Abstract
Introduction
Ustekinumab is an effective treatment of Crohn’s disease (CD). Real-world data addressing the efficacy and safety of ustekinumab are scarce.
Aim
Our aim was to assess the safety and efficacy of ustekinumab in a large national patient cohort.
Methods
A prospective multicenter study, in which we followed patients with active CD treated with ustekinumab for 24 weeks. Induction dose was intravenous ranging from 260 to 520 mg, according to body weight, followed by 90 mg doses given subcutaneously every 8 weeks. Clinical response was defined as a reduction of at least 1 severity category, as defined by Harvey–Bradshaw index (HBI). Patients with HBI < 5 were considered to be in clinical remission. Patients who stopped needing steroids at week 24 were defined as being in steroid-free clinical remission.
Results
A total of 106 CD patients from eight Israeli centers were included. All patients were previously exposed to at least one biological agent. Our cohort consisted of 65 (61.3%) females. Mean age was 41 ± 14 years with an average disease duration of 12.2 ± 8 years. A total of 96 (90.5%) patients continued treatment throughout week 24. Clinical response was observed in 52% of these patients with mean HBI reduction from 8.34 ± 3.8 to 6.8 ± 4.4 at week 24 (p = 0.001). Clinical remission was achieved in 33 patients (31.1%). Moreover, the number of patients requiring steroid treatment was reduced by 66% at week 24. Out of 106 patients, 11 patients (10.4%) discontinued treatment: 3 due to adverse events (2.8%), 7 due to a lack of response, and 1 who was lost to follow-up. Following 24 weeks of treatment, 15 patients reported minor adverse events.
Conclusions
In a large real-world Israeli cohort of non-naïve-to-biological-treatment CD patients, ustekinumab was effective and safe in induction of clinical remission with a significant reduction in the number of patients requiring steroid treatment.
Keywords: Crohn’s disease, IBD, gastroenterology, inflammatory bowel disease, inflammation
Key summary
Ustekinumab has been shown to be effective and safe in Crohn’s disease patients in a number of clinical trials.
Nevertheless, real-life experience with ustekinumab is still required.
In our real-life study, we show a 52% clinical response and a 31.1% clinical remission following 24 weeks.
We present a high safety profile of ustekinumab with only 2.8% of patients having minor adverse events.
Introduction
Ustekinumab (Stelara, Janssen Biotech Inc.) is a monoclonal antibody directed against the p40 subunit common to interleukin (IL)-12 and IL-23. Intravenous (IV) ustekinumab was previously demonstrated to be effective in the induction and maintenance of remission in both anti-tumor necrosis factor (TNF) naïve and experienced Crohn’s disease (CD) patients (UNITI trials).1 Moreover, data analysis from three clinical trials showed that ustekinumab increased endoscopic improvement following 8 weeks.2 Recent phase III studies reported subcutaneous (SC) ustekinumab to maintain clinical response and remission for 92 weeks.3 Furthermore, positive response to ustekinumab was recently shown to be maintained for over 152 weeks of treatment.4 Moreover, among anti-TNF treatment failures, ustekinumab seems to be superior in inducing a clinical response, and equally effective in remission induction, compared with adalimumab.5 Overall, ustekinumab maintained a favorable safety profile in all previous studies. Since ustekinumab has been approved for CD patients only in 2017, real-world studies remain limited. To date, several real-world experience studies have been published.6–10 In a recent retrospective real-world British cohort that included 149 patients, 63% response and 39% remission were reported at week 32, along with 50% reduction in C-reactive protein (CRP) levels.11 In another prospective study of ustekinumab induction, an endoscopic remission rate at week 24 of 7.1% was demonstrated with concurrent clinical remission rate of 39.5%.12 A pooled analysis comparing the effectiveness and safety of these studies was recently performed. This comparison suggested that real-life efficacy and safety results with ustekinumab were similar and even somewhat better than those reported in the CERTIFI and UNITI trials.13 In the last few months another three prospective and retrospective studies confirmed the effectiveness of ustekinumab in biological-exposed CD patients, demonstrating good clinical response and steroid-free clinical remission along with reduced levels of inflammation markers.14–16 Nevertheless, there is still much interest in prospective real-world efficacy data. In the current study, we evaluated the efficacy and safety of ustekinumab for induction of remission of active CD in a multicenter Israeli cohort.
