Abstract
Introduction
Although pancreatic tuberculosis (TB) is traditionally considered to be a rare clinical entity, in recent times, an increase in the number of reports of pancreatic TB has been noted. We conducted a systematic review in order to summarise currently available data on pancreatic TB.
Methods
A comprehensive literature search of Medline, Scopus and ISI Web of Science databases was conducted in order to identify papers reporting cases of pancreatic TB. The eligibility criteria for inclusion in the review required that the studies reported patient(s) affected by pancreatic TB and that individual data on age, sex, clinical presentation and outcome were available.
Results
In total, 116 studies reporting data on 166 patients were included in the analysis. The majority of patients were males (62.1%) diagnosed at a mean age of 41.61 ± 13.95 years. Most cases were diagnosed in Asia (50.0%), followed by North America (22.9%), Europe (20.5%), Africa (4.2%) and South America (2.4%). Human immunodeficiency virus (HIV) infection was diagnosed in 25.3% of those affected. Pancreatic TB most frequently presented itself in the form of a pancreatic mass (79.5%) localised mainly in the head (59.0%) and less frequently in the body (18.2%) and tail (13.4%). Extrapancreatic TB involvement most frequently affected the peripancreatic lymph nodes (47.3%). More than half of patients (55.2%) were subjected to laparotomy, while 21.08% underwent endoscopic ultrasound fine-needle aspiration biopsy. The presence of TB was identified most frequently through histological analysis (59.6%), followed by culture (28.9%), staining (27.7%) and, in a smaller number, by polymerase chain reaction (9.6%) and cytology (6.6%). Almost all patients received anti-tubercular pharmacological therapy (98.2%), while 24.1% underwent surgery. Despite treatment, 8.7% of patients died.
Conclusion
Increased awareness of pancreatic TB is needed, not only in endemic areas but especially in relation to HIV infection and other clinical conditions associated with immunoincompetence.
Keywords: Pancreatitis, tuberculosis, pancreatic mass, pancreatic cancer, diagnosis
Introduction
Mycobacterium tuberculosis infection (TB) still represents one of the major causes of death worldwide, as well as being the leading cause from a single infectious agent.1 It is reported that in 2017, TB caused an estimated 1.6 million deaths.1 Although the vast majority of TB cases are pulmonary, approximately 12.5% are extrapulmonary,2,3 with abdominal TB accounting for 11–16%.4,5 Possible pathways for abdominal TB infection include haematogenous spread from primary pulmonary TB, ingestion of infected milk products, ingestion of infected sputum from pulmonary TB and direct invasion from an adjacent organ.4,6 TB affects various intra-abdominal organs, including the intestines, gastroduodenum, liver, biliary tract spleen and pancreas.
For decades, the pancreas was one of the locations rarely affected by abdominal TB. In large autopsy series on TB patients, Auerbach et al.7 and Bhansali et al.8 reported pancreatic involvement in only 4.7% and in 0% of patients, respectively. However, in recent times, an increase in the number of reports of pancreatic TB has been noted.9–13 This could possibly be due to increased availability of powerful imaging tools and development of different techniques that make obtaining specimens from the pancreas possible. The increased number of immunocompromised individuals, such as those affected by human immunodeficiency virus (HIV) or advanced stage malignant diseases, also plays an important role in this phenomenon. TB has been reported to be the leading infectious cause of death in HIV co-infected patients.14,15 Furthermore, in the USA, abdominal TB has been reported usually to be a manifestation of disseminated disease in HIV-affected individuals.16
Pancreatic TB is known to be a great mimicker, often being misdiagnosed as pancreatic cancer.17 Nevertheless, it is of great importance to diagnose pancreatic TB infection in time and to initiate adequate treatment, not only because pharmacological treatment is reported to be highly effective in most cases17 but also because in many cases costly and risky surgical procedures can be avoided. However, awareness of pancreatic TB is still relatively low among physicians encountering this condition, leading to unsatisfactory treatment results and increased health-care costs.
We conducted a systematic review in order to summarise currently available data on pancreatic TB and to contribute further to raising awareness of this important clinical entity.
Methods
This systematic review has been reported following the PRISMA statement.
A comprehensive literature search of Medline, Scopus and ISI Web of Science databases was conducted in order to identify papers reporting cases of pancreatic TB. The following combination of keywords was used (pancreas OR pancreatic) AND (tuberculosis OR Mycobacterium).
