Abstract
Helicobacter pylori persistently colonizes the human stomach, and is associated with inflammation-induced gastric cancer. Bacterial crosstalk with the host immune system produces various inflammatory mediators and subsequent reactions in the host, but not bacterial clearance. Interleukin-1β (IL-1β) is implicated in gastric cancer development and certain gene polymorphisms play a role in this scenario. Mature IL-1β production depends on inflammasome activation, and the NLRP3 inflammasome is a major driver in H. pylori-infected mice, while recent studies demonstrated the down-regulation of NLRP3 expression in human immune cells, indicating a differential NLRP3 regulation in human vs. mice. In addition to the formation of mature IL-1β or IL-18, inflammasome activation induces pyroptotic death in cells. We demonstrate that H. pylori infection indeed upregulated the expression of pro-IL-1β in human immune cells, but secreted only very low amounts of mature IL-1β. However, application of exogenous control activators such as Nigericin or ATP to infected cells readily induced NLRP3 inflammasome formation and secretion of high amounts of mature IL-1β. This suggests that chronic H. pylori infection in humans manipulates inflammasome activation and pyroptosis for bacterial persistence. This inflammasome deregulation during H. pylori infection, however, is prone to external stimulation by microbial, environmental or host molecules of inflammasome activators for the production of high amounts of mature IL-1β and signaling-mediated gastric tumorigenesis in humans.
Keywords: Inflammasome, NLRP3, Helicobacter pylori, Interleukin-1β
1. Introduction
Helicobacter pylori infection in the global human population is a major health burden in many parts of the world. The advent of antibiotic therapy and improved hygiene protocols drastically reduced the burden in several developed countries in recent decades, but lack of diagnosis and poor hygiene in underdeveloped countries made the condition worse with a high percentage of colonized individuals [1]. A meta-analysis of published data revealed that 44.3% to 60.3% of the global population harbors this bacterium in their stomach [2]. The country-specific prevalence varies from 18.9% in Switzerland to 87.7% in Nigeria [1]. H. pylori colonization causes mild gastritis in every colonized individual, however, 10–20% of cases are associated with the development of peptic ulcers, 1–2% develop gastric cancer and <1% cause gastric mucosa-associated lymphoid tissue lymphoma (MALT) [3,4]. H. pylori is a bacterial carcinogen recognized by the World Health Organization (WHO) due to the association with gastric cancer and MALT lymphoma [5]. The burden of gastric cancer increases every year and 1,033,701 new cases were reported and 782,685 people died worldwide in 2018 as per data of the International Agency for Research on Cancer (IARC) (http://gco.iarc.fr). Once colonized in childhood, H. pylori infection can last for the whole life, if not eradicated by prophylactic measures.
H. pylori colonization induces various pattern recognition receptors (PRRs) of the host to produce robust chemokine and cytokine responses through signal transduction events in different cellular systems in the gastric tissue [6,7,8]. This bacterium utilizes many of its virulence factors to overcome the natural barriers in the host and establish colonization. Highly virulent type-I H. pylori strains harbor the ~40 kb cytotoxicity associated gene pathogenicity island (cagPAI) in their genomes, which codes for a type IV secretion system (T4SS) and the effector protein CagA. T4SS-dependent delivery of CagA leads to phosphorylation by c-Src and c-Abl kinases, and both phosphorylated and non-phosphorylated CagA cause perturbation in signaling, cellular functions, morphology and eventually oncogenesis [9,10,11,12,13]. In contrast, less virulent type-II strains do not encode the cagPAI in their genomes. Moreover, type-I strains are generally implicated in the development of associated pathologies due to increased inflammation in the colonized individuals. In addition, H. pylori secretes a toxin called vacuolating cytotoxin A (VacA), which causes vacuolation of epithelial cells in culture. VacA s1-i1-m1 allele-harboring strains showed maximum vacuolization in epithelial barrier dysfunction and thereby are associated with peptic ulcer and gastric cancer [14,15,16]. Multiple receptors are reported for VacA on epithelial cells, however, CD18 is the only receptor identified in immune cells like T cells [16,17]. VacA binding to host receptors induce several signaling events, mitochondrial damage, and apoptotic cell death [18,19,20,21]. The H. pylori flagellum helps to travel through the thick gastric mucous layer to interact with the gastric epithelium. The hostile acidic gastric environment is generally neutralized by the activity of H. pylori urease and GGT (γ-glutamyl transferase) enzymes. Furthermore, an array of adhesion molecules helps for specific attachment to the epithelium [22,23,24]. The establishment of colonization and PRR responses attract various immune cells, especially a high population of neutrophils to infiltrate into the site of infection [25,26,27]. Furthermore, the immune responses through cellular and soluble mediators lead to the development of adaptive immunity against this bacterium. H. pylori elicits strong T cellular and B cellular responses to mainly develop Th1 and Th17 effector cell populations and strong humoral immunity through antibodies, respectively [26,28,29,30,31]. Macrophages were reported to be differentiated into mixed pro-inflammatory M1 and anti-inflammatory M2 populations during H. pylori infection [32,33,34]. In addition, H. pylori infection causes anti-inflammatory T-regulatory (Treg) cell production to favor suppressed inflammatory reactions and dysregulated gastric microbiota [35,36,37,38].
Interestingly, persistent infection by H. pylori is also beneficial for the human host through suppressing other illnesses including asthma, allergies and inflammatory bowel disease [35,37,39,40]. Host gene polymorphisms are known to have a crucial impact in producing different associated pathologies of H. pylori infection [41,42,43]. In particular, a specific interleukin-1β (IL-1β) gene polymorphism has prominence and was implicated in hypochlorhydria and gastric cancer development [41,44,45,46]. IL-1β signaling works as an inhibitor of acid secretion and causes gastric atrophy, which provides a hotbed for metaplasia and gastric cancer development. Furthermore, it directly induces the proliferation of gastric carcinoma cells [47,48,49,50]. The secreted IL-1β levels correlated with gastric inflammation and gastric carcinogenesis [51,52,53]. In addition, stomach specific expression of mature human IL-1β in mice developed the stepwise progression of gastric inflammation, dysplasia and gastric cancer and Th1 specific immunity promoted this process [54,55]. However, IL-1β deficiency or IL-1 receptor blockade inhibited these processes [54,55]. In concurrence, lymphocyte-deficient mice expressing IL-1β in the stomach exhibited atrophic gastritis, metaplasia and dysplasia, which supports an independent role of IL-1β in gastric carcinogenesis [54]. H. pylori-related MALT lymphomagenesis was reported to be associated with specific intra-tumoral T cell responses. Moreover, translocated CagA mediates B-cell proliferation, leading to lymphomagenesis of MALT lymphomas [56]. In general, the production of mature IL-1β and IL-18 is mediated through the formation of multi-protein scaffolds, called inflammasome and caspase-1 activation [57,58,59], whereas there are some other proteases that can also cleave the pro-forms of these cytokines [60]. Inflammasomes are mainly formed by NLRP1 (Nod-like receptor family, pyrin domain containing 1), NLRP3, NLRC4 (Nod-like receptor family, card domain containing 4), AIM2 (absent in melanoma 2) and Pyrin/TRIM20 (tripartite motif 20) signaling platforms [57,58,59,61]. The NLRP3 inflammasome is the most studied inflammasome, and generally requires two major signaling events for activation, signal-1 and signal-2. The first signal induces the increased expression of components such as NLRP3, ASC (apoptosis associated speck protein), pro-IL-1β, and caspase-1. Finally, inflammasome scaffold formation and activation requires a second signal commonly provided by various microbial, environmental and host molecules such as Nigericin, silica, asbestos, monosodium urate (MSU), ATP or reactive oxygen species (ROS) [62,63,64]. Mice infected with H. pylori were reported to activate the NLRP3 inflammasome and secreted mature IL-1β from immune cells through a TLR2/NOD2-dependent mechanism [39,65]. It should be also noted that most of the inflammasome studies on H. pylori were done in mice or with isolated mouse dendritic cells (DCs), and identified different virulence factors such as cagPAI, VacA, LPS and urease B that are involved in this process [39,65,66]. In addition, chronic H. pylori infection in mice regulates inflammasome activation through a MUC1-dependent mechanism [67]. However, very little data exists on inflammasome formation in H. pylori-infected human cells and some controversy occurs with regard to the involvement of different virulence factors in the mechanism of activation when compared to mice [39,53,65,66]. In addition to this, we recently found that NLRP3 expression regulation occurred in H. pylori-infected human monocytes/macrophages, which was dependent on miR-223-3p and the presence of secreted IL-10 [68]. Consequently, the regulation of the inflammasome by H. pylori in mouse versus human cells is not fully understood. Thus, we aimed here to study in more detail the NLRP3 inflammasome formation and secretion of mature IL-1β in H. pylori-infected human monocytes/macrophages and resolve some of the conflicting data. We have also reconstructed the NLRP3 inflammasome by overexpression of its components in a non-competent HEK293 epithelial cell line (HEK293-NLRP3-INSOME) and used it for the validation of monocyte/macrophage functions. We found that NLRP3 inflammasome formation and activation not occurred during H. pylori infection of human cells, however, which can be surmounted by induction with exogenous second signal activators. This is an important finding that acute infection by H. pylori creates a partial situation for NLRP3 inflammasome activation, which may be completed by the release of high molar ATP, monosodium urate (MSU), ROS, exposure to orally passing agents or anything likely to produce high amounts of oncogenic mature IL-1β. These results can partly explain the clearance of H. pylori in mice versus chronic infection in humans.
2. Results
2.1. Different Clinical H. pylori Strains Induce Upregulated Pro-IL-1β Expression in Infected THP1 Monocytes
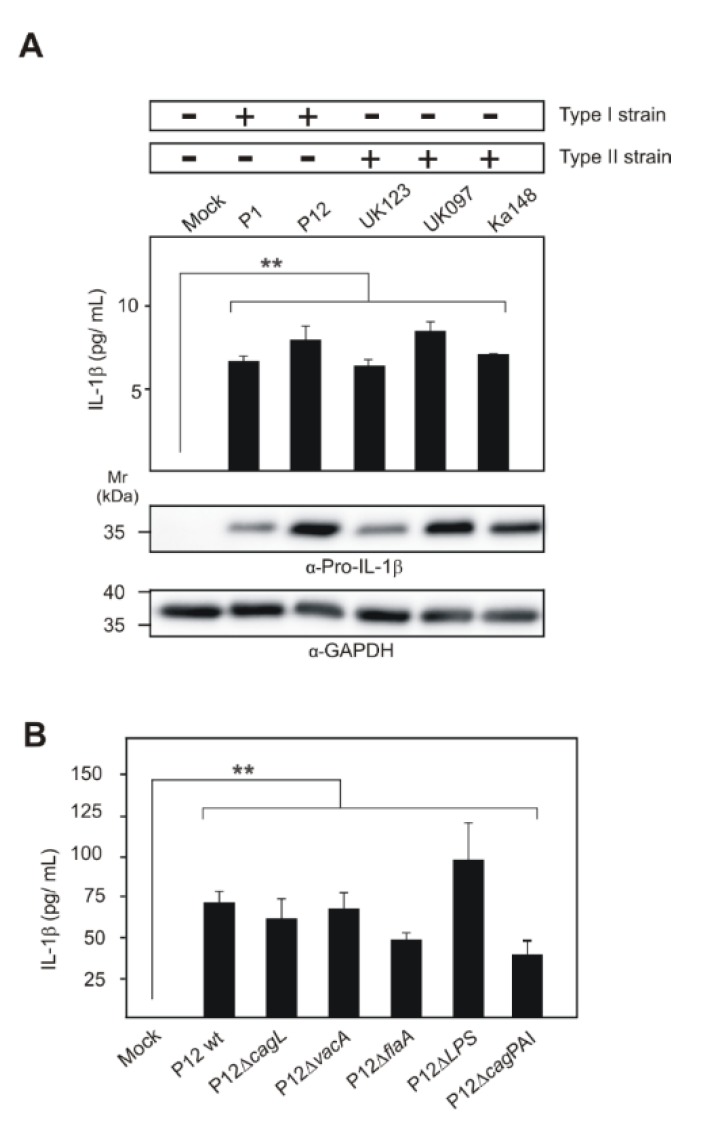
We have previously shown that THP1 monocytes, a commonly used human model cell line for inflammasome activation, constitutively express NLRP3 and pro-caspase-1. Furthermore, NLRP3 expression increased after 6 h of infection with H. pylori, but downregulated at a later period [68]. Thus, we used 6 h of infection in the current inflammasome activation experiments unless stated otherwise. The expression of pro-IL-1β is a prerequisite for mature IL-1β production, and its secretion proceeds through activation of the inflammasome. H. pylori infection-mediated IL-1β expression and secretion have important roles in pathogenesis. We infected THP1 monocytes with H. pylori belonging to the highly virulent type-I (P1 and P12) or less-virulent type-II (UK123, UK097 and Ka148) strains. Both types of H. pylori induced the profound expression of pro-IL-1β in THP1 monocytes at 6 h of infection compared to mock-treated control cells (Figure 1A; Supplementary Figure S1). The relative amounts of pro-IL-1β protein expressions were quantified (Supplementary Figure S1). However, cells infected with both types of H. pylori secreted very small quantities of mature IL-1β (5–10 pg/mL) as determined by standard ELISA (Figure 1A). Moreover, THP1 monocytes infected with P12 wild-type and isogenic mutants in major virulence factors (ΔcagL, ΔvacA, ΔflaA, ΔLPS, ΔcagPAI) secreted similar amounts of mature IL-1β even after 24 h (Figure 1B), which suggests that these factors are not involved in this process. By comparison with reported studies in mice, DCs infected with ΔcagPAI and ΔcagL mutants of P12 strain showed a significant reduction in IL-1β secretion compared to wild-type bacteria (200–800 pg/mL), and furthermore, priming with E. coli LPS drastically increased (above 2000 pg/mL) the IL-1β secretion [39,65]. This shows that H. pylori-infected human and mouse cells exhibit significant differences in cleavage of pro-IL-1β and secretion of the mature form. Therefore, our further studies aimed to investigate inflammasome activation in H. pylori-infected human cells.
