niparko JK, Minor LB. Cochlear implantation in patients with bilateral Ménière’s syndrome. Otol Neurotol. 2003;24(3):397-403. doi: 10.1097/00129492-200305000-00009 [DOI] [PubMed] [Google Scholar]
- 28.Beyea JA, McMullen KP, Harris MS, et al. Cochlear implants in adults: effects of age and duration of deafness on speech recognition. Otol Neurotol. 2016;37(9):1238-1245. doi: 10.1097/MAO.0000000000001162 [DOI] [PubMed] [Google Scholar]
- 29.Derinsu U, Yuksel M, Gecici CR, Ciprut A, Akdeniz E. Effects of residual speech and auditory deprivation on speech perception of adult cochlear implant recipients. Auris Nasus Larynx. 2018. doi: 10.1016/j.anl.2018.06.006 [DOI] [PubMed] [Google Scholar]
- 30.Fabie JE, Keller RG, Hatch JL, et al. Evaluation of outcome variability associated with lateral wall, mid-scalar, and perimodiolar electrode arrays when controlling for preoperative patient characteristics. Otol Neurotol. 2018;39(9):1122-1128. doi: 10.1097/MAO.0000000000001951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holden LK, Firszt JB, Reeder RM, Uchanski RM, Dwyer NY, Holden TA. Factors affecting outcomes in cochlear implant recipients implanted with a perimodiolar electrode array located in scala tympani. Otol Neurotol. 2016;37(10):1662-1668. doi: 10.1097/MAO.0000000000001241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamakura T, Nadol JB Jr. Correlation between word recognition score and intracochlear new bone and fibrous tissue after cochlear implantation in the human. Hear Res. 2016;339:132-141. doi: 10.1016/j.heares.2016.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li PM, Somdas MA, Eddington DK, Nadol JB Jr. Analysis of intracochlear new bone and fibrous tissue formation in human subjects with cochlear implants. Ann Otol Rhinol Laryngol. 2007;116(10):731-738. doi: 10.1177/000348940711601004 [DOI] [PubMed] [Google Scholar]
- 34.Medina MDM, Polo R, Gutierrez A, et al. Cochlear implantation in postlingual adult patients with long-term auditory deprivation. Otol Neurotol. 2017;38(8):e248-e252. doi: 10.1097/MAO.0000000000001257 [DOI] [PubMed] [Google Scholar]
- 35.Plant K, McDermott H, van Hoesel R, Dawson P, Cowan R. Factors predicting postoperative unilateral and bilateral speech recognition in adult cochlear implant recipients with acoustic hearing. Ear Hear. 2016;37(2):153-163. doi: 10.1097/AUD.0000000000000233 [DOI] [PubMed] [Google Scholar]
- 36.Rubinstein JT, Parkinson WS, Tyler RS, Gantz BJ. Residual speech recognition and cochlear implant performance: effects of implantation criteria. Am J Otol. 1999;20(4):445-452. [PubMed] [Google Scholar]
- 37.Durieux N, Vandenput S, Pasleau F. [OCEBM levels of evidence system]. Rev Med Liege. 2013;68(12):644-649. [PubMed] [Google Scholar]
- 38.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hedges LV, Olkin I. Statistical Methods for Meta-Analysis. Vol 20 Academic Press; 1985. [Google Scholar]
- 40.Mukaka MM. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012;24(3):69-71. [PMC free article] [PubMed] [Google Scholar]
- 41.Lee Rodgers J, Nicewander WA. Thirteen ways to look at the correlation coefficient. Am Stat. 1988;42(1):59-66. doi: 10.1080/00031305.1988.10475524 [DOI] [Google Scholar]
- 42.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046-1055. doi: 10.1016/S0895-4356(01)00377-8 [DOI] [PubMed] [Google Scholar]
- 44.Blamey P, Artieres F, Başkent D, et al. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants: an update with 2251 patients. Audiol Neurootol. 2013;18(1):36-47. doi: 10.1159/000343189 [DOI] [PubMed] [Google Scholar]
- 45.Blamey P, Arndt P, Bergeron F, et al. Factors affecting auditory performance of postlinguistically deaf adults using cochlear implants. Audiol Neurootol. 1996;1(5):293-306. doi: 10.1159/000259212 [DOI] [PubMed] [Google Scholar]
- 46.Dierickx C, Jacquemin L, Boon E, et al. Predictive factors of speech understanding in adults with cochlear implants. B-ENT. 2016;12(3):219-226. [PubMed] [Google Scholar]
- 47.Boisvert I, McMahon CM, Tremblay G, Lyxell B. Relative importance of monaural sound deprivation and bilateral significant hearing loss in predicting cochlear implantation outcomes. Ear Hear. 2011;32(6):758-766. doi: 10.1097/AUD.0b013e3182234c45 [DOI] [PubMed] [Google Scholar]
- 48.Moberly AC, Castellanos I, Mattingly JK. Neurocognitive factors contributing to cochlear implant candidacy. Otol Neurotol. 2018;39(10):e1010-e1018. doi: 10.1097/MAO.0000000000002052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hillyer J, Elkins E, Hazlewood C, Watson SD, Arenberg JG, Parbery-Clark A. Assessing cognitive abilities in high-performing cochlear implant users. Front Neurosci. 2019;12(1056):1056. doi: 10.3389/fnins.2018.01056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heydebrand G, Hale S, Potts L, Gotter B, Skinner M. Cognitive predictors of improvements in adults’ spoken word recognition six months after cochlear implant activation. Audiol Neurootol. 2007;12(4):254-264. doi: 10.1159/000101473 [DOI] [PubMed] [Google Scholar]
- 51.Adunka OF, Gantz BJ, Dunn C, Gurgel RK, Buchman CA. Minimum reporting standards for adult cochlear implantation. Otolaryngol Head Neck Surg. 2018;159(2):215-219. doi: 10.1177/0194599818764329 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Duration of hearing loss and word recognition in quiet