The search was limited to human subjects, with language restriction to English studies until 1 July 2018. The snowball strategy, including a manual search of the references listed by studies retrieved from the online databases and from previously published reviews, was also performed in order to identify potential additional studies. The eligibility criteria for inclusion in the review required that that studies reported patient(s) affected by pancreatic TB and that individual data on age, sex, clinical presentation and outcome were available. All study types fulfilling the inclusion criteria, including case studies and case series, were considered for inclusion. Studies were excluded if no clear evidence for the presence of pancreatic TB infection was present.
Data from the included studies were independently extracted by two investigators (NP and MR) and entered into a Microsoft Excel 2010 (Microsoft Corp., Redmond, WA) spreadsheet. Any discrepancies regarding individual study inclusion, data extraction or interpretation were resolved by consulting a third investigator (JML). We extracted the following data: first author name, year of publication, region of manuscript origin, patient sex, age at the diagnosis, presence of risk factors (alcohol, smoking, intravenous drug intake and co-morbidities), clinical characteristics (symptoms and clinical presentation), diagnostics, treatment (pharmacological or surgical) and outcome (cured or died).
Descriptive analysis using proportion and mean ± standard deviation was computed for categorical and quantitative variables, respectively. Analyses were conducted using Stata v13 (StataCorp, College Station, TX).
Results
Figure 1 depicts the flow chart of the search strategy and a diagram for paper selection.
Figure 1.
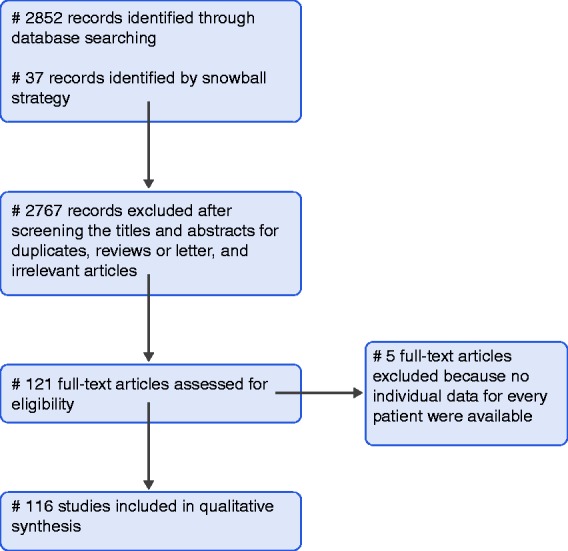
Search strategy and flow diagram for search of databases.
In total, 121 case studies and case series reporting pancreas involvement in TB infection published from 1978 to 2017 were identified. Of those, 84 were identified through a database search, while 37 were identified by the snowball strategy. However, five case series in which data on demographics and clinical presentation were not reported separately for every patient were excluded from the analyses. Finally, 116 studies reporting data on 166 patients were included in the analysis (reference in Supplemental File 1).
Table 1 reports the distribution of demographics and risk factors among 166 patients diagnosed with pancreatic TB (Table 1). The majority of patients were male (62.1%) diagnosed at a mean age of 41.61 ± 13.95 years. Most of the cases were diagnosed in Asia (50.0%), followed by North America (including Australia and New Zealand; 22.9%), Europe (20.5%), Africa (4.2%) and South America (2.4%). Data on the presence of various risk factors, including co-morbidities and lifestyle habits, were available for 87 (52.4%) patients. Of these, 22 (25.3%) were previously or simultaneously diagnosed as HIV-positive, 10.5% were associated with alcohol abuse and 8.1% with intravenous drugs abuse, while 6.9% reported smoking (Table 1). Previous TB infection was present in only 7.3% of patients.
Table 1.
Demographics and risk factors in 166 patients diagnosed with pancreatic tuberculosis.
| Age (years) | 41.61 ± 13.95 |
| Age range (years) | 16–82 |
| Sex | |
| Male | 103 (62.1%) |
| Female | 63 (38.0%) |
| Origin | |
| North America, Australia and New Zealand | 38 (22.8%) |
| Europe | 34 (20.5%) |
| Asia | 83 (50.0%) |
| Africa | 7 (4.2%) |
| South America | 4 (2.4%) |
| Risk factors | |
| Drugs | 7 (8.1%) |
| Alcohol | 9 (10.4%) |
| Smoking | 6 (6.9%) |
| HIV-positive | 22 (22.3%) |
| Previous tuberculosis | 7 (7.3%) |
HIV: human immunodeficiency virus.
