Abstract
The rare autosomal dominant Charcot-Marie-Tooth type 2B (CMT2B) is associated with mutations in the RAB7A gene, involved in the late endocytic pathway. CMT2B is characterized by predominant sensory loss, ulceromutilating features, with lesser-to-absent motor deficits. We characterized clinically and genetically a family harboring a novel pathogenic RAB7A variant and performed structural and functional analysis of the mutant protein. A 39-year-old woman presented with early-onset walking difficulties, progressive distal muscle wasting and weakness in lower limbs and only mild sensory signs. Electrophysiology demonstrated an axonal sensorimotor neuropathy. Nerve biopsy showed a chronic axonal neuropathy with moderate loss of all caliber myelinated fibers. Next-generation sequencing (NGS) technology revealed in the proband and in her similarly affected father the novel c.377A>G (p.K126R) heterozygous variant predicted to be deleterious. The mutation affects the biochemical properties of RAB7 GTPase, causes altered interaction with peripherin, and inhibition of neurite outgrowth, as for previously reported CMT2B mutants. However, it also shows differences, particularly in the epidermal growth factor receptor degradation process. Altogether, our findings indicate that this RAB7A variant is pathogenic and widens the phenotypic spectrum of CMT2B to include predominantly motor CMT2. Alteration of the receptor degradation process might explain the different clinical presentations in this family.
Keywords: RAB7A, Charcot–Marie–Tooth disease type 2B, CMT2B, peripheral sensory neuropathy, NGF, RAB7, mutations, axons, lysosomes, autophagy, neurite outgrowth, endocytosis, EGFR
1. Introduction
Autosomal dominant axonal Charcot–Marie–Tooth disease type 2B (CMT2B) largely overlaps with hereditary sensory-autonomic neuropathies (HSANs) as it is characterized by predominant sensory loss, high frequency of ulcer formations and amputations, with variable motor deficits [1,2,3,4,5,6,7,8,9,10]. Affected patients show severe distal sensory loss particularly for pain and touch, reduced-to-absent deep tendon reflexes, foot deformities, and sometimes distal wasting and weakness mainly in lower limbs [1,2,3,4,5,7,8,9,10]. The disease typically starts during the second or third decade [10] and runs a slowly progressive course [11,12]. Males have a higher occurrence of ulcers [4], suggesting a difference in disease severity according to gender as for HSAN I [13].
CMT2B is caused by heterozygous mutations in RAB7A, with five mutations (L129F, K157N, N161T/I, V162M) reported in patients from eleven families [1,2,3,4,5,7,8,9,10], all displaying the characteristic ulcero-mutilating phenotype with variable motor involvement (Table 1).
Table 1.
RAB7A mutations associated with Charcot–Marie–Tooth type 2B (CMT2B).
| Mutation | Clinical Phenotype | Families/Sporadic Cases | Age of Onset | Reference |
|---|---|---|---|---|
| L129F | CMT2B ulcero-mutilating features |
Three related Austrian families | Adolescence—adulthood | [1,8] |
| K157N | CMT2B ulcerations, osteomyelitis, amputation |
A patient (de novo mutation) |
Adolescence | [3] |
| N161I | CMT2B pain, no muscle atrophy, and (except for the proband) ulcero-mutilating features |
A Chinese family | Teens or later | [5] |
| N161T | CMT2B pain, ulcers, gangrene, and amputations |
An English family | Adolescence | [2] |
| V162M | CMT2B ulcero-mutilating features (except for pt I-2 of the Scottish family who had drop feet but probably no ulcers) |
Five unrelated families (from USA, Scotland, Austria, Belgium, Italy) | Adolescence—adulthood | [1,4,7,9,10] |
| K126R | CMT2 Predominantly motor phenotype with little sensory involvement |
One Italian family | Adolescence | Present paper |
Pathomechanisms, whereby RAB7A mutations lead to CMT2B, are a matter for debate and investigation. RAB7A, hereafter referred to as RAB7, is a member of the Rab family of small GTPases involved in the regulation of vesicular trafficking between early endosomes and lysosomes, controlling transport to the degradative compartments in the endocytic pathway and lysosome biogenesis [14]. RAB7 modulates the Endoplasmic Reticulum (ER) morphology by controlling the ER homeostasis and ER stress [15]. Crosstalk occurring at mitochondria-lysosome contact sites regulated by Rab7 has also been recently demonstrated [16].
Although ubiquitously expressed, RAB7 has specific functions in neurons, where it regulates retrograde axonal trafficking and signaling of neurotrophin receptors, as well as neurite outgrowth [17,18]. Furthermore, RAB7 regulates cell migration by influencing integrin trafficking and vimentin assembly [19] and cortical neurons’ migration during development [20].
Interestingly, RAB7 has specific effectors in neurons as co-immunoprecipitates with the neurotrophin receptor TrkA (Tropomyosin-receptor-kinase A) and interacts directly with the intermediate filament protein peripherin [18,21]. Therefore, it is not surprising that mutations in RAB7 cause a disease restricted to neurons, although it is unclear why sensory neurons are so selectively vulnerable.
Previous biochemical characterization of four CMT2B-causative RAB7 mutants showed increased dissociation rate constant (Koff,) for nucleotides and lower GTPase activity per binding event [22,23,24]. Overexpression of these mutant proteins inhibits neurite outgrowth in several cell lines [25,26]. Furthermore, these RAB7 mutant proteins display stronger interaction with some RAB7 effector proteins, including RILP (RAB-interacting lysosomal protein), required for lysosomal transport towards the microtubule organizing center (MTOC) by inducing dynein-dynactin motors recruitment [27].
RAB7 and RILP control degradation of the epidermal-growth-factor receptor (EGFR), a member of the receptor tyrosine-kinase family involved in regulating cell proliferation, survival, differentiation and migration [28,29]. Importantly, EGF seems also to have important neurotrophic functions [30,31].
Previous experiments on EGFR degradation obtained on cells transiently or stably transfected with CMT2B-causing RAB7 mutants gave conflicting results: transient expression of these mutants demonstrated normal or increased EGFR degradation [22,23] while stable transfection revealed inhibition [32].
Here, we report a family with a novel CMT2B phenotype with motor predominance and absence of ulcers and mutilations, carrying a novel pathogenic RAB7 variant (c.377A>G, p.K126R) which is absent in global databases, affects a highly conserved amino-acid in the GTPase domain of Rab7, is predicted to be pathogenic by in silico analysis, and is transmitted as an autosomal dominant trait. We performed extensive biochemical and functional studies, which confirmed its pathogenic role.
2. Materials and Methods
2.1. Patients
We evaluated clinically and electrophysiologically (standard procedures) one healthy and two affected family members (Figure 1A). Informed consent was obtained for all procedures from study participants.
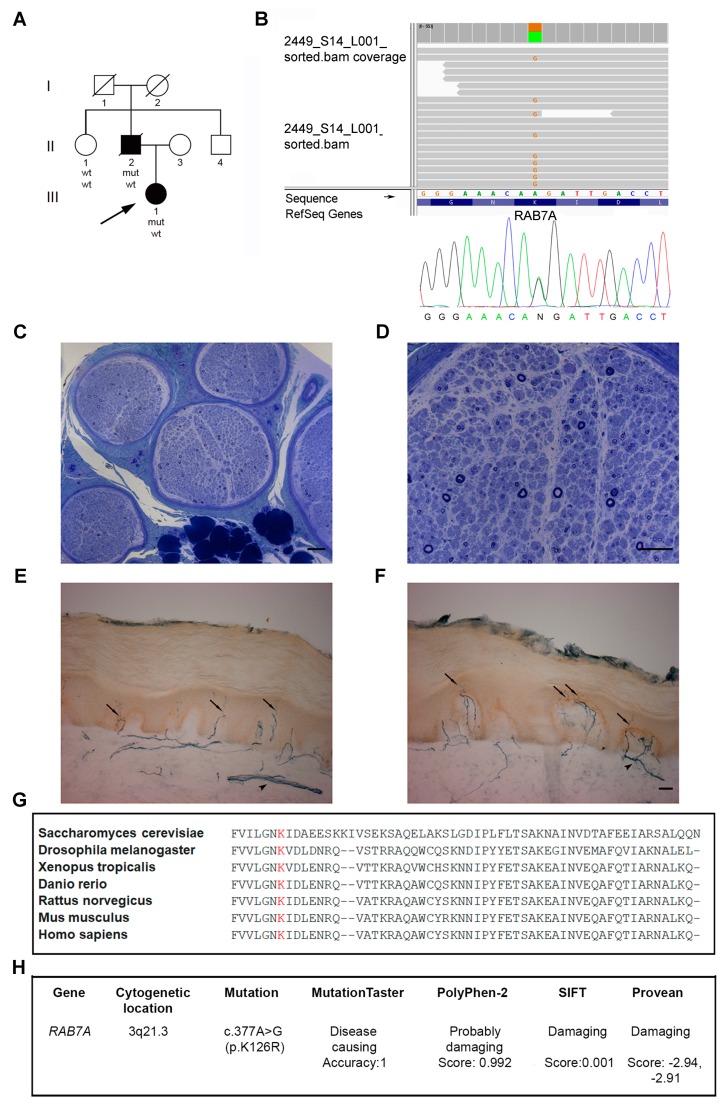
Figure 1.