Figure 1.
Induced expression of pro-IL-1β in H. pylori-infected THP1 monocytes. The first signal of NLRP3 inflammasome activation requires the optimal production of inflammasome components including pro-IL-1β, which is not generally expressed in immune cells without induction. Different wild-type strains including type-I and type-II H. pylori were infected for 6 h to analyze the pro-IL-1β expression and secretion (A). Furthermore, H. pylori P12 wild-type and isogenic mutants were also used to identify the effect on secretion at 24 h of infection with THP1 monocytes (B). GAPDH protein immunoblots were used as loading reference. ** p ≤ 0.01.
2.2. Comparison of Canonical NLRP3 Inflammasome Activation with that of H. pylori-Infected Cells
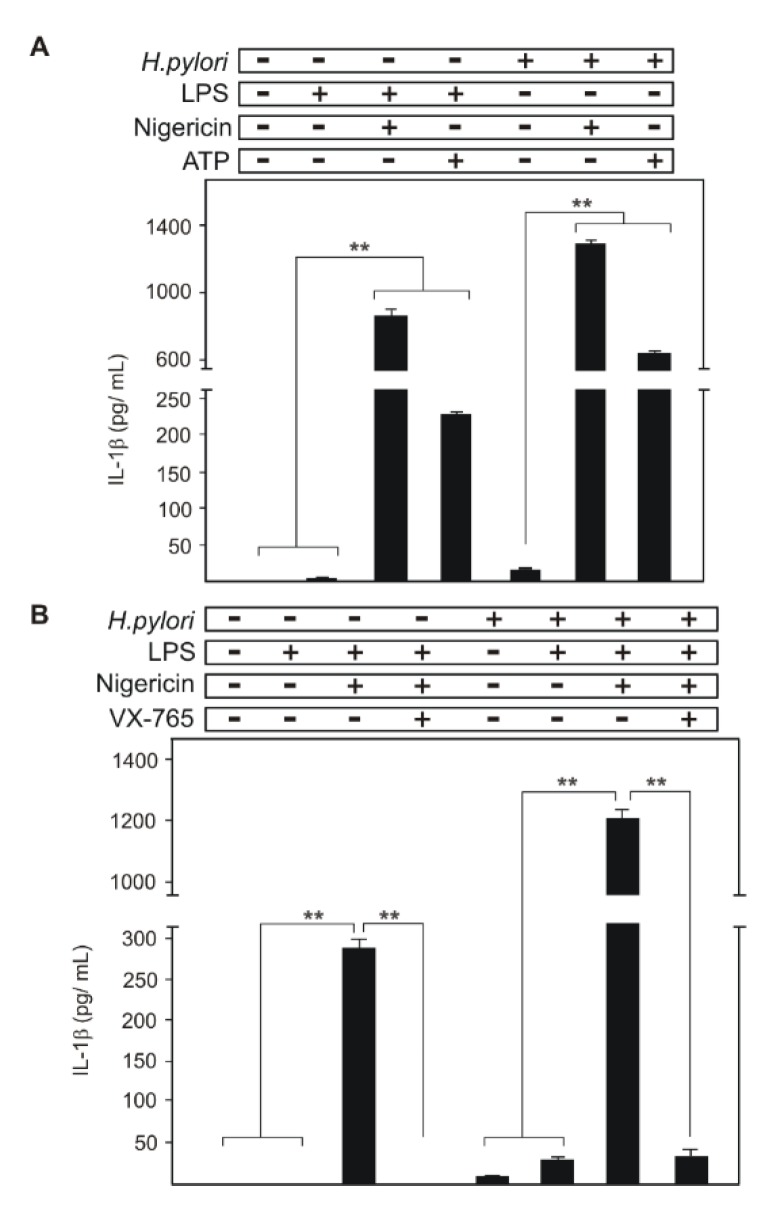
We next analyzed whether differences exist between canonical NLRP3 inflammasome activation in THP1 monocytes versus H. pylori infection. NLRP3 inflammasome formation and activation was carried out by treatment with E. coli LPS (as signal-1) followed by addition of 10 µM Nigericin or 5 mM ATP (as signal-2), and determined mature IL-1β production and secretion by ELISA. As expected, E. coli LPS-treated cells induced with Nigericin or ATP secreted significantly high amounts of mature IL-1β (Figure 2A). However, cells treated with E. coli LPS or H. pylori alone secreted no or only small amounts of mature IL-1β (Figure 2A; data not shown). As above, H. pylori infection of THP1 monocytes secreted significantly less mature IL-1β, thus we hypothesized that signal-2 for activation is missing upon infection. In this context, we treated H. pylori-infected cells with signal-2 activators, and surprisingly, Nigericin or ATP addition significantly increased the secretion of mature IL-1β, which complies with our hypothesis that H. pylori lacks the signal-2 for activation of NLRP3 inflammasome in THP1 cells (Figure 2A). This result is also in line with NLRP3 inflammasome activation-mediated secretion in mouse DCs after infection or treatment with H. pylori and LPS/ATP, respectively [66]. In fact, this shows interference of this important innate immune response by H. pylori in human cells. Moreover, we analyzed H. pylori/Nigericin inflammasome activation for the involvement of caspase-1, as other caspases and proteases were also implicated in the cleavage of pro-IL-1β [60]. THP1 monocytes were treated with caspase-1 inhibitor VX-765 (10 µM) 15 min before LPS treatment or infection with H. pylori, which followed Nigericin-mediated NLRP3 inflammasome activation. The VX-765 pre-treatment completely abolished caspase-1-dependent mature IL-1β secretion in LPS/Nigericin treated cells (Figure 2B). However, we noted small amounts of IL-1β secretion from VX-765 treated cells exposed to H. pylori/Nigericin, which is equivalent to the IL-1β production solely by infection.
Figure 2.
NLRP3 inflammasome activation in THP1 monocytes was compared with H. pylori infection at 6 h of treatment. E. coli LPS/Nigericin and LPS/ATP were used as signal-1/signal-2 for canonical activation of the NLRP3 inflammasome in THP1 monocytes as described in the text (A). H. pylori infection secreted low amounts of IL-1β when compared with canonical NLRP3 inflammasome activation-mediated mature IL-1β secretion. Moreover, E. coli LPS/H. pylori co-treatment showed a corresponding increase in IL-1β secretion but this was not comparable with the inflammasome activation response (B). However, H. pylori-infected cells treated with signal-2 activators Nigericin or ATP induced the production of a high amount of mature IL-1β secretion (A,B). The caspase-1 inhibitor VX-765 inhibited all NLRP3 inflammasome driven IL-1β secretion in LPS treated or H. pylori-infected cells after activation but infected cells maintained basic secretion (B). ** p ≤ 0.01.
2.3. Inflammasome Activation in H. pylori-Infected NLRP3 and Caspase-1 Knockout THP1 Monocytes
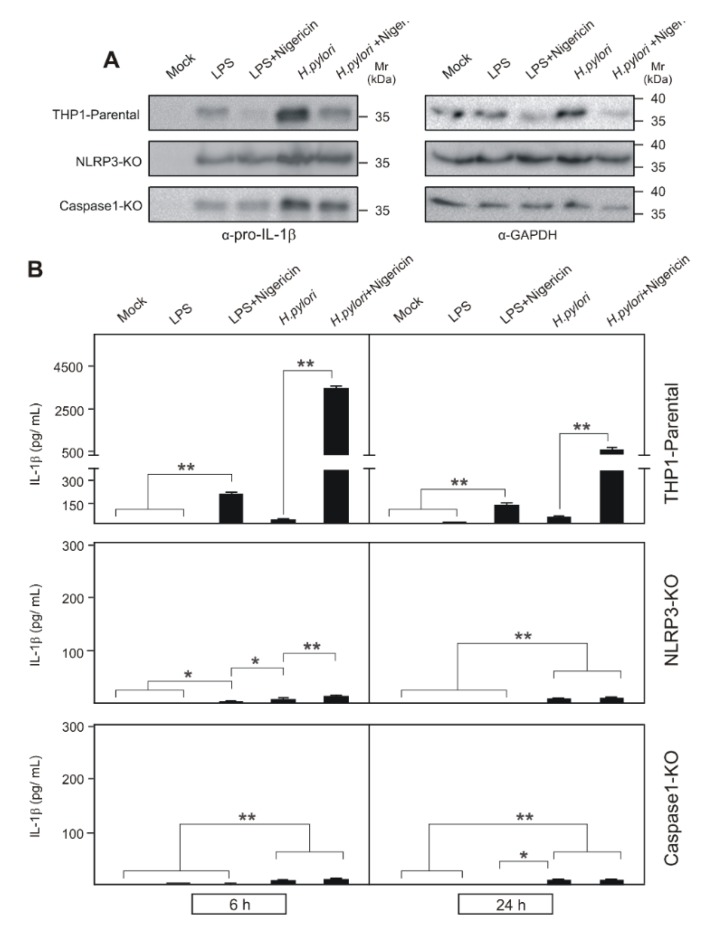
To further substantiate our findings, NLRP3 and caspase-1 were knocked-out in THP-1 by CRISPR/Cas9 technology and named as NLRP3-KO or CASP1-KO cells, respectively. LPS-treated and H. pylori-infected NLRP3-KO and CASP1-KO THP1 cells were used to investigate inflammasome activation compared to parental control cells. H. pylori infection of NLRP3-KO and CASP1-KO cells upregulated pro-IL-1β expression in similar levels observed for parental cells (Figure 3A; Supplementary Figure S2). Active caspase-1 also targets GAPDH [69], being cleaved in THP1 parental cells after Nigericin treatment, while not in NLRP3-KO or caspase-1-KO cells, indicating the stimulation of pyroptotic cell death. Nigericin-induced activation of the NLRP3 inflammasome in LPS-treated NLRP3-KO or CASP1-KO cells did not yield any significant mature IL-1β secretion at 6 h compared to parental cells (Figure 3B). Although, H. pylori infection of these knockout cells secreted small amounts of IL-1β as in parental cells, which again confirms that this low secretion is not dependent on NLRP3 inflammasome and caspase-1 activation (Figure 3B). Furthermore, LPS/Nigericin-treated parental cells drastically reduced the mature IL-1β secretion at 24 h when compared with early phase induction, which is implicated for decreased NLRP3 expression in the late phase observed in previous studies [68,70]. H. pylori/Nigericin-treated cells also exhibited a similar decrease in mature IL-1β secretion at later periods of infection due to both decreased NLRP3 expression [68] and inflammasome activation. However, infection-induced small amounts of IL-1β secretion gradually increased at a later time point, which again suggests an inflammasome/caspase-1 independent mechanism for this in human THP-1 cells.