Table 2 reports symptoms and clinical presentations in the patients included in the analysis. Most frequently, patients with pancreatic TB reported pain (74.8%), followed by weight loss (51.6%), fever (46.5%) and jaundice (20.0%). Pancreatic TB most frequently presented itself in the form of a pancreatic mass (79.5%) localised mainly in the pancreatic head (59.0%) and less frequently in the body (18.2%), tail (13.4%) or neck (1.8%). Pancreatic abscesses were present in 12.1% of patients, while 6.6% and 6.0% of patients presented with chronic or acute pancreatitis, respectively. Extrapancreatic TB involvement most frequently affected the peripancreatic lymph nodes (47.3%), followed by the spleen (8.3%), intestines (8.2%), liver (6.9%) and lungs (6.3%).
Table 2.
Clinical characteristics in 166 patients diagnosed with pancreatic tuberculosis.
| Symptoms | |
| Fever | 74 (46.5%) |
| Pain | 119 (74.8%) |
| Weight loss | 82 (51.6%) |
| Jaundice | 31 (20.0%) |
| Diarrhoea | 5 (3.1%) |
| Clinical presentation | |
| Pancreatic mass | 132 (79.5%) |
| Pancreatic head mass | 98 (59.0%) |
| Pancreatic neck mass | 3 (1.8%) |
| Pancreatic body mass | 30 (18.2%) |
| Pancreatic tail mass | 22 (13.4%) |
| Acute pancreatitis | 10 (6.0%) |
| Chronic pancreatitis | 11 (6.6%) |
| Pancreatic abscess | 20 (12.1%) |
| Pseudocyst | 3 (1.8%) |
| Extrapancreatic involvement | |
| Peripancreatic lymph nodes | 69 (47.3%) |
| Pulmonary | 9 (6.3%) |
| Intestine | 12 (8.2%) |
| Liver | 10 (6.9%) |
| Spleen | 12 (8.3%) |
The diagnostics that patients with pancreatic TB underwent are reported in Table 3. Almost all patients underwent computed tomography (CT; 89.8%), followed by abdominal ultrasound (55.2%), endoscopic ultrasound (EUS; 27.7%) and endoscopic retrograde cholangiopancreatography (24.4%). A substantial proportion of patients underwent EUS-guided fine-needle aspiration biopsy (EUS-FNA; 21.1%), but only a few underwent CT-guided FNA (7.8%). More than half of patients with pancreatic TB (55.2%) were subjected to laparatomy.
Table 3.
Diagnostics in 166 patients diagnosed with pancreatic tuberculosis.
| Imaging | |
| Abdominal ultrasonography | 91 (55.2%) |
| Computed tomography | 149 (89.8%) |
| Computed tomography FNA | 13 (7.8%) |
| Magnetic resonance | 5 (3.0%) |
| Magnetic resonance cholangiopancreatography | 6 (3.6%) |
| Endoscopic retrograde cholangiopancreatography | 40 (24.2%) |
| Endoscopic ultrasound | 46 (27.7%) |
| Endoscopic ultrasound FNA | 35 (21.1%) |
| Laparatomy | 91 (55.2%) |
| Laboratory | |
| Tuberculin test positive | 28 (68.3%) |
| Elevated lipase | 5 (31.3%) |
| Elevated amylase | 15 (26.8%) |
| Tuberculosis identification method | |
| Culture | 48 (28.3%) |
| Staining | 46 (27.7%) |
| Histology | 99 (59.6%) |
| Cytology | 11 (6.6%) |
| PCR | 16 (9.6%) |
FNA: fine-needle aspiration; PCR: polymerase chain reaction.
Laboratory analysis results were only available for a few patients (Table 3). Of those, 68.3% displayed a positive skin tuberculin test, while elevated lipase and amylase levels were present in 31.3% and 26.8%, respectively. The presence of TB was identified most frequently through histological analysis (59.7%), followed by culture (28.9%), staining (27.7%) and, in a smaller number, by polymerase chain reaction (PCR; 9.6%) and cytology (6.6%).
