Abstract
Chronic mesenteric ischaemia is a severe and incapacitating disease, causing complaints of post-prandial pain, fear of eating and weight loss. Even though chronic mesenteric ischaemia may progress to acute mesenteric ischaemia, chronic mesenteric ischaemia remains an underappreciated and undertreated disease entity. Probable explanations are the lack of knowledge and awareness among physicians and the lack of a gold standard diagnostic test. The underappreciation of this disease results in diagnostic delays, underdiagnosis and undertreating of patients with chronic mesenteric ischaemia, potentially resulting in fatal acute mesenteric ischaemia. This guideline provides a comprehensive overview and repository of the current evidence and multidisciplinary expert agreement on pertinent issues regarding diagnosis and treatment, and provides guidance in the multidisciplinary field of chronic mesenteric ischaemia.
Keywords: Median arcuate ligament syndrome, atherosclerosis, mesenteric arteries, mesenteric artery stenting, coeliac artery release
Introduction
Chronic mesenteric ischaemia is a severe and incapacitating disease, causing complaints of post-prandial pain, fear of eating and weight loss. Chronic mesenteric ischaemia can progress to acute mesenteric ischaemia (AMI), a much dreaded and often lethal complication. Nevertheless, chronic mesenteric ischaemia remains an underappreciated, underdiagnosed and undertreated disease entity, mainly due to lack of knowledge and awareness among physicians. The increased incidence of cardiovascular disease in the elderly population and the rise in the prevalence of obesity and diabetes mellitus is likely to contribute to an increasing incidence of chronic mesenteric ischaemia. Although weight loss is still a consistent finding in patients with chronic mesenteric ischaemia, modern, faster diagnostic workup compared to the pre-computed tomography (CT) era has lowered the proportion of chronic mesenteric ischaemia patients who are underweight at diagnosis.1,2 Some patients may still be overweight at diagnosis, while others will have a normal body mass index (BMI) at diagnosis but were overweight at the onset of symptoms, which usually precede diagnosis by at least six months.3 Hence, the misconception that patients with chronic mesenteric ischaemia are all cachectic, as stated in older textbooks, is a diagnostic pitfall leading to further diagnostic delay and no longer applies in the current clinical context.
Even though criteria and recommendations for mesenteric ischaemia have been formulated by radiology, interventional radiology and vascular surgery societies, a multidisciplinary guideline covering the full multidisciplinary spectrum of chronic mesenteric ischaemia and suitable to the needs of all physicians involved in the care for chronic mesenteric ischaemia patients is urgently needed.4–6
United European Gastroenterology (UEG) acknowledged the need for a multidisciplinary guideline by supporting this guideline with a UEG Activity Grant. Other organizations including European Association for Gastroenterology, Endoscopy and Nutrition (EAGEN), European Society of Gastrointestinal and Abdominal Radiology (ESGAR), Cardiovascular and Interventional Radiological Society of Europe (CIRSE), Dutch Mesenteric Ischemia Study group (DMIS) and national societies such as Netherlands Association of Hepatogastroenterologists (NVMDL) and Hellenic Society of Gastroenterology (HSG) also recognised the need for a multidisciplinary guideline. We therefore jointly aimed to develop a guideline that provides a comprehensive overview and repository of current evidence and expert agreement, and offers guidance to physicians involved in the multidisciplinary field of gastrointestinal (GI) diseases.
Methodology
This multidisciplinary clinical consensus guideline consists of recommendations for the management of chronic mesenteric ischaemia. The grading of recommendations assessment development and evaluation (GRADE) method was used to assess the quality of evidence and indicate the strength of recommendation (Table 1).7 The multidisciplinary European expert panel was composed of experts publishing on chronic mesenteric ischaemia within the last 10 years and experts recommended by supporting organizations (CIRSE, EAGEN, ESGAR, DMIS, HSG and NVMDL). The expert panel comprised six gastroenterologists, seven radiologists (six interventional, one diagnostic), eight vascular surgeons, a physiologist and an angiologist.
Table 1.