Figure 3.
Expression of pro-IL-1β in THP1 parental, THP1 NLRP3-KO and THP1 Caspase-1-KO monocytes after E. coli LPS treatment, H. pylori infection and NLRP3 inflammasome activation with Nigericin were shown in Western blots in comparison with control GAPDH as loading reference (A). GAPDH, a known target for active caspase-1 [69] is also cleaved in THP1 parental cells but not in NLRP3-KO or caspase-1-KO cells after Nigericin treatment, which suggests pyroptotic death (A). NLRP3 inflammasome activation is evident with cleavage of pro-IL-1β and secretion of high amount of mature IL-1β from THP1 parental monocytes but not in NLRP3-KO or caspase-1-KO cells after Nigericin treatment, however, both knockout cells secreted basic background secretion after H. pylori infection, which again confirms the inflammasome or caspase-1-independent secretion of IL-1β by H. pylori (B). * p ≤ 0.05; ** p ≤ 0.01.
2.4. Activation of NLRP3 Inflammasome in Human Primary M1 and M2 Macrophages during H. pylori Infection
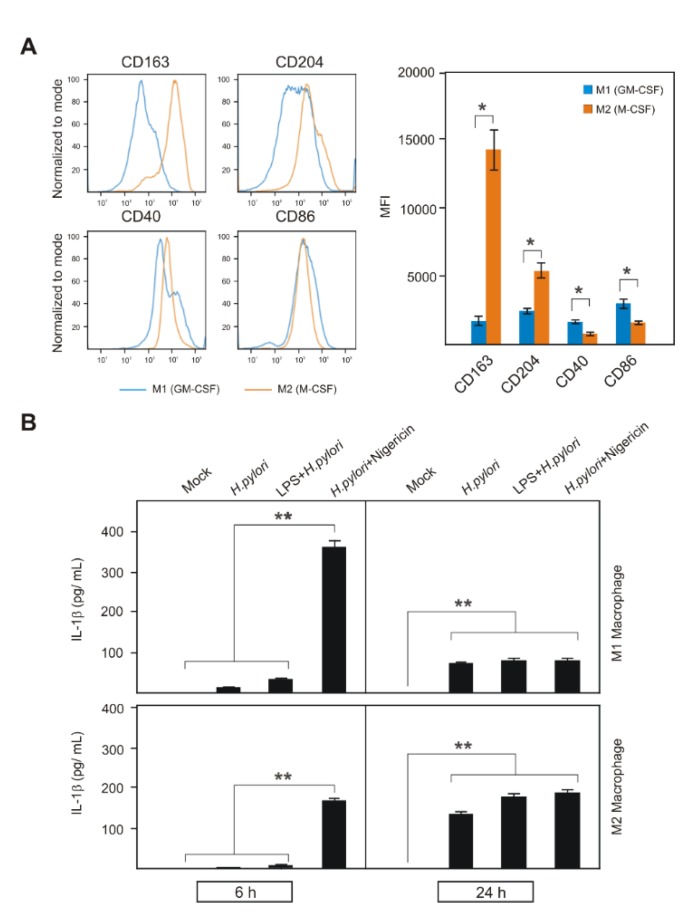
Mixed populations of pro- and anti-inflammatory M1 and M2 macrophages, respectively, were reported to be present in the gastric mucosa of H. pylori-infected individuals [32,33,34]. To test their importance, peripheral blood monocytes were isolated from three volunteers and differentiated to M1 and M2 macrophages, which was confirmed by flow cytometric analysis of overexpression of M1 markers (CD40 and CD86) and M2 markers (CD163 and CD204) (Figure 4A). These cells were used for studying H. pylori-, LPS/H. pylori- and H. pylori/Nigericin-induced IL-1β expression and secretion. As observed in THP1 cells, H. pylori- and LPS/H. pylori-infected primary M1 and M2 cells secreted IL-1β, which gradually increased between 6 h to 24 h of infection (Figure 4B). Furthermore, Nigericin-induced NLRP3 inflammasome activation in H. pylori-infected M1 and M2 macrophages significantly secreted high amounts of mature IL-1β (Figure 4B). Furthermore, M1 macrophages secreted higher amounts of mature IL-1β in H. pylori/Nigericin-treated cells at the early phase, but decreased at the later time point, which again confirms the downregulation of NLRP3 expression in infected primary cells [68]. Moreover, Nigericin-induced NLRP3 activation in infected M2 macrophages significantly increased mature IL-1β secretion at an early time point (Figure 4B). Whereas, this level was maintained at the later time point, but it was not highly significant from infected cells without Nigericin treatment. H. pylori infection of M2 macrophages gradually increased significant IL-1β secretion at the later time point, but were not highly responsive to Nigericin treatment, which also supports NLRP3 expression regulation in M2 macrophages Thus, pro- and anti-inflammatory M1 and M2 macrophages, respectively, and the THP1 cell line, exhibited a similar regulation in NLRP3 inflammasome activation-mediated mature IL-1β secretion at the later time point (Figure 4B). Together, H. pylori induces the first signal of NLRP3 inflammasome in human primary cells and the THP1 cell line; however, it lacks a proper second signal activation and mature IL-1β secretion.
Figure 4.
Primary human M1 and M2 macrophages express significantly increased amounts of M1 markers CD40, CD86 and M2 markers CD163, CD204 by flow cytometry (A). These M1 and M2 macrophages were infected with H. pylori, co-treated with LPS and further treated with Nigericin to analyze NLRP3 inflammasome activation and mature IL-1β secretion (B). M1 and M2 macrophages showed a response that was similar to the THP1 monocytes. * p ≤ 0.05; ** p ≤ 0.01.
2.5. NLRP3 Inflammasome Characteristic in H. pylori-Infected Cells
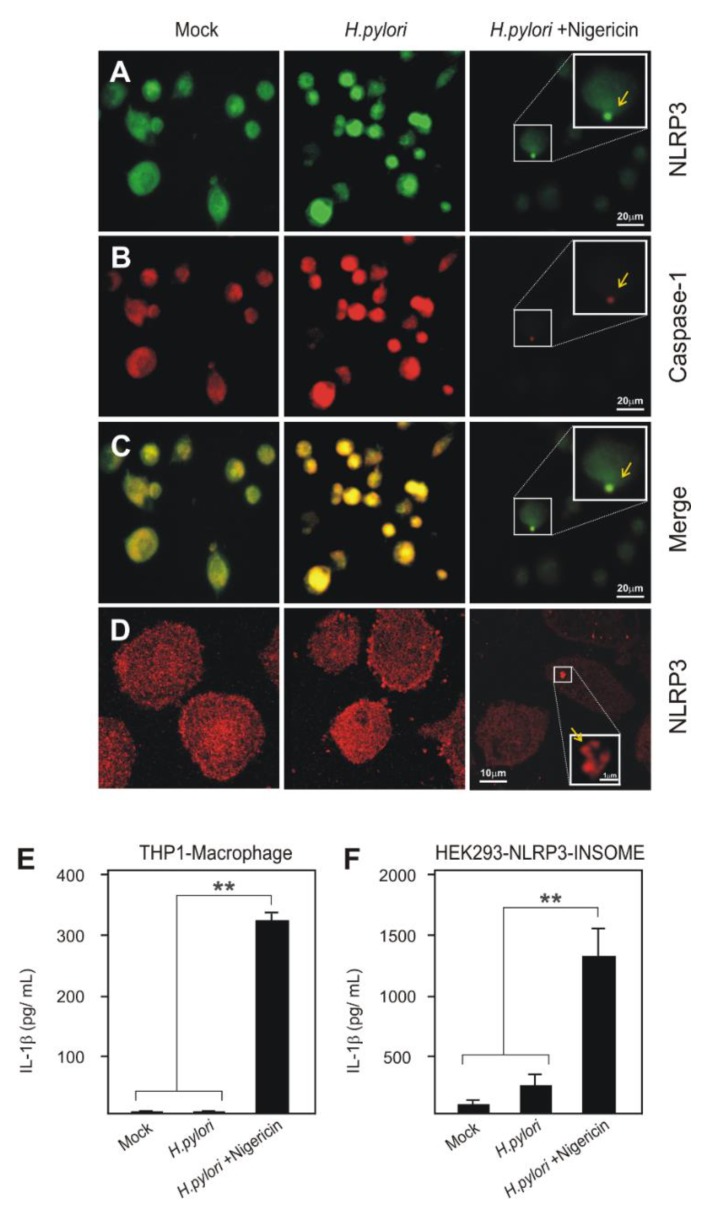
We next infected PMA-differentiated THP1 macrophages with H. pylori for investigating NLRP3 inflammasome formation at 6 h. After infection, THP1 macrophages were fixed and stained using immuno-fluorescence labelling against NLRP3 and caspase-1 for visualization by widefield fluorescence microscopy. The careful analysis of H. pylori-infected cells and mock control cells did not reveal typical NLRP3 inflammasome speck formation, but a homogenous distribution of the protein. However, the infected cells treated with signal-2 inducer, Nigericin, clearly formed the expected inflammasome speck (Figure 5A). In addition, the labelling for pro-caspase-1 merged at the corresponding spot of the NLRP3 inflammasome speck in H. pylori/Nigericin-treated THP1 macrophages, which confirms the close interaction of both of these molecules for active inflammasome formation (Figure 5B,C). The detailed characteristic of NLRP3 inflammasome formed in these cells was further visualized by super resolution STED (stimulated emission depletion) microscopy. As seen in the widefield fluorescence microscope, H. pylori-infected, and mock-treated control cells did not show the organization of the inflammasome and cells had a normal size and appearance. After induction with Nigericin in H. pylori-infected cells, NLRP3 inflammasome was visualized at super resolution in the characteristic donut shape (Figure 5D) [71], which confirmed the need for the exogenous second signal in NLRP3 inflammasome formation during H. pylori infection.
Figure 5.
The immuno-fluorescence images of NLRP3, caspase-1 and merged in H. pylori-infected, H. pylori/Nigericin-treated and mock treated control cells of PMA differentiated THP1 macrophages (A–C). The NLRP3 inflammasome formation is indicated with yellow arrows. The super resolution STED microscopy of the NLRP3 and inflammasome formation in H. pylori-infected, H. pylori/Nigericin-treated and mock treated control cells with more clarity showing donut-shaped inflammasome speck (D). The mature IL-1β secretion from THP1 macrophages and reconstructed HEK293-NLRP3-INSOME secretion after infection and Nigericin treatment were determined in parallel by ELISA (E,F). ** p ≤ 0.01.
NLRP3 inflammasome activation in the Nigericin-treated infected (H. pylori/Nigericin) cells was confirmed by the significantly high secretion of mature IL-1β (Figure 5E). Next, we transfected inflammasome-deficient HEK293 epithelial cells with eGFP-NLRP3 inflammasome constructs, generating HEK293-NLRP3-INSOME cells, which was previously described for mouse cells and NLRs [72,73]. The basic inflammasome response was not drastically changed in these cells upon infection with H. pylori, but Nigericin treatment significantly increased the secretion of mature IL-1β (Figure 5F). These data further confirm the inability of H. pylori to establish proper NLRP3 inflammasome functions in human cells.