Pedigree, DNA sequencing, nerve, and skin biopsy of the proband. (A) Family pedigree. (B) Next-Generation Sequencing and Sanger chromatogram of the proband with the heterozygous c.377A>G (p.K126R) variant in the RAB7 gene. (C,D) Sural nerve biopsy from the 18-year-old proband. (C) Semithin section stained with toluidine blue showing a uniform and moderate loss of fibers. (D) At higher magnification, no degenerating or regenerating fibers were observed. Scale bars: C = 100 μm; D = 50 μm. Skin biopsies from the 38-year-old proband (E) and a 52-year-old healthy female individual (F), taken at the medial side of the proximal phalanx of the index finger. (E,F) Immunostaining with anti-protein gene product 9.5 antibodies (PGP9.5) showed a minimal reduction of the intraepidermal nerve fiber (IENF) density in the proband (E) as compared to control (F). Arrows indicate intra-epidermal nerve fibers and arrowheads indicate dermal nerve bundles. Scale bars: E, F = 50 μm. (G) The CLUSTAL multiple sequence alignment by MUSCLE (3.8) shows the conservation of lysine amino-acid at position 126 during evolution [35]. (H) In silico analysis: RAB7K126R variant was predicted to be pathogenic by major online programs.
The index case underwent a biopsy of sural nerve biopsy which was processed for histological and ultrastructural examination [33]. 3-mm skin biopsies were taken (from shoulder and the lateral aspect of the proximal phalanx of the index finger) for fibroblast culture, immunohistochemistry, and intraepidermal nerve fiber (IENF) count [34].
2.2. Gene Sequence Analysis
The proband’s DNA was analyzed by Next Generation Sequencing (NGS) technology with a probe-based customized panel for CMT and related disorders (Illumina Nextera Rapid Capture Custom kit, Illumina Inc., San Diego, CA, USA), (Tables S1–S3). Sequencing was performed using the NGS MiSeq sequencer (Illumina Inc.). The entire RAB7 gene-targeted region (6 coding exons and 25 bp of flanking introns) was sequenced by NGS with a depth of coverage >20×. The sequence variant was confirmed in proband and her father by the Sanger method (Figure 1B).
2.3. Mutagenesis and Plasmid Construction
Most constructs used in this study have been described previously [14,22,29]. RAB7K126R mutant was constructed using the QuickChange XL Site-Directed Mutagenesis Kit (Stratagene, San Diego, CA, USA). The oligonucleotides used to generate the mutant were 5′-GTGTTGGGAAACAGGATTGACCTCG-3′ and 5′-CGAGGTCAATCCTGTTTCCCAACAC-3′. The mutant RAB7K126R plasmids were obtained using RAB7wt cDNA previously cloned in pcDNA3-2xHA or pET-16b vector in frame with DNA coding for hemagglutinin (HA) or poly-His tag, respectively.
2.4. Antibodies
Western blotting analysis (WB): anti-HA (1:500, sc-805), anti-peripherin (1:500, sc-28539) and anti-vinculin (1:10000, sc-25336) were from Santa Cruz Biotechnology, Santa Cruz, CA, USA; anti-actin (1:5000, ab-8224) and anti-tubulin (1:6000, clone B512), were from Sigma-Aldrich, St-Louis, MO, USA, while anti-EGFR (1:2000, 20-ES04) was from Fitzgerald, Concord, MA, USA and anti-RILP (1:400, 13574–1-AP) from Proteintech, Rosemont, IL, USA. Immunofluorescence analysis: anti-early endosome antigen 1 (EEA1, 1:1000, ab70521, Abcam), anti-HA (1:500, ab9110, Abcam), anti-EGFR (1:100, 20-ES04, Fitzgerald). Secondary antibodies conjugated to fluorochromes for immunofluorescence or horseradish peroxidase (HRP) were from Invitrogen (Carlsbad, CA, USA), Santa Cruz Biotechnology or Fitzgerald. Immunohistochemistry: myelin basic protein (MBP, 1:100, ab7349, Abcam), anti-peripherin (1:1000, ab4666, Abcam), anti-vasoactive intestinal peptide (VIP, 1:400, ab22736, Abcam), anti-neurofilament 200 (NF-H, 1:400, N0142, Sigma-Aldrich), anti-PGP9.5 (1:500, MCA4750GA, Bio-Rad, Hercules, CA, USA), anti-EGFR (1:100, 20-ES04, Fitzgerald). Secondary antibodies Alexa Fluor-conjugated (Jackson ImmunoResearch, Cambridge, UK) were employed.
2.5. Cells and Transfection
Neuro2A and NCI H1299 cells, fibroblasts from CMT2B patients and control were grown in DMEM supplemented with 10% or 15% FBS, 2 mM L-glutamine, 100 U/mL penicillin and 10 mg/mL streptomycin.
Transfection was performed using Metafectene PRO reagent (Biontex, München, DE) according to manufacturer’s instructions. Cells were analyzed 24 h after transfection.
2.6. Western Blotting and Co-Immunoprecipitation
Cells were processed for SDS-PAGE and WB as previously described [22]. Frozen sural nerves were pulverized and sonicated in lysis buffer (95 mM NaCl, 25 mMTris-HCl, pH 7.4, 10 mM EDTA, 2% SDS, and protease inhibitors) and lysates were subjected to WB [20].
Densitometry analysis was performed using the NIH ImageJ program or Gene Tools from Syngene and normalized to appropriate loading controls signal intensity. For immunoprecipitation, we used the anti-HA affinity gel (Ezview Red Anti-HA E6779, Sigma-Aldrich) according to the manufacturer’s instruction.
2.7. Confocal Immunofluorescence Microscopy
Cells grown on 12-mm round glass coverslips were fixed, permeabilized with 0.1% Triton X-100 for 5 min at room temperature and incubated with primary and secondary antibodies as previously described [21]. Cells were viewed with a Zeiss LSM700 confocal microscope. Zen 2011 software (Carl Zeiss, Oberkochen, Germany) was used for image capture and to calculate the weighted colocalization coefficient of EGFR and EEA1.
Skin biopsies were sectioned (50 μm) using a standard cryostat (Bio-Optica, Milan, Italy). Immunohistochemistry assays were performed following a standard free-floating protocol. Fluorescent images were acquired with Leica TSC SP8 confocal microscope (Leica Microsystems, Wetzlar, Germany).
2.8. Nucleotide Dissociation and GTPase Assay
Nucleotide exchange assay and determination of Koff were performed as previously reported [36]. The GTPase assay was performed as previously described [37]. GTP hydrolysis was quantified as a GDP signal relative to GDP+GTP signal [37].
2.9. EGFR Degradation Assay
To measure EGFR degradation cells were treated and processed as previously described [22].
2.10. Neurite Outgrowth Assay
Differentiation of Neuro2A was triggered by serum withdrawal as previously described [25]. Cells were co-transfected with the pEGFPC1 vector (to express GFP and visualize the entire cell) and with a plasmid for expression of HA-tagged RAB7wt or mutant proteins, fixed, permeabilized and stained [19]. For each experiment, to determine the percentage of cells bearing neurites (>50 μm), approximately 100 transfected cells were counted in at least 10 randomly chosen visual fields. We compared the number of cells bearing neurites longer than 50 μm between control cells and cells expressing HA-tagged RAB7wt and mutant proteins.
2.11. Real-Time PCR
Total RNA was isolated using the RNeasy Micro kit (Qiagen, Hilden, Germany) and retrotranscription was made using SuperScript II Reverse Transcriptase (Invitrogen) according to the manufacturer’s instructions.
Quantitative real-time PCR was carried out with Power SYBR Green (Applied Biosystems, Foster City, USA) using Applied Biosystems 7900HT Fast Real-time PCR System. The primers used were GAPDH forward 5′-GGTGGTCTCCTCTGACTTCAACA-3′ and reverse 5′-GTTGCTGTAGCCAAATTCGTTGT-3′; EGFR forward 5′-GGCAGGAGTCATGGGAGAA-3′ and reverse: 5′-GCGATGGACGGGATCTTAG-3′ from Eurofins Genomics (Ebersberg, Germany). The thermal profile used: 1 cycle of 2 min at 50 °C; 1 cycle of 10 min at 95 °C; 40 cycles of 15 s at 95 °C, 1 min at 55 °C; 1 cycle of 15 s at 95 °C and 15 s at 60 °C. The relative expression level was calculated using the comparative CT method and expressed as “fold change.” The relative quantification was considered significant when there was a minimum of two-fold change.
2.12. Molecular Modeling Studies
Wild type RAB7 structure was retrieved from the Protein Data Bank (pdb code 1T91) and used to generate the model of RAB7K126R protein (Pymol, The PyMOL Molecular Graphics System, Version 1.2r3pre, Schrödinger, LLC). Molecular dynamics simulations were performed within MOE (MOE, Chemical Computing Group Inc., Montreal H3A 2R7 Canada).
2.13. Statistical Analysis
Data were statistically analyzed using Student’s t-test or χ2 test (* p < 0.05, ** p < 0.01 and *** p < 0.001). Experiments were performed at least in triplicate.
3. Results
3.1. Patients
Patients’ clinical features are reported in Table 2.
Table 2.
Clinical features of patients.
| Patients | Proband | Father |
| Age at examination, yrs. | 39 | 55 |
| Onset age/symptoms | 14/Cramps, gait difficulties |
11/Gait difficulties |
| Motor symptoms legs | Complete footdrop, AFOs | Complete footdrop, AFOs |
| Sensory symptoms legs | Slight sensory loss feet | N |
| Proximal/Distal weakness UL | −/+- (4+; 4+) | −/+ (2;1) |
| Proximal/Distal weakness LL | −/+ (0;3) | −/+ (0;0) |
| Ankle deep tendon reflexes | Absent | Absent |
| Pinprick UL/LL | Normal/Ankles | Normal/Normal |
| Joint position sense UL/LL | Normal/Normal | Normal/Toes |
| Vibration sense UL/LL | Normal/Toes | Normal/Ankles |
| Pes cavus | N | Y |
| Additional features | Mild toenail dystrophy |
Hand tremor |
| Ulnar nerve SAP amplitude | 5.9 µV (ulnar nerve) |
0.8 µV (median nerve) |
| CMTES | 9/28 | 11/28 |
Motor weakness based on Medical Research Council Scale: upper limb distal weakness assessed by first dorsal interosseous and abductor pollicis brevis; upper limb proximal weakness assessed by deltoids, biceps brachii and triceps. Lower limb distal weakness assessed by anterior tibialis and gastrocnemius; lower limb proximal weakness assessed by iliopsoas and quadriceps muscles. Values are based on the side that gave the worst score. Abbreviations: AFO = ankle-foot orthosis; UL = upper limbs; LL = lower limbs; N = no; Y = yes; SAP = sensory action potential; CMTES = Charcot–Marie–Tooth examination score; + = weakness present; - = weakness absent; +- = mild weakness.
