Abstract
Objective
The purpose of this study was to investigate whether immediate or delayed tailored DAIR treatment based on microbial species is the optimal treatment for acute post-operative periprosthetic joint infection (PJI).
Methods
A multicenter retrospective study was conducted to identify patients who underwent debridement, antibiotics, and implant retention (DAIR) for PJI. Decision analysis modeling was employed to determine the treatment strategy that yielded the greatest patient outcome.
Results
316 patients who underwent DAIR for PJI were identified.
Conclusion
The decision analysis model determined that the optimal treatment strategy is to perform an immediate DAIR to achieve the greatest QALY outcomes in TKA and THA patients with acute PJI.
Keywords: Periprosthetic joint infection, Decision analysis, Total joint arthroplasty, Irrigation and debridement
1. Introduction
The incidence of periprosthetic joint infection (PJI) is increasing as the number of total hip (THA) and total knee (TKA) arthroplasties being performed increases worldwide.1 This devastating and costly complication remains one of the most vexing challenges that patients, orthopaedic surgeons, and infectious disease physicians face. While there have been improvements in identifying risk factors and diagnostic testing for this disease, the three core treatment modalities that include debridement, antibiotics, and implant retention (DAIR), single stage exchange, and two stage exchange have not fundamentally changed in decades. Irrigation, debridement, and component retention with or without modular component exchange remains the most attractive of these options due to its relative simplicity, lower morbidity, lower cost, and preservation of host bone.2 Unfortunately, the success rates for controlling PJI with irrigation and debridement (I&D) procedures vary widely from 0 to 89%.3 Most studies have reported disappointing results, particularly with staphylococcal infections. Many authors have identified factors that may improve the success rates of DAIR, such as early surgical treatment and the presence of less virulent organisms that may be more amenable to this treatment (eg. non-staphylococcal infections).4,5
Conventional culture techniques generally require 48 hours or more of incubation to properly identify microbial species and sensitivities.6 Many surgeons believe that a delay of surgical treatment for PJI that extends beyond 48 hours may jeopardize the potential success of a DAIR procedure.7,8 Many also understand that a DAIR procedure in the face of a staphylococcal infection has a low likelihood of being successful and that a more aggressive treatment, such as two-stage exchange, may be a better option.9 This scenario often leaves surgeons in a quandary in trying to decide whether it is better for patients to delay surgical treatment until the infecting organism(s) has been identified and therefore be able to tailor treatment based on this information, or to urgently operate without this information risking performing a procedure with low likelihood of success, such as DAIR in the face of a staphylococcal infection. The treatment that maximizes infection control while also minimizing morbidity, mortality, and repeated surgical interventions should result in the optimal outcome for patients.
Decision-analysis modeling is a powerful tool that is now commonly used to help inform a number of medical and orthopaedic decision-making processes including the cost-effectiveness of unicompartmental arthroplasty versus TKA,10 the cost-effectiveness of antibiotic-laden cement for THA,11 and the surgical treatment of early post-operative infections in THA.12 Timing of surgical treatments are particularly well-suited to decision-analysis modeling because the choice to undergo surgery is a discrete decision that produces one or more outcomes with a specific probability. Each outcome can then be quantified by its desirability (i.e., perfect health may be highly desirable and bed rest may be most undesirable), and placed into a mathematical algorithm that calculates which decision has the highest probability of resulting in the most desirable outcome.
The purpose of this study is to report the infection control rate of a debridement and implant retention strategy for acute PJI of THA or TKA categorized by timing of treatment and infecting organism across multiple institutions. Decision and sensitivity analysis modeling is used then to estimate probable quality-of-life outcomes in two hypothetical clinical scenarios. The first scenario is where DAIR is performed on all patients within 48 hours independent of the infecting organism. The second scenario is a strategy where a treatment delay of 48 hours for the sake of infecting organism identification is employed and where DAIR is performed only on non-staphylococcal organisms and staphylococcal infections are treated with a two-stage exchange.