Methods
Patients with active CD treated with ustekinumab were prospectively followed up for 24 weeks. Patients discontinuing treatment before week 24 for adverse events (AEs) or primary non-responders were included as well. Induction dose was 260 to 520 mg IV, according to body weight, followed by 90 mg SC every 8 weeks. Clinical response was defined as a drop of at least 1 severity category, as defined by the Harvey–Bradshaw index (HBI). Mild disease was classified as HBI of 5 to < 8, moderate as 8 to < 16, and severe as 16 or more. Clinical remission was defined as HBI < 5. Corticosteroid usage was assessed through week 24 in patients treated with corticosteroids at the beginning of the trial. Steroid-free remission was considered as the percentage of patients who experienced a clinical remission and stopped steroid treatment.
Low serum CRP concentration was categorized into a cutoff of <0.5 mg/L. Active perianal disease was defined as any actively draining perianal fistula. Active extra intestinal manifestation (EIM) was defined as any clinically significant arthralgia, uveitis, or any CD-related skin manifestation. At week 8, data was available for 88 patients. The primary outcome was clinical response at week 24. Secondary outcomes included clinical remission, steroid usage, steroid-free clinical remission at week 24, the effect on EIMs and safety.
Statistical analysis
Patient characteristics are shown as frequency counts for categorical variables. For continuous variables without normal distribution, calculations of medians and interquartile ranges (IQRs) were performed. Differences over time were estimated at three time points: at the beginning of treatment, following 8 weeks and following 24 weeks. Statistical differences between patient’s clinical information at baseline and at 24 weeks were calculated for those who continued throughout the trial period. Categorical variables were compared using Chi-square test or Fisher’s exact test. Continuous variables were analyzed by Wilcoxon signed ranks test or paired samples t-test, according to variable distribution. All tests were two tailed and significance was defined as p-value < 0.05. The data were analyzed using software package for statistics (IBM SPSS version 25).
Ethical consideration
The study was approved on February 2019 by the institutional review board (No. 20/19) of Shaare Zedek Medical Center. Due to the nature of our study, informed consent was not required.
Results
Patient population
A total of 106 CD patients from eight Israeli centers were enrolled in the study. The majority of patients (96/106, 90.6%), continued the treatment throughout week 24. The clinical and demographic characteristics are detailed in Table 1. The cohort included 41 patients (39%) with mild disease activity, 62 patients (58%) with moderate disease activity, and 3 patients (3%) with severe disease. Eleven patients did not complete the 24 week follow-up period and thus were considered as dropouts; however, they were included in the final analysis. Of these patients, three were due to AEs (2.8%), seven were due to lack of response and one patient was lost to follow-up.
Table 1.
Clinical and demographic characteristics of Crohn’s disease patients treated with ustekinumab.
| n (%) | |
|---|---|
| Age (mean ± SD), years | 41 ± 14 |
| Gender | |
| Male | 41 (38.6) |
| Disease duration (mean ± SD), years | 12.2 ± 8 |
| Disease behavior | |
| B1 (inflammatory) | 41 (38) |
| B2 (stricturizing) | 33 (30.5) |
| B3 (penetrating) | 34 (31.5) |
| Disease location | |
| L1 (ileal) | 49 (45.4) |
| L2 (colonic) | 15 (13.8) |
| L3 (ileocolic) | 44 (40.8) |
| Perianal surgery | 7 (6.5) |
| Previous related Crohn’s disease surgery | |
| Ileocolic resection | 19 (18) |
| Hemicolectomy | 10 (9.4) |
| Small intestine resection | 8 (8) |
| Other | 10 (9.4) |
| None | 59 (55.6) |
| Background diseases | 35 (33) |
| Previous conventional therapy | |
| Immunomodulators | 45 (41.7) |
| Concomitant immunomodulators | 29 (26.9) |
| 5-ASA | 23 (21.3) |
| None | 11 (10.1) |
| Previous biological treatment | |
| One anti-TNF agent | 21 (19.4) |
| Two anti-TNF agents | 19 (17.5) |
| Anti-TNF and vedolizumab | 68 (63.0) |
The mean disease duration was 12.2 ± 8 years. A total of 47 patients (44%) had a previous surgery, while 19 patients (18%) previously underwent ileocolic resection. All patients had at least one previous anti-TNF exposure. In total, 80 patients (75%) failed on at least two prior biological treatments (e.g. adalimumab, infliximab or vedolizumab). A total of 49 patients (46%) had additional background diseases, such as psoriasis (7%), rheumatological disorders (4.7%) and metabolic disorders (3.7%).