The proband (III-1) (Figure 1A), a 39-year-old female, at age 14 years started complaining of calf muscle cramps, progressive walking difficulties, distal muscle wasting and weakness, recurrent ankle sprains and balance issues. At age 31 years, she underwent bilateral foot surgery for Achilles tendon lengthening and toes straightening. Afterward, she started wearing ankle-foot orthoses (AFOs). She reported no symptoms in upper limbs and minimal sensory loss in the feet. Neurological examination showed steppage gait, mild wasting, and weakness of intrinsic hand muscles (MRC 4+), moderate distal atrophy in lower limbs, severe paresis of toe and foot dorsiflexors (MRC 0), and moderate foot plantar-flexion weakness (MRC 3). She had mild pinprick sensory loss in the feet, slightly reduced vibration sense at the great toe, and absent ankle jerks. She had neither ulcers nor amputations. CMTES was 9/28.
Nerve conduction studies (NCS) showed no motor responses in the peroneal nerves, decreased compound muscle action potential (CMAP) amplitude in the tibial nerve (0.4 mV), the reduced amplitude of right ulnar nerve (5.9 µV) sensory action potential (SAP), unobtainable sural nerve SAP, with normal findings at other examined motor and sensory nerves in upper limbs. Nerve conduction velocities (NCVs) were preserved. EMG showed severe neurogenic changes in lower limb distal muscles.
Nerve biopsy (age 18) showed a chronic axonal neuropathy, with moderate and diffuse loss of myelinated fibers of all calibers (Figure 1C); residual fibers had normal myelin sheaths. There were no signs of ongoing degeneration or regeneration (Figure 1D). Electron microscopy confirmed the histologic findings disclosing some collagen pockets or denervated, flattened, Schwann cell processes indicating loss of unmyelinated fibers.
Skin IENF density (age 38) was 5.9 fibers/mm, showing only a minimal difference (Figure 1E) with a gender-matched control (6.4 fibers/mm, Figure 1F).
Her father (II-2) (Figure 1A) had symptom onset at age 11 years, manifesting progressive foot-drop and walking difficulties, with later occurrence of wasting and weakness of hand muscles, difficulties in doing buttons, and balance loss. He did neither undergo foot surgery nor complain of positive sensory symptoms. Neurological examination at age 55 years showed pes cavus, ataxic and steppage gait, positive Romberg sign, moderate hand tremor, severe distal limb wasting and weakness, with intrinsic hand muscles MRC = 1–2 and complete loss of foot movements, absent ankle jerks, reduced position sense at the great toes, and vibration sense at the feet. Neither ulcers nor amputations were present. CMTES was 11/28 and CMTNS 17/36. NCS was consistent with a severe axonal motor-sensory polyneuropathy: the absence of sensory and motor responses in lower limbs, markedly reduced amplitude of the median CMAP (0.1 mV), ulnar (0.8 µV) and radial (4.4 µV) SAPs. He died of acute leukemia a few months later.
Proband’s aunt (II-1) (Figure 1A) had no history of neuropathy and normal examination and NCS. The grandparents were reported to have no symptoms and died in their 60s (I-2) or 70s (I-1); the uncle (II-4) was asymptomatic and refused clinical examination and genetic testing.
3.2. Identification of a Novel RAB7 Mutation Causing CMT2B
NGS analysis revealed a heterozygous missense variant in RAB7, c.377A>G (p.K126R), in proband and affected father which was absent in the unaffected aunt and the major gene variant databases (Single Nucleotide Polymorphism database 150, 1000 Genomes, Exome Variant Server, Exome Aggregation Consortium, Genome Aggregation Database) (Table S2). The variant, confirmed in both patients by Sanger sequencing (Figure 1B), is located in a highly conserved amino-acid in the GTPase domain (Figure 1G) and predicted to be deleterious by in silico analysis (Figure 1H).
3.3. Characterization of the Biochemical Properties of the RAB7K126R Mutant Protein
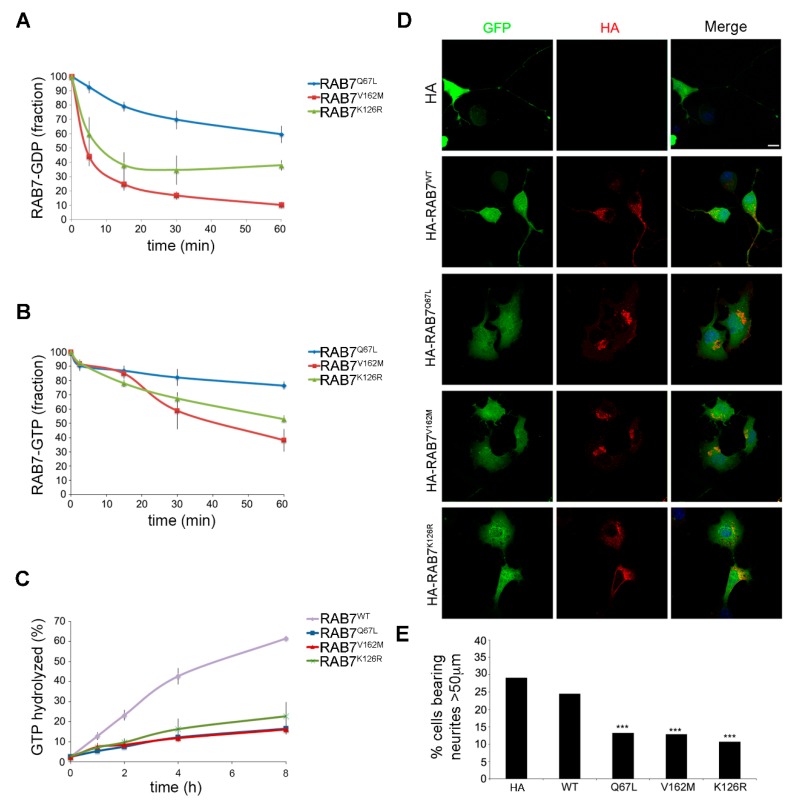
To study the biochemical properties of the RAB7K126R mutant, we analyzed the dissociation rate constants (Koff) for GDP and GTP. We bacterially expressed His-tagged RAB7Q67L (a constitutively active mutant, impaired in GTP hydrolysis that displays nucleotide Koff similar to RAB7wt), RAB7V162M (a previously characterized CMT2B mutant) and the new RAB7K126R mutant and purified them by affinity chromatography. Then, we determined the Koff for GDP and GTP measuring radioactivity loss over time (Figure 2A,B). As expected, RAB7Q67L showed GDP and GTP Koff similar to previously published data on RAB7wt [22,36] while the RAB7V162M CMT2B mutant protein displayed higher Koff for both GTP and GDP, as previously reported [22]. Interestingly, also the RAB7K126R, similarly to the other CMT2B-causing mutant proteins, exhibited Koff values significantly higher for GDP and GTP (Figure 2A,B).
Figure 2.
Biochemical and biological properties of the RAB7K126R mutant protein. (A,B) Dissociation of guanine nucleotides from RAB7K126R, RAB7Q67L, and RAB7V162M. Purified proteins were incubated with [3H]GDP (A) or [3H]GTP (B), then a 100× excess of cold competitor (GDP or GTP) was added and dissociation of nucleotide was monitored for 1h to calculate Koff. (C) The measure of GTPase activity of RAB7wt, RAB7K126R, RAB7Q67L, and RAB7V162M purified proteins per binding event. Proteins were loaded with [32P]GTP and then GTP hydrolysis was monitored during 8 h adding 100× fold excess of unlabeled GTP. (D,E) To analyze the effect of RAB7K126R on neurite outgrowth, Neuro2A cells were co-transfected with the indicated HA-tagged RAB7wt and mutant expressing constructs and with the pEGFPC1 plasmid to visualize entire cell and incubated for 24 h in serum-free medium to induce cell differentiation. (D) Confocal microscopy images of a field of cells per condition are shown. Scale bar = 10 μm. (E) Quantification of neurite outgrowth in Neuro2A cells expressing different RAB7wt and mutant proteins. Neurites were defined as processes longer than 50 μm. The percentage of cells bearing neurites was calculated as the number of cells with neurites divided by the total number of cells. We compared the number of cells bearing neurites longer than 50 μm between control cells (cells transfected with pEGFPC1 and HA empty plasmid) and cells expressing RAB7wt and mutant proteins. We show a statistical analysis of three independent experiments (χ2 test, *** p < 0.001).
We also measured GTPase activity per binding event by loading the purified His-tagged RAB7wt, RAB7Q67L, RAB7V162M and RAB7K126R with 32P-GTP (Figure 2C). The RAB7 estimated hydrolysis rate constant was consistent with previous reports [22,36]. Moreover, as previously reported, the RAB7Q67L and the CMT2B-causing RAB7V162M mutants showed lower GTPase activity [22]. Notably, also the RAB7K126R showed impaired GTPase activity per binding event similarly to the RAB7V162M mutant (Figure 2C).
Altogether these data demonstrate that the RAB7K126R mutant displays higher nucleotide Koff (particularly high for GDP) and impaired GTP hydrolysis per binding event, similarly to previously analyzed CMT2B-causing mutant proteins [22,23,24].