2. Materials and methods
A retrospective study was conducted across two health care systems from January 1st, 2001 to December 31st, 2017 with institutional review board approval. Patients included for analysis were those who underwent a first-time DAIR procedure for acute PJI of a primary THA or TKA. PJI was confirmed by laboratory and culture data consistent with the current MSIS criteria at the time of treatment.13 Exclusion criteria included patients with duration of symptoms greater than 7 days prior to DAIR as reported in the patient's medical chart, patients in which exact duration of symptoms was not reported, and patients with less than two-year minimum follow-up. The study period from 2001 to 2017 was stratified into 4 quartiles (2001–2004, 2005–2008, 2009–2012, 2013–2017) and infection control rates of each of these periods was compared between one another to observe whether it improved over the course of the study.
The reported EMR results were categorized according to I&D timing [2 days or less (immediate I&D) or 3–7 days (delayed I&D)] from the diagnosis of PJI. A two-day cut off time was chosen based on the approximate time required for conventional culture techniques to isolate many staphylococcal organisms.6,14 Patients were also categorized according to the infecting organism (staphylococcal species versus other) and the joint involved (knee versus hip). Polymicrobial PJIs in which culture results included staphylococcal organisms were considered in the staphylococcal group. Infection control rates after I&D for each group was calculated separately and reported.
Infection control for this study was defined as the reported resolution of infection after one DAIR and without the use of antibiotic suppression.
2.1. Decision analysis
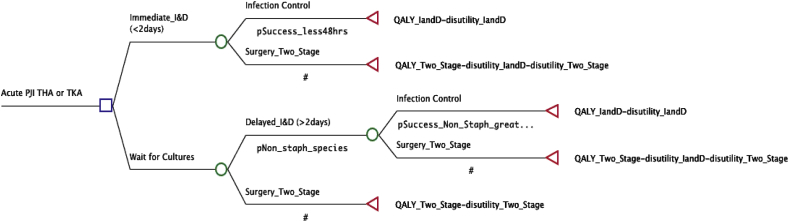
The decision tree model and sensitivity analysis were built and executed using TreeAge Pro 2017 software (Williamstown, MA, USA). The decision tree models the hypothetical clinical scenario of a patient with an acute PJI. From the root node (diagnosis of acute PJI), two treatments are available: an immediate I&D (within 2 days from onset of symptoms) with or without modular exchange, or delayed treatment (surgical treatment 3–7 days after symptom onset; Fig. 1). The model assumes the data obtained from waiting for cultures would dictate the initial surgical treatment with a non-staphylococcal infection undergoing a delayed I&D as the initial treatment and a staphylococcal species infection, due to its accepted low infection control rate with I&D, undergoing a two-stage exchange procedure.
Fig. 1.
Model decision tree is shown. The initial decision is found at the left side of the tree at the root node where one of two treatment protocols is chosen. The tree then sequentially progresses from left to right based on probabilities of treatment results.
The model assumes that success of a given procedure required a 1-year period or greater without additional surgery for treatment of the infection. If the patient fails an I&D, whether or not delayed, the patient would then undergo a two-stage exchange.12,15, 16, 17 This model allows patients to undergo a maximum of one I&D and one two stage revision, with two-stage revision considered a terminating state. This algorithm did not model the possibility of failed two-stage exchange as this scenario has been modeled previously.18 The primary model analysis was for both THA and TKA infections combined, but also a secondary analysis of only THA infections and TKA infections was performed.
Utility in decision-analysis modeling quantifies the desirability of the final outcome of each pathway through the tree, with the value of 1 being most desirable. In this model, utilities for treatment of an infected prosthesis were based on the quality-of-life database compiled by the Institute for Clinical Research and Health Policy Studies.19 Disutility in decision-analysis modeling represents the undesirability of an outcome and is often a negative number (termed disutility toll) used to estimate the negative impact of an undesirable outcome on the terminal utility value, in this model, quality-adjusted life years. Failure of treatment causing subsequent reoperation gave rise to an additional deduction (disutility toll) on the final value. This deduction attempts to account for the disabilities sustained from reoperation and the generally lower quality-of life following multiple operations on the same joint. Disutility values were derived from previous decision-analysis modeling, with an open I&D assumed to be −0.1, similar to the disutility of the primary surgery.12
Sensitivity analysis is used in conjunction with decision-analysis modeling to help mitigate the uncertainty of the estimates and avoid resultant bias in results. The threshold value is defined as the value of the parameter that drives a change in the resultant decision. In this analysis, the rate of infection control with an immediate I&D was subjected to a one-way sensitivity analysis through a range of 0–89%, as previously reported.3
2.2. Surgical technique and postoperative care
Given that twenty-three surgeons performed the DAIR procedures analyzed in this study, there was no universally administered surgical protocol. However the standard of care across all institutions in this study included synovectomy coupled with modular component irrigation and exchange. Following surgery, an antibiotic protocol would be planned with consultation of an infectious disease specialist, typically consisting of 6 weeks of organism-specific intravenous antibiotic therapy.
