Abstract
Background:
Early adulthood is a critical period when young men involved in antisocial behavior (AB) may desist. Factors including marriage and employment have been shown to predict desistance, but little work has examined whether biological factors (e.g., neural reactivity) predict deflections from life-long AB trajectories.
Methods:
We examined the continuity of, or desistance from, AB in early adulthood using group-based trajectories of AB across adolescence in a sample of 242 men from low-income, urban families. We examined contextual factors (romantic relationship quality, employment, neighborhood danger) and neural factors (amygdala reactivity to fearful faces, ventral striatum reactivity to reward) as moderators of the continuity of AB from adolescence (age 10–17) into early adulthood (age 22–23), and whether these pathways differed by race.
Results:
High relationship satisfaction and employment at age 20 predicted decreased AB at age 22–23, but only among men with adolescent-onset/moderate AB trajectories. Ventral striatum reactivity predicted continued AB, but only among African-American men with early-starting AB. Amygdala reactivity to fearful faces was related to later AB for those in the early-starting group, but in divergent directions depending on race: amygdala reactivity to fearful faces was positively related to AB in European-Americans and negatively related to AB among African-Americans.
Conclusions:
Contextual factors only predicted deflections of AB in those engaged in late-starting, moderate levels of AB, whereas neural factors predicted continued AB only in those with early-starting, severe AB, and in divergent ways based on participant race. Though there is limited power to infer causality from this observational design, research on desistance broadly can contribute to informing personalized interventions for those engaged in serious adolescence AB.
Keywords: Antisocial Behavior, Desistance, Conduct Disorder, Amygdala, Ventral Striatum
Antisocial behavior (AB), which includes aggression and rule breaking, is a large public health concern because these behaviors are highly prevalent (>10% of teenagers) and confer negative financial and emotional consequences for victims and society (Foster & Jones, 2005; Nock, Kazdin, Hiripi, & Kessler, 2006). AB is fairly stable (Loeber, 1991), with stability correlations as high as .5 from ages 5 to 30 (Huesmann, Eron, Lefkowitz, & Walder, 1984). This stability belies the heterogeneity in course for AB, with a wealth of literature showing that, compared to youth who begin AB in adolescence, children with childhood-onset AB have more severe, stable, and chronic AB into adulthood, as well as poorer health, social, and employment outcomes (Moffitt, 2006; Moffitt, Caspi, Harrington, & Milne, 2002). Despite the stability of AB, a potential “turning point” for youth involved in AB is early adulthood (Laub & Sampson, 1993). During the early 20s, social contexts change dramatically with increases in independence, autonomy, and responsibility. For some youth, increased autonomy can further entrench the individual into a life of crime. However, for others, this period may present opportunities to change behavioral trajectories (Chung, Little, & Steinberg, 2005).
Research during early adulthood suggests that changes in context, such as those brought about by the presence of positive romantic relationships and employment, may promote desistance from AB, potentially by engaging the emerging adult in productive, supportive relationships that increase time spent in prosocial activities (Burt et al., 2010; Laub, Nagin, & Sampson, 1998; Laub & Sampson, 1993). Conversely, research suggests that negative contexts, such as living in a deprived neighborhood, may impede efforts to desist (Foster & Brooks-Gunn, 2013). Identifying factors that predict deflections of earlier trajectories of AB can help to inform interventions and juvenile justice policy (Mulvey et al., 2004). However, few studies have examined whether these factors are similarly protective across race, especially given that African-American men face many unique structural and contextual challenges compared to European-American men that may undermine attempts to desist (Mays, Cochran, & Barnes, 2007; Mulvey et al., 2010). Thus, the first aim of the present study was to examine whether promotive contextual factors at age 20 predicted changes in the trajectory of AB across adolescence to AB in early adulthood (age 22–23) in European- and African-American young men raised in low-income, urban environments, and whether race moderated these effects.
In addition to contextual factors, identifying person-level predictors of desistance can inform personalized interventions. An important potential person-level predictor of desistance versus persistence is variation in neural reactivity, but little work has examined neural factors as longitudinal predictors of desistance from (or persistence of) AB. Reactivity of the amygdala, particularly to social signals of distress (fearful faces), and reactivity of the ventral striatum (VS) to reward have been central to etiological theories of AB (Blair, Leibenluft, & Pine, 2014; Hyde, Shaw, & Hariri, 2013). Disruptions in cortiolimbic and cortiostriatal systems are thought to explain why those engaging in AB show less fear and empathy towards victims (amygdala dysfunction) and why those high on AB show a reward-dominant behavior style (VS dysfunction) (Blair et al., 2014; Hyde et al., 2013). In support of these theories, increasing numbers of studies have linked amygdala reactivity and VS reactivity to AB cross-sectionally (Buckholtz et al., 2010; Hyde et al., 2013; Viding, Sebastian, et al., 2012). In the current sample, lower amygdala reactivity to fearful faces and lower VS reactivity to reward are related to greater concurrent AB at age 20 (Hyde et al., 2016; Murray, Shaw, Forbes, & Hyde, 2017). However, reactivity in these neural regions may be important, not just to understanding the onset and maintenance of AB, but also to understanding desistance from AB, particularly among those with the most severe AB. In fact, a recent “neuro-prediction” study showed that neural reactivity predicted future re-arrest (vs. desistance) in an incarcerated sample (Aharoni et al., 2013). Thus, the second aim of the current study was to examine whether variation in amygdala reactivity to fearful faces and VS reactivity to reward, predicted changes in pathways from adolescent trajectories of AB to later AB during early adulthood.
The overall goal of the present study was to examine contextual and neural factors that predict desistance, by examining whether these factors predicted deflections from trajectories of AB across adolescence (low, late-starting, and early-starting) to AB at age 22–23. As each of the contextual and neural factors examined in this study have been previously shown to increase risk for AB or decrease the probability of desistance, we hypothesized that young men living in dangerous neighborhoods, who were unemployed, without a satisfying relationship, and who exhibited lower amygdala reactivity to threat and lower VS reactivity to reward would show persistent AB from adolescence to early adulthood. In contrast, we hypothesized that living in a safer neighborhood, being employed, having a satisfying romantic relationship, and neural profiles marked by less risk for AB, would predict desistance from AB. Moreover, given theories that adolescent-onset AB trajectories may be more malleable (Moffitt, 2006) and, in contrast, that brain-behavior relationships may vary by the severity of psychopathology (Plichta & Scheres, 2014), we hypothesized that predictors of desistance would vary by AB trajectory group. However, given the lack of literature from studies that have explored desistance among youth with adolescent-onset AB, we considered it this an exploratory hypothesis. We examined these questions among a sample of racially diverse men followed since early childhood, who grew up in low-income, urban families, so that we could examine how these processes unfold in a context risky for AB. Moreover, we examined whether AB desistance versus persistence would differ between European- and African-American youth based on the additional stressors faced by African-American youth which may impede the protective effects of promotive contextual factors. Given recent findings from the current sample showing that the relationship between neural reactivity and concurrent AB were moderated by race (Hyde et al., 2016), but with no prior research to guide these analyses, we also considered moderation of these pathways by race to be exploratory.