3.4. Expression of the RAB7K126R Mutant Inhibits Neurite outgrowth in Neuro2A Cells
To analyze the impact of the RAB7K126R mutant on a neuronal-specific process, we studied the effect on neurite outgrowth in neuroblastoma Neuro2A cells, which were transfected with the pEGFP plasmid to visualize the cells and therefore, the neurites. Cells were co-transfected with the HA empty plasmid (control cells) or with plasmids encoding HA-tagged RAB7wt, RAB7Q67L, RAB7V162M, or RAB7K126R. After transfection and differentiation, the cells were processed for immunofluorescence analysis using anti-HA antibodies to identify transfected cells and scored for the presence of neurites longer than 50 μm (Figure 2D). About 30% of control cells had long neurite after serum withdrawal and similar values were obtained also in cells overexpressing RAB7wt (Figure 2E). In contrast, as expected, expression of the RAB7Q67L or RAB7V162M mutant resulted in a strong neurite outgrowth inhibition (Figure 2E), consistent with previous results [25]. Similarly, RAB7K126R mutant expression considerably inhibited neurite outgrowth, by about 60% (Figure 2E).
3.5. Analysis of Peripherin and RILP, two known Downstream RAB7 Effectors
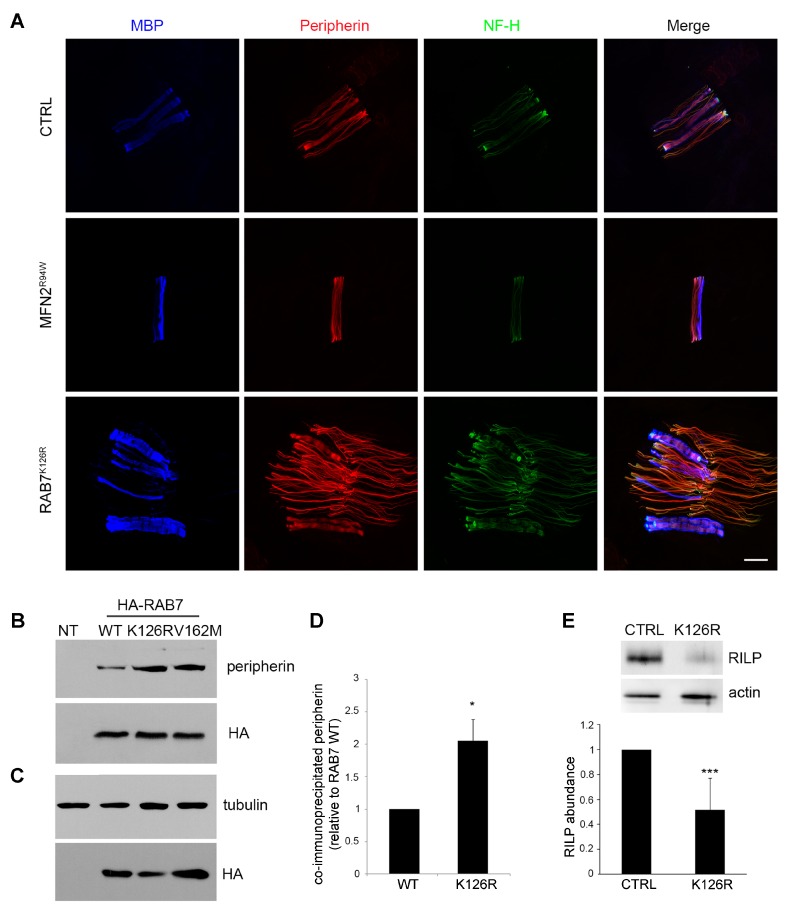
RAB7 interacts with peripherin, an intermediate filament protein expressed mainly in peripheral nerves, and CMT2B-causing mutants display a stronger interaction compared to RAB7wt [21]. Immunohistochemical study for peripherin in association with anti-MBP and anti-NF-H antibodies showed a markedly increased expression of peripherin and NF-H in the proband compared to a CMT2A patient (MFN2R94W, axonal neuropathy control) and a healthy control (Figure 3A).
Figure 3.
Analysis of two RAB7 interactors: peripherin and RAB-interacting lysosomal protein (RILP). (A) Immunohistochemical staining was performed on skin biopsy section using antibodies direct to peripherin, neurofilament-200, and MBP (myelinated nervous fibers) in the proband, in a patient with a mutation in MFN2 (axonal neuropathy), and in a healthy control. Scale bar = 20 μm in all images. (B) HA-tagged RAB7wt and mutant proteins were expressed in Neuro2A cells, as indicated, immunoprecipitated with an anti-HA antibody and subjected to WB using anti-peripherin and anti-HA antibodies. (C) Total extracts of Neuro2A cells, overexpressing the indicated proteins, were analyzed using anti-HA and anti-tubulin antibody to evaluate expression levels of the proteins. (D) Quantification of immunoprecipitated peripherin. Values are the mean of three independent experiments ± s.e.m.. The intensities were quantified by densitometry, normalized against the amount of RAB7wt or RAB7K126R, and plotted as a percentage of the intensities obtained using RAB7wt (set to 1). Data were analyzed statistically using Student’s t-test. Values of cells expressing RAB7K126R were found to be significantly different from the values of cells expressing RAB7wt (* p < 0.05). (E) Lysates of the sural nerve from a healthy individual (CTRL) and proband carrying the RAB7K126R mutation were analyzed by immunoblotting using anti-RILP and anti-actin antibodies. The intensities of the RILP bands were quantified using Gene Tools from Syngene and normalized against actin. The intensity of the RILP band in CTRL cells has been set to 1. Data represent the mean ± s.e.m. of six independent experiments. Statistical analysis was performed using Student’s t-test. *** p < 0.001.
To investigate how the RAB7K126R mutant interacts with the peripherin, we performed a co-immunoprecipitation experiment (Figure 3B,C). Neuro 2A cells were transfected with the empty plasmid or with constructs encoding HA-tagged RAB7wt, RAB7K126R, and RAB7V162M and then lysed. HA-tagged proteins were immunoprecipitated and subjected to WB using anti-HA and anti-peripherin antibodies. As expected, peripherin was co-immunoprecipitated by HA-tagged RAB7wt (Figure 3B). The RAB7V162M mutant was able to co-immunoprecipitate peripherin more efficiently, in accordance with previous data [21]. Similarly, peripherin was more efficiently co-immunoprecipitated by the RAB7K126R mutant as compared to RAB7wt (Figure 3B–D).
As RAB7, in association with RILP, controls the late endocytic pathway [21], we decided to analyze RILP abundance in proband’s sural nerve lysate. Similarly to what described for the disease-causing RAB7N161T mutant in an immunostaining experiment [2], the amount of RILP was reduced by almost half (Figure 3E).
3.6. Expression of the RAB7K126R Mutant Inhibits Ligand-Induced EGFR Degradation
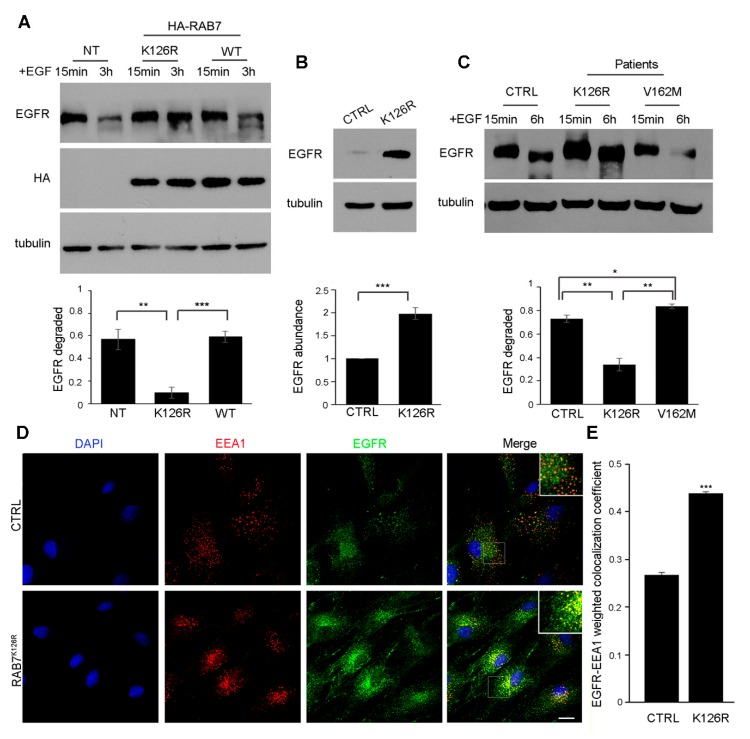
To establish if the RAB7K126R mutant affects EGFR degradation, a cellular process normally regulated by RAB7 [28] (Figure S1), we performed an EGFR degradation assay (Figure 4A). Quantification revealed that, in agreement with previous data [22,23], about 60% of EGFR was degraded in control cells and in cells expressing RAB7wt (Figure 4A). In contrast, the expression of RAB7K126R strongly inhibited degradation that was significantly different from RAB7wt (Figure 4A). Thus, with respect to EGFR degradation, the RAB7K126R seems to behave differently from the previously discovered CMT2B-causative mutant proteins as a transient expression of these mutants increased EGFR degradation [22,23].
Figure 4.