3. Results
Three hundred sixteen patients with PJI were identified that met the inclusion criteria. The overall infection control rate for THA and TKA was 57.0%. Patients treated within 2 days of symptom presentation had an infection control rate of 59.5% and those treated between 3 and 7 days had an infection control rate of 52.6% (p = 0.23). Patients with staphylococcal infections had an infection control rate of 50.6% which was significantly lower than the infection control rate of 57.1% observed in the non-staphylococcal species group (p = 0.03). The proportion of patients with a staphylococcal infection was similar between the early treatment and delayed treatment cohorts (p = 0.1). The extracted data used as the baseline probabilities in the decision analysis are reported in Table 1. The estimated outcome utilities are reported in Table 2.
Table 1.
Infection Control Rates with DAIR from EMR Review: Surgical timing, Infecting organism and Joint involved.
| Number of patients | Infection Control Rate | |
|---|---|---|
| Surgical Timing | ||
|
200 (63%) | 59.5% |
|
124 | 53.2% |
|
76 | 69.7% |
|
116 (37%) | 52.6% |
|
69 | 44.9% |
|
47 | 63.8% |
|
25 | 56% |
|
22 | 72.7% |
| ORGANISM | ||
|
156 (49.4%) | 50.6% |
|
91 (28.8%) | 57.1% |
|
69 | |
| JOINT | ||
|
190 (60.1%) | 51.1% |
|
92 | 43.5% |
|
54 | 58.2% |
|
44 | |
|
126 (39.9%) | 65.9% |
|
64 | 60.9% |
|
62 | 80.0% |
Table 2.
Model variables.
| EMR Review | Reference | ||
|---|---|---|---|
| Probabilities | |||
| Infection Control with I&D < 48 h of symptoms | 0.595 | ||
| Incidence of Non-Staphyloccocal PJI | 0.571 | ||
| Infection Control with I&D for Non-Staphylococcal Species treated >48 h | 0.638 | ||
| Utilities | |||
| Procedure | Quality of Life | ||
| Open Debridement/Retention of Prosthesis | 0.93 | CEVR26 | |
| Two-stage exchange | 0.85 | CEVR26 | |
| Disutility of Revision | |||
| Procedure | Toll | ||
| Open Debridement/Retention of Prosthesis | −0.1 | Bedair et al.12 | |
| Two-stage exchange | −0.2 | Bedair et al.12 | |
When stratified by date of surgery quartiles, the infection control rate was observed to increase from 50% to 64% when comparing the first quartile (2001–2004) to the fourth quartile (2014–2017) respectively, however the interquartile rates were not found to be different from one another at the 5% level.
Using the data obtained from the EMR, the treatment strategy with the greatest health-related quality of life for a patient presenting with PJI after THA or TKA, was the strategy to immediately perform DAIR without waiting for culture data to identify the infecting organism (Expected value (EV) 0.7166 vs. 0.6949). Analysis restricted to THA only (Expected value (EV) 0.7452 vs. 0.7010) or TKA only (Expected value (EV) 0.6990 vs. 0.6793) did not change the preferred strategy. One-way sensitivity analysis for combined THA and TKA PJIs with EMR data indicated that when the probability of infection control with an immediate DAIR varies from 0 to 89%, the control rate would need to fall below 52% for delayed treatment strategy to yield the greatest utility (Fig. 2).
Fig. 2.
One-way sensitivity analysis using EMR data exploring the impact that uncertainty in the infection control rate has on preferred strategy.