Explanation of definitions of grading of recommendations assessment development and evaluation (GRADE) score used by the GRADE method.
| GRADE | Explanation | Definition strength of recommendation | Definition quality of evidence |
|---|---|---|---|
| 1A | Strong recommendation High quality of evidence |
Benefits clearly outweigh risks and burdens, or vice versa | Further research is very unlikely to change our confidence in the estimate of effect |
| 1B | Strong recommendation Moderate quality of evidence |
Benefits clearly outweigh risks and burdens, or vice versa | Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate |
| 1C | Strong recommendation Low quality of evidence |
Benefits clearly outweigh risks and burdens, or vice versa | Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate |
| 1D | Strong recommendation Very low quality of evidence |
Benefits clearly outweigh risks and burdens, or vice versa | Any estimate of effect is very uncertain |
| 2A | Weak recommendation High quality of evidence |
Trade-offs between benefits and risks and burdens are closely balanced | Further research is very unlikely to change our confidence in the estimate of effect |
| 2B | Weak recommendation Moderate quality of evidence |
Trade-offs between benefits and risks and burdens are closely balanced | Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate |
| 2C | Weak recommendation Low quality of evidence |
Trade-offs between benefits and risks and burdens are closely balanced | Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate |
| 2D | Weak recommendation Very low quality of evidence |
Trade-offs between benefits and risks and burdens are closely balanced | Any estimate of effect is very uncertain |
Formulation of draft recommendations was based on an overview of the evidence – answering predefined research questions – and current clinical practice. The modified Delphi method was used to improve recommendations and to reach consensus.8 A total of three anonymous voting rounds were held, the first round during a plenary expert panel meeting, while the second and third rounds consisted of an online survey. Experts voted by rating their agreement with a recommendation on a nine-point Likert scale, where ‘1’ meant complete disagreement and ‘9’ meant complete agreement. Consensus was reached when ≥70% scored 7–9 and ≤15% scored 1–3.9 A recommendation was excluded when ≥70% scored 1–3 and ≤15% scored 7–9 or when consensus was not reached after the third voting round. A detailed description of the methods used to develop this guideline and an overview of the results of the voting process can be found in Supplementary Material Document A.
Results of the systematic literature review and modified Delphi method
Arterial anatomy and pathophysiology
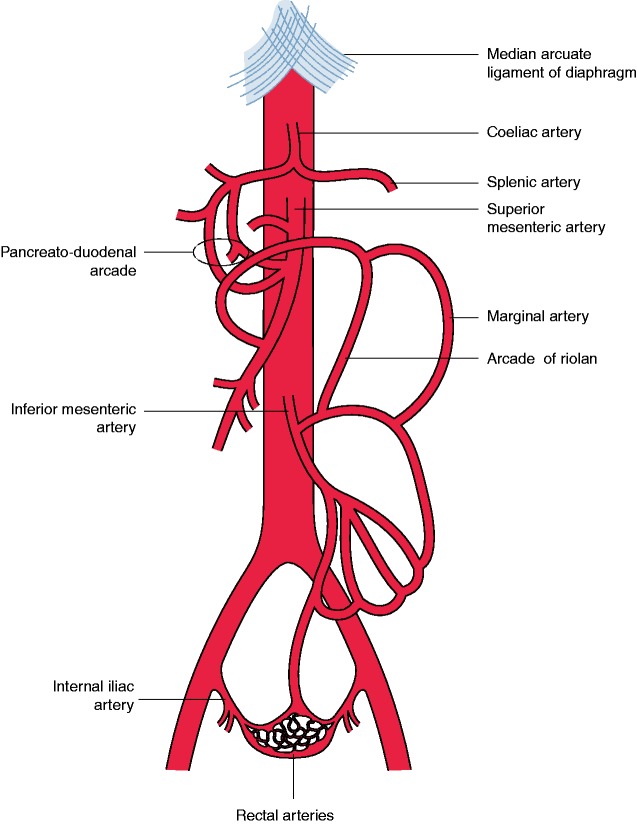
The arterial mesenteric circulation is provided by three major abdominal aortic branches, from cranial to caudal: coeliac artery (CA), superior mesenteric artery (SMA) and inferior mesenteric artery (IMA) (Figure 1). The CA supplies blood to the liver, spleen, pancreas, stomach, duodenal bulb and the descending duodenum proximal to the major papilla.10 The SMA provides blood to the duodenum distal to the major papilla, jejunum, ileum, ascending colon and proximal two-thirds of the transverse colon. The IMA distributes blood to the distal one-third of the transverse colon, descending colon, sigmoid and rectum. An extensive collateral network connecting the CA, SMA and IMA guarantees blood supply and protects the gut against ischaemia. The mesenteric circulation is characterised by a large variety in local anatomy. The most frequently observed and most important collaterals are discussed below. The CA and SMA are connected by the pancreato-duodenal arcade. The superior part of this arcade is formed by the gastroduodenal artery – originating from the common hepatic branch of the CA – and divides in an anterior and posterior superior pancreatoduodenal artery.11 The connecting anterior and posterior branches of the inferior pancreatoduodenal artery originate from the SMA. Two possible collaterals connect the SMA and IMA. The marginal artery connects the middle colic artery of the SMA with the ascending branch of the left colic artery of the IMA.12 The arcade of Riolan connects the SMA and IMA with a more medial course and does not run in the proximity of the colon. However, a consistent presence of this centrally communicating collateral artery has been disputed.13 The IMA and internal iliac arteries are connected by anastomoses of the superior and inferior rectal arteries.