Expression of RAB7K126R inhibits ligand-induced EGFR degradation affecting EGFR intracellular trafficking. (A) NCI-H1299 cells overexpressing HA-RAB7K126R or HA-RAB7wt were treated with cycloheximide for 1 h and stimulated for 15 min or 3 h with EGF. Cells were then lysed and analyzed by WB using a specific anti-EGFR antibody to detect undegraded EGFR, and anti-HA and anti-tubulin antibodies to verify RAB7 overexpression and equal loading, respectively. The amount of EGFR degraded at 3 h was quantified and plotted as a percentage of the respective intensity at 15 min set to 1. The error bars represent the s.e.m. of three independent experiments. According to a Student’s t-test, * p < 0.05; ** p < 0.01. (B) Lysates of dermal fibroblasts from a healthy individual (CTRL) and CMT2B patients carrying the RAB7K126R mutation were analyzed by immunoblotting using anti-EGFR and anti-tubulin antibodies. The intensities of the EGFR bands were quantified using NIH ImageJ and normalized against tubulin. The intensity of the EGFR band in CTRL cells has been set to 1. Data represent the mean ± s.e.m. of six experiments. Student’s t-test, *** p < 0.001. (C) Fibroblasts derived from control and CMT2B patients were incubated with cycloheximide and stimulated with EGF for the indicated times. Cell lysates were analyzed by immunoblotting with antibodies against EGFR and tubulin. Densitometric analysis was performed with NIH ImageJ normalizing against tubulin. Data represent the mean ± s.e.m. of at least three experiments. Student’s t-test, * p < 0.05; ** p < 0.01. (D) Dermal fibroblasts from CTRL and the CMT2B proband carrying the RAB7K126R mutation incubated with cycloheximide and stimulated with EGF for 3 h were subjected to immunofluorescence analysis using specific anti-EGFR (green) and anti-EEA1 (red) antibodies followed by the appropriate secondary antibodies. Scale bar = 20 μm. (E) Data represent the mean ± s.e.m. of at least 50 cells of three independent experiments. Statistical analyses were performed using Student’s t-test with control fibroblasts as a referring sample. *** p < 0.001.
3.7. EGFR Amount, Degradation and Intracellular Distribution are Altered in Patient Fibroblasts Carrying the RAB7K126R Mutation
To validate data obtained by transiently expressing the CMT2B-causing RAB7 mutant proteins we performed the assay in skin fibroblasts from the proband and a control. We evaluated first the total EGFR amount in the absence of EGF stimulation. Interestingly, in fibroblasts carrying the RAB7K126R mutation the amount of EGFR was higher compared to control cells (Figure 4B), while EGFR transcript quantification by RT-PCR revealed no significant change in EGFR mRNA levels (data not shown), thus suggesting a difference in EGFR degradation. Therefore, we performed an EGFR degradation assay and, notably, we found that EGFR degradation was strongly inhibited in fibroblasts carrying the RAB7K126R mutation as compared to control cells (Figure 4C). In contrast, EGFR degradation was increased in fibroblasts carrying the V162M mutation (Figure 4C), consistently with previous data [22].
Altogether, these data suggest that the accumulation of EGFR in RAB7K126R is mainly due to impaired protein degradation.
To determine whether the impaired EGFR degradation is due to trafficking alteration we performed immunofluorescence analysis of patient and control fibroblasts using specific antibodies against EGFR and a marker of the early endosomes EEA1 (Figure 4D). Interestingly, EGFR intracellular distribution differed between control and patient cells: in patient cells, the amount of EGFR colocalizing with EEA1 was strongly increased, suggesting impaired trafficking of EGFR to late endocytic organelles (Figure 4E).
3.8. EGFR Amount is Altered in Patient Tissues
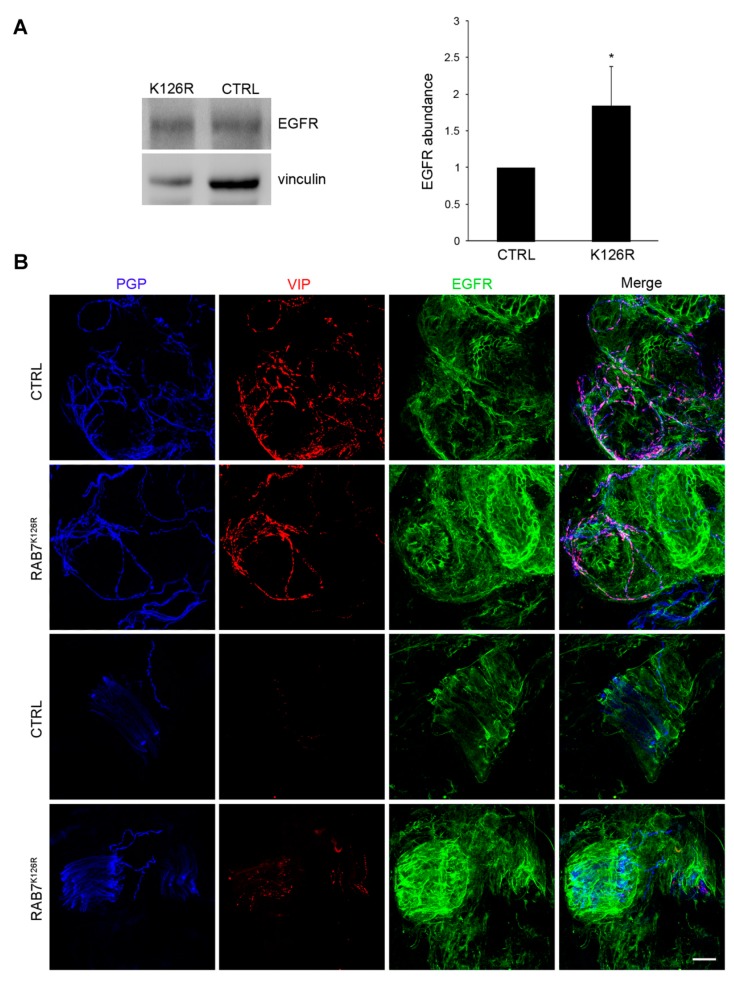
We performed WB of sural nerve lysates from the proband and a gender-matched healthy control and we found an almost twofold increase in EGFR expression (Figure 5A), in agreement with in vitro results. EGFR expression was also studied in skin biopsy sections: a qualitative analysis of confocal images, stained using antibodies against EGFR, PGP9.5 (pan-axonal marker), and VIP (sympathetic cholinergic fibers’ marker), showed a widespread receptor overexpression in the proband, including areas around nerve fibers (Figure 5B).
Figure 5.
Expression of EGFR in proband sural nerve and skin biopsy sections. (A) Sural nerves from the proband and a gender-matched healthy control were lysed and EGFR protein expression was analyzed in both samples by WB using a specific anti-EGFR antibody. Antibody against vinculin was employed as a loading control. Densitometric analysis was performed with Gene Tools from Syngene. Data represent the mean ± s.e.m. of four experiments. The intensity of the EGFR band in CTRL cells has been set to 1. Statistical analysis was performed using Student’s t-test. * p < 0.05. (B) Confocal microscopy study of a skin biopsy from the proband and a healthy control using a specific anti-EGFR antibody. Nervous fibers are labeled with PGP9.5 (pan-axonal marker) and VIP (sympathetic cholinergic fibers’ marker) antibodies. Scale bar = 20 μm in all images.
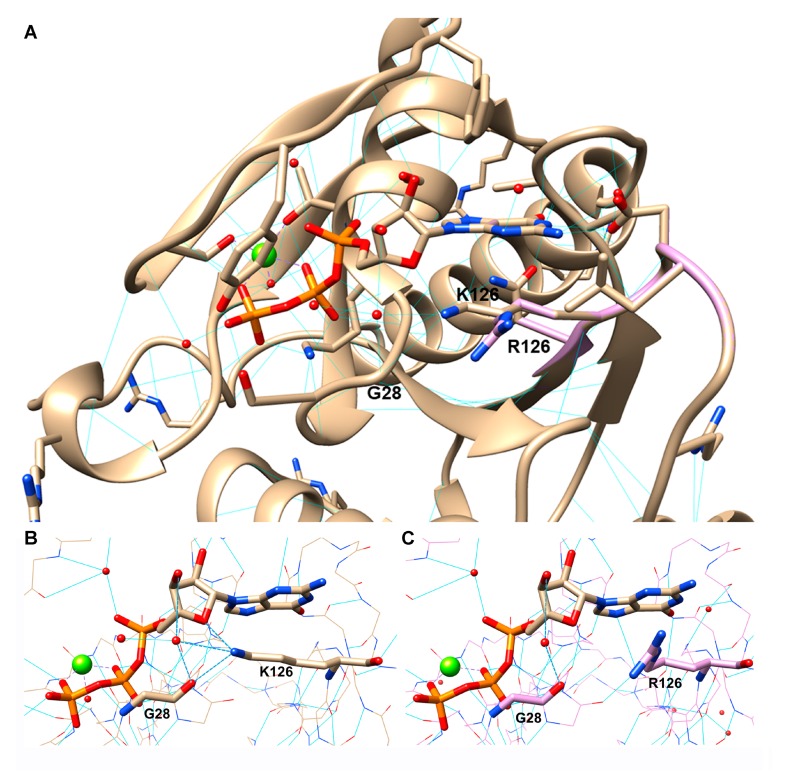
3.9. RAB7K126R Computational Model
Residue 126 is located in the GTPase binding site, where the lysine side chain forms two H-bonds with the nucleotide. The first H-bond is made with the ribose ring oxygen, while the second one is an H-bond bridging the amino group with the hydroxyl group on position 4 of the sugar, via a conserved water molecule (Figure 6A). Interestingly, this H-bond bridge involves glycine 28 too, contributing to stabilize not only GTP inside its pocket but also the pocket secondary structure. According to the 3D model, the arginine side chain is unable to perform this H-bonds network, being differently oriented due to the bigger volume of its terminal guanidine group. Thus, RAB7K126R mutation, as previously reported,33 seems to reduce the GTP anchoring inside the protein and consequently to determine an impaired RAB7 functionality. Short molecular dynamics simulations performed on both wild type and mutated GTP bound complexes confirm these observations (Figure 6B,C).
Figure 6.
Structural insights into the disease-causing RAB7K126R mutant. (A) Comparison between the wild type (pdb code 1T91) and the virtual model of the mutated protein, colored in light brown and light pink, respectively. The RAB7K126R mutation is located in the GTPase domain. (B,C) The H-bond interactions performed by K126 (B) or R126 (C), GTP, a conserved water molecule and G28. The protein is colored in light brown (B) or light pink (C). Bold dashed blue lines highlight the specific H bond net performed by residue 126, GTP, G28, and water. Light blue lines indicate H-bonds. Magnesium ion is represented as a green sphere and water molecules are represented as red spheres.