Efficacy
At week 24, 55/106 patients responded, and 33/106 achieved clinical remission (Figure 1). Of the 37 patients who were treated with steroids at the beginning of the study, 21 (57%) were discontinued treatment, while 4 (11%) achieved a steroid-free remission on week 24. In total, only 16 patients (15%) received corticosteroid treatment at week 24. In addition, mean HBI significantly improved from a baseline score of 8.34 ± 3.8 to 6.9 ± 4.6 at week 8 and to 6.8 ± 4.4 at week 24 (p = 0.001). Similarly, the median HBI score was also decreased from 8 (IQR 6–10) at baseline to 7 (IQR 4–10) at week 24 (p < 0.001). CRP measurements were analyzed in the patients who completed follow-up at week 24. Of these patients, the percentage of patients with low CRP levels was significantly increased from 16% to 29.7% at week 8 and to 32.9% at week 24, suggesting a reduced inflammatory state (p = 0.007, Figure 2(a)).
Figure 1.
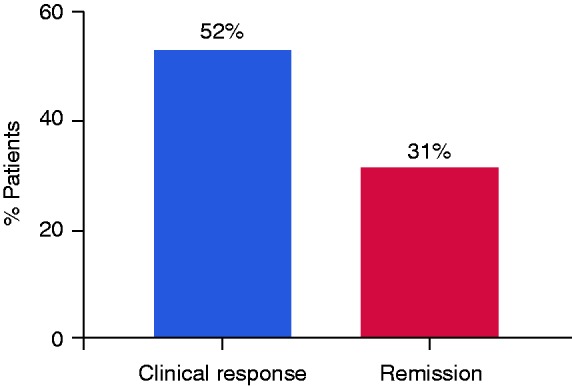
Clinical outcomes of Crohn’s disease patients treated with ustekinumab. Clinical response was defined as a drop reduction of at least 1 severity category by the Harvey–Bradshaw index (HBI). Patients with HBI < 5 were considered to be in clinical remission.
Figure 2.
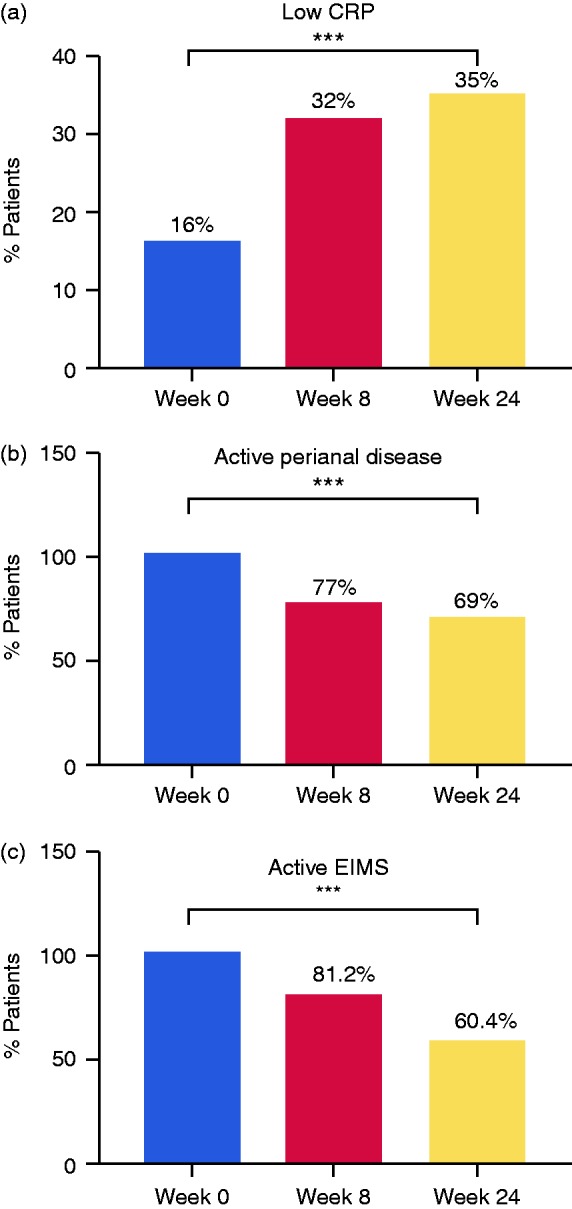
Changes in biochemical and disease-associated activity markers during ustekinumab treatment. (a) Low serum C-reactive protein (CRP) concentration was categorized into a cutoff of < 0.5 mg/L. (b) Active perianal disease was defined as any actively draining perianal fistula. (c) Active extra intestinal manifestation (EIM) was defined as any clinically significant arthralgia, uveitis, or any Crohn’s disease–related skin manifestation. ***p < 0.001.
Furthermore, we found that 14 of the patients in remission achieved a clinical remission (HBI < 5) as well as low CRP levels (42%). Median CRP levels decreased from 1.7 mg/L (IQR 0.65–4.10 mg/L) at baseline to 0.95 mg/L (IQR 0.31–2.93 mg/L) at week 24 (p = 0.005). Interestingly, we did not find any correlation between effectiveness, either by HBI decrease or by percentage of patients with low CRP levels, to prior use of vedolizumab.
Active perianal disease and active EIMs were also evaluated. Active perianal disease was observed in 26 patients at the beginning of the study; 20 patients (18.8%) remained with an active disease at week 8, while 18 patients (16.9%) remained with the perianal disease at week 24, (p < 0.001, Figure 2(b)). Similarly, active EIMs were reported in 48 patients (45.2%) at baseline. This number was reduced to 39 patients at week 8 and to 29 at week 24 (p < 0.001, Figure 2(c)).
Safety
Throughout the study period only three patients (2.8%) discontinued treatment due to AEs. Two patients experienced an exacerbation of arthralgia (one in week 7 and one in week 23). The third patient ceased therapy due to skin eruption and cough.
Altogether, 15 patients experienced AEs, most of them minor. At 8 weeks of treatment, eight AEs were reported and an additional seven AEs were reported by 24 weeks. All AEs are summarized in Table 2.