Figure 1.
Anatomy of the arterial mesenteric circulation.
Mesenteric blood flow increases by up to 30–150% after a meal, as oxygen demand increases significantly during digestion.14 Patients with earlier stages of chronic mesenteric ischaemia frequently report symptoms after eating, symptoms that can be explained by the increased oxygen demand exceeding the supply of oxygenated blood after a meal. Patients with more advanced stages of chronic mesenteric ischaemia usually experience permanent abdominal symptoms, which are aggravated by eating, because even pre-prandial blood supply is insufficient.
Prevalence and aetiology
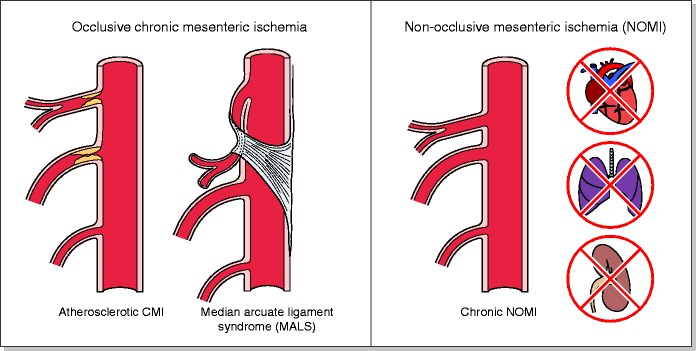
Chronic mesenteric ischaemia is caused by either occlusive mesenteric ischaemia or non-occlusive mesenteric ischaemia (NOMI) (Figure 2). The prevalence of both occlusive and non-occlusive ischaemia is currently unknown, but chronic mesenteric ischaemia may not be as rare as frequently stated in older literature. Mesenteric artery stenosis is a frequent finding, with a reported prevalence in post-mortem and duplex ultrasound studies of 6–29% and may be as high as 67% in persons aged 80 years or older.14–21 Nevertheless, only a minority of patients with a mesenteric artery stenosis develop chronic mesenteric ischaemia, since the gut is protected against ischaemia by the abundant collateral circulation.22,23
Figure 2.
The most frequent causes of occlusive chronic mesenteric ischaemia (CMI) and chronic non-occlusive mesenteric ischaemia (NOMI).
MALS: median arcuate ligament syndrome.
Occlusive chronic mesenteric ischaemia can be caused by atherosclerosis, median arcuate ligament syndrome (MALS), vasculitis or by mesenteric venous thrombosis (MVT). Atherosclerosis is the most common cause of occlusive chronic mesenteric ischaemia and is most frequently seen in females (65–72%).1,14 The cause of this female predisposition is unknown, but awareness is important to avoid diagnostic delays in female patients. Risk factors associated with atherosclerotic chronic mesenteric ischaemia are smoking, hypertension, diabetes, hypercholesterolemia and a (family) history of cardiovascular disease.1,3,24–26
MALS, previously known as Dunbar syndrome or coeliac artery compression syndrome, is a rare and controversial cause of occlusive chronic mesenteric ischaemia. MALS is defined as a symptomatic eccentric compression of the CA by the median arcuate ligament (MAL), which is a fibrous arch uniting the diaphragmatic crura (Figure 3). The exact prevalence of MALS is unknown, but compression of the CA by the MAL is present in 3.4–7.3% of asymptomatic patients in whom imaging is performed for other indications.20,27–29 Compression of the CA by the MAL is frequently observed in individuals with a high origin of the CA from the aorta or a lower insertion of the MAL. Since the position of the diaphragm and thus the MAL changes during respiration, the severity of CA compression varies. Compression is most severe during maximal expiration. MALS is more prevalent in young women (four female: one male) and in patients with a low BMI.30
Figure 3.