Almost all patients received anti-tubercular pharmacological therapy (98.2%), while 24.1% underwent surgery (Table 4). After a mean reported follow-up of 16.49 ± 22.13 months, 91.3% were considered to be cured, while 12 (8.7%) died.
Table 4.
Outcome and treatment in 166 patients diagnosed with pancreatic tuberculosis.
| Therapy | |
| Pharmacological | 159 (98.2%) |
| Surgical | 39 (24.1%) |
| Follow-up (months) | 16.49 ± 22.13 |
| Outcome | |
| Cured | 126 (91.3%) |
| Died | 12 (8.7%) |
Discussion
By pooling currently available data, our systematic review depicts the profile of typical patients with pancreatic TB, including demographics, symptoms, clinical presentations, diagnostics, therapies and outcomes.
On average, the majority of patients diagnosed with pancreatic TB were male in the fifth decade of life. Most patients were diagnosed in Asia, in line with the burden of global TB being associated with low socio-economic conditions.1 However, almost half of the patients with pancreatic TB included in this systematic review were diagnosed in developed countries such as North America and Europe (including Australia and New Zealand). Ethnicity and immigration data were not available for most of the patients included, and it is reasonable to consider that some of these cases were immigrants. However, as the pancreas is considered to be an extremely hostile environment for TB due to the anti-mycobacterial effect of pancreatic lipases and deoxyribonucleases,18 the involvement of such an organ in TB infection points to pronounced immunodeficiency as a predisposing condition. Limited data were available regarding the presence of risk factors among the patients included, but it was noteworthy that a substantial percentage of patients suffered from acquired immune deficiency syndrome or were HIV-positive. A sub-analysis not reported in the Results showed that more than 60% of patients with HIV were diagnosed in North America and Europe. The role of HIV infection in the development of pancreatic TB has been previously recognised.19 Our study further emphasises this association, especially in Western countries. Based on our systematic review, two main types of patients with pancreatic TB can be differentiated: one from areas of endemic TB infection, and another associated with immunodeficiency predominantly caused by HIV infection and present in the more economically developed world. This is in line with previous reports associating abdominal TB with disseminated HIV in individuals born in Western countries,16 as well as non-HIV-infected immigrants coming from endemic areas.20
Considering the symptoms present in patients with pancreatic TB, our results revealed abdominal pain to be predominant, followed by fever, weight loss and jaundice. Although some of the symptoms can be explained by the systemic effects of TB infection, they are in large part a consequence of pancreatic involvement and are also present in other clinical entities affecting the pancreas.21 Furthermore, if we take into consideration the fact that almost 80% of pancreatic TB cases manifested themselves as pancreatic masses, the picture of pancreatic TB as a great mimicker, often misdiagnosed as pancreatic cancer, becomes clearer. Indeed, although almost 90% of the patients in our review underwent abdominal CT, and a smaller proportion other powerful diagnostic tools such as magnetic resonance and EUS, more than 50% subsequently underwent laparatomy, attesting to a misdiagnosis, mainly as pancreatic cancer, or that there was a diagnostic dilemma that could not be resolved in another way. In addition, as less than 10% of patients in our review were previously diagnosed with TB of any other organ at the time of diagnosis, the diagnostic ‘trap’ is complete. Furthermore, concerning the extrapancreatic involvement of TB at the time of diagnosis, imaging revealed enlarged peripancreatic lymph nodes in most patients, which is also a typical finding in pancreatic cancer.21,22 Few patients exhibited the pulmonary lesions characteristic of TB. Taken together, these findings depict the relative ease with which pancreatic TB is misdiagnosed, even when all diagnostic tools are available.
Our analysis did not show serum lipase or amylase levels to be of significant diagnostic value in pancreatic TB, as these were only elevated in a limited number of cases. However, when conducted, a tuberculin skin test was positive in almost 70% of cases, showing that this simple and cheap diagnostic tool could make a great difference to patients with suspected pancreatic TB, at least in countries where TB is not endemic. Taking into consideration the latest guidelines for the diagnosis of TB,23 we advise a tuberculin skin test, or interferon-gamma release assay where available, to be performed initially in all cases where pancreatic TB is suspected. Furthermore, according to European Union standards for TB care, obtaining appropriate specimens for microbiological analysis is essential for the diagnosis of extrapulmonary TB. Although in the past century most tissue or liquid specimens were acquired during surgical procedures24–26 or even during autopsies,27,28 an increased number of cases in recent decades were diagnosed using less invasive methods with a good yield, such as EUS-FNA.29,30 Evaluating the performance of different diagnostic and sampling tools for the diagnosis of pancreatic TB is beyond the scope of this systematic review. However, based on what we encountered when attempting to address this important issue, and the overall performance of EUS-FNA to obtain adequate samples for diagnostics of pancreatic masses31as well as EUS performance to obtain samples suitable for culture in different locations,32 we propose that in the case of suspected pancreatic TB, after initial testing with a tuberculin skin test or interferon-gamma release assay, EUS-FNA should precede any other invasive sampling or treatment.