2.6. Reconstructed NLRP3 Inflammasome Speck Induction by a Known Bacterial Activator and H. pylori
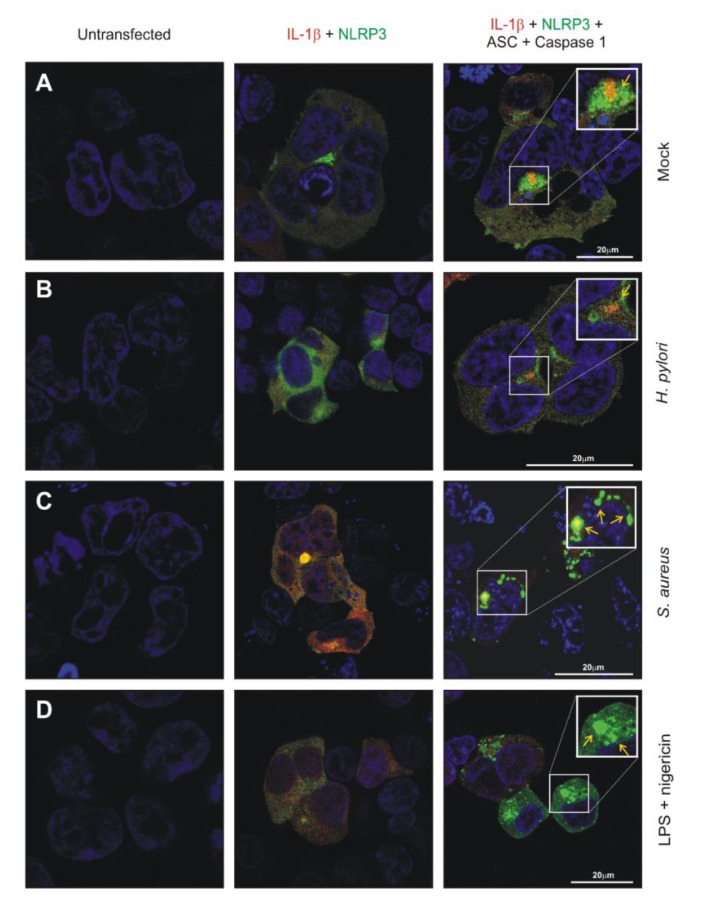
HEK293-NLRP3-INSOME cells were used for further analysis on understanding the inflammasome activation dynamics. Activation of NLRP3 inflammasome in HEK293-NLRP3-INSOME cells with the bacterial activator Staphylococcus aureus and LPS/Nigericin were compared with that of H. pylori infection. The eGFP-NLRP3 and mCherry-pro-IL-1β were visualized in the mock control, LPS/Nigericin treated, H. pylori, and S. aureus infected HEK293-NLRP3-INSOME cells by confocal laser scanning fluorescence microscopy (CLSM) (Figure 6A–D). Interestingly, S. aureus-infected and LPS/Nigericin-treated cells induced more NLRP3 inflammasome specks merged with the mCherry-pro-IL-1β, while H. pylori-infected and mock-treated cells maintained similar speck-free features (Figure 6A–D).
Figure 6.
Confocal laser scanning microscopy was used to analyze the reconstructed HEK293-NLRP3-INSOME cells in mock control (A), H. pylori-infected (B), S. aureus-infected (C) and E. coli LPS/Nigericin-treated (D). EGFP-NLRP3 and mCherry-pro-IL-1β were visualized using their characteristic colors and NLRP3 inflammasome in various conditions mentioned above were marked with yellow arrows. The respective column heading shows the transfection status of each cell before or after infection or treatment.
2.7. Impact of Known H. pylori Virulence Factors
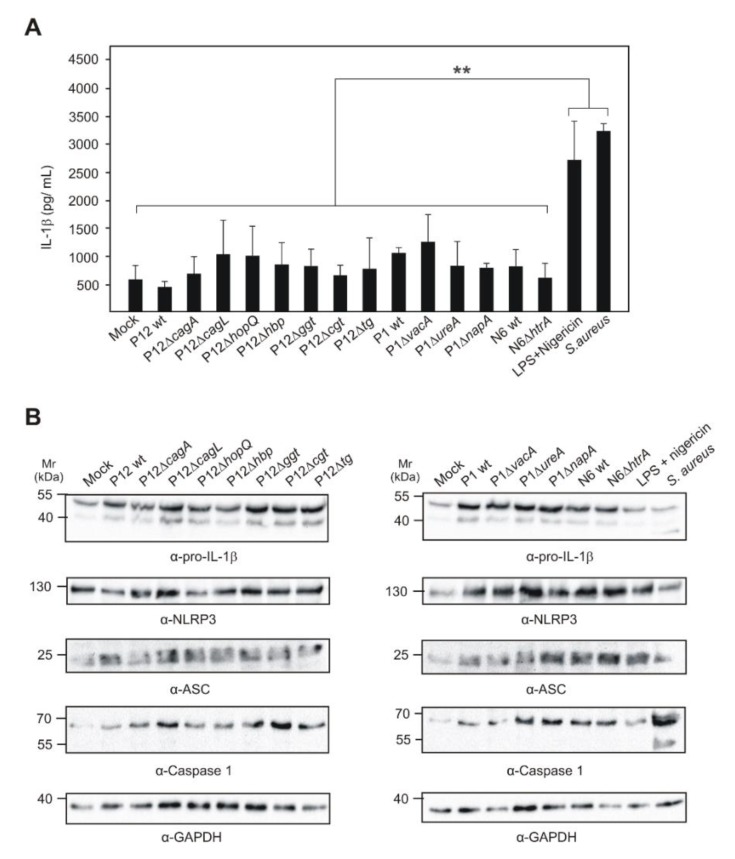
As next we asked if major reported H. pylori virulence factors may inhibit inflammasome formation and mature IL-1β secretion in human target cells. To test this idea, three different type-I H. pylori wild-type strains (P12, P1 and N6) and their respective isogenic mutants of eleven important virulence factors, including ∆cagA, ∆cagL, ∆hopQ, ∆hbp, ∆ggt, ∆tg, ∆cgt, ∆vacA, ∆ureA, ∆napA or ∆htrA, were applied to infect HEK293-NLRP3-INSOME cells. However, the secreted mature IL-1β concentration from H. pylori wild-type and isogenic mutants were not significantly different to mock-treated control cells, which ruled out the involvement of these genes in inhibiting NLRP3 inflammasome function (Figure 7A). As positive controls, S. aureus-infected and LPS/Nigericin-treated cells significantly upregulated the mature IL-1β secretion in HEK293-NLRP3-INSOME cells when compared to H. pylori infection (Figure 7A). Furthermore, the expression of all inflammasome components was confirmed in H. pylori- and S. aureus-infected. LPS/Nigericin-treated HEK293-NLRP3-INSOME cells upon Western blotting (Figure 7B; Supplementary Figure S3). Finally, S. aureus-infected THP1 monocytes secreted very high amounts of mature IL-1β in comparison with H. pylori infection, which further proves the validity of the above results (Figure 8). Thus, we identified here for the first time that H. pylori is unable to activate the NLRP3 inflammasome in human immune cells and secretes low amounts of IL-1β through a caspase-1 independent mechanism.
Figure 7.
HEK293-NLRP3-INSOME cells infected with three different type-I strains and their respective isogenic mutants were used to analyze further activation of the NLRP3 inflammasome and were compared with LPS/Nigericin-treated or S. aureus-infected cells (A). The inflammasome components expression was further confirmed by Western blot in the mock control, H. pylori-infected, E. coli LPS/Nigericin-treated and S. aureus-infected cells (B). GAPDH protein immunoblots were used as a loading reference. ** p ≤ 0.01.
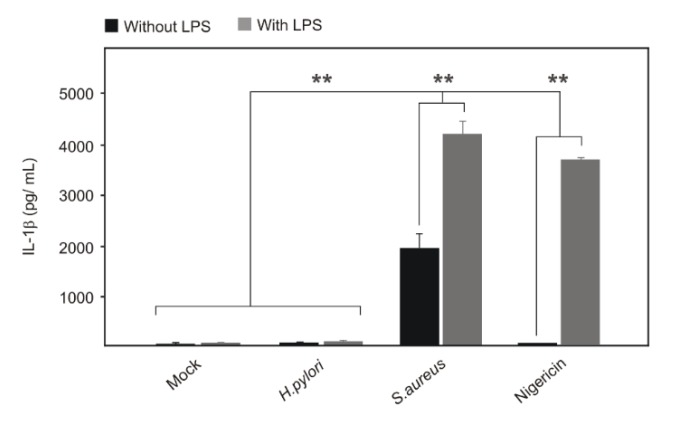
Figure 8.
THP1 monocytes were infected with S. aureus and the level of mature IL-1β secretion was compared with LPS/Nigericin treatment and H. pylori infection. S. aureus infection secreted comparable levels of LPS/Nigericin treatment, which shows canonical NLPR3 inflammasome activation by this bacterial activator in monocytes and confirms the inability of H. pylori to activate this innate immune function. ** p ≤ 0.01.
3. Discussion
Inflammasomes are multi-protein scaffolds formed by intracellular innate immune receptors including NLRs, AIM2 or TRIM20 for the activation of caspase-1 and cleavage of pro-forms of IL-1β and IL-18 to its mature functional molecules [57,58,59]. NLRP3 is the most studied inflammasome type and follows a two-step activation mechanism. The first step starts with the increased production of required inflammasome components for scaffold formation and activation [64,74]. The second step of NLRP3 inflammasome activation occurs by different factors discussed above, however, several research groups concluded that these factors function through key events such as K+ efflux, Ca2+ signaling, mitochondrial damage, lysosome rupture or ROS [64,74,75]. Furthermore, a new mechanism was implicated in NLRP3 activation by its recruitment to phosphatidylinositol-4-phosphate regions on the dispersed trans Golgi network through ionic bonding of conserved basic amino acid residues [76]. Certain phosphorylation events in NLRP3 also keep the molecule inactive, which were reported to be lost under chronic inflammatory conditions like CAPS (cryopyrin-associated periodic syndromes) and inflammatory bowel disease [77,78]. H. pylori induces the production of several cytokines including IL-1β after infection of humans or mice. There are several reports showing that H. pylori activated the NLRP3 inflammasome during mouse infection [39,65,66]. NLRP3 also showed non-inflammasomal function in H. pylori infection by maintaining the population of CD11b+ DCs in the gastrointestinal tissues [79]. H. pylori infection also impacted the NLRP3 inflammasome function in mice through MUC1, which happened through suppression of NF-κB signaling [67]. In fact, MUC1 expression in macrophages limits gastritis through regulation of the NLRP3 inflammasome [67]. However, very few studies are available on the NLRP3 inflammasome activation in human cell systems during H. pylori infection. In our previous report, we showed that NLRP3 protein expression is downregulated in human immune cells through miRNA-223-3p and IL-10 upon H. pylori infection [68]. Therefore, the present study dissected the NLRP3 inflammasome formation upon H. pylori infection of different human immune cells.
Human immune cells infected with different type-I and type-II H. pylori strains were analyzed for NLRP3 inflammasome function through the expression level of pro-IL-1β, NLRP3 inflammasome formation and secretion of mature IL-1β. Both type-I and type-II strains induced the upregulated expression of pro-IL-1β, but secreted very little mature IL-1β in THP1 monocytes after infection. In addition to the cagPAI, other specific virulence factors such as CagL, VacA, LPS, and urease B were also implicated in NLRP3 inflammasome activation by infected mice [39,65,66]. THP1 monocytes infected with these and other isogenic mutants of H. pylori virulence factors also secreted very little IL-1β, which suggests a different mechanism in human cells. As the amounts of secreted IL-1β were surprisingly very low, we compared the canonical NLRP3 inflammasome activation (E. coli LPS + Nigericin or ATP) with that during H. pylori infection. Canonical inflammasome activation yielded a high amount of mature IL-1β in comparison with H. pylori-infected cells. This demonstrates that our cell system is functionally intact for NLRP3 inflammasome activation, however, H. pylori infection failed to activate the same. There are two possibilities occurring here: (i) H. pylori infection lacks a proper second signal activation or (ii) irreversibly inhibits NLRP3 inflammasome formation. To test these possibilities, we treated H. pylori-infected cells with Nigericin or ATP, which interestingly secreted very high amounts of IL-1β and ruled out irreversible inhibition of inflammasome formation by this pathogen. Furthermore, we tried to confirm that mature IL-1β secretion is dependent on caspase-1 activation. Thus, we treated cells with VX-765, a caspase-1 inhibitor, which almost completely inhibited NLRP3 inflammasome-mediated IL-1β secretion during canonical activation in LPS-treated and H. pylori-infected THP1 monocytes; however, infected cells secreted very small amounts, even after caspase-1 inhibition. Conclusively, this shows that H. pylori-infected cells lacked a proper second signal for inflammasome activation, at least in infected THP1 monocytes, but secreted small amounts of IL-1β by a caspase-1 independent mechanism.