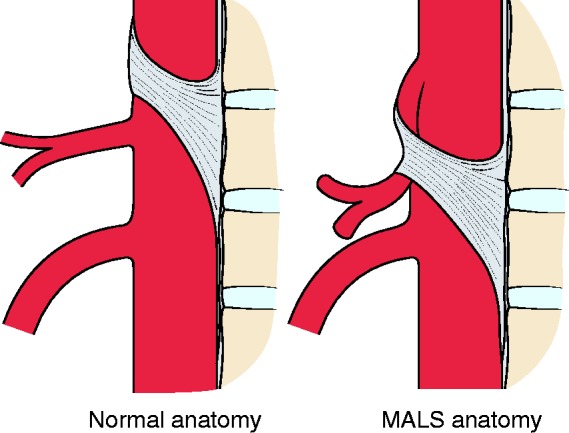
Celiac artery compression in median arcuate ligament syndrome (MALS).
Vasculitis is a rare cause of occlusive chronic mesenteric ischaemia, though it should be considered in chronic mesenteric ischaemia patients because a different therapeutic approach is needed. Involvement of the mesenteric arteries and consequent chronic mesenteric ischaemia are most frequently seen in patients with polyarteritis nodosa, immunoglobulin (Ig)A vasculitis and Takayasu arteritis.31 Mesenteric artery involvement is less frequently reported in rheumatoid arthritis-associated vasculitis, anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis, systemic lupus erythematosus, Behçet’s disease and giant cell arteritis.32,33
MVT can cause AMI due to an outflow obstruction, thereby compromising the mesenteric circulation. Chronic mesenteric ischaemia caused by MVT is rare, since venous collaterals usually form rapidly, but chronic mesenteric ischaemia can occur in patients with chronic MVT.34,35 Due to the rarity of MVT as a cause of chronic mesenteric ischaemia it is not discussed in detail in this guideline and the reader is referred to guidelines by Björck et al. and Kearon et al.6,36
Chronic NOMI was recognised as a disease entity after the introduction of functional tests; by detecting mucosal ischaemia in patients with typical symptoms in the absence of mesenteric artery stenoses or occlusions. The exact pathophysiology of chronic NOMI is unknown, although insufficient mesenteric (micro) circulation seems the most probable explanation. Chronic NOMI is characterised by low-grade ischaemia and is associated with cardiac forward failure, pulmonary hypertension, severe chronic obstructive pulmonary disease, vasospasms of the mesenteric arteries, low-flow states (e.g. patients with chronic kidney disease on dialysis) and severe anaemia.14,24,37 Prevalence of chronic NOMI is unknown, but cohort studies – performed in expert centres using functional testing as an integral part of the diagnostic trajectory – report chronic NOMI in 13–29% of chronic mesenteric ischaemia patients.14,24 Similar to other aetiological causes of chronic mesenteric ischaemia, chronic NOMI patients experience gradually increasing symptoms, such as postprandial pain, fear of eating, weight loss, nausea and diarrhoea. Acute NOMI, in contrast, is characterised by more severe ischaemia, which can result in transmural ischaemia and bowel perforation due to intestinal infarction.6,38 Patients with acute NOMI often present with a more sudden onset of abdominal pain, abdominal distension and, in the advanced stage, signs of peritoneal irritation.6,38 Acute NOMI is generally seen in critically ill patients, for example due to massive burns or severe sepsis with a need for vasopressor therapy, after cardiac surgery, severe cardiac failure with a need for massive inotropic support or hypotension during or following renal replacement therapy.6
Clinical presentation
Abdominal pain with postprandial worsening, starting 10–30 min after a meal and lasting 1–2 h, is a typical presenting symptom in chronic mesenteric ischaemia patients (74–100%).1,3,24,26,39 To avoid postprandial pain, 90% of patients adapt their eating pattern by eating smaller portions. Food avoidance occurs in more advanced stages and is caused by fear of eating, even though patients have a normal feeling of hunger.3 Weight loss is frequently reported (61–94%) and since weight loss is the most alarming feature, further examination should be performed in all patients presenting with this symptom.1,3,24,26,39 Other possible complaints are diarrhoea (19–61%), nausea (5–84%), and worsening of abdominal pain during exercise (43–76%).1,3,24,26,39 Physical examination reveals an abdominal bruit in 17–87% of patients, but can be completely normal.26,39
The ‘classic triad’ of chronic mesenteric ischaemia, consisting of postprandial pain, weight loss and an abdominal bruit, is found in only a minority of chronic mesenteric ischaemia patients (22%).39 Even when present, the predictive value of the classic triad is limited. Studies have shown a 60% probability of chronic mesenteric ischaemia when the classic triad is present, with an area under the curve of 0.62.3,40
Chronic mesenteric ischaemia patients are at risk of developing acute-on-chronic mesenteric ischaemia, e.g. in cases with a ruptured atherosclerotic plaque. Acute-on-chronic mesenteric ischaemia should be considered when the abdominal symptoms of a chronic mesenteric ischaemia patient progress, since urgent revascularization might be needed in these patients.6