Methods
Participants
Participants were part of the Pitt Mother & Child Project, an ongoing longitudinal study of 310 low-income boys and their families (Shaw, Hyde, & Brennan, 2012). Target children and mothers were seen almost yearly from age 1.5–23. Assessments included questionnaires, interaction tasks, clinical interviews, and an fMRI scan at age 20. Most visits were in the home or lab, except for data collected at age 16 and 23, which were via a phone interview. Attrition to the age 22 and 23 visits was low for such a long-term study (252 participants, 81%, at age 22). However, the fMRI component at age 20 introduced several sources of data loss resulting in a sample size of 167 for the emotional faces task and 144 for the reward reactivity task (Supplemental Methods, Supplemental Tables 1 & 2). Participants provided informed consent, were reimbursed after assessments, and procedures were approved by the University of Pittsburgh IRB.
Behavioral Measures
Self-reported AB.
To assess self-reported AB, we used the Self-Report of Delinquency Questionnaire (SRD; Huizinga & Elliott, 1986), which was administered from ages 10–20 containing age-appropriate items (33 items for ages 10–12, 62 items for ages 15–17, and 53 at ages 22 and 23; see Hyde et al., 2015). During adulthood, we removed 14 items relating to drug use to create a measure of AB that was not influenced by substance use (age 22, α=.80; age 23, α=.85). We also used the 7 item AB subscale of Self-Report Psychopathy Short-Form (SRP-SF) (Gordts, Uzieblo, Neumann, Van den Bussche, & Rossi, 2017) that was administered at age 22 (see Dotterer et al., 2016).
Antisocial personality disorder symptoms (age 22).
To assess clinically meaningful AB at age 22, symptoms of Antisocial Personality Disorder (APD) were assessed and summed using the SCID-II (First, Gibbon, Spitzer, & Benjamin, 1997) (for more details see Hyde, Burt, Shaw, Donnellan, & Forbes, 2015; Shaw et al., 2012).
Adolescent trajectories of AB (ages 10 – 17).
Within a previous study in this sample (Shaw et al., 2012), SRD scores at ages 10, 11, 12, 15, 16, and 17 were used to create trajectory groups using semi-parametric group-based mixture modeling with Proc Traj in SAS 9.2 (Jones, Nagin, & Roeder, 2001). These analyses yielded 4 distinct groups: low (n=171; 63%), late-starting/moderate (n=54; 20%), early high/late “desisting” (n=15; 6%), and early increasing/high (n=28; 10%). These groups have been used in multiple studies in this sample and have discriminated court involvement and clinical diagnoses of conduct disorder at age 17, and APD and substance use at age 20 (Hyde et al., 2015; Shaw et al., 2012). Consistent with all prior work in this sample, we combined the “early high/late desisting” group with the “increasing/high” group to create a “high” group representing child-onset AB. We have consistently combined these groups because the “early high/late desisting” group was relatively small and this group had similar levels of antecedent risk across childhood and similar levels of AB at age 20 as the “early increasing/high” group (Hyde et al., 2015; Shaw et al., 2012). Moreover, boys in this “early high/late desisting group” self-reported as being highest on being “good at telling lies others believe” during adolescence and over 60% had juvenile court involvement, suggesting that they were involved in meaningful levels of AB across adolescence. Additionally, at ages 22–23, these two groups reported the same levels of AB across all 4 measures of AB (all ps>.15). Thus, we concluded that the indication of “desistance” in adolescence in this group actually reflected under-reporting of AB during late adolescence. Accordingly, in the current study, we examined three adolescent AB trajectory groups: low (n=162; 67%), late-moderate (n=48; 20%), and high (n=32; 13%), which also reflect the main groups identified in most longitudinal studies of AB across adolescence (Moffitt, 2006). Given our interest in examining moderation by race, we focused on two specific racial groups that had the highest representation in the sample: European-American and African-American and included the 242 participants who were originally included in AB trajectories construction (Shaw et al., 2012) and reported belonging to one of these racial groups.
Moderating variables
Contextual factors (age 20).
First, we examined neighborhood dangerousness using the 14-item dangerousness scale from the Me and My Neighborhood questionnaire that assesses perceptions of different facets of neighborhood quality (α=.89) (Shaw et al., 2012). Second, we examined relationship satisfaction using 5-items from the Quality of Marriage Index (α=.96; see Supplemental Methods for items) (Norton, 1983). If males were not in a relationship, they were assigned a 0 as their score for relationship satisfaction (findings remained when treating no relationship as “missing”). Note that we coded the variable in this way as our goal was to examine both the presence of a romantic partner (based on research showing the simple presence of a romantic relationship as protective: Burt et al., 2010) and whether this relationship was positive (based on research highlighting the importance of the relationship being positive: Laub et al., 1998). Third, we examined employment assessed via a demographic interview (i.e., employed part-time or full-time vs. unemployed).
Neural factors (age 20).
Amygdala reactivity to fearful faces was measured using an emotional faces task with four blocks of perceptual face processing interleaved with five blocks of sensorimotor control (Hariri, Tessitore, Mattay, Fera, & Weinberger, 2002), previously shown to correlate with AB cross-sectionally in this sample (Supplemental Methods; Hyde et al., 2016). VS reactivity to reward anticipation was measured using a slow event-related card-guessing game that elicits large effects in the VS (Forbes et al., 2009; Forbes, Shaw, & Dahl, 2007). We focused on reward anticipation, which has been previously shown to correlate with AB in this sample (Supplemental Methods; Murray et al., 2017).
As described previously (Hyde et al., 2016), blood oxygenation level–dependent (BOLD) functional images were acquired on a Siemens 3-T Trio scanner using gradient-echo echoplanar imaging. Preprocessing, artifact detection, and evaluation of signal coverage in regions of interest were completed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/; Supplemental Methods). Linear contrasts employing canonical hemodynamic response functions were used to estimate condition-specific BOLD activation for individuals. Individual contrast images were used in second-level random effects models to determine both mean amygdala reactivity to fearful faces (versus shapes) and VS reactivity to anticipation of reward (versus baseline) using one-sample t-tests. Contrast specific BOLD parameter estimates were extracted for use in MPlus v7.2 from activated clusters within an anatomically defined regions of interest in the bilateral amygdala (AAL atlas) (Gard et al., 2017) and VS (PickAtlas v2.4: two 10-mm spheres around +/−12, 12, −10) (Murray et al., 2017).