4. Discussion
DAIR is a common approach to the treatment of acute PJI, however this approach has had highly variable results due to the multitude of factors involved in this complex disease. The role of this treatment modality needs to be further refined as it is clear that some patients may benefit from this, while others do not.2 The overall success rate of controlling PJI in this study with irrigation and debridement was 57.0%. In patients with staphylococcal infection, the success rate was lower than those without staphylococcal infections (50.6% vs. 57.1%). Delayed DAIR infection control rates were found to be lower than those for early DAIR (52.6% vs. 59.5%). However, simply looking at infection control rates of different treatment protocols does not always indicate the course of action that maximizes patient outcomes and quality of life. While the species of the infecting organism has been borne out to be important in the literature in regard to treatment success, it is not clear whether waiting for culture results potentially improves outcomes. This decision analysis groups relevant data across multiple institutions to analyze a hypothetical clinical scenario in an attempt to understand the implications of basing treatment of PJI on the infecting organism.
There are several limitations to this study largely related to the nature of a retrospective patient review and decision modeling. Findings from any retrospective study must be interpreted with caution and prospective studies are needed to corroborate our findings. Similar to other PJI studies, a prospective study observing the infection control rate could be difficult due to the low incidence of PJI. We also analyzed cases over a large time period (16 years), over which technological and surgical technique advances may have improved the infection control rate. However, we addressed this issue by comparing the infection control rate of 4 distinct time periods over the course of our retrospective study, and found that the rates of each of the four periods were similar. Decision analysis is a relatively simple model that attempts to address complex clinical problems, and does not take into account the multiple permutations of each individual clinical scenario. As such, decision analysis provides more of a framework by which to evaluate different treatments rather than estimating the outcome of a particular scenario.
The overall success rate of irrigation and debridement with fixed component retention for PJI was 57.0% from the retrospective chart review in this analysis is similar to other studies published on this topic. Fehring et al. observed a success rate of only 37% with DAIR in patients who developed PJI less than 90 days following the index procedure.21 Koyonos et al. investigated the results of DAIR at three different time points from the index procedure, which they defined as acute post-operatively, acute late, and chronic.22 The success rates were 31%, 44%, and 28%, respectively and mirrored data by Ferhing et al. Urish et al. performed a retrospective review of two hundred sixteen cases of DAIR for TKA PJI and observed an estimated infection control rate of 42.6% at four years.23 Grammatopolous et al. performed a similar study for hip PJI and observed an infection control rate of 68% following the first DAIR and 85% when multiple DAIR's were performed.24 In a systematic review of this topic, Kunutsor et al. found in 2387 periprosthetic infections of the hip and knee, the average infection control rate was 75.4%.20
The infecting organism and the time to treatment both appear to be critical in the success of I&D. Odum et al. reported that in patients with PJI due to staphylococcal species, the success rates of irrigation and debridement ranged from 24 to 27%.5 Marculescu et al. reported a 1.77 times higher failure rate with DAIR if symptoms of PJI were present for greater than 8 days.25
When the combined data of THA and TKA from the retrospective EMR review was used in our decision analysis, the preferred treatment was to perform an immediate DAIR without waiting for cultures to identify the infecting organism. This decision to immediately perform a DAIR procedure remained the same when “THA only” and “TKA only” cohorts were used in the decision analysis. The difference in the expected outcome of each of the strategies was small, but clearly reflects the heterogeneity in the reported data on this treatment for PJI.
It is interesting to consider why there appears to be a difference from our institutions and those reported in the literature on the rates of infection control, which in turn can alter the recommendations based on the model analysis. Our chart review data is from two large academic medical centers with access to dedicated arthroplasty surgeons and infectious disease specialists who both have significant experience treating PJI. This may represent the optimal conditions for treatment and thus reflect the best possible outcome. Therefore, our results may not be able to be generalized to all healthcare settings and may help to explain the increased success rate observed amongst our patients compared to other studies reporting DAIR success rates.21, 22, 23 The data reported in other studies is likely a heterogenous mix of patients, providers, and medical centers with varying levels of resources. This could reflect the more realistic conditions of those treating PJI outside of tertiary care centers. These parameters acutely reflect the power of the sensitivity analysis in decision models. There cannot be singular recommendations as thresholds for changing treatment strategies vary by local conditions. As reported in this sensitivity analysis based on the EMR data, if the infection control rate falls below 52%, a delayed approach may result in the optimal clinical outcome. Clinicians should be aware of their local conditions and PJI control rates and could potentially use these findings to help council patients as to the strategy that may offer the best clinical outcome.