| GRADE | Expert agreement | |
|---|---|---|
| Recommendation 1 | ||
| Chronic mesenteric ischaemia should be considered in patients with unexplained postprandial abdominal pain, weight loss (>5% body weight), adapted eating pattern (to avoid abdominal complaints) or diarrhoea. | 1C | 100% |
| Recommendation 2 | ||
| The absence of the classical triad of chronic mesenteric ischaemia (i.e. postprandial pain, weight loss and abdominal bruit) does not exclude a diagnosis of chronic mesenteric ischaemia. | 1C | 91% |
Diagnostic criteria
Treatment decisions are based on diagnostic criteria. The gold standard diagnosis of chronic mesenteric ischaemia used in the literature is relief of symptoms after revascularization. Chronic mesenteric ischaemia, however, remains a diagnostic challenge with a wide differential diagnosis including chronic pancreatitis, coeliac disease, duodenal ulcers, abdominal malignancies and irritable bowel syndrome. Exclusion of alternative diagnoses should be performed, preferably by a gastroenterologist. The expert panel recommends performing at least an upper GI endoscopy and abdominal imaging (CT scan/magnetic resonance imaging (MRI) scan). Chronic pancreatitis should be considered in all patients, because presenting symptoms and risk factors (e.g. smoking, hypertriglyceridaemia) of chronic pancreatitis are similar to presenting symptoms and risk factors of chronic mesenteric ischaemia. The diagnostic work-up of chronic pancreatitis can be found in the chronic pancreatitis guideline by Dominguez-Munoz et al.41 Coeliac disease should be considered and ruled out in all patients presenting with weight loss, especially since diagnostic tests are relatively easy to perform. Colonoscopy is considered mandatory in patients with diarrhoea – to exclude colorectal carcinoma and other ileal and colonic causes of diarrhoea – and should be considered in elderly patients with symptoms indicating colonic disease or ischaemia. Colonoscopy seems to be of limited value in patients with upper GI symptoms.
Patients suspected of having chronic mesenteric ischaemia should be discussed by a multidisciplinary expert panel (consisting of at least a gastroenterologist, (interventional) radiologist and vascular surgeon) to evaluate the compatibility of history, presence or absence of significant mesenteric artery stenosis on imaging, absence of an alternative diagnosis and, when available, results of a functional test.
Considering the number of stenotic or occluded mesenteric arteries is important when making treatment decisions. The SMA is often regarded as the most important mesenteric artery in the framework of chronic mesenteric ischaemia, since blood flow to the GI tract is predominantly provided by the SMA.14 However, no consistent evidence is available describing higher clinical success rates after revascularization of single-vessel SMA stenosis compared to single-vessel CA stenosis.42 The IMA is considered the least important mesenteric artery and single-vessel stenosis of the IMA is deemed clinically trivial, which is supported by results of endovascular abdominal aortic aneurysm repairs.14 Occlusion of the IMA during endovascular aneurysm repair rarely results in mesenteric ischaemia when the CA and SMA are patent. The probability of chronic mesenteric ischaemia and clinical success of revascularization is higher in patients with multivessel disease than in patients with single-vessel disease. Clinical success of revascularization ranges from 90–100% in patients with involvement of two or three mesenteric arteries.39,43,44 Hence, the expert panel considers chronic mesenteric ischaemia likely in patients with unexplained abdominal symptoms and significant stenoses in both CA and SMA. Further functional testing is not required for the presumptive diagnosis of chronic mesenteric ischaemia in these patients.
A more careful approach is warranted in single-vessel disease. Two small prospective cohort studies consisting of 37 and 50 patients, respectively, reported clinical success in 73–76% of symptomatic patients with single-vessel disease of either CA or SMA, despite a full workup including a functional test and discussion in a multidisciplinary team.39,42 When a functional test is unavailable, the expert panel suggests that the presence of a compatible history, i.e. postprandial abdominal pain and either weight loss or an adapted eating pattern, is essential for the presumptive diagnosis of chronic mesenteric ischaemia in patients with single-vessel disease.