Covariates.
We controlled for maternal education and family income based on a demographic interview at 18 months, and explored race (European-American vs. African-American) as a moderator.
Analytic strategy
Aim 1: Examine how membership in adolescent AB trajectory groups predicts the AB outcomes at ages 22–23.
Using confirmatory factor analysis, we established that the four measures of AB at ages 22–23 formed a single latent construct of AB (Supplemental Figure 1). We tested the longitudinal relationship between adolescent AB groups and AB at ages 22–23 using a MANCOVA model in SPSS v24 with Bonferroni-corrected post-hoc t-tests, as well as with the same dummy-coded structural equation path model used in all other analyses (see below).
Aim 2: Examine how different contextual and neural factors moderate the stability of adolescent AB trajectory groups to AB factor scores at ages 22–23.
We ran two models to examine the interacting effects of the contextual and neural domains using Mplus v7.2 (Muthén & Muthén, 2014). We used full information maximum likelihood estimation with robust standard errors (MLR) which allows for analysis with missing data (final N=242 for all analyses) and which provides estimation with less assumptions about the normality of the data than ML estimation (e.g., it can accommodate data that is not normally distributed/with skew). For each domain, we specified a model with the latent AB factor at ages 22–23 as the dependent variable. The independent variables were the main effects of being in the high or late-moderate AB groups (i.e., dummy codes relative to the low group), each contextual or neural factor, and the interaction between AB group membership and these moderators (Supplemental Figure 3). Significant interactions were examined using simple slope and region-of-significance analyses using an online tool (Preacher, Curran, & Bauer, 2006). Moreover, we follow recommendations for the transparent reporting of interactions (Roisman et al., 2012; Widaman et al., 2012) by reporting the amount of data coverage in regions of significance analyses (i.e., we report the number of participants whose score was in the significant region of the interaction).
Results
Descriptive statistics and bivariate correlations between study variables are presented in Supplemental Table 3 (African-American participants) and 4 (European-American participants).
Do youth with early-starting and adolescent-onset AB across adolescence show continued AB during early adulthood?
As shown in Supplemental Table 5 and Supplemental Figure 2, at age 22–23, men in the high and late-moderate adolescent groups showed significantly higher AB versus those in the low group. Men in the high group also demonstrated higher AB than those in the late-moderate group.
Do contextual and neural factors moderate links between adolescent AB trajectories and early adult AB?
Although we found main effects of group membership and contextual and neural factors on later AB (Tables 1–2), we concentrate our presentation of the Results only on significant interactions between group membership (i.e., evidence of deflections or exacerbations from risky AB trajectories).
Table 1.
Main and Interactive effects of adolescent AB groups and contextual factors at age 20 on AB at ages 22–23
| AB (ages 22–23) | |||
|---|---|---|---|
| B | SE | β | |
| Covariates | |||
| Race | −0.01 | 0.10 | −0.01 |
| Income | 0.07 | 0.06 | 0.07 |
| Mother Education | 0.01 | 0.06 | 0.01 |
| Main Effects of adolescent AB group and contexts | |||
| Late-moderate vs. Low | 0.25 | 0.14 | 0.25† |
| High vs. Low | 0.54 | 0.24 | 0.54* |
| Employment | 0.01 | 0.10 | 0.01 |
| Relationship Satisfaction | 0.14 | 0.11 | 0.14 |
| Neighborhood Danger | 0.20 | 0.13 | 0.20 |
| Two-way interactions probing contextual moderators and race | |||
| Late-moderate × Race | 0.03 | 0.18 | 0.03 |
| High × Race | 0.19 | 0.28 | 0.19 |
| Employment × Race | −0.06 | 0.11 | −0.06 |
| Relationship Satisfaction × Race | −0.07 | 0.09 | −0.07 |
| Neighborhood Danger × Race | −0.01 | 0.11 | −0.01 |
| Late-moderate × Employment | −0.26 | 0.11 | −0.26* |
| High × Employment | −0.21 | 0.18 | −0.21 |
| Late-moderate × Relationship Satisfaction | −0.30 | 0.15 | −0.30* |
| High × Relationship Satisfaction | −0.12 | 0.27 | −0.12 |
| Late-moderate × Neighborhood Danger | −0.03 | 0.16 | −0.03 |
| High × Neighborhood Danger | −0.15 | 0.23 | −0.15 |
| Three-way interactions probing the effect of race | |||
| Late-moderate × Employment × Race | 0.12 | 0.18 | 0.12 |
| High × Employment × Race | −0.18 | 0.28 | −0.18 |
| Late-moderate × Relationship Satisfaction × Race | 0.12 | 0.10 | 0.12 |
| High × Relationship Satisfaction × Race | 0.10 | 0.14 | 0.10 |
| Late-moderate × Neighborhood Danger × Race | 0.01 | 0.15 | 0.01 |
| High × Neighborhood Danger × Race | −0.02 | 0.19 | −0.02 |
Note.
p < .001,
p < .01,
p < .05,
p<.10; N=242
Table 2.
Main and interactive effects of adolescent AB groups and neural factors at age 20 on AB at ages 22–23
| AB (ages 22–23) | |||
|---|---|---|---|
| B | SE | β | |
| Covariates | |||
| Race | −0.37 | 0.17 | −0.10* |
| Income | 0.00 | 0.00 | 0.03 |
| Mother education | 0.02 | 0.05 | 0.01 |
| Main Effects of adolescent AB group and neural reactivity | |||
| Late-moderate vs. low | 0.08 | 0.32 | 0.02 |
| High vs. low | 1.70 | 0.29 | 0.32*** |
| Amygdala reactivity to fear | −0.74 | 0.34 | −0.20 |
| vs reactivity to reward anticipation | 0.30 | 0.55 | 0.05 |
| Two-way interactions probing neural moderators and race | |||
| Late-moderate × race | 0.56 | 0.40 | 0.10 |
| High × race | −1.22 | 0.45 | −0.15* |
| Amygdala reactivity × race | 0.62 | 0.36 | 0.12 |
| VS reactivity × race | −0.85 | 0.64 | −0.08 |
| Late-moderate × amygdala reactivity | 0.94 | 0.50 | 0.12 |
| High × amygdala reactivity | −5.29 | 0.94 | −0.44*** |
| Late-moderate × VS Reactivity | −0.70 | 1.36 | −0.05 |
| Three-way interactions probing the effect of race | |||
| High × VS reactivity | −6.92 | 1.15 | −0.28*** |
| Late-moderate × amygdala reactivity × race | −1.63 | 0.67 | −0.15* |
| High × amygdala × race | 8.22 | 1.11 | 0.58*** |
| Late-moderate × VS reactivity × race | 1.18 | 1.88 | 0.05 |
| High × VS × race | 7.78 | 1.80 | 0.21** |
Note.
p < .001,
p < .01,
p < .05; N=242
Contextual factors.