Table 2.
Adverse events in Crohn’s disease patients treated with ustekinumab.
| Adverse events | Week 8 (n) | Week 24 (n) |
|---|---|---|
| Headache | 0 | 1 |
| Weakness | 1 | 0 |
| Skin eruption | 2 | 1 |
| Abdominal pain | 0 | 1 |
| Dizziness | 1 | 1 |
| Arthralgia | 3 | 1 |
| Flu-like symptoms | 1 | 0 |
| Weight gain | 0 | 1 |
| Fever | 0 | 1 |
Discussion
Ustekinumab was first approved in Israel during 2017 as a third-line treatment for CD, and since 2018 as a second-line therapy after failure of at least one anti-TNF agent. In our national real-world cohort of Israeli CD patients, ustekinumab was effective for induction of clinical response, remission and steroid-free remission following weeks 24 of treatment. According to treatment guidelines, all patients were previously exposed to at least one biological agent prior to ustekinumab. After 24 weeks of treatment, clinical response was achieved in the majority of patients, while clinical remission was achieved in about one-third of these patients. Of the patients receiving steroid treatment, 57% achieved a steroid-free regimen, although the number of patients was quite limited. Noticeably, the therapeutic effect was already achieved by week 8, and was maintained throughout week 24, emphasizing the expected low rates of a secondary loss of response to ustekinumab. Generally, our results are in line with previously published real-world studies.7–9,17–20 However, there are variabilities in trial periods, the definition of primary outcomes and other parameters, such as clinical response. Very recently, three new real-world studies showed high effectiveness in anti-TNF/vedolizumab-exposed CD patients. In the Spanish ENEIDA registry, clinical remission was achieved at weeks 8 and 14 by 47% and 58% of patients, respectively, with 48% of patients having steroid-free remission at week 14. Also in this study, previous exposure to vedolizumab did not interfere with the response to the drug.15 In the ICC prospective multicenter study, which included 21 patients, steroid-free remission rates at weeks 24 and 52 reached 38.2% and 37.1%, respectively. Furthermore, after 24 weeks, 36% of patients achieved clinical remission of perianal fistulas.14 In another observational, retrospective multicenter study, after 1 year 42.1% and 25.7% of patients experienced clinical response and clinical remission, respectively, and 38.8% and 24.3% had achieved steroid-free clinical response and remission, respectively.16 In the McGill cohort, SC ustekinumab was given to 38 anti-TNF failures and initial clinical response was achieved among 74% of patients. This response was maintained in most patients for up to 12 months.17 A large real-world study with ustekinumab by Ma et al. included 167 CD patients, with clinical response defined as a decrease in HBI by ≥ 3 compared with baseline, with complete steroid tapering. The group reported 39% responders, while 