There was great heterogeneity among cases included in our review regarding the methods applied for TB identification. Although histology prevailed, staining and culture were also performed and, in recent years, PCR. What is interesting is that in most cases, multiple identification methods were applied, probably in order for the atypical finding of pancreatic TB to be confirmed.
When a diagnosis of pancreatic TB was set, almost all patients commenced anti-tubercular pharmacological therapy, while in some cases, surgery was still needed. In any case, treatment results were satisfactory, as pancreatic TB caused death in only a few cases. This fact further emphasises the importance of a rapid diagnosis of pancreatic TB, as efficient treatment options are available even for immunocompromised patients.
Very limited data were available on pancreatic TB and the endocrine and exocrine function of the pancreas. Patients with diabetes mellitus (DM) are at higher risk of developing TB,33 suggesting that DM impairs the immune responses necessary to counter the proliferation of TB.34–36 Subsequent studies reported that patients with pulmonary TB are at higher risk of DM than those with extrapulmonary TB.37 The relationship between DM and TB is a two-way street, where DM can also be caused, or worsened, by the destruction of pancreatic parenchyma. Few reports demonstrate DM in patients with pancreatic TB at the time of diagnosis.38,39 Nevertheless, it can be expected for DM to occur in some patients with pancreatic TB during follow-up, for which no data were available. As for the effect of pancreatic TB on the exocrine function of the pancreas, data are even sparser. Although the very first patient ever reported to be treated with pancreatic extracts was one with TB and pancreatic exocrine insufficiency,40 no author of any of the studies reviewed referred to the exocrine function of the pancreas in patients with pancreatic TB. Further research is needed in order to assess the effect of pancreatic TB on both exocrine and endocrine functions of the pancreas.
The main limitation of our systematic review is the great heterogeneity among the studies included, not only in the data available for extraction and subsequent synthesis but also in which data types were reported. Even in case series reporting findings from a single institution or system, data considering individual patients were sometimes not reported individually and uniformly. Furthermore, a substantial number of cases were published in languages other than English, preventing inclusion in the current review. The differing availability of diagnostic tools in different health-care settings also needs to be considered, as this could lead to pancreatic TB patients following different clinical pathways. However, to our knowledge, this is the first systematic review addressing the important clinical entity of pancreatic TB – a subject to which the authors wish to draw attention.
In conclusion, although pancreatic TB is still a relatively rare clinical entity, increased awareness of its existence is needed, not only in endemic areas but especially in relation to HIV infection and other clinical conditions associated with immunoincompetence. Hopefully, this will contribute to shortening the time from clinical presentation to diagnosis, resulting in a more timely initiation of adequate therapy and better treatment outcomes. Furthermore, increased awareness of pancreatic TB among clinicians might reduce the health-care costs associated with unnecessary diagnostic tests, but also spare patients from risky and inadequate surgical procedures.
Supplemental Material
Supplemental material, UEG902353 Supplemental Material for Pancreatic tuberculosis: A systematic review of symptoms, diagnosis and treatment by Nikola Panic, Hartwig Maetzel, Milutin Bulajic, Mihailo Radovanovic and J-Matthias Löhr in United European Gastroenterology Journal
Acknowledgements
NP was supported by a mobility grant from the European Pancreatic Club. He is further supported by Pancreas 2000 – an educational programme for future pancreatologists.
Declaration of conflicting interests
The authors have no financial or other conflicts of interest to disclose.
Funding
The authors received no financial support for the research, authorship and/or publication of this article.
ORCID iD
J-Matthias Löhr https://orcid.org/0000-0002-7647-198X
Supplemental material
Supplemental material for this article is available online.