A presumptive diagnosis of chronic NOMI is suggested by a combination of compatible symptoms and preferably a positive functional test, in the absence of significant mesenteric artery stenoses. Very low quality single-centre cohort studies have reported improvement of symptoms in 56–63% of patients with a presumptive diagnosis of chronic NOMI treated with a vasodilating agent.14,24 Since chronic NOMI is often associated with haemodialysis, severe cardiac or severe pulmonary disease, treatment of these patients should preferably be discussed in a multidisciplinary setting in order to address the underlying condition.
| GRADE | Expert agreement | |
|---|---|---|
| Recommendation 3 | ||
| To exclude alternative diagnoses at least the following diagnostic tests must be performed: upper gastrointestinal endoscopy and abdominal imaging (CT scan/MRI scan). Depending on age and symptoms colonoscopy should be considered, but is mandatory in patients with diarrhoea. | 1D | 91% |
| Recommendation 4 | ||
| A presumptive diagnosis of occlusive chronic mesenteric ischaemia is based on a combination of compatible history, significant mesenteric artery stenosis on radiological imaging and, preferably, a positive functional test. Results should be discussed in an expert multidisciplinary setting by at least a gastroenterologist, vascular surgeon and (interventional) radiologist. | 1C | 78% |
| Recommendation 5 | ||
| In patients with unexplained abdominal symptoms and significant stenoses of the CA and SMA, the probability of chronic mesenteric ischaemia is high and, consequently, a functional test is not required. | 1B | 87% |
| Recommendation 6 | ||
| For the presumptive diagnosis of chronic mesenteric ischaemia in patients with single-vessel stenosis of CA or SMA, after proper exclusion of alternative diagnoses and no available functional test, the following symptoms should be present: postprandial abdominal pain and either weight loss (>5% body weight) or an adapted eating pattern. | 2D | 91% |
| Recommendation 7 | ||
| (a) A presumptive diagnosis of chronic NOMI is based on a combination of compatible symptoms, absence of significant mesenteric artery stenoses and, preferably, a positive functional test.(b) In presumptive chronic NOMI patients with severe cardiac disease, pulmonary disease or in dialysis patients, underlying causes and treatment should be discussed with the respective specialists. | 2D | 87% |
Diagnostic modalities
Defining haemodynamically significant mesenteric artery stenosis
Imaging is an essential part of the work-up of patients suspected of having chronic mesenteric ischaemia. The main purposes are detection of mesenteric artery stenoses or occlusions and exclusion of alternative diagnoses. The definition of a significant or clinically relevant mesenteric artery stenosis is still under debate. A reduction of luminal diameter in the range of 50–75% is used to define a significant stenosis, but comparative data on the clinical success of treatment in varying degrees of stenoses is not available39,42,45–50 The type and number of affected vessels and consequences of overtreatment versus undertreatment are important when setting the definitions for mesenteric artery stenoses.
In patients with single-vessel disease the probability of mistakenly omitting treatment is thought to be low. By contrast, the probability of overtreating patients without true chronic mesenteric ischaemia is substantial and may expose these patients to complications, potentially without any compensating benefits from treatment. Therefore, a ≥70% stenosis of either CA or SMA could be considered relevant in presumed symptomatic single-vessel disease. In patients with more than one stenotic or occluded artery, unsuccessful treatment is relatively infrequent, but the burden and possible consequences of mistakenly denying treatment are substantial (e.g. development of AMI, poor quality of life, etc.). Comparing the surface area and blood flow volumes of the SMA and CA, it is apparent that the surface area and postprandial flow of the SMA are both approximately 33% higher.14,51 Therefore, a SMA stenosis of 50% is more likely to become haemodynamically relevant, especially when part of the blood volume is diverted through the collateral circulation to provide blood to other stenotic mesenteric arteries. The expert panel suggests that a ≥50% stenosis of the SMA could be considered relevant in symptomatic patients with extensive multivessel disease.
| GRADE | Expert agreement | |
|---|---|---|
| Recommendation 8 | ||
| In symptomatic patients with single-vessel disease of either the CA or SMA, a ≥70% stenosis could be considered relevant. | 2D | 87% |
| Recommendation 9 | ||
| In symptomatic patients with extensive multivessel mesenteric artery disease, a ≥50% stenosis of the SMA could be considered relevant. | 2D | 78% |
Radiological imaging
Computed tomography angiography (CTA) and contrast-enhanced magnetic resonance angiography (CE-MRA) have replaced conventional angiography as the gold-standard imaging test for patients suspected of having chronic mesenteric ischaemia. Two investigator-blinded cohort studies evaluated the accuracy of detecting ≥50% and ≥70% stenoses of mesenteric arteries by CTA while using angiography as a reference.45,52 CTA had a sensitivity of 100% and a specificity of 95–100%. Advantages of CTA include its reproducibility, low interobserver variation, guidance of treatment planning and the potential detection of extravascular signs of possible AMI or alternative diagnoses. Signs of AMI or acute-on-chronic mesenteric ischaemia are mostly non-specific and may include bowel lumen dilatation (paralysis), bowel wall thickening and mesenteric fat stranding (oedema) combined with an obstruction of mesenteric arteries may be an early sign of on-going intestinal ischaemia, whereas decreased bowel wall enhancement and pneumatosis suggest more advanced AMI.53 Disadvantages of CTA are radiation exposure and the risk of contrast-induced nephropathy. A reduction of both radiation exposure and dosage of contrast agents can be achieved by performing a dual energy CTA.54 A CTA after injection of an adequate amount of intravenous (i.v.) contrast (1.5–2 ml/kg bodyweight with a concentration of >300 mg iodine/ml contrast media; flow rate 3.5–4 ml/s) with ≤1 mm acquisition slice thickness in arterial and venous/portal venous phase is recommended in patients with suspected chronic mesenteric ischaemia, to reliably assess the patency of the mesenteric vasculature and possible signs of AMI.