The very small amounts of IL-1β secretion by H. pylori-infected human cells can also be dependent on non-canonical NLRP3 inflammasome-mediated activation of other caspases [80,81,82]. To investigate this possibility, we infected CRISPR/Cas9 knockout THP1 monocytes for NLRP3 and caspase-1 to analyze IL-1β secretion. Either of the infected and LPS-treated knockout cell lines induced the expression of pro-IL-1β. Furthermore, H. pylori-infected cells secreted small amounts of IL-1β as in the parental THP1 cells. The Nigericin-induced second signal produced high amounts of mature IL-1β secretion both in infected and LPS-treated parental THP1 cells; however, NLRP3-KO and caspase-1-KO cells failed to activate NLRP3 inflammasome-mediated mature IL-1β secretion under both conditions as expected. This confirmed two scenarios: (i) the basic IL-1β secretion in H. pylori-infected cells is independent both of the NLRP3 inflammasome and caspase-1 activity, and (ii) in Nigericin- or ATP-treated infected cells, secretion of mature IL-1β is dependent on NLRP3 and caspase-1. Since THP-1 is an immortal cancer cell line, we next included primary human immune cells in our studies. Primary M1 and M2 macrophages were prepared from patients followed by infection with H. pylori for indicated time periods, which also secreted low levels of mature IL-1β as observed in parental and knockout THP-1 monocytes. However, Nigericin-treatment significantly increased mature IL-1β secretion. Thus, we speculate that H. pylori infection of humans in vivo might not be different to that observed in human cell systems in vitro.
The visualization of NLRP3 inflammasome formation and structure is another powerful tool to confirm the above results. We used PMA-differentiated THP1 macrophages to visualize the NLRP3 inflammasome in H. pylori infection and subsequent Nigericin treatment. Immuno-fluorescence microscopic pictures showed that H. pylori/Nigericin-treated cells revealed clear NLRP3 inflammasome speck formation, where both NLRP3 and caspase-1 co-localized in flattened elongated cells, which suggests inflammasome activation and pyroptosis. However, mock-treated control and H. pylori-infected THP1 macrophages maintained similar characteristic shapes and no clear signs of NLRP3 inflammasome specks. The super resolution STED microscopy for NLRP3 inflammasome speck formation in H. pylori/Nigericin-treated cells showed characteristic donut-shaped specks [71,76] and also secreted significantly high amounts of mature IL-1β in THP1 macrophages. Furthermore, we reconstructed the NLRP3 inflammasome by transient expression of all components including pro-IL-1β in HEK293 epithelial cells and H. pylori infection did not significantly change the basic mature IL-1β secretion, whereas Nigericin treatment highly increased the secretion. This finally confirmed that H. pylori infection of human monocytes/macrophages clearly activates the first signal but is unable to fully activate the NLRP3 inflammasome.
There are bacteria such as S. aureus that are known to activate the NLRP3 inflammasome [83,84,85]. We therefore compared S. aureus with H. pylori on induction of the NLRP3 inflammasome in reconstructed HEK293 cells. In concurrence with our above data, H. pylori infection did not change basic IL-1β secretion levels; however, S. aureus-infected and LPS/Nigericin-treated cells increased the reconstructed NLRP3 inflammasome activity to significantly enhance the amounts of mature IL-1β secretion. Moreover, we explored the possibility that major virulence factors of H. pylori could eventually inhibit the activation of the NLRP3 inflammasome. However, infection of three different wild-type strains and 11 well-known isogenic mutants did not reveal any critical effect on inflammasome function and mature IL-1β secretion. Moreover, confocal microscopy images showed increased inflammasome specks in S. aureus-infected and LPS/Nigericin-treated cells when compared to H. pylori-infected and mock control cells. Altogether, these data confirmed the inability of H. pylori to induce proper NLRP3 inflammasome formation in human cells. Based on the IL-1β secretion in studied cells and microscopic inflammasome analysis, it was plausible that no other inflammasome types were activated in H. pylori-infected cells. The increased expression of pro-IL-1β in monocytes/macrophages or other cells by H. pylori infection, make them vulnerable to NLRP3 inflammasome activation through increased concentration of ATP, MSU, ROS or environmental factors or co-infection with other inflammasome activating bacteria. The regulation of NLRP3 expression and activation during H. pylori infection may control the increased tissue destruction in the gastric mucosa and ensure bacterial survival and persistence. Together, our study created a new perspective on inflammasome manipulation by H. pylori and that may support chronic infection in humans. Therefore, more studies with higher animal models and patients are needed to understand the clinical perspective and possibilities for therapeutic intervention.
4. Materials and Methods
4.1. Bacterial Strains and Culture
Helicobacter pylori wild-type strains of type I (P1, P12, N6) and type II (UK123, UK097, Ka148) were used in this study. The P12ΔcagA, ΔcagL, ΔcagPAI, ΔflaA, Δggt, Δhbp, ΔhopQ, Δhp1191 (LPS), Δcgt, Δtg and ΔvacA, as well as P1ΔnapA, ΔureA and ΔvacA and N6ΔhtrA isogenic mutants were created by insertion of a chloramphenicol or kanamycin resistance gene cassette, respectively, and were used in this study [86,87,88,89]. H. pylori were grown on horse serum agar plates supplemented with vancomycin (10 µg/mL), nystatin (1 µg/mL) and trimethoprim (5 µg/mL), and chloramphenicol (4 µg/mL) or kanamycin (8 µg/mL) for selection of isogenic mutants. All plates were incubated at 37 °C for 2 days in an anaerobic jar containing a campygen gas mix (Oxoid, Wesel/Germany) [88,90]. H. pylori grown on plates was harvested and resuspended in BHI (brain heart infusion) broth using a sterile cotton swab (Carl Roth, Karlsruhe/Germany). The bacterial concentration was measured as the optical density at 600 nm (OD600nm) using an Eppendorf spectrophotometer. The respective eukaryotic cells grown in medium without antibiotics and antimycotics were infected with H. pylori at a multiplicity of infection (MOI) of 100 [68,88]. The mock treated control cells were incubated with equal amounts of BHI broth. Staphylococcus aureus wild-type strain RN6390 cultured in LB broth until OD600nm = 0.6–0.8 at 37 °C and 600 rpm was also used for infection [91].
4.2. Cell Line Cultures
THP-1 (ATCC-TIB-202) monocytic leukemia cells were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS; Thermo Fisher Scientific, Dreieich/Germany), and 1% antibiotic and antimycotic solution (Sigma- Aldrich, St. Louis, MO, USA) in a humidified incubator at 37 °C with 5% CO2 [68,88,92]. Before infection, LPS (Sigma-Aldrich, St. Louis, MO, USA) or VX-765 inhibitor (Invivogen, Toulouse/France) treatment, cells were washed with PBS at pH 7.4 and the required number of cells were cultured in plates with antibiotic- and antimycotic-free medium. The CRISPR/Cas9 generated NLRP3 or caspase-1 knockout THP-1 cells (kind gift from Prof. Veit Hornung, Ludwig-Maximilians University, Munich, Germany) were also maintained or prepared as parental THP1 monocytes mentioned before. For macrophages, THP-1 monocytes were differentiated with 40 nM of Phorbol 12-Myristate 13-Acetate (PMA; Sigma-Aldrich, St. Louis, MO, USA) in complete RPMI-1640 medium for 48 h culture with daily replenishment of fresh medium and cells were also grown on glass cover slips for microscopy. Differentiated THP1 macrophages were rested in culture medium without PMA for another 48 h to attain the morphological and functional status of the macrophages [68,93]. Then, the cells were washed with PBS at pH 7.4 before adding antibiotic- and antimycotic-free medium for H. pylori infection or LPS treatment.
Human epithelial HEK293 cells (ATCC-CRL-1573) were cultured in Dulbecco’s modified Eagle medium (DMEM; Thermo Fisher Scientific, Dreieich/Germany) supplemented with 10% FBS (Thermo Fisher Scientific, Dreieich/Germany) and 1% antibiotic or antimycotic solution, respectively (Sigma-Aldrich, St. Louis, MO, USA) at 37 °C and 5% CO2 [88]. To increase the adherence of HEK293 cells, the used dishes were pre-coated with 0.01% poly-L-lysine (Sigma-Aldrich, St. Louis, MO, USA) at 37 °C one day prior to seeding. For transfection, 2.0 × 105 cells were seeded in 12-well plates one day prior to transfection.
4.3. Preparation of M1 and M2 Macrophages Differentiation and Culture
PBMCs (peripheral blood mononuclear cells) were isolated by density gradient centrifugation of buffy coat preparations from the peripheral blood of volunteers (Deutsches Rotes Kreuz, Erlangen/Germany). Monocytes were isolated by adherence on plastic and cultured in the presence of 50 ng/mL GM-CSF (Berlex, USA) to generate M1 macrophages, or in the presence of 50 ng/mL M-CSF (R&D systems, Minneapolis/USA) to obtain M2 macrophages. Macrophages were detached with 1 mM EDTA (Sigma-Aldrich, St. Louis, MO, USA) solution after 6 days of culture. The phenotype was evaluated by expression of surface markers CD86, CD40, CD163 and CD204 by flow cytometry. The following antibodies were used for flow cytometry: CD163-BV421 (clone: GHI/61, BioLegend, Fell/Germany), CD40-FITC (clone: 5C3, BioLegend, Fell/Germany), CD86-PE (clone: IT2.2, BioLegend, Fell/Germany) and CD204-APC (clone: 351615, R&D systems, Minneapolis/USA).
4.4. NLRP3 Inflammasome Reconstruction by Transient Transfection and Expression of Components
The NLRP3 inflammasome was reconstructed in HEK293 cells with some modifications as described elsewhere [72]. We transfected 200 ng of pmCherry-C1-proIL1B, 200 ng of pEGFP-C2-NLRP3 (a gift from Christian Stehlik, Addgene plasmid # 73955; http://n2t.net/addgene:73955; RRID:Addgene_73955) [94], 20 ng of pcDNA3-Myc-ASC (a gift from Christian Stehlik, Addgene plasmid # 73952; http://n2t.net/addgene:73952; RID:Addgene_73952) [95] and 100 ng of pCI-caspase1 (a gift from Kate Fitzgerald, Addgene plasmid # 41552; http://n2t.net/addgene:41552; RRID:Addgene_41552) [96] using Lipofectamine 3000 (Thermo Fisher Scientific, Dreieich/Germany) following the manufacturer’s protocol. mCherry-pro-IL-1β was constructed by ligating the pro-IL-1β coding sequence from pCellFree_G03 IL1B (a gift from Kirill Alexandrov, Addgene plasmid # 67066; http://n2t.net/addgene:67066; RRID: Addgene_67066) [97] in-frame into a pmCherry-C1 vector backbone. Finally, the sequence was verified by sequencing. The cells were washed twice 16 h post transfection and grown in medium without antibiotics or antimycotics but supplemented with FBS.
4.5. SDS-PAGE and Immunoblotting
Cell lysates from harvested infected and non-infected immune cells were prepared by adding equal amounts of 2 x SDS-PAGE lysis buffer and boiling for 5 min. Polyacrylamide gels (6–12%) were used to resolve the proteins by electrophoresis, which were blotted on Immobilon-P membranes (Millipore, Massachusetts/USA) after that. The blotted membranes were blocked in TBST buffer with 5% skimmed milk or BSA for 1 hour at room temperature as described [88,92,98]. Anti-IL-1β (R&D systems, Minneapolis/USA), anti-NLRP3, anti-ASC (Adipogen, San Diego/USA), anti-caspase-1 and anti-GAPDH (Santa Cruz Biotechnology, Dallas/USA) primary antibodies were used for detection. As secondary antibodies, horseradish peroxidase-conjugated anti-goat polyvalent rabbit immunoglobulin, anti-rabbit or anti-mouse polyvalent goat immunoglobulin, respectively, were used (Thermo Fisher Scientific, Dreieich/Germany). Antibody detection was performed with the Amersham ECL Prime chemiluminescence Western blot kit (GE Healthcare, Chicago/USA) as described [88,98,99]. The band intensities were quantified using ImageLab Software 5.0 (BioRad, Hercules/USA).