References
- 1.World Health Organization.. Global tuberculosis report, Geneva, Switzerland: World Health Organization, 2018. [Google Scholar]
- 2.Wang HS, Chen WS, Su WJ, et al. The changing pattern of intestinal tuberculosis: 30 years’ experience. Int J Tuberc Lung Dis 1998; 2: 569–574. [PubMed] [Google Scholar]
- 3.Misra SP, Misra V, Dwivedi M, et al. Colonic tuberculosis: clinical features, endoscopic appearance and management. J Gastroenterol Hepatol 1999; 14: 723–729. [DOI] [PubMed] [Google Scholar]
- 4.Aston NO. Abdominal tuberculosis. World J Surg 1997; 21: 492–499. [DOI] [PubMed] [Google Scholar]
- 5.Singhal A, Gulati A, Frizell R, et al. Abdominal tuberculosis in Bradford, UK: 1992–2002. Eur J Gastroenterol Hepatol 2005; 17: 967–971. [DOI] [PubMed] [Google Scholar]
- 6.Horvath KD, Whelan RL. Intestinal tuberculosis: return of an old disease. Am J Gastroenterol 1998; 93: 692–696. [DOI] [PubMed] [Google Scholar]
- 7.Auerbach O. Acute generalized miliary tuberculosis. Am J Pathol 1944; 20: 121–136. [PMC free article] [PubMed] [Google Scholar]
- 8.Bhansali SK. Abdominal tuberculosis. Experiences with 300 cases. Am J Gastroenterol 1977; 67: 324–337. [PubMed] [Google Scholar]
- 9.Singhai P, Gadhadh R, Joshi S, et al. Isolated pancreatic tuberculosis in an immunocompetent host. J Assoc Physicians India 2017; 65: 98–100. [PubMed] [Google Scholar]
- 10.Sun PJ, Lin Y, Cui XJ. Isolated pancreatic tuberculosis with elevated CA 19-9 levels masquerading as a malignancy: a rare case report and literature review. Medicine (Baltimore) 2018; 97: e13858–e13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Irfan M, Thiavalappil F, Nagaraj J, et al. Tuberculous pancreatitis complicated by ruptured splenic artery pseudoaneurysm. Monaldi Arch Chest Dis 2013; 79: 134–135. [DOI] [PubMed] [Google Scholar]
- 12.Falkowski AL, Graber J, Haack HG, et al. Isolated pancreatic tuberculosis: a case report and radiological comparison with cystic pancreatic lesions. J Radiol Case Rep 2013; 7: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ali M, Shaukat A, Al-Suwaidi Z, et al. Tuberculosis of pancreas, the first case reported from Qatar. Int J Mycobacteriol 2019; 8: 101–103. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization.. Global tuberculosis report, Geneva, Switzerland: World Health Organization, 2015. [Google Scholar]
- 15.Hesseling AC, Rabie H. Tuberculosis and HIV remain major causes of death in African children. Int J Tuberc Lung Dis 2016; 20: 996–997. [Google Scholar]
- 16.Bhargava D. Abdominal tuberculosis: current status. Apollo Med 2007; 4: 287–291. [Google Scholar]
- 17.Sharma V, Rana SS, Kumar A, et al. Pancreatic tuberculosis. J Gastroenterol Hepatol 2016; 31: 310–318. [DOI] [PubMed] [Google Scholar]
- 18.Knowles KF, Saltman D, Robson HG, et al. Tuberculous pancreatitis. Tubercle 1990; 71: 65–68. [DOI] [PubMed] [Google Scholar]
- 19.Meesiri S. Pancreatic tuberculosis with acquired immunodeficiency syndrome: a case report and systematic review. World J Gastroenterol 2012; 18: 720–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Probert CSJ, Jayanthi V, Wicks AC, et al. Epidemiological study of abdominal tuberculosis among Indian migrants and the indigenous population of Leicester, 1972–1989. Gut 1992; 33: 1085–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fogel EL, Shahda S, Sandrasegaran K, et al. A multidisciplinary approach to pancreas cancer in 2016: a review. Am J Gastroenterol 2017; 112: 537–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu LC, Goggins MG, Fishman EK. Diagnosis and detection of pancreatic cancer. Cancer J 2017; 23: 333–342. [DOI] [PubMed] [Google Scholar]