4. Discussion
CMT2B has long been considered mainly a sensory neuropathy with a large overlap with the HSANs. Only five RAB7 mutations have been reported, all involving the GTP binding domain and associated with a mainly sensory phenotype.
We identified and characterized a novel missense variant (p.K126R) in RAB7 associated with predominantly motor CMT2 in the two affected family members. Notably, both subjects had remarkable muscle wasting and weakness with only minor sensory signs, in keeping with CMT2 with motor predominance, never linked previously to RAB7 mutations. All previous families showed ulcers and amputations, with variable motor involvement (Table 1). Lower occurrence of ulcers is reported in CMT2B females [4], but the motor predominant phenotype was shown also by the father, ruling out a gender effect.
The p.K126R variant affects, like the other CMT2B mutations, the RAB7 GTP-binding domain and its pathogenicity is supported by the involved site, segregation analysis, and in silico predictions. The lysine 126 is very conserved not only in RAB7 during evolution (Figure 1G) but also in many other GTPases as this amino-acid is part of the switch II region; it is one of the amino-acids forming the guanine binding pocket and it is a crucial amino-acid residue involved in the hydrogen bond with the nucleotide ribose oxygen [38]. Importantly, mutations in this amino-acid residue are predicted to have an increased rate of dissociation of the nucleotides favoring the active GTP-bound state [38]. Despite arginine shares with lysine basic and charged features, it impairs the GTP pocket architecture. The bulkier side chain of arginine exceeds lysine volume and is thus unable to perform the same specific interactions with GTP and some amino acid residues (water bridging with glycine 28) in the surrounding pocket (Figure 6B,C).
We carried out a series of functional experiments to demonstrate the RAB7K126R pathogenic role and look for differences with other RAB7 mutants, to explain the distinctive predominantly motor phenotype. Our studies confirmed that the mutation affects a series of RAB7 properties, very similar to previously characterized mutants, but with at least one important difference.
First, biochemical data indicate that the mutant protein has an increased Koff for both nucleotides and particularly high for GDP (Figure 2A), inhibiting also GTPase activity per binding event (Figure 2C), similarly to previous uncovered CMT2B-causing RAB7 mutant proteins [22,23,24]. Interestingly, the corresponding mutation in the HRAS gene, a gene encoding a member of the RAS superfamily of small GTPases as the RAB7 gene, is associated with the Costello syndrome, a disease causing an increased risk of developing tumors in different organs further supporting pathogenicity of this variant [39].
Second, expression of RAB7K126R strongly inhibits neurite outgrowth, compared to the other CMT2B-causing RAB7 mutants (Figure 2D) [25,26]. As the onset of CMT2B occurs in the second or third decade, similarly to many other CMT types, development appears to be unaffected. However, the mutant proteins can rather impact on axonal regeneration, considering that this process recapitulates all the stages of neuronal differentiation [40]. Indeed, the detrimental action of RAB7 mutants could be counteracted by other factors during development, but with age, these factors could become less effective and a decrease in regeneration capabilities may contribute to the progressive axonal loss underlying clinical manifestations.
Third, the RAB7K126R mutant protein interacts more strongly with the intermediate filament peripherin (Figure 3B–D), as for already reported RAB7 mutations. Peripherin is important for neuronal morphology, maturation, and differentiation, but also axonal regeneration [41,42]. RAB7 affects peripherin assembly [21] and altered RAB7-peripherin interaction could impair axonal regeneration thus contributing to nerve degeneration. Notably, we found increased labeling of peripherin in skin biopsy where numerous peripherin-positive filamentous structures were seen in contrast to the few seen in controls (Figure 3A).
With respect to both findings, it is noteworthy that the nerve biopsy showed—at least for this single sensory nerve—no evidence of regenerating clusters despite the moderate loss of myelinated fibers (Figure 1D).
Fourth, RAB7K126R mutant protein strongly reduces RILP downstream effector levels (Figure 3E), similarly to RAB7N161T [2]. Notably, in previous functional studies, siRNA-mediated RILP depletion was sufficient to cause strong effects on EGFR degradation and the biogenesis of late endosomes [27,43].
Fifth, our data demonstrate also that expression of the RAB7K126R mutant protein causes inhibition of EGFR degradation in contrast to previously studied CMT2B-RAB7 mutants [22,23]. Indeed, expression of the RAB7L129F, RAB7K157N, RAB7N161T, and RAB7V162M mutants did not reveal any inhibition of EGFR degradation but rather a small and reproducible, though not statistically significant, increase [22,23]. Furthermore, analysis of EGFR degradation in RAB7V162M fibroblasts revealed an increase of EGFR degradation compared to control fibroblasts (Figure 4C), while, expression of RAB7K126R caused EGFR degradation inhibition (Figure 4A), which was further confirmed in patient fibroblasts (Figure 4C). In addition, the total EGFR amount was strongly increased in the RAB7K126R patient compared to control fibroblasts (Figure 4B). These findings were confirmed by WB on the sural nerve (Figure 5A) and immunohistochemistry on skin biopsy which showed increased EGFR staining in the proband compared to the control although primarily in the surround of nerve fibers (Figure 5B). Three hours after internalization most of EGFR was still in early endosomes in patient cells, demonstrating that impaired EGFR trafficking to late endosomes and lysosomes causes inhibition of degradation and accumulation of EGFR (Figure 4D,E).
It is tempting to speculate that the prominent motor symptoms observed in our patients might be related to the differences in EGFR amount and degradation rate in contrast with previously studied CMT2B mutants associated with the typical sensory-predominant phenotype. EGF signaling mediated by EGFR is important for development and maintenance of various tissues including the nervous system, and EGFR is expressed in differentiated post-mitotic neurons suggesting pleiotropic functions, although the possibility for this receptor to exert distinct actions on different kind of neurons (e.g., motor versus sensory) is still unclear. Besides its key role in regulating neural stem cell proliferation, self-renewal, differentiation, and migration, EGF has neurotrophic and neuromodulatory functions on different kinds of neurons, increasing neuronal survival [31]. EGFR signaling is also required for proper cutaneous innervation during development and it seems to limit axonal outgrowth and branching [44]. Interestingly, after central nervous system (CNS) injuries and diseases, activation of the EGFR pathway triggers quiescent astrocytes to become reactive and destructive to neurons [45,46]. After spinal cord injury, EGFR activates astrocytes that represent a physical barrier to axon regeneration, expressing and secreting molecules that inhibit nerve growth [47]. Consistently, rats subjected to weight-drop spinal cord injury treated with a potent EGFR inhibitor to the injured area show motor and sensory function improvement [48]. Lack of EGFR expression is associated with progressive neurodegenerative disorders in mice [49,50]. Overexpression of EGFR is associated with cancer (high-grade glioma) [51]. Moreover, EGFR has a crucial role in the neurometabolic axis seen in the aging process [52]. Glia and endothelial cells demonstrate induced expression of EGFR after an acute injury or chronic neurodegeneration and there are multiple and mechanistically diverse routes by which the EGFR may be involved in the neuronal aspect of aging-related disorders and neurodegeneration. Consistently, it has been demonstrated that inhibition of EGFR enhances peripheral nerve regeneration after injury [30,53,54].
Thus, the inhibited degradation and the consequent accumulation of EGFR observed in RAB7K126R cells could cause an increase in EGFR signaling leading to inhibition of axonal regeneration. The above-mentioned absence of nerve regeneration at nerve biopsy in our patient is of interest in this context.
EGFR has also been implicated in the expansion of the peripheral nervous system (PNS) progenitor cells, including Schwann cell precursors [55]. After peripheral nerve injury, EGFR expression is upregulated and promotes proliferation and migration of Schwann cells [56]. Importantly, EGFR hyperactivation induces a peripheral neurodegenerative disease as transgenic mice overexpressing the EGFR ligand epigen show a phenotype similar to CMT1A and CMT4F animal models [57].
Although there are at present no data regarding a specific role of EGFR in motoneurons, literature data on EGF signaling and EGFR in both CNS and PNS, suggest that the inhibition of EGFR degradation could be a possible explanation for the different clinical presentation in our family compared to the previously studied CMT2B families.
Recently, Wong et al. showed a role for RAB7 in driving the interactions between lysosomes and mitochondria, opening a new field of investigations for unraveling RAB7 functions [16]. We did not find any evidence of mitochondrial abnormalities in the proband’s sural nerve, but it will be interesting to study the role of mitochondria in CMT2B in the future and to verify whether these organelles play a key role in the phenotype and its variants.
5. Conclusions
We demonstrated that the behavior of the RAB7K126R protein was similar to the previously studied CMT2B-causative RAB7 mutated proteins in all investigated functions except EGFR degradation, as RAB7K126R showed inhibited degradation of the receptor. It is tempting to speculate that such difference might explain the distinctive clinical presentation in this family, but further studies are needed. This report expands the phenotypic spectrum of CMT2B: RAB7 has to be added to the list of genes causing the classic CMT2 phenotype with motor predominance and must be considered in the genetic work-up of these patients.
Acknowledgments
We thank the patients who participated in the study. We thank Claudia Ciano and Vidmer Scaioli for performing nerve conduction studies.