First, we found a significant interaction between membership in the late-moderate group and employment (Table 1). Employment was specifically protective for those in the late-moderate group, as those in the late-moderate group who were employed (n=28, 63% of the group reported data in the region of significance) showed significantly lower AB at ages 22–23 than those in the late-moderate group who were unemployed (n=16, 36% of the group reported data in the region of significance) (Figure 1). Employment was not protective for those in the high group, but for those in the late-moderate trajectory employment predicted 20% less AB at age 22–23.
Figure 1.
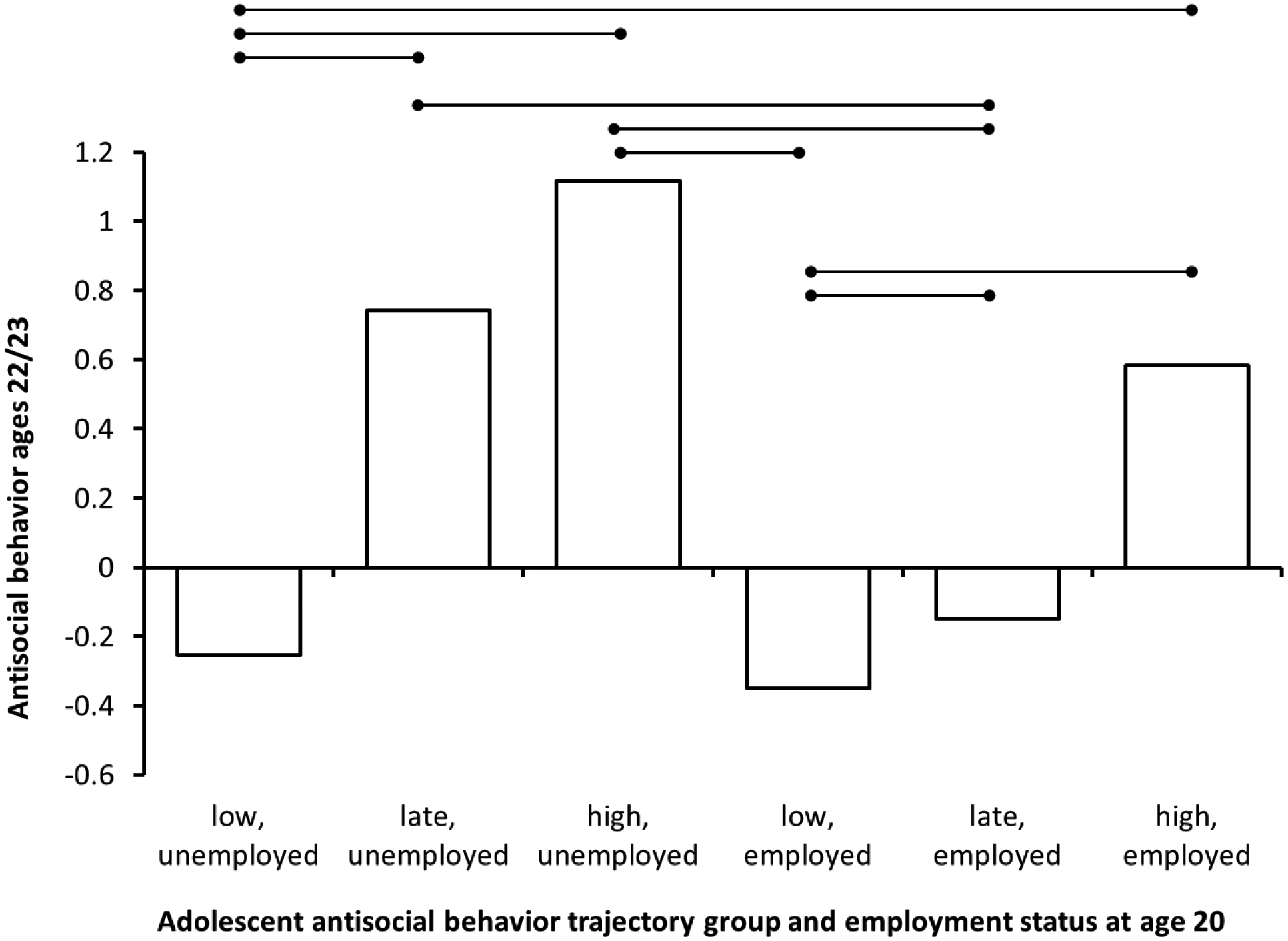
Employment predicts desistance of AB particularly among males showing late-moderate trajectories of AB across adolescence.
Note. N=242. Significant between group contrasts all significant at p<.05 (Bonferroni corrected). Bars connected by a line are significantly different. See Supplemental Table 6 for individual estimates.
Second, we found a similar interaction between relationship satisfaction at age 20 and late-moderate AB group membership (Table 1), whereby relationship satisfaction was protective, but only for those in the late-moderate group (Supplemental Figure 4). Regions of significance analyses showed that low levels of relationship satisfaction or not being in a relationship were related to higher AB for the late-moderate group (n=29, 67%), whereas greater relationship satisfaction was protective (n=13, 30%), predicting levels of AB as low as the low AB group.
Finally, neighborhood danger did not moderate AB trajectories, nor did any pathways vary by race.
Neural factors.
We found that amygdala reactivity to fearful faces predicted later AB, but only in the high AB group and in divergent directions based on race (i.e., a 3-way interaction; Table 2). Among African-American males, lower amygdala reactivity was related to higher AB, but only among those who were in the high AB group (Figure 2; Supplemental Table 7). The regions of significance showed that low amygdala reactivity to fear predicted greater AB in the high group (< .15, though n=3 in the region of significance) and, though regions of significance implied that high amygdala reactivity (> .75) was protective for the high AB group, no participants had scores in this range. In contrast, among European-American participants in the high group, having higher amygdala reactivity was related to more AB at ages 22–23 (> −.04, n=7). Although regions of significance implied that having very low amygdala reactivity was protective (below −.59), no participants had valid scores in this range in the high AB subsample.