60.3% of patients achieved a clinical response at both 3 and 6 months.9 In a Spanish multicenter open labeled study, 116 patients with refractory CD were included. Clinical response was achieved in 84% of patients and the clinical benefit, defined as clinical improvement that included both remission and response, after 6 weeks, 12 weeks and at the end of the follow-up, was 76%, 64% and 58%, respectively.19 In a retrospective chart review on patients with refractory CD, 46% reached clinical response, 35% achieved clinical remission, 76% demonstrated endoscopic response and 24% achieved complete endoscopic remission while treated with ustekinumab.18 In another retrospective observational study, SC ustekinumab induced response in nearly 66% of anti-TNF failures after 3 months. This clinical benefit was associated with biologic and endoscopic response.6 However, in another study that investigated associations between concentrations of ustekinumab and endoscopic outcomes among CD patients, 80.7% of patients at week 26 had clinical response, 66.1% had clinical remission and 50% had steroid-free remission. It was found that higher concentrations of ustekinumab were correlated with higher endoscopic score and with lower CRP levels.21 Finally in a recent retrospective study in which 57 CD patients were included, 35.1% achieved steroid-free clinical remission, 10.5% achieved steroid-free response and 54.4% were non-responders.22 Thus, the rates of clinical response and clinical remission in our study are in line with the ones described in the above studies and are in accordance with the recently published pooled analysis by Engel and colleagues.13 With respect to AEs, the safety in our study was rather good. Only 3 patients stopped treatment due to AEs and, overall, the AEs that were reported by another 15 patients were minor. Sandborn et al. assessed the safety parameters up to 36 weeks and the rate of AEs was similar between the ustekinumab and the placebo group.23 In addition, ustekinumab has not been fundamentally evaluated in perianal CD. In our study, about 30% reduction in patients with perianal disease by week 24 was reported. These promising significant findings should be further examined in order to assess complete fistula healing. Unfortunately, fecal calprotectin measurements and regular endoscopic evaluations were available for only a very small number of patients and therefore were not analyzed. Other limitations include low power for steroid-free remission and missing CRP results and clinical scores for some time points.
In conclusion, our results support short-term efficacy and safety of ustekinumab in real-world practice. In our study, we show a favorable safety profile of ustekinumab that is consistent with previously published studies. Long-term effects in refractory CD patients should be further evaluated.
Acknowledgements
We thank Rivka Farkash and Gali Epstein for their assistance in analyzing the data and Aviya Hoyda for data management.