List of Abbreviations
AFO: ankle-foot orthosis; CMAP: compound muscle action potential; CMT2B: Charcot–Marie–Tooth type 2B; CMTES/CMTNSv2: CMT Examination/Neuropathy Score version 2; CNS: central nervous system; DTT: dithiothreitol; EEA1: early endosome antigen 1; EGFR: epidermal growth factor receptor; ESCRT-II: endosomal sorting complex required for transport; HA: hemagglutinin; HRP: horseradish peroxidase; HSANs: hereditary sensory and autonomic neuropathies; IENF: intraepidermal nerve fiber; MAF: minor allele frequency; MBP: myelin basic protein; MRC: Medical Research Council; NCS: nerve conduction studies; NF-H: high molecular weight neurofilament; MTOC: microtubule organizing center; NGS: Next Generation Sequencing; NCV: nerve conduction velocities; PGP9.5: protein gene product 9.5; PNS: peripheral nervous system; PTEN: phosphatase and tensin homolog; RAB7A: Ras-related in brain 7a; RILP: RAB-interacting lysosomal protein; SAP: sensory action potential; TBC1D15: Tre-2-Budding Uninhibited by Benzimidazole-Cell Division Cycle 16 Domain, Domain Family Member 15; TrkA: Tropomyosin receptor kinase A; VIP: vasoactive intestinal peptide; VPS22: vacuolar-sorting protein 22.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4409/9/4/1028/s1, Figure S1: Endocytosis pathway as shown by the KEGG database (modified) [58,59,60], Table S1: List of genes analyzed (Charcot–Marie–Tooth disease and related disorders), Table S2: Rare genetic variants (MAF < 1%) found in the analyzed genes of the proband, Table S3: Common genetic variants (MAF ≥ 1%) found in the analyzed genes of the proband.
Author Contributions
Conceptualization: P.S., M.D.L., D.P., and C.B. Project administration, funding, and supervision: D.P. and C.B. Investigation, data curation, and/or validation: P.S., M.D.L., V.N., C.P., R.R., G.P., M.M.R., J.M.P., T.C., G.M.F., P.F., E.C., R.L., G.L., S.M., and F.T. Writing—original draft: P.S., M.D.L., P.F., D.P., and C.B.; Writing—review and editing: all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Telethon Italy (Grant GGP16037 to C.B. and GUP13006 to D.P.) and Associazione Italiana per la Ricerca sul Cancro (AIRC Investigator Grant 2016 N.19068 to C.B.). R.R. was supported by Consorzio Interuniversitario Biotecnologie (CIB) with a travel grant. D.P., C.P., P.S., and G.P. are part of the Inherited Neuropathy Consortium (INC), which is within the NCATS Rare Diseases Clinical Research Network (RDCRN) and is supported also by the Muscular Dystrophy Association (MDA) and the Charcot-Marie-Tooth American Association (CMTA). D.P., C.P., P.S., M.M.R., R.L., G.L., S.M., and F.T. are members of the European Reference Network for Rare Neuromuscular Diseases (ERN EURO-NMD).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Verhoeven K., De Jonghe P., Coen K., Verpoorten N., Auer-Grumbach M., Kwon J.M., FitzPatrick D., Schmedding E., De Vriendt E., Jacobs A., et al. Mutations in the small gtp-ase late endosomal protein rab7 cause charcot-marie-tooth type 2b neuropathy. Am. J. Hum. Genet. 2003;72:722–727. doi: 10.1086/367847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Houlden H., King R.H., Muddle J.R., Warner T.T., Reilly M.M., Orrell R.W., Ginsberg L. A novel rab7 mutation associated with ulcero-mutilating neuropathy. Ann. Neurol. 2004;56:586–590. doi: 10.1002/ana.20281. [DOI] [PubMed] [Google Scholar]
- 3.Meggouh F., Bienfait H.M., Weterman M.A., de Visser M., Baas F. Charcot-marie-tooth disease due to a de novo mutation of the rab7 gene. Neurology. 2006;67:1476–1478. doi: 10.1212/01.wnl.0000240068.21499.f5. [DOI] [PubMed] [Google Scholar]
- 4.Manganelli F., Pisciotta C., Provitera V., Taioli F., Iodice R., Topa A., Fabrizi G.M., Nolano M., Santoro L. Autonomic nervous system involvement in a new cmt2b family. J. Peripher. Nerv. Syst. 2012;17:361–364. doi: 10.1111/j.1529-8027.2012.00415.x. [DOI] [PubMed] [Google Scholar]
- 5.Wang X., Han C., Liu W., Wang P., Zhang X. A novel rab7 mutation in a chinese family with charcot-marie-tooth type 2b disease. Gene. 2014;534:431–434. doi: 10.1016/j.gene.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 6.Pareyson D., Saveri P., Piscosquito G. Charcot-marie-tooth disease and related hereditary neuropathies: From gene function to associated phenotypes. Curr. Mol. Med. 2014;14:1009–1103. doi: 10.2174/1566524014666141010154205. [DOI] [PubMed] [Google Scholar]
- 7.Kwon J.M., Elliott J.L., Yee W.C., Ivanovich J., Scavarda N.J., Moolsintong P.J., Goodfellow P.J. Assignment of a second charcot-marie-tooth type ii locus to chromosome 3q. Am. J. Hum. Genet. 1995;57:853–858. [PMC free article] [PubMed] [Google Scholar]
- 8.Auer-Grumbach M., De Jonghe P., Wagner K., Verhoeven K., Hartung H.P., Timmerman V. Phenotype-genotype correlations in a cmt2b family with refined 3q13-q22 locus. Neurology. 2000;55:1552–1557. doi: 10.1212/WNL.55.10.1552. [DOI] [PubMed] [Google Scholar]
- 9.De Jonghe P.V.T., FitzPatrick D., Spoelders P., Martin J.J., Van Broeckhoven C. Mutilating neuropathic ulcerations in a chromosome 3q13-q22 linked charcot-marie-tooth disease type 2b family. J. Neurol. Neurosurg. Psychiatry. 1997;62:570–573. doi: 10.1136/jnnp.62.6.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Auer-Grumbach M., Wagner K., Timmerman V., De Jonghe P., Hartung H.P. Ulcero-mutilating neuropathy in an austrian kinship without linkage to hereditary motor and sensory neuropathy iib and hereditary sensory neuropathy I loci. Neurology. 2000;54:45–52. doi: 10.1212/WNL.54.1.45. [DOI] [PubMed] [Google Scholar]
- 11.Houlden H., King R., Blake J., Groves M., Love S., Woodward C., Hammans S., Nicoll J., Lennox G., O’Donovan D.G., et al. Clinical, pathological and genetic characterization of hereditary sensory and autonomic neuropathy type 1 (hsan i) Brain. 2006;129:411–425. doi: 10.1093/brain/awh712. [DOI] [PubMed] [Google Scholar]
- 12.Auer-Grumbach M., De Jonghe P., Verhoeven K., Timmerman V., Wagner K., Hartung H.P., Nicholson G.A. Autosomal dominant inherited europathies with prominent sensory loss and mutilations: A review. Arch. Neurol. 2003;60:329–334. doi: 10.1001/archneur.60.3.329. [DOI] [PubMed] [Google Scholar]
- 13.Kugathasan U., Evans M.R.B., Morrow J.M., Sinclair C.D.J., Thornton J.S., Yousry T.A., Hornemann T., Suriyanarayanan S., Owusu-Ansah K., Lauria G., et al. Development of mrc centre mri calf muscle fat fraction protocol as a sensitive outcome measure in hereditary sensory neuropathy type 1. J. Neurol. Neurosurg. Psychiatry. 2019;90:895–906. doi: 10.1136/jnnp-2018-320198. [DOI] [PubMed] [Google Scholar]
- 14.Bucci C., Thomsen P., Nicoziani P., McCarthy J., van Deurs B. Rab7: A key to lysosome biogenesis. Mol. Biol. Cell. 2000;11:467–480. doi: 10.1091/mbc.11.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mateus D., Marini E.S., Progida C., Bakke O. Rab7a modulates er stress and er morphology. Biochim. Biophys. Acta Mol. Cell Res. 2018;1865:781–793. doi: 10.1016/j.bbamcr.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Wong Y.C., Ysselstein D., Krainc D. Mitochondria-lysosome contacts regulate mitochondrial fission via rab7 gtp hydrolysis. Nature. 2018;554:382–386. doi: 10.1038/nature25486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deinhardt K., Salinas S., Verastegui C., Watson R., Worth D., Hanrahan S., Bucci C., Schiavo G. Rab5 and rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron. 2006;52:293–305. doi: 10.1016/j.neuron.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 18.Saxena S., Bucci C., Weis J., Kruttgen A. The small gtpase rab7 controls the endosomal trafficking and neuritogenic signaling of the nerve growth factor receptor trka. J. Neurosci. 2005;25:10930–10940. doi: 10.1523/JNEUROSCI.2029-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Margiotta A., Progida C., Bakke O., Bucci C. Rab7a regulates cell migration through rac1 and vimentin. Biochim. Biophys. Acta. 2017;1864:367–381. doi: 10.1016/j.bbamcr.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 20.Kawauchi T., Sekine K., Shikanai M., Chihama K., Tomita K., Kubo K., Nakajima K., Nabeshima Y., Hoshino M. Rab gtpases-dependent endocytic pathways regulate neuronal migration and maturation through n-cadherin trafficking. Neuron. 2010;67:588–602. doi: 10.1016/j.neuron.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Cogli L., Progida C., Thomas C.L., Spencer-Dene B., Donno C., Schiavo G., Bucci C. Charcot-marie-tooth type 2b disease-causing rab7a mutant proteins show altered interaction with the neuronal intermediate filament peripherin. Acta Neuropathol. 2013;25:257–272. doi: 10.1007/s00401-012-1063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spinosa M.R., Progida C., De Luca A., Colucci A.M.R., Alifano P., Bucci C. Functional characterization of rab7 mutant proteins associated with charcot-marie-tooth type 2b disease. J. Neurosci. 2008;28:1640–1648. doi: 10.1523/JNEUROSCI.3677-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Luca A., Progida C., Spinosa M.R., Alifano P., Bucci C. Characterization of the rab7k157n mutant protein associated with charcot-marie-tooth type 2b. Biochem. Biophys. Res. Commun. 2008;372:283–287. doi: 10.1016/j.bbrc.2008.05.060. [DOI] [PubMed] [Google Scholar]