As the role of irrigation and debridement for PJI after THA or TKA becomes further defined, these observations confirm that one of the most critical elements influencing outcomes is the identity of the infecting microbe which may be more important to predicting success than the immediacy of surgical treatment. Though the observed differences in this model favoring delayed treatment are small, one may appreciate these findings as evidence that waiting on treatment until the clinical picture is complete does not appear to be harmful to the patient's ultimate outcome. Furthermore, approaching this problem with the most amount of information possible allows the patient and treating physicians to come to a shared decision with respect to proposed treatments and the patient's wishes.
Disclosures/conflicts of interest/funding
One of the authors (HSB) has received funding from Smith & Nephew, Exactech, Zimmer Biomet, and Wolter Kluwer. Another author (RS) has received royalties from Smith & Nephew, is a paid consultant for Smith & Nephew and Intelijoint, owns stock in Intelijoint and Gauss Surgical, receives research support from Smith & Nephew and Intelijoint, is on the editorial board for Journal of Arthroplasty and Arthoplasty Today, and is a board member for AAHKS and AAOS. The remaining authors have no competing interests or funding to declare.
Ethics statement
This study was approved by our institutional review board.
References
- 1.Kurtz S.M., Lau E., Schmier J., Ong K.L., Zhao K., Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23:984–991. doi: 10.1016/j.arth.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 2.Jiranek W.A., Waligora A.C., Hess S.R., Golladay G.L. Surgical treatment of prosthetic joint infections of the hip and knee: changing paradigms? J Arthroplasty. 2015;30:912–918. doi: 10.1016/j.arth.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Romanò C.L., Manzi G., Logoluso N., Romanò D. Value of debridement and irrigation for the treatment of peri-prosthetic infections. A systematic review. Hip Int. J. Clin. Exp. Res. Hip Pathol. Ther. 2012;22(Suppl 8):S19–24. doi: 10.5301/HIP.2012.9566. [DOI] [PubMed] [Google Scholar]
- 4.Hartman M.B., Fehring T.K., Jordan L., Norton H.J. Periprosthetic knee sepsis. The role of irrigation and debridement. Clin Orthop. 1991;113–118 [PubMed] [Google Scholar]
- 5.Odum S.M., Fehring T.K., Lombardi A.V. Periprosthetic Infection Consortium. Irrigation and debridement for periprosthetic infections: does the organism matter? J Arthroplasty. 2011;26:114–118. doi: 10.1016/j.arth.2011.03.031. [DOI] [PubMed] [Google Scholar]
- 6.Schäfer P., Fink B., Sandow D., Margull A., Berger I., Frommelt L. Prolonged bacterial culture to identify late periprosthetic joint infection: a promising strategy. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2008;47:1403–1409. doi: 10.1086/592973. [DOI] [PubMed] [Google Scholar]
- 7.Brandt C.M., Sistrunk W.W., Duffy M.C. Staphylococcus aureus prosthetic joint infection treated with debridement and prosthesis retention. Clin Infect Dis. 1997;24:914–919. doi: 10.1093/clinids/24.5.914. [DOI] [PubMed] [Google Scholar]
- 8.Son W.S., Shon O.-J., Lee D.-C., Park S.-J., Yang H.S. Efficacy of open debridement and polyethylene exchange in strictly selected patients with infection after total knee arthroplasty. Knee Surg Relat Res. 2017;29:172–179. doi: 10.5792/ksrr.16.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mont M.A., Waldman B., Banerjee C., Pacheco I.H., Hungerford D.S. Multiple irrigation, debridement, and retention of components in infected total knee arthroplasty. J Arthroplasty. 1997;12:426–433. doi: 10.1016/s0883-5403(97)90199-6. [DOI] [PubMed] [Google Scholar]
- 10.Slover J., Espehaug B., Havelin L.I. Cost-effectiveness of unicompartmental and total knee arthroplasty in elderly low-demand patients. A Markov decision analysis. J. Bone Joint Surg. Am. 2006;88:2348–2355. doi: 10.2106/JBJS.E.01033. [DOI] [PubMed] [Google Scholar]