Figure 2.
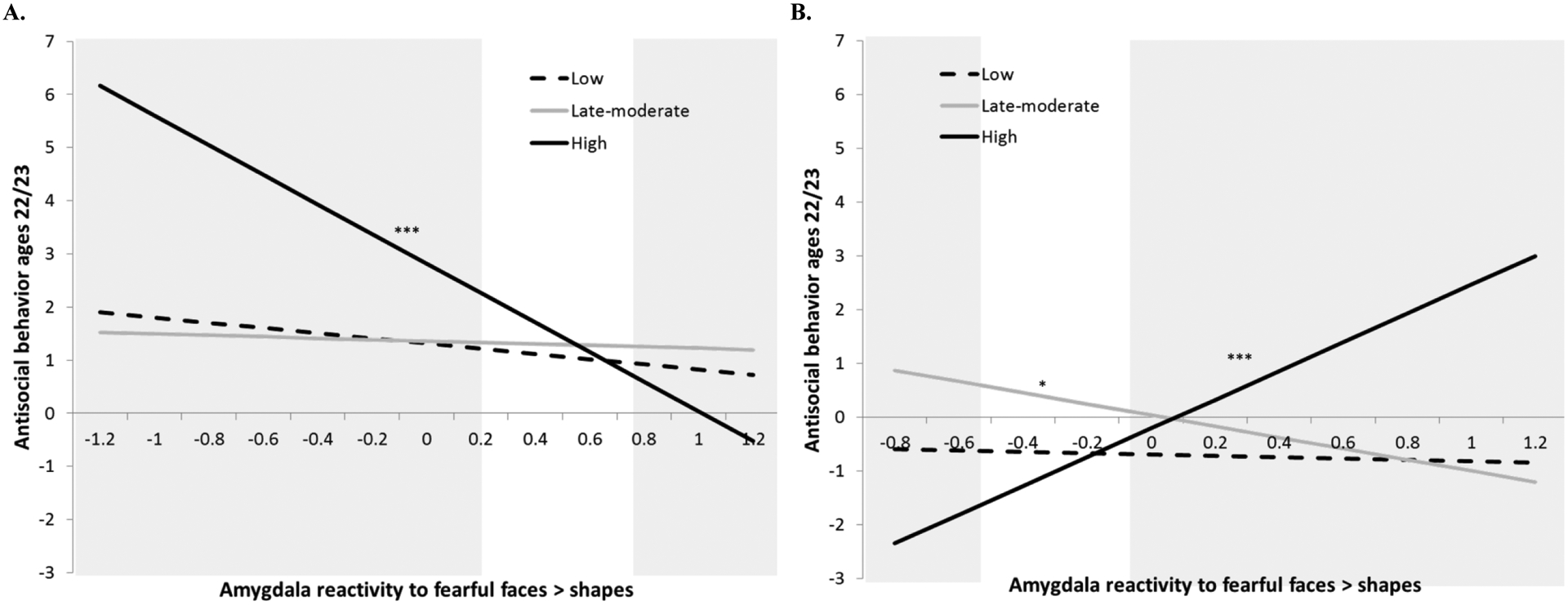
Differential effects of amygdala reactivity to threat on later AB among European-American versus African-American males who showed high AB in adolescence
***p<.001, *p<.05. N=242. Models controlled for income and maternal education. A. For African-American males who had high adolescent AB, there was a signficant inverse relationship between amygdala reactivity to fearful faces at age 20 and antisocial behavior at ages 22–23 (B= -2.79, SE=.80, β=.67, p<.001). The region of significance was for values of amygdala reactivity up to .15 and higher thant .75 (shown in grey shading). B. For European-American males who had high adolescent AB, greater amygdala reactivity to fearful faces at age 20 was related to more AB at ages 22–23 (B=2.68, SE=.55, β=.86, p<.001). For European-American males who showed late-moderate AB in adolescence, lower amygdala reactivity to fearful faces at age 20 was protective against future AB (B=-.1.04, SE=.39, β=-.54, p<.01), although the interaction of being in the late-moderate group x amygdala reactivity to fear was only significant at a trend-leve, limiting what can be inferred from this significant slope (see Supplemental Table 6).The region of significance was for values of amygdala reactivity below -.59 and above -.05 (shown in grey shading).
Finally, we found a significant three-way interaction between the high AB group, race, and VS reactivity to reward. Among African-American participants in the high AB group, lower reward-related VS reactivity predicted more AB at ages 22–23 (< 0.11, n=5). There was no “protective” level of VS reactivity to reward for African-American men in the high AB group, and no significant relationships in the European-American men (Figure 3).
Figure 3.
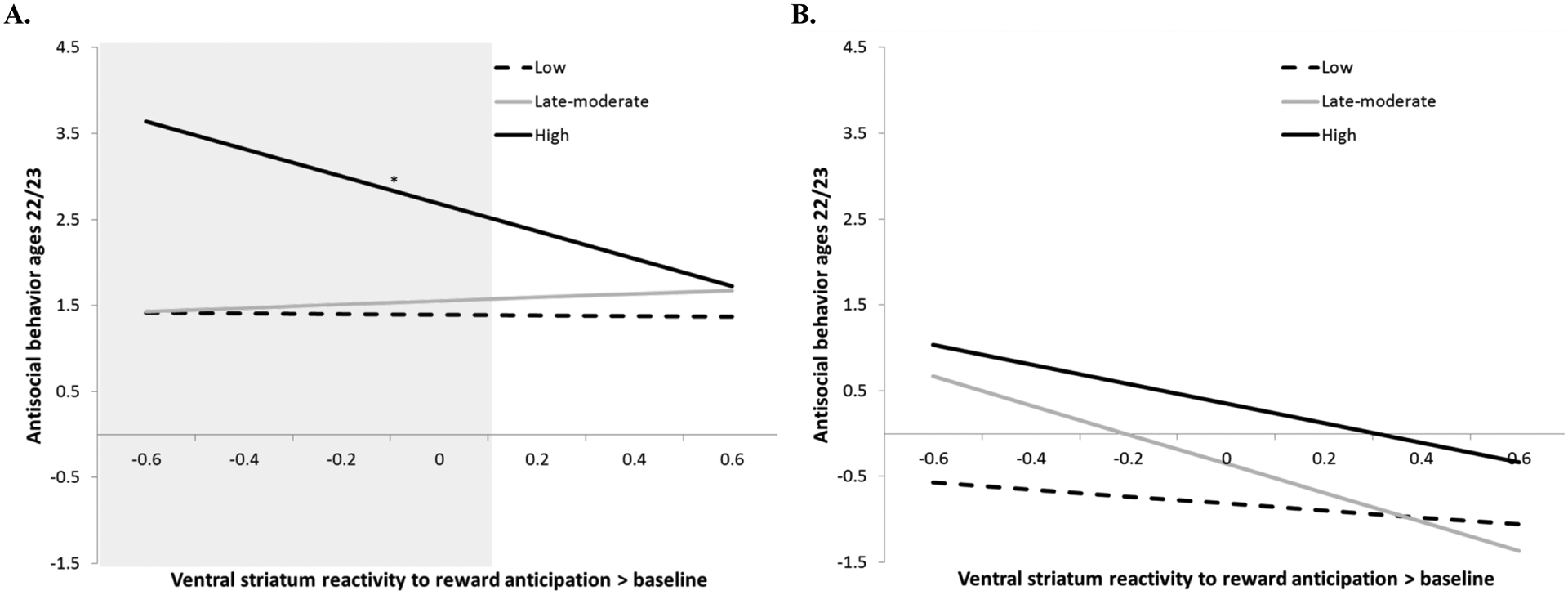
Lower ventral striatum reactivity to reward is related to higher AB among African-American males who showed high AB in adolescence
Note. *p<.05. N=242. Model controlled for income and maternal education (see Supplemental Table 6). A. For African-American males who showed high AB across adolescence, there were significant inverse relationships between VS reactivity to reward anticipation at age 20 and AB at ages 22–23 (B=−1.60, SE=.76, β=−.36, p<.05). The region of significance was for values of VS reactivity below 0.11 (shown in grey shading). There was no moderating effect of VS reactivity on pathways from adolescent AB group to AB at ages 22–23 among European-American participants.