4.6. Immunofluorescence Microscopy
The mock-treated control and infected cells were fixed with 3.8% PFA, which followed the standard protocol steps of washes, permeabilization and staining with corresponding fluorescently labelled antibodies [100]. After fixing, the cells were permeabilized with 0.25% Triton X100 for 1 min and blocked with 5% BSA in PBS for 1h. Anti-NLRP3 (Adipogen, San Diego, CA, USA) and anti-caspase-1 (Novus Biologicals, Centennial, CO, USA) were used as primary antibodies for staining and mounted with 50% vectashield (Vector Labs, Peterborough/ United Kingdom) in glycerin. FITC conjugated anti-mouse and TRITC conjugated anti-rabbit were used as secondary antibodies to visualize under the fluorescence microscope (Leica DMRE7, Leica Microsystems, Wetzlar/Germany). Separate images were taken in the corresponding channels and images were obtained by LAS AF computer software (Leica Microsystems, Wetzlar/Germany). Final images for publication were processed using ImageJ software (National Institute of Health, Bethesda/USA).
Confocal laser scanning microscopy (CSLM) of HEK293 cells was done by following this method. The cells were prepared as above and stained with DAPI (4′,6-diamidino-2-phenylindole, Roth, Germany) before being mounted with vectashield as above. These samples were studied using a Leica DMI4000B fluorescence microscope and different lasers (Leica Microsystems, Wetzlar/Germany) for visualizing DNA, eGFP-NLRP3 and mCherry-Pro-IL-1β [101]. Images were acquired by LAS AF computer software (Leica Microsystems, Wetzlar/Germany) and final images were processed as above.
4.7. STED Super Resolution Microscopy
The cells were processed and fixed as described above and were used for STED super resolution microscopy. The mounting agent was specially prepared as 86% glycerol consisting of 2.5% diazabicylco-2-2-2-octan (DABCO) for STED microscopy. Anti-NLRP3 (Santa Cruz, Dallas, TX/USA) antibody was used as primary antibody and a goat anti-rabbit antibody conjugated with Abberior Star Red (Abs. max 638 nm and Fluo. max 655 nm) served as the secondary antibody for visualization in the Abberior Instruments 775 STED microscope (https://www.abberior-instruments.com/products/expert-line/775-sted/) facility at Optical Imaging Centre Erlangen (OICE), Friedrich Alexander University Erlangen-Nuremberg. The samples were pulsed with 640 nm laser for initial visualization, followed by 775 nm wavelength laser for stimulated emission depletion for super resolution images, which gives the characteristic of cellular structures at nm ranges [102]. The acquired images were processed as above.
4.8. Quantification of Cytokines
The supernatants of H. pylori infected, and non-infected cells were collected and centrifuged at 12,000× g in a cold centrifuge at 4 °C to remove bacteria or debris before storing at −80 °C until assayed. Human IL-1β concentrations in the supernatant were determined by standard ELISA, with commercially available assay kits, described by the manufacturer (Becton Dickinson, Heidelberg/Germany).
4.9. Statistical Analysis
All experiments were repeated at least three times with similar results. The data were evaluated using one-way ANOVA followed by Tukey’s post hoc test with GraphPad statistical software. P values of p ≤ 0.05 (*), p ≤ 0.01 (**), p ≤ 0.001 (***) and p ≤ 0.0001 (****) were considered as statistically significant.
5. Conclusions
Helicobacter pylori is known to be associated with inflammation after colonizing the gastric mucosa. We found that H. pylori infection with various clinical isolates in human immune cells did not induce increased secretion of mature IL-1β secretion but rather low amounts were secreted. In mice models, H. pylori infection induced NLRP3 inflammasome activation and mature IL-1β secretion. However, infection in humans or cell cultures are not conclusive as described in the previous studies. We showed that NLRP3 inflammasome formation is not induced in infected human immune cells and epithelial cells expressing reconstructed inflammasome, when compared to known chemical and bacterial activators; LPS with Nigericin or ATP and Staphylococcus aureus, respectively. Upregulated expression and secretion of IL-1β was one of the causative factors for hyperproliferation of gastric epithelial cells and oncogenesis. Because H. pylori avoids NLRP3 inflammasome activation despite upregulated expression of pro-IL-1β and NLRP3 in immune cells, the pathogen slows down the host immunity through regulating massive production of mature IL-1β. This process may be crucial for bacterial colonization, survival and persistence. Externally added second signal activators such as Nigericin or ATP reversed this block, thus this gives novel options for therapeutic eradication of H. pylori.
Acknowledgments
We thank Wilhelm Brill and Nina Rottmann (FAU Erlangen) for excellent technical assistance, and Veit Hornung for the NLRP3 or caspase-1 knockout THP-1 cell lines. We also thank Mark Achtman (Warwick Medical School, University of Warwick, UK) and Sebastian Suerbaum (Max von Pettenkofer Institute Munich, Germany) for providing the H. pylori type-II strains UK123 and UK097, Anna Zawilak-Pawlik (Hirszfeld Institute of Immunology and Experimental Therapy, Wrocław, Poland) for the N6∆htrA gene mutant as well as Alexandra Gruss (Institut MICrobiologie del’ALImentation au Service, Institut National de la Recherche Agronomique, France) for S. aureus strain RN6390. The general advice by Mohammed Lamkanfi on the NLRP3 inflammasome (Ghent University, Belgium) was appreciated very much, too. We are also grateful to the help on STED and Confocal microscopies by Tristan Nowak (OICE, FAU Erlangen) and Aileen Harrer (FAU Erlangen), respectively. We acknowledge the support by Deutsche Forschungsgemeinschaft and Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU) within the funding program Open Access Publishing.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/12/4/803/s1. Table S1: H. pylori strains and mutants used in this study.
Author Contributions
Conceptualization, S.K.P. and S.B; methodology S.K.P., N.B., H.B. and S.B.; investigation, S.K.P., N.B. and H.B.; writing—original draft preparation, S.K.P.; writing—review and editing, S.K.P., S.B., N.B. and H.B.; visualization, S.K.P.; supervision, S.K.P.; project administration, S.K.P. and S.B.; funding acquisition, S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by DFG-grant A04 in CRC-1181 to S.B and post-doctoral fellowship to S.K.P.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Hooi J.K.Y., Lai W.Y., Ng W.K., Suen M.M.Y., Underwood F.E., Tanyingoh D., Malfertheiner P., Graham D.Y., Wong V.W.S., Wu J.C.Y., et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. 2017;153:420–429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 2.Sjomina O., Pavlova J., Niv Y., Leja M. Epidemiology of Helicobacter pylori infection. Helicobacter. 2018;23:e12514. doi: 10.1111/hel.12514. [DOI] [PubMed] [Google Scholar]
- 3.Wroblewski L.E., Peek R.M., Wilson K.T. Helicobacter pylori and Gastric Cancer: Factors That Modulate Disease Risk. Clin. Microbiol. Rev. 2010;23:713–739. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bauer B., Meyer T.F. The Human Gastric Pathogen Helicobacter pylori and Its Association with Gastric Cancer and Ulcer Disease. Ulcers. 2011;2011:1–23. doi: 10.1155/2011/340157. [DOI] [Google Scholar]
- 5.Polk D.B., Peek R.M. Helicobacter pylori: Gastric cancer and beyond. Nat. Rev. Cancer. 2010;10:403–414. doi: 10.1038/nrc2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castaño-RodrÃ-guez N., Kaakoush N.O., Mitchell H.M. Pattern-Recognition Receptors and Gastric Cancer. Front. Immunol. 2014;5:5. doi: 10.3389/fimmu.2014.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pachathundikandi S.K., Lind J., Tegtmeyer N., El-Omar E.M., Backert S. Interplay of the Gastric Pathogen Helicobacter pylori with Toll-Like Receptors. Biomed. Res. Int. 2015;2015:192420. doi: 10.1155/2015/192420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meliț L.E., Mărginean C.O., Mărginean C.D., Mărginean M.O. The Relationship between Toll-like Receptors and Helicobacter pylori -Related Gastropathies: Still a Controversial Topic. J. Immunol. Res. 2019;2019:1–10. doi: 10.1155/2019/8197048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selbach M., Moese S., Hauck C.R., Meyer T.F., Backert S. Src Is the Kinase of the Helicobacter pylori CagA Protein in Vitro and In Vivo. J. Biol. Chem. 2002;277:6775–6778. doi: 10.1074/jbc.C100754200. [DOI] [PubMed] [Google Scholar]
- 10.Tammer I., Brandt S., Hartig R., König W., Backert S. Activation of Abl by Helicobacter pylori: A Novel Kinase for CagA and Crucial Mediator of Host Cell Scattering. Gastroenterology. 2007;132:1309–1319. doi: 10.1053/j.gastro.2007.01.050. [DOI] [PubMed] [Google Scholar]
- 11.Ohnishi N., Yuasa H., Tanaka S., Sawa H., Miura M., Matsui A., Higashi H., Musashi M., Iwabuchi K., Suzuki M., et al. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc. Natl. Acad. Sci. USA. 2008;105:1003–1008. doi: 10.1073/pnas.0711183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tegtmeyer N., Wittelsberger R., Hartig R., Wessler S., Martinez-Quiles N., Backert S. Serine Phosphorylation of Cortactin Controls Focal Adhesion Kinase Activity and Cell Scattering Induced by Helicobacter pylori. Cell Host Microbe. 2011;9:520–531. doi: 10.1016/j.chom.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Mueller D., Tegtmeyer N., Brandt S., Yamaoka Y., De Poire E., Sgouras D., Wessler S., Torres J., Smolka A., Backert S. c-Src and c-Abl kinases control hierarchic phosphorylation and function of the CagA effector protein in Western and East Asian Helicobacter pylori strains. J. Clin. Investig. 2012;122:1553–1566. doi: 10.1172/JCI61143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atherton J.C., Cao P., Peek R.M., Tummuru M.K.R., Blaser M.J., Cover T.L. Mosaicism in Vacuolating Cytotoxin Alleles of Helicobacter pylori: Association of specific vacA types with cytotoxin production and peptic ulceration. J. Biol. Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 15.Palframan S.L., Kwok T., Gabriel K. Vacuolating cytotoxin A (VacA), a key toxin for Helicobacter pylori pathogenesis. Front. Cell. Infect. Microbiol. 2012;2:2. doi: 10.3389/fcimb.2012.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foegeding N., Caston R., McClain M., Ohi M., Cover T. An Overview of Helicobacter pylori VacA Toxin Biology. Toxins. 2016;8:173. doi: 10.3390/toxins8060173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sewald X., Gebert-Vogl B., Prassl S., Barwig I., Weiss E., Fabbri M., Osicka R., Schiemann M., Busch D.H., Semmrich M., et al. Integrin Subunit CD18 Is the T-Lymphocyte Receptor for the Helicobacter pylori Vacuolating Cytotoxin. Cell Host Microbe. 2008;3:20–29. doi: 10.1016/j.chom.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Nakayama M., Kimura M., Wada A., Yahiro K., Ogushi K., Niidome T., Fujikawa A., Shirasaka D., Aoyama N., Kurazono H., et al. Helicobacter pylori VacA Activates the p38/Activating Transcription Factor 2-mediated Signal Pathway in AZ-521 Cells. J. Biol. Chem. 2004;279:7024–7028. doi: 10.1074/jbc.M308898200. [DOI] [PubMed] [Google Scholar]