- 11.Cummins J.S., Tomek I.M., Kantor S.R., Furnes O., Engesaeter L.B., Finlayson S.R.G. Cost-effectiveness of antibiotic-impregnated bone cement used in primary total hip arthroplasty. J. Bone Joint Surg. Am. 2009;91:634–641. doi: 10.2106/JBJS.G.01029. [DOI] [PubMed] [Google Scholar]
- 12.Bedair H., Ting N., Bozic K.J., Della Valle C.J., Sporer S.M. Treatment of early postoperative infections after THA: a decision analysis. Clin Orthop. 2011;469:3477–3485. doi: 10.1007/s11999-011-2119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zmistowski B., Della Valle C., Bauer T.W., Malizos K.N., Alavi A., Bedair H., Booth R.E., Choong P., Deirmengian C., Ehrlich G.D., Gambir A., Huang R., Kissin Y., Kobayashi H., Kobayashi N., Krenn V., Lorenzo D., Marston S.B., Meermans G., Perez J., Ploegmakers J.J., Rosenberg A., Simpfendorfer C., Thomas P., Tohtz S., Villafuerte J.A., Wahl P., Wagenaar F.-C., Witzo E. Diagnosis of periprosthetic joint infection. J Orthop Res. 2014;32(Suppl 1):S98–107. doi: 10.1002/jor.22553. null. [DOI] [PubMed] [Google Scholar]
- 14.Butler-Wu S.M., Burns E.M., Pottinger P.S. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011;49:2490–2495. doi: 10.1128/JCM.00450-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garvin K.L., Hanssen A.D. Infection after total hip arthroplasty. Past, present, and future. J. Bone Joint Surg. Am. 1995;77:1576–1588. doi: 10.2106/00004623-199510000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Hanssen A.D., Spangehl M.J. Treatment of the infected hip replacement. Clin Orthop. 2004:63–71. doi: 10.1097/00003086-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Tsukayama D.T., Estrada R., Gustilo R.B. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J. Bone Joint Surg. Am. 1996;78:512–523. doi: 10.2106/00004623-199604000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Parisi T.J., Konopka J.F., Bedair H.S. What is the long-term economic societal effect of periprosthetic infections after THA? A markov analysis. Clin Orthop. 2017;475:1891–1900. doi: 10.1007/s11999-017-5333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anon . Institute for Clinical Research & Health Policy Studies, Tufts Medical Center; 2009. The Center for the Evaluation of Value and Risk in Health (CEVR). The Cost-Effectiveness Analysis Registry.http://www.cearegistry.org Available at: [Google Scholar]
- 20.Kunutsor S.K., Beswick A.D., Whitehouse M.R., Wylde V., Blom A.W. Debridement, antibiotics and implant retention for periprosthetic joint infections: a systematic review and meta-analysis of treatment outcomes. J Infect. 2018;77:479–488. doi: 10.1016/j.jinf.2018.08.017. [DOI] [PubMed] [Google Scholar]
- 21.Fehring T.K., Odum S.M., Berend K.R. Failure of irrigation and débridement for early postoperative periprosthetic infection. Clin Orthop. 2013;471:250–257. doi: 10.1007/s11999-012-2373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koyonos L., Zmistowski B., Della Valle C.J., Parvizi J. Infection control rate of irrigation and débridement for periprosthetic joint infection. Clin Orthop. 2011;469:3043–3048. [Google Scholar]
- 23.Urish K.L., Bullock A.G., Kreger A.M. A multicenter study of irrigation and debridement in total knee arthroplasty periprosthetic joint infection: treatment failure is high. J Arthroplasty. 2018;33:1154–1159. doi: 10.1016/j.arth.2017.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grammatopoulos G., Kendrick B., McNally M. Outcome following debridement, antibiotics, and implant retention in hip periprosthetic joint infection—an 18-year experience. J Arthroplasty. 2017;32:2248–2255. doi: 10.1016/j.arth.2017.02.066. [DOI] [PubMed] [Google Scholar]
- 25.Marculescu C.E., Berbari E.F., Hanssen A.D. Outcome of prosthetic joint infections treated with debridement and retention of components. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2006;42:471–478. doi: 10.1086/499234. [DOI] [PubMed] [Google Scholar]
- 26.Anon . Institute for Clinical Research & Health Policy Studies, Tufts Medical Center; 2009. The Center for the Evaluation of Value and Risk in Health (CEVR). The Cost-Effectiveness Analysis Registry.http://www.cearegistry.org Available at: [Google Scholar]