Discussion
By combining rich measures of context and neural reactivity during socioemotional and reward processing in a longitudinal sample of 242 young men, we identified factors that predicted deflections from the continuity in AB from adolescence to early adulthood. Specifically we found that relationship satisfaction and employment were potentially important protective factors for men who had followed an adolescent-onset trajectory of AB (late-moderate group). In addition, neural reactivity to fearful faces was a predictor of AB pathways for men in the high, early-starting AB trajectory group in divergent directions depending on race: For African-Americans, greater amygdala reactivity to fearful faces was protective, whereas for European-Americans, greater reactivity predicted increased AB. Finally, lower VS reactivity to reward predicted increased AB, but only for African-American men in the high AB trajectory group. These results highlight the utility of examining both contextual and neural variables as factors that may promote persistence or desistance of AB into young adulthood.
Contextual factors
Two well-researched factors associated with desistance, the presence of a positive romantic relationship and employment, predicted desistance from AB, but only for those with adolescent-onset AB (i.e., late-moderate group). Thus, these factors may be more effective for young men with less entrenched AB. It is important to note that we examined perceived relationship quality, not marriage which was the focus of past studies (Burt et al., 2010; Laub et al., 1998). Thus, for the more severe, high AB group a more formalized, long-term relationship (e.g., marriage) may be necessary to promote desistance. Additionally, it could be that early-starting trajectories are more intractable and less malleable in the face of positive relationships (Moffitt, 2006, 2018). Of course, one alternate interpretation, given the use of a self-report measures of relationship quality, is that early (versus late) starters may have different biases in their reporting of relationship quality. Similarly, it is possible that later-onset delinquents who have higher intelligence and more adaptive personal characteristics are able to both desist from crime and enter into good social relations and employment. Thus, desisting and forming social bonds may not be related causally; a key limitation to our observational design. Importantly however, these findings did not vary by race, suggesting that a positive romantic relationship and employment predicts outcomes similarly regardless of the unique risk factors (e.g., discrimination) faced by African-American men.
Neural factors
When examining neural reactivity to fearful faces, we found that greater amygdala reactivity increased the likelihood of persistence in the high AB group among European-American men, whereas for African-American men, lower amygdala reactivity predicted persistence. The dramatic divergence across race is consistent with previous work in this sample (Hyde et al., 2016), where we found that cross-sectional relationships between lower amygdala reactivity to fearful faces and AB were strongest among African-American participants. Because the task presents African-American men with faces of people who appear to be of European origin, we have interpreted our findings as consistent with research suggesting that amygdala reactivity to emotional faces may vary dramatically to in-group versus out-group members (Hart et al., 2000; Phelps et al., 2000). Thus, our findings suggest that, at this critical developmental juncture, the extent to which European-American men with long histories of AB are hyper-reactive to distress in members of their in-group, predicts their continued persistence in AB, whereas for African-American men, lower reactivity to distress in out-group members predicts persistence.
Although our goal was to examine neural reactivity as a statistical moderator, based on the categorical nature of the trajectory groups, we graphed the interactions with neural reactivity on the x-axis (rather than as the grouping variable). This approach also clarifies that among African-American participants, amygdala reactivity to fearful faces is negatively correlated with AB within the high AB group. Whereas the direction of this relationship is consistent with past work in this sample (Hyde et al., 2016), it contrasts with much of the previous literature that has linked AB to greater amygdala reactivity (at least when not accompanied by high levels of callous-unemotional traits which were not related to amygdala reactivity in this sample) (Hyde et al., 2013; Viding, Fontaine, & McCrory, 2012). However, few previous studies have examined race as a moderator and most have examined extreme, clinical samples with participants of European-origin. Our current findings suggest that, consistent with the literature, amygdala reactivity to fearful faces may be positively related to AB, but only in individuals of European descent with severe AB, which likely matches the sample composition of several previous studies in this area (e.g., Viding, Sebastian, et al., 2012).