- 24.McCray B.A., Skordalakes E., Taylor J.P. Disease mutations in rab7 result in unregulated nucleotide exchange and inappropriate activation. Hum. Mol. Genet. 2010;19:1033–1047. doi: 10.1093/hmg/ddp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cogli L., Progida C., Lecci R., Bramato R., Krüttgen A., Bucci C. Cmt2b-associated rab7 mutants inhibit neurite outgrowth. Acta Neuropathol. 2010;120:491–501. doi: 10.1007/s00401-010-0696-8. [DOI] [PubMed] [Google Scholar]
- 26.Yamauchi J., Torii T., Kusakawa S., Sanbe A., Nakamura K., Takashima S., Hamasaki H., Kawaguchi S., Miyamoto Y., Tanoue A. The mood stabilizer valproic acid improves defective neurite formation caused by charcot-marie-tooth disease-associated mutant rab7 through the jnk signaling pathway. J. Neurosci. Res. 2010;88:3189–3197. doi: 10.1002/jnr.22460. [DOI] [PubMed] [Google Scholar]
- 27.Progida C., Malerød L., Stuffers S., Brech A., Bucci C., Stenmark H. Rilp is required for proper morphology and function of late endosomes. J. Cell Sci. 2007;120:3729–3737. doi: 10.1242/jcs.017301. [DOI] [PubMed] [Google Scholar]
- 28.Ceresa B.P., Bahr S.J. Rab7 activity affects epidermal growth factor: Epidermal growth factor receptor degradation by regulating endocytic trafficking from the late endosome. J. Biol. Chem. 2006;281:1099–1106. doi: 10.1074/jbc.M504175200. [DOI] [PubMed] [Google Scholar]
- 29.Cantalupo G., Alifano P., Roberti V., Bruni C.B., Bucci C. Rab-interacting lysosomal protein (rilp): The rab7 effector required for transport to lysosomes. EMBO J. 2001;20:683–693. doi: 10.1093/emboj/20.4.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xian C.J., Zhou X.F. Egf family of growth factors: Essential roles and functional redundancy in the nerve system. Front. Biosci. 2004;9:85–92. doi: 10.2741/1210. [DOI] [PubMed] [Google Scholar]
- 31.Yamada M., Ikeuchi T., Hatanaka H. The neurotrophic action and signalling of epidermal growth factor. Prog. Neurobiol. 1997;51:19–37. doi: 10.1016/S0301-0082(96)00046-9. [DOI] [PubMed] [Google Scholar]
- 32.Basuray S., Mukherjee S., Seaman M.N., Wandinger-Ness A. Rab7 mutants associated with charcot-marie-tooth disease cause delayed growth factor receptor transport and altered endosomal and nuclear signaling. J. Biol. Chem. 2013;288:1135–1149. doi: 10.1074/jbc.M112.417766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fabrizi G.M., Simonati A., Morbin M., Cavallaro T., Taioli F., Benedetti M.D., Edomi P., Rizzuto N. Clinical and pathological correlations in charcot-marie-tooth neuropathy type 1a with the 17p11.2p12 duplication: A cross-sectional morphometric and immunohistochemical study in twenty cases. Muscle Nerve. 1998;21:869–877. doi: 10.1002/(SICI)1097-4598(199807)21:7<869::AID-MUS4>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 34.Lauria G., Hsieh S.T., Johansson O., Kennedy W.R., Leger J.M., Mellgren S.I., Nolano M., Merkies I.S., Polydefkis M., Smith A.G., et al. European federation of neurological societies/peripheral nerve society guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the european federation of neurological societies and the peripheral nerve society. Eur. J. Neurol. 2010;17:e903–e949. doi: 10.1111/j.1468-1331.2010.03023.x. [DOI] [PubMed] [Google Scholar]
- 35.Edgar R.C. Muscle: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shapiro A.D., Riederer M.A., Pfeffer S.R. Biochemical analysis of rab9, a ras-like gtpase involved in protein transport from late endosomes to the trans golgi metwork. J. Biol. Chem. 1993;268:6925–6931. [PubMed] [Google Scholar]
- 37.Stenmark H., Parton R.G., Steele-Mortimer O., Lutcke A., Gruenberg J., Zerial M. Inhibition of rab5 gtpase activity stimulates membrane fusion in endocytosis. EMBO J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kjeldgaard M., Nyborg J., Clark B.F. The gtp binding motif: Variations on a theme. FASEB J. 1996;10:1347–1368. doi: 10.1096/fasebj.10.12.8903506. [DOI] [PubMed] [Google Scholar]
- 39.Kerr B., Delrue M.A., Sigaudy S., Perveen R., Marche M., Burgelin I., Stef M., Tang B., Eden O.B., O’Sullivan J., et al. Genotype-phenotype correlation in costello syndrome: Hras mutation analysis in 43 cases. J. Med. Genet. 2006;43:401–405. doi: 10.1136/jmg.2005.040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahar M., Cavalli V. Intrinsic mechanisms of neuronal axon regeneration. Nat. Rev. Neurosci. 2018;19:323–337. doi: 10.1038/s41583-018-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kirkcaldie M.T.K., Dwyer S.T. The third wave: Intermediate filaments in the maturing nervous system. Mol. Cell Neurosci. 2017;84:68–76. doi: 10.1016/j.mcn.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Parlakian A., Paulin D., Izmiryan A., Xue Z., Li Z. Intermediate filaments in peripheral nervous system: Their expression, dysfunction and diseases. Rev. Neurol. 2016;172:607–613. doi: 10.1016/j.neurol.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 43.Progida C., Spinosa M., De Luca A., Bucci C. Rilp interacts with the vps22 component of the escrt-ii complex. Biochem. Biophys. Res. Commun. 2006;347:1074–1079. doi: 10.1016/j.bbrc.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Maklad A., Nicolai J.R., Bichsel K.J., Evenson J.E., Lee T.C., Threadgill D.W., Hansen L.A. The egfr is required for proper innervation to the skin. J. Invest. Dermatol. 2009;129:690–698. doi: 10.1038/jid.2008.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu B., Neufeld A.H. Activation of epidermal growth factor receptors in astrocytes: From development to neural injury. J. Neurosci. Res. 2007;85:3523–3529. doi: 10.1002/jnr.21364. [DOI] [PubMed] [Google Scholar]
- 46.Liddelow S.A., Guttenplan K.A., Clarke L.E., Bennett F.C., Bohlen C.J., Schirmer L., Bennett M.L., Munch A.E., Chung W.S., Peterson T.C., et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith G.M., Strunz C. Growth factor and cytokine regulation of chondroitin sulfate proteoglycans by astrocytes. Glia. 2005;52:209–218. doi: 10.1002/glia.20236. [DOI] [PubMed] [Google Scholar]
- 48.Erschbamer M., Pernold K., Olson L. Inhibiting epidermal growth factor receptor improves structural, locomotor, sensory, and bladder recovery from experimental spinal cord injury. J. Neurosci. 2007;27:6428–6435. doi: 10.1523/JNEUROSCI.1037-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sibilia M., Wagner E.F. Strain-dependent epithelial defects in mice lacking the egf receptor. Science. 1995;269:234–238. doi: 10.1126/science.7618085. [DOI] [PubMed] [Google Scholar]
- 50.Sibilia M., Steinbach J.P., Stingl L., Aguzzi A., Wagner E.F. A strain-independent postnatal neurodegeneration in mice lacking the egf receptor. EMBO J. 1998;17:719–731. doi: 10.1093/emboj/17.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hatanpaa K.J., Burma S., Zhao D., Habib A.A. Epidermal growth factor receptor in glioma: Signal transduction, neuropathology, imaging, and radioresistance. Neoplasia. 2010;12:675–684. doi: 10.1593/neo.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siddiqui S., Fang M., Ni B., Lu D., Martin B., Maudsley S. Central role of the egf receptor in neurometabolic aging. Int. J. Endocrinol. 2012;2012:739428. doi: 10.1155/2012/739428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hendry J.M., Alvarez-Veronesi M.C., Placheta E., Zhang J.J., Gordon T., Borschel G.H. Erbb2 blockade with herceptin (trastuzumab) enhances peripheral nerve regeneration after repair of acute or chronic peripheral nerve injury. Ann. Neurol. 2016;80:112–126. doi: 10.1002/ana.24688. [DOI] [PubMed] [Google Scholar]
- 54.Placheta E., Hendry J.M., Wood M.D., Lafontaine C.W., Liu E.H., Alvarez Veronesi M.C., Frey M., Gordon T., Borschel G.H. The erbb2 inhibitor herceptin (trastuzumab) promotes axonal outgrowth four weeks after acute nerve transection and repair. Neurosci. Lett. 2014;582:81–86. doi: 10.1016/j.neulet.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 55.Williams J.P., Wu J., Johansson G., Rizvi T.A., Miller S.C., Geiger H., Malik P., Li W., Mukouyama Y.S., Cancelas J.A., et al. Nf1 mutation expands an egfr-dependent peripheral nerve progenitor that confers neurofibroma tumorigenic potential. Cell Stem Cell. 2008;3:658–669. doi: 10.1016/j.stem.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gu Y., Chen C., Yi S., Wang S., Gong L., Liu J., Gu X., Zhao Q., Li S. Mir-sc8 inhibits schwann cell proliferation and migration by targeting egfr. PLoS ONE. 2015;10:e0145185. doi: 10.1371/journal.pone.0145185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dahlhoff M., Emrich D., Wolf E., Schneider M.R. Increased activation of the epidermal growth factor receptor in transgenic mice overexpressing epigen causes peripheral neuropathy. Biochim. Biophys. Acta. 2013;1832:2068–2076. doi: 10.1016/j.bbadis.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 58.Kanehisa M., Goto S. Kegg: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kanehisa M., Sato Y., Furumichi M., Morishima K., Tanabe M. New approach for understanding genome variations in kegg. Nucleic Acids Res. 2019;47:D590–D595. doi: 10.1093/nar/gky962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kanehisa M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 2019;28:1947–1951. doi: 10.1002/pro.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.