Declaration of conflicting interests
AB-GS reports consulting/advisory fees from Takeda, Janssen, Neopharm, Pfizer and AbbVie; speaker/teaching fees from Takeda, Janssen, AbbVie, Neopharm and Rafa Laboratories; research support from Takeda and Janssen. MW reports consulting fees from AbbVie, Janssen, Takeda, Pfizer, Medtronic and Neopharm; speaker fees from AbbVie, Janssen and Takeda. DS reports consulting/advisory fees from Takeda, Janssen, AbbVie, Check-Cap, Neopharm, Pfizer and Medigus; speaker fees from Takeda, Janssen and AbbVie. EZ reports research support and consulting fees from Janssen, AbbVie, Takeda, Neopharm and Pfizer. YC reports grant support from AbbVie, Takeda, Janssen, Medtronic and Protalix; speaker fees from AbbVie, Takeda, Janssen and Ferring. HY reports consulting/speaker fees from AbbVie, Janssen, Takeda, Pfizer, Falk Pharma, Neopharm and Given Imaging. NM reports research support from Takeda and Janssen; consulting fees from Janssen, Pfizer, Neopharm and BiomX; speaker fees from AbbVie, Pfizer and Takeda. SB-H reports research support from Takeda, AbbVie, Celltrion, Pfizer and Janssen; consulting fees from Janssen, Takeda, AbbVie, Celltrion, SBH, GSK, Ferring and Pfizer. RE reports consulting/speaker fees from AbbVie, Takeda, Janssen and Medtronic. ID reports research support from Pfizer and Altman Research; consulting/advisory fees from Takeda, Janssen, AbbVie, Celltrion, Arena Pharmaceuticals, Neopharm, Roche/Genentech, Pfizer, Medtronic, Gilead, Ferring, Rafa Laboratories, Given Imaging, MSD, Protalix and Sublimity Therapeutics; speaker/teaching fees from Takeda, Janssen, AbbVie, Ferring, Falk Pharma, Given Imaging, Roche/Genentech, Pfizer, Celltrion and MSD. EG reports consulting fees from Janssen, Takeda, AbbVie and Digma Medical. UK reports research support from Takeda, Janssen and Medtronic; speaker/advisory fees from AbbVie, Takeda, Janssen, MSD, Falk Pharma and Medtronic. The remaining authors have no conflicting interests to declare.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Ethics approval
The study was approved on February 2019 by the institutional review board (No. 20/19) of Shaare Zedek Medical Center.
Informed consent
Due to the nature of the study, informed consent was not required.
References
- 1.Feagan BG, Sandborn WJ, Gasink C, et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2016; 375: 1946–1960. [DOI] [PubMed] [Google Scholar]
- 2.Rutgeerts P, Gasink C, Chan D, et al. Efficacy of ustekinumab for inducing endoscopic healing in patients with Crohn’s disease. Gastroenterology 2018; 155: 1045–1058. [DOI] [PubMed] [Google Scholar]
- 3.Sandborn WJ, Rutgeerts P, Gasink C, et al. Long-term efficacy and safety of ustekinumab for Crohn’s disease through the second year of therapy. Aliment Pharmacol Ther 2018; 48: 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanauer SB, Sandborn WJ, Feagan BG, et al. IM-UNITI: 3 year efficacy, safety, and immunogenicity of ustekinumab treatment of Crohn’s disease. J Crohns Colitis 2020; 14: 23–32. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed Z, Venkata K, Zhang N, et al. Comparative effectiveness of ustekinumab versus adalimumab in induction of clinical response and remission in Crohn’s disease: experience of a real-world cohort at a tertiary care inflammatory bowel disease referral center. Gastroenterology Res 2019; 12: 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wils P, Bouhnik Y, Michetti P, et al. Subcutaneous ustekinumab provides clinical benefit for two-thirds of patients with Crohn’s disease refractory to anti-tumor necrosis factor agents. Clin Gastroenterol Hepatol 2016; 14: 242–250.e241–242. [DOI] [PubMed] [Google Scholar]
- 7.Greenup AJ, Rosenfeld G, Bressler B. Ustekinumab use in Crohn’s disease: a Canadian tertiary care centre experience. Scand J Gastroenterol 2017; 52: 1354–1359. [DOI] [PubMed] [Google Scholar]