Limitations
The current study had several strengths, including a relevant, well-characterized sample at high risk for AB followed prospectively from early childhood, use of contextual and neural measures, assessment of adolescent trajectories of AB to examine desistance, and prospective neuro-prediction of later AB. However, several important limitations merit consideration: First, the sample is composed of young men from low-income, urban families; findings may not generalize to other populations. Second, although our sample is relatively large for a neuroimaging study, we found relatively small cell sizes for specific trajectories of AB as moderated by race (though on par with many clinical neuroimaging studies of AB) and did have substantial attrition with respect to the neural measures. These smaller cell sizes may have limited our power to detect smaller effects (Dawson & Richter, 2006), particularly given the difficulties in detecting small to medium interaction effects in samples smaller than 500 participants (McClelland & Judd, 1993). Third, we found a relatively small number of participants with data within the regions of significance for several of the interactions. We highlight the limited data coverage through the use of transparent reporting approaches that are highly recommended (Roisman et al., 2012; Widaman et al., 2012), though rarely used, when reporting results from interaction models. Thus, while our findings were consistent with the hypotheses, the results should be seen as exploratory and must be replicated in larger samples. Of course, the challenge to replication is that few current datasets include a larger sample size with neuroimaging and longitudinal data across adolescence with greater power to test the current hypotheses (although see the forthcoming ABCD study); a point highlighted in a recent review of AB trajectories (Moffitt, 2018). Fourth, we examined the brain as a statistical moderator versus the brain as a predictor of behavior. Although the amygdala and VS are implicated in the etiology of AB (i.e., as “predictors”), our study addressed whether individual variability in these circuits magnifies or buffers AB pathways once they are started (i.e., as “moderators” while controlling for the main effects of neural reactivity on AB). Relatedly, we use the term “moderators” through the paper in a statistical sense (i.e., an interaction term). However, only race meets true definitions of a moderator (e.g., our moderators were not measured at baseline, are not uncorrelated with independent variables: Chmura Kraemer, Kiernan, Essex, & Kupfer, 2008; Kraemer, Stice, Kazdin, Offord, & Kupfer, 2001). Factors such as marriage, employment, and neural reactivity are all likely influenced by the past trajectory of AB and the contextual factors that predict trajectories of AB. Moreover, these factors change over time. Thus, although we selected factors that chronologically appeared between adolescent trajectories of AB and the later outcome of AB in early adulthood, these “turning points” should be seen as varying factors that may “predict” desistance in the statistical and descriptive sense and may not be stable, causal factors.
Conclusions
In sum, our study addresses a novel question about how both contextual and neural factors predict the potential for desistance in high-risk males transitioning into early adulthood. Contextual factors appeared to be important in predicting the desistance of youth with moderate and late-onset AB, whereas neural factors related to the cortiolimbic and corticostriatal circuits predicted both desistance and persistence among those in early-starting trajectories of AB. The results bridge findings across social and biological approaches and highlight the potential to identify who and why some individuals are at most risk for chronic AB.
Supplementary Material
Key Points:
Contextual factors (e.g., marriage, employment) have been shown to predict desistance in antisocial behavior in early adulthood, but little work has also examined neural factors that predict desistance
Relationship quality and employment predicted decreased antisocial behavior in adulthood, but only for men who were in moderate/adolescent-onset trajectories of antisocial behavior
Amygdala reactivity to fearful faces predicted increased antisocial behavior, but only among men with early onset/high trajectories of antisocial behavior and in divergent ways based on participant race
Lower ventral striatum reactivity to reward predicted increased antisocial behavior, but only among African-American men with early-onset trajectories of antisocial behavior
Acknowledgements:
This research was supported by grants R01 MH050907, R01 MH01666, and K05 DA25630 to Daniel S. Shaw, and R01 DA02622 to Daniel S. Shaw and Erika E. Forbes. Luke W. Hyde was supported by NSF 12-614 and Rebecca Waller was supported by T32 AA007477-24A1. We are grateful to the work of the staff of the Pitt Mother & Child Project for their many years of service, and to our study families for sharing their lives with us and making the research possible.
Abbreviations:
- AB
Antisocial Behavior
- APD
Antisocial Personality Disorder
- BOLD
Blood Oxygenation Level–Dependent
- VS
Ventral Striatum
Footnotes
Conflicts of interest: All authors report no biomedical financial interests or potential conflicts of interest.
References
- Aharoni E, Vincent GM, Harenski CL, Calhoun VD, Sinnott-Armstrong W, Gazzaniga MS, & Kiehl KA (2013). Neuroprediction of future rearrest. Proceedings of the National Academy of Sciences, 110(15), 6223–6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJR, Leibenluft E, & Pine DS (2014). Conduct Disorder and Callous–Unemotional Traits in Youth. New England Journal of Medicine, 371(23), 2207–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Benning SD, Li R, … Shelby ES (2010). Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nature Neuroscience, 419–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, Donnellan MB, Humbad MN, Hicks BM, McGue M, & Iacono WG (2010). Does marriage inhibit antisocial behavior?: An examination of selection vs causation via a longitudinal twin design. Archives of General Psychiatry, 67(12), 1309–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmura Kraemer H, Kiernan M, Essex M, & Kupfer DJ (2008). How and why criteria defining moderators and mediators differ between the Baron & Kenny and MacArthur approaches. Health Psychology, 27(2S), S101–S108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HL, Little M, & Steinberg L (2005). The transition to adulthood for adolescents in the juvenile justice system: A developmental perspective In Osgood DW, Foster EM, Flanagan C, & Ruth GR (Eds.), On your own without a net: The transition to adulthood for vulnerable populations (pp. 68–91). Chicago, IL: University of Chicago Press [Google Scholar]
- Dawson JF, & Richter AW (2006). Probing three-way interactions in moderated multiple regression: development and application of a slope difference test. Journal of Applied Psychology, 91(4), 917–926. [DOI] [PubMed] [Google Scholar]
- Dotterer HL, Waller R, Neumann CS, Shaw DS, Forbes EE, Hariri AR, & Hyde LW (2016). Examining the Factor Structure of the Self-Report of Psychopathy Short-Form Across Four Young Adult Samples. Assessment, 1073191116640355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, & Benjamin LS (1997). User’s guide for the structured clinical interview for DSM-IV axis II personality disorders: SCID-II: American Psychiatric Pub. [Google Scholar]
- Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, … Dahl RE (2009). Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. American Journal of Psychiatry, 166(1), 64–73. doi: 10.1176/appi.ajp.2008.07081336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Shaw DS, & Dahl RE (2007). Alterations in reward-related decision making in boys with recent and future depression. Biological psychiatry, 61(5), 633–639. [DOI] [PubMed] [Google Scholar]