Several studies suggest CE-MRA could be a good alternative for the detection of CA and SMA stenoses, as it shows 100% sensitivity, 91–100% specificity and almost perfect interobserver agreement.52,55–58 Small studies dating from before 2002 suggest CE-MRA has difficulties detecting smaller vessels, but major improvements in magnetic resonance (MR) image quality have solved this issue.57–59 Clear benefits of CE-MRA over other imaging modalities are the absence of radiation exposure and contrast-induced nephropathy. These factors should be considered when performing imaging in young patients or patients with impaired renal function. A disadvantage of CE-MRA is the long duration of the examination, which could result in a poor image quality due to artefacts caused by breathing or patient movements. The expert panel considers CE-MRA the test of choice in patients with contraindications for CTA, for example patients with a severe allergy for the prescribed contrast agent or impairment of renal function.
Duplex ultrasound (DUS) of the mesenteric arteries had been extensively studied and used in clinical practice for decades. DUS can image the proximal mesenteric arteries but is often difficult to perform and may be negatively affected by abdominal fat and overlying intestinal gas. A study of the application of DUS in 324 patients reported interpretability as good in 66–68% of cases, moderate in 11–18%, and not interpretable in 15–23% of cases.60 Various prospective and retrospective studies have determined blood flow velocity cut-offs for significant CA and SMA stenoses by comparing DUS, as performed by experienced technicians, with angiography. A wide range of cut-offs were reported, with peak systolic velocities (PSVs) ranging between 200–320 cm/s in the CA and 205–400 cm/s in the SMA (sensitivity 58–100% and specificity 48–100%).47,49,60–63 Mesenteric artery PSV and end diastolic velocity (EDV) are strongly influenced by inspiration and expiration.64 This should be considered when interpreting study results, since most studies do not report whether DUS was performed during inspiration or expiration. Even though the sensitivity and specificity of DUS is inferior to the sensitivity and specificity of CTA or CE-MRA, it remains a valuable imaging technique because the costs of DUS are relatively low, contrast agents are not needed and radiation exposure is avoided. Hence, the expert panel concludes that DUS – when performed by an experienced technician – might be used as a screening method to exclude significant proximal mesenteric artery stenosis. When DUS is positive, CTA or CE-MRA is required to confirm the presence of a significant stenosis and to guide treatment planning.
Based on the diagnostic performance of non-invasive imaging modalities and the known complications of angiography (e.g. pseudoaneurysm, dissection, bleeding, etc.), the expert panel recommends that angiography be reserved for therapeutic purposes only.
Radiological imaging in suspected MALS
In MALS, the severity of CA stenosis is influenced by respiration. A cohort study, including 78 patients with clinical signs of MALS and previous imaging, performed angiography at both maximal inspiration and maximal expiration.65 The study found that during expiration CA compression was present in 100% of patients, whereas CA compression was present in only 56% of patients during inspiration. Standard protocol abdominal CT or MR imaging during inspiration only is therefore unsuitable when the goal is to exclude MALS.
Features suggesting CA compression on CTA or CE-MRA are an eccentric stenosis of the CA, a hook-shaped appearance of the CA and variation in the severity of the stenosis between inspiration and expiration. Evidence supporting the feasibility of CTA to detect CA compression is limited to very low quality retrospective studies which reported the presence of CA compression on CTAs performed for other indications.66,67 No evidence on feasibility of CE-MRA exists for this indication. Two studies, including six and 19 patients with CA compression, respectively, performed DUS during both inspiration and expiration.68,69 These studies reported a sensitivity of 83–95% and specificity of 89–100%.