- 19.Hisatsune J., Yamasaki E., Nakayama M., Shirasaka D., Kurazono H., Katagata Y., Inoue H., Han J., Sap J., Yahiro K., et al. Helicobacter pylori VacA Enhances Prostaglandin E2 Production through Induction of Cyclooxygenase 2 Expression via a p38 Mitogen-Activated Protein Kinase/Activating Transcription Factor 2 Cascade in AZ-521 Cells. Infect. Immun. 2007;75:4472–4481. doi: 10.1128/IAI.00500-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radin J.N., González-Rivera C., Ivie S.E., McClain M.S., Cover T.L. Helicobacter pylori VacA Induces Programmed Necrosis in Gastric Epithelial Cells. Infect. Immun. 2011;79:2535–2543. doi: 10.1128/IAI.01370-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akazawa Y., Isomoto H., Matsushima K., Kanda T., Minami H., Yamaghchi N., Taura N., Shiozawa K., Ohnita K., Takeshima F., et al. Endoplasmic Reticulum Stress Contributes to Helicobacter pylori VacA-Induced Apoptosis. PLoS ONE. 2013;8:e82322. doi: 10.1371/journal.pone.0082322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat. Rev. Gastroenterol. Hepatol. 2010;7:629–641. doi: 10.1038/nrgastro.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Backert S., Neddermann M., Maubach G., Naumann M. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2016;21:19–25. doi: 10.1111/hel.12335. [DOI] [PubMed] [Google Scholar]
- 24.Chang W.-L., Yeh Y.-C., Sheu B.-S. The impacts of H. pylori virulence factors on the development of gastroduodenal diseases. J. Biomed. Sci. 2018;25:68. doi: 10.1186/s12929-018-0466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunn B.E., Cohen H., Blaser M.J. Helicobacter pylori. Clin. Microbiol. Rev. 1997;10:720–741. doi: 10.1128/CMR.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White J.R., Winter J.A., Robinson K. Differential inflammatory response to Helicobacter pylori infection: Etiology and clinical outcomes. J. Inflamm. Res. 2015;8:137–147. doi: 10.2147/JIR.S64888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gobert A.P., Wilson K.T. The Immune Battle against Helicobacter pylori Infection: NO Offense. Trends Microbiol. 2016;24:366–376. doi: 10.1016/j.tim.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson K.T., Crabtree J.E. Immunology of Helicobacter pylori: Insights into the failure of the immune response and perspectives on vaccine studies. Gastroenterology. 2007;133:288–308. doi: 10.1053/j.gastro.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Backert S., Naumann M. What a disorder: Proinflammatory signaling pathways induced by Helicobacter pylori. Trends Microbiol. 2010;18:479–486. doi: 10.1016/j.tim.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Blaser N., Backert S., Pachathundikandi S.K. Immune Cell Signaling by Helicobacter pylori: Impact on Gastric Pathology. Springer; New York, NY, USA: 2019. [DOI] [PubMed] [Google Scholar]
- 31.Pachathundikandi S.K., Blaser N., Backert S. Mechanisms of Inflammasome Signaling, microRNA Induction and Resolution of Inflammation by Helicobacter pylori. In: Backert S., editor. Molecular Mechanisms of Inflammation: Induction, Resolution and Escape by Helicobacter pylori. Volume 421. Springer International Publishing; Cham, Switzerland: 2019. pp. 267–302. [DOI] [PubMed] [Google Scholar]
- 32.Quiding-Järbrink M., Raghavan S., Sundquist M. Enhanced M1 Macrophage Polarization in Human Helicobacter pylori-Associated Atrophic Gastritis and in Vaccinated Mice. PLoS ONE. 2010;5:e15018. doi: 10.1371/journal.pone.0015018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gobert A.P., Verriere T., Asim M., Barry D.P., Piazuelo M.B., de Sablet T., Delgado A.G., Bravo L.E., Correa P., Peek R.M., et al. Heme Oxygenase-1 Dysregulates Macrophage Polarization and the Immune Response to Helicobacter pylori. J. Immunol. 2014;193:3013–3022. doi: 10.4049/jimmunol.1401075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beceiro S., Radin J.N., Chatuvedi R., Piazuelo M.B., Horvarth D.J., Cortado H., Gu Y., Dixon B., Gu C., Lange I., et al. TRPM2 ion channels regulate macrophage polarization and gastric inflammation during Helicobacter pylori infection. Mucosal Immunol. 2017;10:493–507. doi: 10.1038/mi.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oertli M., Noben M., Engler D.B., Semper R.P., Reuter S., Maxeiner J., Gerhard M., Taube C., Müller A. Helicobacter pylori γ-glutamyl transpeptidase and vacuolating cytotoxin promote gastric persistence and immune tolerance. Proc. Natl. Acad. Sci. USA. 2013;110:3047–3052. doi: 10.1073/pnas.1211248110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaebisch R., Mejías-Luque R., Prinz C., Gerhard M. Helicobacter pylori cytotoxin-associated gene A impairs human dendritic cell maturation and function through IL-10-mediated activation of STAT3. J. Immunol. 2014;192:316–323. doi: 10.4049/jimmunol.1302476. [DOI] [PubMed] [Google Scholar]
- 37.Hussain K., Letley D.P., Greenaway A.B., Kenefeck R., Winter J.A., Tomlinson W., Rhead J., Staples E., Kaneko K., Atherton J.C., et al. Helicobacter pylori-Mediated Protection from Allergy Is Associated with IL-10-Secreting Peripheral Blood Regulatory T Cells. Front. Immunol. 2016;7:720. doi: 10.3389/fimmu.2016.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brawner K.M., Kumar R., Serrano C.A., Ptacek T., Lefkowitz E., Morrow C.D., Zhi D., Kyanam-Kabir-Baig K.R., Smythies L.E., Harris P.R., et al. Helicobacter pylori infection is associated with an altered gastric microbiota in children. Mucosal Immunol. 2017;10:1169–1177. doi: 10.1038/mi.2016.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koch K.N., Hartung M.L., Urban S., Kyburz A., Bahlmann A.S., Lind J., Backert S., Taube C., Müller A. Helicobacter urease-induced activation of the TLR2/NLRP3/IL-18 axis protects against asthma. J. Clin. Investig. 2015;125:3297–3302. doi: 10.1172/JCI79337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Engler D.B., Reuter S., van Wijck Y., Urban S., Kyburz A., Maxeiner J., Martin H., Yogev N., Waisman A., Gerhard M., et al. Effective treatment of allergic airway inflammation with Helicobacter pylori immunomodulators requires BATF3-dependent dendritic cells and IL-10. Proc. Natl. Acad. Sci. USA. 2014;111:11810–11815. doi: 10.1073/pnas.1410579111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amieva M.R., El-Omar E.M. Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology. 2008;134:306–323. doi: 10.1053/j.gastro.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Atherton J.C., Blaser M.J. Coadaptation of Helicobacter pylori and humans: Ancient history, modern implications. J. Clin. Investig. 2009;119:2475–2487. doi: 10.1172/JCI38605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salama N.R., Hartung M.L., Müller A. Life in the human stomach: Persistence strategies of the bacterial pathogen Helicobacter pylori. Nat. Rev. Microbiol. 2013;11:385–399. doi: 10.1038/nrmicro3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.El-Omar E.M., Carrington M., Chow W.H., McColl K.E., Bream J.H., Young H.A., Herrera J., Lissowska J., Yuan C.C., Rothman N., et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 45.Furuta T., El-Omar E.M., Xiao F., Shirai N., Takashima M., Sugimurra H. Interleukin 1β polymorphisms increase risk of hypochlorhydria and atrophic gastritis and reduce risk of duodenal ulcer recurrence in Japan. Gastroenterology. 2002;123:92–105. doi: 10.1053/gast.2002.34156. [DOI] [PubMed] [Google Scholar]
- 46.Hong J.-B., Zuo W., Wang A.-J., Lu N.-H. Helicobacter pylori Infection Synergistic with IL-1β Gene Polymorphisms Potentially Contributes to the Carcinogenesis of Gastric Cancer. Int. J. Med. Sci. 2016;13:298–303. doi: 10.7150/ijms.14239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Apte R.N., Dotan S., Elkabets M., White M.R., Reich E., Carmi Y., Song X., Dvozkin T., Krelin Y., Voronov E. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006;25:387–408. doi: 10.1007/s10555-006-9004-4. [DOI] [PubMed] [Google Scholar]
- 48.Resende C., Regalo G., Durães C., Pinto M.T., Wen X., Figueiredo C., Carneiro F., Machado J.C. Interleukin-1B signalling leads to increased survival of gastric carcinoma cells through a CREB-C/EBPβ-associated mechanism. Gastric Cancer. 2016;19:74–84. doi: 10.1007/s10120-014-0448-x. [DOI] [PubMed] [Google Scholar]
- 49.Bent R., Moll L., Grabbe S., Bros M. Interleukin-1 Beta—A Friend or Foe in Malignancies? Int. J. Mol. Sci. 2018;19:2155. doi: 10.3390/ijms19082155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mantovani A., Barajon I., Garlanda C. IL-1 and IL-1 regulatory pathways in cancer progression and therapy. Immunol. Rev. 2018;281:57–61. doi: 10.1111/imr.12614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kato S., Onda M., Yamada S., Matsuda N., Tokunaga A., Matsukura N. Association of the interleukin-1 beta genetic polymorphism and gastric cancer risk in Japanese. J. Gastroenterol. 2001;36:696–699. doi: 10.1007/s005350170033. [DOI] [PubMed] [Google Scholar]
- 52.Rad R., Prinz C., Neu B., Neuhofer M., Zeitner M., Voland P., Becker I., Schepp W., Gerhard M. Synergistic Effect of Helicobacter pylori Virulence Factors and Interleukin-1 Polymorphisms for the Development of Severe Histological Changes in the Gastric Mucosa. J. Infect. Dis. 2003;188:272–281. doi: 10.1086/376458. [DOI] [PubMed] [Google Scholar]
- 53.Kameoka S., Kameyama T., Hayashi T., Sato S., Ohnishi N., Hayashi T., Murata-Kamiya N., Higashi H., Hatakeyama M., Takaoka A. Helicobacter pylori induces IL-1β protein through the inflammasome activation in differentiated macrophagic cells. Biomed. Res. 2016;37:21–27. doi: 10.2220/biomedres.37.21. [DOI] [PubMed] [Google Scholar]
- 54.Tu S., Bhagat G., Cui G., Takaishi S., Kurt-Jones E.A., Rickman B., Betz K.S., Penz-Oesterreicher M., Bjorkdahl O., Fox J.G., et al. Overexpression of Interleukin-1β Induces Gastric Inflammation and Cancer and Mobilizes Myeloid-Derived Suppressor Cells in Mice. Cancer Cell. 2008;14:408–419. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shigematsu Y., Niwa T., Rehnberg E., Toyoda T., Yoshida S., Mori A., Wakabayashi M., Iwakura Y., Ichinose M., Kim Y.-J., et al. Interleukin-1β induced by Helicobacter pylori infection enhances mouse gastric carcinogenesis. Cancer Lett. 2013;340:141–147. doi: 10.1016/j.canlet.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 56.Kuo S.-H., Wu M.-S., Yeh K.-H., Lin C.-W., Hsu P.-N., Chen L.-T., Cheng A.-L. Novel Insights of Lymphomagenesis of Helicobacter pylori-Dependent Gastric Mucosa-Associated Lymphoid Tissue Lymphoma. Cancers. 2019;11:547. doi: 10.3390/cancers11040547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.von Moltke J., Ayres J.S., Kofoed E.M., Chavarría-Smith J., Vance R.E. Recognition of Bacteria by Inflammasomes. Annu. Rev. Immunol. 2013;31:73–106. doi: 10.1146/annurev-immunol-032712-095944. [DOI] [PubMed] [Google Scholar]
- 58.Lamkanfi M., Dixit V.M. Mechanisms and Functions of Inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 59.Backert S., editor. Inflammasome Signaling and Bacterial Infections. Volume 397. Springer International Publishing; Cham, Switzerland: 2016. Current Topics in Microbiology and Immunology. [Google Scholar]
- 60.Afonina I.S., Müller C., Martin S.J., Beyaert R. Proteolytic Processing of Interleukin-1 Family Cytokines: Variations on a Common Theme. Immunity. 2015;42:991–1004. doi: 10.1016/j.immuni.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 61.Gavrilin M.A., Abdelaziz D.H.A., Mostafa M., Abdulrahman B.A., Grandhi J., Akhter A., Abu Khweek A., Aubert D.F., Valvano M.A., Wewers M.D., et al. Activation of the Pyrin Inflammasome by Intracellular Burkholderia cenocepacia. J. Immunol. 2012;188:3469–3477. doi: 10.4049/jimmunol.1102272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perregaux D., Gabel C.A. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J. Biol. Chem. 1994;269:15195–15203. [PubMed] [Google Scholar]