- Foster EM, & Jones DE (2005). The high costs of aggression: Public expenditures resulting from conduct disorder. American Journal of Public Health, 95(10), 1767–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster H, & Brooks-Gunn J (2013). Neighborhood influences on antisocial behavior during childhood and adolescence In Gibson C & Krohn M (Eds.), Handbook of life-course criminology (pp. 69–90). New York, NY: Springer. [Google Scholar]
- Gard AM, Waller R, Shaw DS, Forbes EE, Hariri AR, & Hyde LW (2017). The Long Reach of Early Adversity: Parenting, Stress, and Neural Pathways to Antisocial Behavior in Adulthood. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2(7), 582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordts S, Uzieblo K, Neumann C, Van den Bussche E, & Rossi G (2017). Validity of the Self-Report Psychopathy Scales (SRP-III full and short versions) in a community sample. Assessment, 24(3), 308–325. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Tessitore A, Mattay VS, Fera F, & Weinberger DR (2002). The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage, 17(1), 317–323. [DOI] [PubMed] [Google Scholar]
- Hart AJ, Whalen PJ, Shin LM, McInerney SC, Fischer H, & Rauch SL (2000). Differential response in the human amygdala to racial outgroup vs ingroup face stimuli. Neuroreport, 11(11), 2351–2354. [DOI] [PubMed] [Google Scholar]
- Huesmann LR, Eron LD, Lefkowitz MM, & Walder LO (1984). Stability of aggression over time and generations. Developmental Psychology, 20(6), 1120. [Google Scholar]
- Huizinga D, & Elliott DS (1986). Reassessing the reliability and validity of self-report delinquency measures. Journal of quantitative criminology, 2(4), 293–327. [Google Scholar]
- Hyde LW, Burt SA, Shaw DS, Donnellan MB, & Forbes EE (2015). Early starting, aggressive and callous-unemotional? Examining overlap and predictive utility of antisocial behavior subtypes. Journal of Abnormal Psychology, 124, 329–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde LW, Shaw DS, & Hariri AR (2013). Neuroscience, developmental psychopathology and youth antisocial behavior: Review, integration, and directions for research. Developmental Review, 33, 168–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde LW, Shaw DS, Murray L, Gard A, Hariri AR, & Forbes EE (2016). Dissecting the Role of Amygdala Reactivity in Antisocial Behavior in a Sample of Young, Low-Income, Urban Men. Clinical Psychological Science, 4(3), 527–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BL, Nagin DS, & Roeder K (2001). A SAS procedure based on mixture models for estimating developmental trajectories. Sociological methods & research, 29(3), 374–393. [Google Scholar]
- Kraemer HC, Stice E, Kazdin A, Offord D, & Kupfer D (2001). How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. American Journal of Psychiatry, 158(6), 848–856. [DOI] [PubMed] [Google Scholar]
- Laub JH, Nagin DS, & Sampson RJ (1998). Trajectories of change in criminal offending: Good marriages and the desistance process. American Sociological Review, 63(2), 225–238. [Google Scholar]
- Laub JH, & Sampson RJ (1993). Turning points in the life course: Why change matters to the study of crime. Criminology, 31(3), 301–325. [Google Scholar]
- Loeber R (1991). Antisocial behavior: more enduring than changeable? Journal of the American Academy of Child and Adolescent Psychiatry, 30(3), 393–397. [DOI] [PubMed] [Google Scholar]
- Mays VM, Cochran SD, & Barnes NW (2007). Race, race-based discrimination, and health outcomes among African Americans. Annual Review of Psychology, 58, 201–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland GH, & Judd CM (1993). Statistical difficulties of detecting interactions and moderator effects. Psychological Bulletin, 114(2), 376–390. [DOI] [PubMed] [Google Scholar]
- Moffitt TE (2006). Life-course-persistent versus adolescence-limited antisocial beahvior In Cicchetti D & Cohen DJ (Eds.), Developmental Psychopathology: Vol. 3, Risk, disorder and adaptation (pp. 570–598). Hoboken, NJ: Wiley. [Google Scholar]
- Moffitt TE (2018). Male antisocial behaviour in adolescence and beyond. Nature Human Behaviour, 10.1038/s41562-41018-40309-41564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Harrington H, & Milne BJ (2002). Males on the life-course-persistent and adolescence-limited antisocial pathways: follow-up at age 26 years. Development and Psychopathology, 14(1), 179–207. [DOI] [PubMed] [Google Scholar]
- Mulvey EP, Steinberg L, Fagan J, Cauffman E, Piquero AR, Chassin L, … Hecker T (2004). Theory and research on desistance from antisocial activity among serious adolescent offenders. Youth Violence and Juvenile Justice, 2(3), 213–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvey EP, Steinberg L, Piquero AR, Besana M, Fagan J, Schubert C, & Cauffman E (2010). Trajectories of desistance and continuity in antisocial behavior following court adjudication among serious adolescent offenders. Development and Psychopathology, 22(2), 453–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L, Shaw DS, Forbes EE, & Hyde LW (2017). Reward-Related Neural Correlates of Antisocial Behavior and Callous–Unemotional Traits in Young Men. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2(4), 346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, & Muthén BO (2014). Mplus User’s Guide: Seventh Edition Los Angeles, CA: Muthén & Muthén. [Google Scholar]
- Nock MK, Kazdin AE, Hiripi E, & Kessler RC (2006). Prevalence, subtypes, and correlates of DSM-IV conduct disorder in the National Comorbidity Survey Replication. Psychological Medicine, 36(5), 699–710. doi: 10.1017/S0033291706007082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton R (1983). Measuring marital quality: A critical look at the dependent variable. Journal of Marriage and the Family, 45, 141–151. [Google Scholar]
- Phelps EA, O’Connor KJ, Cunningham WA, Funayama ES, Gatenby JC, Gore JC, & Banaji MR (2000). Performance on indirect measures of race evaluation predicts amygdala activation. Journal of Cognitive Neuroscience, 12(5), 729–738. [DOI] [PubMed] [Google Scholar]
- Plichta MM, & Scheres A (2014). Ventral–striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: A meta-analytic review of the fMRI literature. Neuroscience & Biobehavioral Reviews, 38, 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, & Bauer DJ (2006). Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of educational and behavioral statistics, 31(4), 437–448. [Google Scholar]
- Roisman GI, Newman DA, Fraley RC, Haltigan JD, Groh AM, & Haydon KC (2012). Distinguishing differential susceptibility from diathesis–stress: Recommendations for evaluating interaction effects. Development and Psychopathology, 24(2), 389–409. [DOI] [PubMed] [Google Scholar]
- Shaw DS, Hyde LW, & Brennan LM (2012). Predictors of Boys’ Antisocial Trajectories from Toddlerhood through Adolescence. Development and Psychopathology, 24, 871–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viding E, Fontaine NMG, & McCrory EJ (2012). Antisocial behaviour in children with and without callous-unemotional traits. Journal of the Royal Society of Medicine, 105(5), 195–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viding E, Sebastian CL, Dadds MR, Lockwood PL, Cecil CAM, De Brito SA, & McCrory EJ (2012). Amygdala Response to Preattentive Masked Fear in Children With Conduct Problems: The Role of Callous-Unemotional Traits. American Journal of Psychiatry, 169(10), 1109–1116. [DOI] [PubMed] [Google Scholar]
- Widaman KF, Helm JL, Castro-Schilo L, Pluess M, Stallings MC, & Belsky J (2012). Distinguishing ordinal and disordinal interactions. Psychological methods, 17(4), 615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