- 23.Lewinsohn DM, Leonard MK, LoBue PA, et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention clinical practice guidelines: diagnosis of tuberculosis in adults and children. Clin Infect Dis 2017; 64: 111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ladas SD, Vaidakis E, Lariou C, et al. Pancreatic tuberculosis in non-immunocompromised patients: reports of two cases, and a literature review. Eur J Gastroenterol Hepatol 1998; 10: 973–976. [DOI] [PubMed] [Google Scholar]
- 25.Desai DC, Swaroop VS, Mohandas KM, et al. Tuberculosis of the pancreas: report of three cases. Am J Gastroenterol 1991; 86: 761–763. [PubMed] [Google Scholar]
- 26.Fischer G, Spengler U, Neubrand M, et al. Isolated tuberculosis of the pancreas masquerading as a pancreatic mass. Am J Gastroenterol 1995; 90: 2227–2230. [PubMed] [Google Scholar]
- 27.Crook LD, Johnson FP Jr. Tuberculosis of the pancreas: a case report. Tubercle 1988; 69: 148–151. [DOI] [PubMed] [Google Scholar]
- 28.Stambler JB, Klibaner MI, Bliss CM, et al. Tuberculous abscess of the pancreas. Gastroenterology 1982; 83: 922–925. [PubMed] [Google Scholar]
- 29.Song TJ, Lee SS, Park DH, et al. Yield of EUS-guided FNA on the diagnosis of pancreatic/peripancreatic tuberculosis. Gastrointest Endosc 2009; 69: 484–491. [DOI] [PubMed] [Google Scholar]
- 30.Raghavan P, Rajan D. Isolated pancreatic tuberculosis mimicking malignancy in an immunocompetent host. Case Rep Med 2012; 2012: 501246–501246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puli SR, Bechtold ML, Buxbaum JL, et al. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass?: A meta-analysis and systematic review. Pancreas 2013; 42: 20–26. [DOI] [PubMed] [Google Scholar]
- 32.Fritscher-Ravens A, Ghanbari A, Topalidis T, et al. Granulomatous mediastinal adenopathy: can endoscopic ultrasound-guided fine-needle aspiration differentiate between tuberculosis and sarcoidosis? Endoscopy 2011; 43: 955–961. [DOI] [PubMed] [Google Scholar]
- 33.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med 2008; 5: e152–e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martens GW, Arikan MC, Lee J, et al. Tuberculosis susceptibility of diabetic mice. Am J Respir Cell Mol Biol 2007; 37: 518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viardot A, Grey ST, Mackay F, et al. Potential antiinflammatory role of insulin via the preferential polarization of effector T cells toward a T helper 2 phenotype. Endocrinology 2007; 148: 346–353. [DOI] [PubMed] [Google Scholar]
- 36.Stalenhoef JE, Alisjahbana B, Nelwan EJ, et al. The role of interferon-gamma in the increased tuberculosis risk in type 2 diabetes mellitus. Eur J Clin Microbiol Infect Dis 2008; 27: 97–103. [DOI] [PubMed] [Google Scholar]
- 37.Pande T, Huddart S, Xavier W, et al. Prevalence of diabetes mellitus amongst hospitalized tuberculosis patients at an Indian tertiary care center: a descriptive analysis. PLoS One 2018; 13: e0200838–e0200838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patankar T, Prasad S, Laxminarayan R. Diabetes mellitus: an uncommon manifestation of pancreatic tuberculosis. J Assoc Physicians India 1999; 47: 938–939. [PubMed] [Google Scholar]
- 39.Loya AC, Prayaga AK, Sundaram C, et al. Cytologic diagnosis of pancreatic tuberculosis in immunocompetent and immunocompromised patients: a report of 2 cases. Acta Cytol 2005; 49: 97–100. [DOI] [PubMed] [Google Scholar]
- 40.Fles JA. Ein Fall von Diabetes mellitus mit Atrophie der Leber und des Pancreas. Arch f Holländ Beitr z Natur- u Heilkunde 1864; 3: 187–205. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, UEG902353 Supplemental Material for Pancreatic tuberculosis: A systematic review of symptoms, diagnosis and treatment by Nikola Panic, Hartwig Maetzel, Milutin Bulajic, Mihailo Radovanovic and J-Matthias Löhr in United European Gastroenterology Journal