- 63.Dostert C., Pétrilli V., Van Bruggen R., Steele C., Mossman B.T., Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.He Y., Hara H., Núñez G. Mechanism and Regulation of NLRP3 Inflammasome Activation. Trends Biochem. Sci. 2016;41:1012–1021. doi: 10.1016/j.tibs.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim D.-J., Park J.-H., Franchi L., Backert S., Núñez G. The Cag pathogenicity island and interaction between TLR2/NOD2 and NLRP3 regulate IL-1β production in Helicobacter pylori infected dendritic cells: Immunity to infection. Eur. J. Immunol. 2013;43:2650–2658. doi: 10.1002/eji.201243281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Semper R.P., Mejías-Luque R., Groß C., Anderl F., Müller A., Vieth M., Busch D.H., Prazeres da Costa C., Ruland J., Groß O., et al. Helicobacter pylori—Induced IL-1β Secretion in Innate Immune Cells Is Regulated by the NLRP3 Inflammasome and Requires the Cag Pathogenicity Island. J. Immunol. 2014;193:3566–3576. doi: 10.4049/jimmunol.1400362. [DOI] [PubMed] [Google Scholar]
- 67.Ng G.Z., Menheniott T.R., Every A.L., Stent A., Judd L.M., Chionh Y.T., Dhar P., Komen J.C., Giraud A.S., Wang T.C., et al. The MUC1 mucin protects against Helicobacter pylori pathogenesis in mice by regulation of the NLRP3 inflammasome. Gut. 2016;65:1087–1099. doi: 10.1136/gutjnl-2014-307175. [DOI] [PubMed] [Google Scholar]
- 68.Pachathundikandi S.K., Backert S. Helicobacter pylori controls NLRP3 expression by regulating hsa-miR-223-3p and IL-10 in cultured and primary human immune cells. Innate Immun. 2018;24:11–23. doi: 10.1177/1753425917738043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shao W., Yeretssian G., Doiron K., Hussain S.N., Saleh M. The Caspase-1 Digestome Identifies the Glycolysis Pathway as a Target during Infection and Septic Shock. J. Biol. Chem. 2007;282:36321–36329. doi: 10.1074/jbc.M708182200. [DOI] [PubMed] [Google Scholar]
- 70.Gurung P., Li B., Subbarao Malireddi R.K., Lamkanfi M., Geiger T.L., Kanneganti T.-D. Chronic TLR Stimulation Controls NLRP3 Inflammasome Activation through IL-10 Mediated Regulation of NLRP3 Expression and Caspase-8 Activation. Sci. Rep. 2015;5:14488. doi: 10.1038/srep14488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Man S.M., Hopkins L.J., Nugent E., Cox S., Gluck I.M., Tourlomousis P., Wright J.A., Cicuta P., Monie T.P., Bryant C.E. Inflammasome activation causes dual recruitment of NLRC4 and NLRP3 to the same macromolecular complex. Proc. Natl. Acad. Sci. USA. 2014;111:7403–7408. doi: 10.1073/pnas.1402911111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shi H., Murray A., Beutler B. Reconstruction of the Mouse Inflammasome System in HEK293T Cells. BIO-PROTOCOL. 2016;6:6. doi: 10.21769/BioProtoc.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Compan V., López-Castejón G. Functional Reconstruction of NLRs in HEK293 Cells. In: Di Virgilio F., Pelegrín P., editors. NLR Proteins. Volume 1417. Springer; New York, NY, USA: 2016. pp. 217–221. [DOI] [PubMed] [Google Scholar]
- 74.Kelley N., Jeltema D., Duan Y., He Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019;20:3328. doi: 10.3390/ijms20133328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Muñoz-Planillo R., Kuffa P., Martínez-Colón G., Smith B.L., Rajendiran T.M., Núñez G. K+ Efflux Is the Common Trigger of NLRP3 Inflammasome Activation by Bacterial Toxins and Particulate Matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen J., Chen Z.J. PtdIns4P on dispersed trans-Golgi network mediates NLRP3 inflammasome activation. Nature. 2018;564:71–76. doi: 10.1038/s41586-018-0761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mortimer L., Moreau F., MacDonald J.A., Chadee K. NLRP3 inflammasome inhibition is disrupted in a group of auto-inflammatory disease CAPS mutations. Nat. Immunol. 2016;17:1176–1186. doi: 10.1038/ni.3538. [DOI] [PubMed] [Google Scholar]
- 78.Spalinger M.R., Kasper S., Gottier C., Lang S., Atrott K., Vavricka S.R., Scharl S., Gutte P.M., Grütter M.G., Beer H.-D., et al. NLRP3 tyrosine phosphorylation is controlled by protein tyrosine phosphatase PTPN22. J. Clin. Investig. 2016;126:1783–1800. doi: 10.1172/JCI83669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arnold I.C., Zhang X., Urban S., Artola-Borán M., Manz M.G., Ottemann K.M., Müller A. NLRP3 Controls the Development of Gastrointestinal CD11b + Dendritic Cells in the Steady State and during Chronic Bacterial Infection. Cell Rep. 2017;21:3860–3872. doi: 10.1016/j.celrep.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 80.Schmid-Burgk J.L., Gaidt M.M., Schmidt T., Ebert T.S., Bartok E., Hornung V. Caspase-4 mediates non-canonical activation of the NLRP3 inflammasome in human myeloid cells: Innate immunity. Eur. J. Immunol. 2015;45:2911–2917. doi: 10.1002/eji.201545523. [DOI] [PubMed] [Google Scholar]
- 81.Gurung P., Burton A., Kanneganti T.-D. NLRP3 inflammasome plays a redundant role with caspase 8 to promote IL-1β–mediated osteomyelitis. Proc. Natl. Acad. Sci. USA. 2016;113:4452–4457. doi: 10.1073/pnas.1601636113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen M., Xing Y., Lu A., Fang W., Sun B., Chen C., Liao W., Meng G. Internalized Cryptococcus neoformans Activates the Canonical Caspase-1 and the Noncanonical Caspase-8 Inflammasomes. J. Immunol. 2015;195:4962–4972. doi: 10.4049/jimmunol.1500865. [DOI] [PubMed] [Google Scholar]
- 83.Mariathasan S., Weiss D.S., Newton K., McBride J., O’Rourke K., Roose-Girma M., Lee W.P., Weinrauch Y., Monack D.M., Dixit V.M. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 84.Craven R.R., Gao X., Allen I.C., Gris D., Wardenburg J.B., McElvania-TeKippe E., Ting J.P., Duncan J.A. Staphylococcus aureus α-Hemolysin Activates the NLRP3-Inflammasome in Human and Mouse Monocytic Cells. PLoS ONE. 2009;4:e7446. doi: 10.1371/journal.pone.0007446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Melehani J.H., James D.B.A., DuMont A.L., Torres V.J., Duncan J.A. Staphylococcus aureus Leukocidin A/B (LukAB) Kills Human Monocytes via Host NLRP3 and ASC when Extracellular, but Not Intracellular. PLoS Pathog. 2015;11:e1004970. doi: 10.1371/journal.ppat.1004970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Backert S., Ziska E., Brinkmann V., Zimny-Arndt U., Fauconnier A., Jungblut P.R., Naumann M., Meyer T.F. Translocation of the Helicobacter pylori CagA protein in gastric epithelial cells by a type IV secretion apparatus. Cell. Microbiol. 2000;2:155–164. doi: 10.1046/j.1462-5822.2000.00043.x. [DOI] [PubMed] [Google Scholar]
- 87.Backert S., Schwarz T., Miehlke S., Kirsch C., Sommer C., Kwok T., Gerhard M., Goebel U.B., Lehn N., Koenig W., et al. Functional Analysis of the cag Pathogenicity Island in Helicobacter pylori Isolates from Patients with Gastritis, Peptic Ulcer, and Gastric Cancer. Infect. Immun. 2004;72:1043–1056. doi: 10.1128/IAI.72.2.1043-1056.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kumar Pachathundikandi S., Brandt S., Madassery J., Backert S. Induction of TLR-2 and TLR-5 expression by Helicobacter pylori switches cagPAI-dependent signalling leading to the secretion of IL-8 and TNF-α. PLoS ONE. 2011;6:e19614. doi: 10.1371/journal.pone.0019614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zawilak-Pawlik A., Zarzecka U., Żyła-Uklejewicz D., Lach J., Strapagiel D., Tegtmeyer N., Böhm M., Backert S., Skorko-Glonek J. Establishment of serine protease htrA mutants in Helicobacter pylori is associated with secA mutations. Sci. Rep. 2019;9:11794. doi: 10.1038/s41598-019-48030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wiedemann T., Hofbaur S., Tegtmeyer N., Huber S., Sewald N., Wessler S., Backert S., Rieder G. Helicobacter pylori CagL dependent induction of gastrin expression via a novel αvβ5-integrin–integrin linked kinase signalling complex. Gut. 2012;61:986–996. doi: 10.1136/gutjnl-2011-300525. [DOI] [PubMed] [Google Scholar]
- 91.Rigoulay C., Entenza J.M., Halpern D., Widmer E., Moreillon P., Poquet I., Gruss A. Comparative Analysis of the Roles of HtrA-Like Surface Proteases in Two Virulent Staphylococcus aureus Strains. Infect. Immun. 2005;73:563–572. doi: 10.1128/IAI.73.1.563-572.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moese S., Selbach M., Zimny-Arndt U., Jungblut P.R., Meyer T.F., Backert S. Identification of a tyrosine-phosphorylated 35 kDa carboxy-terminal fragment (p35CagA) of the Helicobacter pylori CagA protein in phagocytic cells: Processing or breakage? Proteomics. 2001;1:618–629. doi: 10.1002/1615-9861(200104)1:4<618::AID-PROT618>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 93.Daigneault M., Preston J.A., Marriott H.M., Whyte M.K.B., Dockrell D.H. The Identification of Markers of Macrophage Differentiation in PMA-Stimulated THP-1 Cells and Monocyte-Derived Macrophages. PLoS ONE. 2010;5:e8668. doi: 10.1371/journal.pone.0008668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khare S., Dorfleutner A., Bryan N.B., Yun C., Radian A.D., de Almeida L., Rojanasakul Y., Stehlik C. An NLRP7-Containing Inflammasome Mediates Recognition of Microbial Lipopeptides in Human Macrophages. Immunity. 2012;36:464–476. doi: 10.1016/j.immuni.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bryan N.B., Dorfleutner A., Rojanasakul Y., Stehlik C. Activation of Inflammasomes Requires Intracellular Redistribution of the Apoptotic Speck-Like Protein Containing a Caspase Recruitment Domain. J. Immunol. 2009;182:3173–3182. doi: 10.4049/jimmunol.0802367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hornung V., Ablasser A., Charrel-Dennis M., Bauernfeind F., Horvath G., Caffrey D.R., Latz E., Fitzgerald K.A. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gagoski D., Polinkovsky M.E., Mureev S., Kunert A., Johnston W., Gambin Y., Alexandrov K. Performance benchmarking of four cell-free protein expression systems: Benchmarking of Cell-Free Systems. Biotechnol. Bioeng. 2016;113:292–300. doi: 10.1002/bit.25814. [DOI] [PubMed] [Google Scholar]
- 98.Tegtmeyer N., Hartig R., Delahay R.M., Rohde M., Brandt S., Conradi J., Takahashi S., Smolka A.J., Sewald N., Backert S. A Small Fibronectin-mimicking Protein from Bacteria Induces Cell Spreading and Focal Adhesion Formation. J. Biol. Chem. 2010;285:23515–23526. doi: 10.1074/jbc.M109.096214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Traverso F.R., Bohr U.R.M., Oyarzabal O.A., Rohde M., Clarici A., Wex T., Kuester D., Malfertheiner P., Fox J.G., Backert S. Morphologic, Genetic, and Biochemical Characterization of Helicobacter Magdeburgensis, a Novel Species Isolated from the Intestine of Laboratory Mice: Characterization of a Novel Helicobacter Species. Helicobacter. 2010;15:403–415. doi: 10.1111/j.1523-5378.2010.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brandt S., Kwok T., Hartig R., Konig W., Backert S. NF- B activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc. Natl. Acad. Sci. USA. 2005;102:9300–9305. doi: 10.1073/pnas.0409873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Harrer A., Bücker R., Boehm M., Zarzecka U., Tegtmeyer N., Sticht H., Schulzke J.D., Backert S. Campylobacter jejuni enters gut epithelial cells and impairs intestinal barrier function through cleavage of occludin by serine protease HtrA. Gut Pathog. 2019;11:4. doi: 10.1186/s13099-019-0283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stoldt S., Wenzel D., Kehrein K., Riedel D., Ott M., Jakobs S. Spatial orchestration of mitochondrial translation and OXPHOS complex assembly. Nat. Cell Biol. 2018;20:528–534. doi: 10.1038/s41556-018-0090-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.