- 8.Ma C, Fedorak RN, Kaplan GG, et al. Long-term maintenance of clinical, endoscopic, and radiographic response to ustekinumab in moderate-to-severe Crohn’s disease: real-world experience from a multicenter cohort study. Inflamm Bowel Dis 2017; 23: 833–839. [DOI] [PubMed] [Google Scholar]
- 9.Ma C, Fedorak RN, Kaplan GG, et al. Clinical, endoscopic and radiographic outcomes with ustekinumab in medically-refractory Crohn’s disease: real world experience from a multicentre cohort. Aliment Pharmacol Ther 2017; 45: 1232–1243. [DOI] [PubMed] [Google Scholar]
- 10.Wils P, Bouhnik Y, Michetti P, et al. Long-term efficacy and safety of ustekinumab in 122 refractory Crohn’s disease patients: a multicentre experience. Aliment Pharmacol Ther 2018; 47: 588–595. [DOI] [PubMed] [Google Scholar]
- 11.Gadhok R, Rao R, Honap S, et al. P318 ustekinumab: early experience and medium-term outcomes from a UK multi-centre real-world cohort. J Crohns Colitis 2019; 13: S260–S260. [Google Scholar]
- 12.Verstockt B, Dreesen E, Noman M, et al. Ustekinumab exposure-outcome analysis in Crohn’s disease only in part explains limited endoscopic remission rates. J Crohns Colitis 2019; 13: 864–872. [DOI] [PubMed] [Google Scholar]
- 13.Engel T, Yung DE, Ma C, et al. Effectiveness and safety of ustekinumab for Crohn’s disease; systematic review and pooled analysis of real-world evidence. Dig Liver Dis 2019; 51: 1232–1240. [DOI] [PubMed] [Google Scholar]
- 14.Biemans VBC, van der Meulen-de Jong AE, van der Woude CJ, et al. Ustekinumab for Crohn’s disease: results of the ICC registry, a nationwide prospective observational cohort study. J Crohns Colitis 2020; 14: 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iborra M, Beltran B, Fernandez-Clotet A, et al. Real-world short-term effectiveness of ustekinumab in 305 patients with Crohn’s disease: results from the ENEIDA registry. Aliment Pharmacol Ther 2019; 50: 278–288. [DOI] [PubMed] [Google Scholar]
- 16.Liefferinckx C, Verstockt B, Gils A, et al. Long-term clinical effectiveness of ustekinumab in patients with Crohn’s disease who failed biologic therapies: a national cohort study. J Crohns Colitis 2019; 13: 1401–1409. [DOI] [PubMed] [Google Scholar]
- 17.Kopylov U, Afif W, Cohen A, et al. Subcutaneous ustekinumab for the treatment of anti-TNF resistant Crohn’s disease–the McGill experience. J Crohns Colitis 2014; 8: 1516–1522. [DOI] [PubMed] [Google Scholar]
- 18.Harris KA, Horst S, Gadani A, et al. Patients with refractory Crohn’s disease successfully treated with ustekinumab. Inflamm Bowel Dis 2016; 22: 397–401. [DOI] [PubMed] [Google Scholar]
- 19.Khorrami S, Ginard D, Marin-Jimenez I, et al. Ustekinumab for the treatment of refractory Crohn’s disease: the Spanish experience in a large multicentre open-label cohort. Inflamm Bowel Dis 2016; 22: 1662–1669. [DOI] [PubMed] [Google Scholar]
- 20.Lightner AL, McKenna NP, Tse CS, et al. Postoperative outcomes in ustekinumab-treated patients undergoing abdominal operations for Crohn’s disease. J Crohns Colitis 2018; 12: 402–407. [DOI] [PubMed] [Google Scholar]
- 21.Battat R, Kopylov U, Bessissow T, et al. Association between ustekinumab trough concentrations and clinical, biomarker, and endoscopic outcomes in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2017; 15: 1427–1434.e2. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann P, Krisam J, Wehling C, et al. Ustekinumab: “Real-world” outcomes and potential predictors of nonresponse in treatment-refractory Crohn’s disease. World J Gastroenterol 2019; 25: 4481–4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sandborn WJ, Gasink C, Gao LL, et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med 2012; 367: 1519–1528. [DOI] [PubMed] [Google Scholar]


