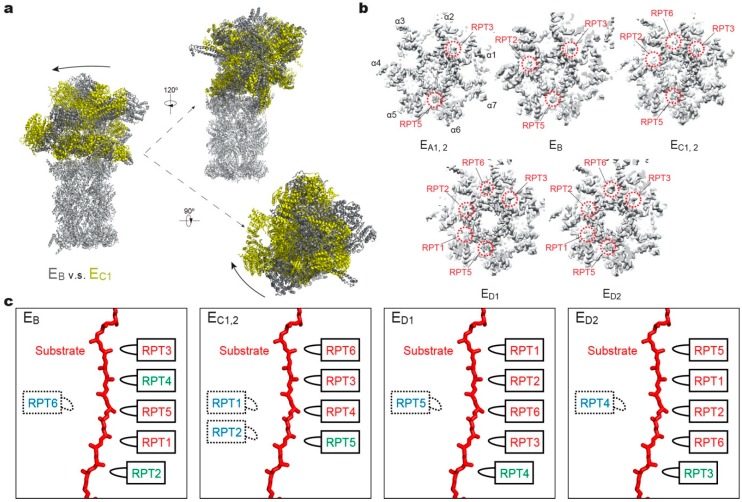
Figure 3.
Structural features of the substrate-bound human 26S proteasome in different conformational states. (a) Conformational switching of the RP during the transition from state EB (dark gray; PDB ID: 6MSE) to EC1 (yellow; PDB ID: 6MSG), with the CP (gray) aligned. (b) Cutaway surface representations of the RP–CP interface in different states. The red dashed circles highlight the densities of the RPT C-terminal tails that are inserted into the α-pockets of the CP. (c) Schematics showing the relative locations of the pore-1 loops of six RPT subunits along the vertical axis. RPT subunits in ATP-bound (red), ADP-bound (green) and apo-like (blue) states are depicted alongside the substrate from top to bottom and subunits that are disengaged from the substrate are placed on the left side. States EA1, EA2 and EC1 are omitted here, as their ATPase structures are identical to that of EB and EC2, respectively.