Based on the lack of comparative studies showing superiority of one technique over another, DUS, CTA and CE-MRA are all considered appropriate. MALS is most often suspected and diagnosed in young patients, in whom harmful effects of radiation should be avoided. We recommend an inspiratory and expiratory DUS or CE-MRA, with ≤2 mm slices and 3D reconstructions, in patients of younger age and suspected MALS. A protocol for radiologists, containing technical details of imaging in chronic mesenteric ischaemia patients, is provided in Supplementary Material Document B.
| GRADE | Expert agreement | |
|---|---|---|
| Recommendation 10 | ||
| In patients with suspected chronic mesenteric ischaemia, a CTA (≤1 mm acquisition slice thickness, arterial and venous/portal venous phase) should be performed. | 1C | 91% |
| Recommendation 11 | ||
| CE-MRA is the diagnostic test of choice in case of a contraindication for CTA. | 1C | 87% |
| Recommendation 12 | ||
| Duplex ultrasound – when performed by an experienced technician – might be used as a screening method to exclude significant proximal mesenteric artery stenosis. Additional CTA or MRA imaging is required for patients with a positive duplex ultrasound. | 2C | 78% |
| Recommendation 13 | ||
| Angiography should be reserved for therapeutic purposes. | 1C | 100% |
| Recommendation 14 | ||
| CA compression in MALS can be diagnosed by inspiration/expiration duplex ultrasound, CTA or CE-MRA. In patients of younger age, suspected of having MALS, both duplex ultrasound and CE-MRA (≤2 mm slices with 3D reconstructions) in inspiration and expiration are recommended imaging techniques. | 1D | 74% |
Functional testing
Functional tests have been developed to characterise chronic intestinal ischaemia and prove insufficiency of the collateral circulation.70 The most extensively described functional tests are tonometry and visible light spectroscopy (VLS). Tonometry measures the intraluminal partial pressure of carbon dioxide (PCO2) in the stomach and small bowel by positioning two balloons at the end of a nasogastric tube and nasojejunal tube. Studies have shown that intraluminal PCO2 is closely related to local arterial PCO2 and indicative of mucosal ischaemia. Two types of tonometry have been described and validated: exercise tonometry and 24-hour tonometry. Exercise tonometry uses a standardised exercise protocol to elicit ischaemia and has a 78–97% sensitivity and 69–92% specificity.71,72 During 24-hour tonometry, meals are used to elicit ischaemia, while PCO2 levels are monitored during a 24-hour period. The sensitivity of this test is 76–92% and specificity 77–94%.40,70 Major disadvantages of tonometry are the invasiveness (nasogastric tubes), long duration and interference of gastric acid and acids in food. Unfortunately, the disappearance of this test is expected in the near future, since tonometry equipment is no longer produced.
VLS is performed during upper GI endoscopy and measures mucosal oxygen saturation in the antrum, duodenal bulb and descending duodenum. Studies of VLS in chronic mesenteric ischaemia patients report a 90% sensitivity, 60% specificity and fair to good intraobserver and interobserver agreement.73,74 VLS is quicker and less cumbersome than tonometry, but ischaemia cannot be provoked during VLS. A recent study that performed VLS measurements both preprandial and 45 min after luminal feeding in the stomach found no additional discriminative value of postprandial VLS measurements.75 Other disadvantages of VLS are interference of bile and the requirement to switch off endoscopic light during measurements, causing difficulties in maintaining the position of the probe during peristaltic waves.76
The expert panel acknowledges the value of ischaemia detection by a discriminative functional test in patients with suspected chronic mesenteric ischaemia. Though of proven value in literature, current functional tests have serious limitations and are not widely available throughout Europe. The expert panel recognises the need for a more potent and widely available functional test.
Blood tests
Biomarkers for chronic mesenteric ischaemia are not currently available. Two small prospective cohort studies using a diagnostic work-up, including a functional test, have evaluated the diagnostic potential of markers such as lactate, D-dimer, lactate dehydrogenase, C-reactive protein, leucocytes, intestinal fatty acid binding protein and lipopolysaccharide.77,78 None of these markers were sufficiently discriminative and suitable for clinical practice. The clinical value of lactate, obtained from a peripheral vein, to rule out any form of intestinal ischaemia is still a widespread misconception. A cohort study measuring lactate before a meal and 60 and 240 min after a meal revealed the poor sensitivity (34%) of lactate in chronic mesenteric ischaemia patients.77 Similar sensitivities of lactate have been shown in a systematic review of patients presenting with AMI, in whom ischaemia is even more extensive and severe.79 Though nonspecific, normal D-dimer levels can exclude AMI (sensitivity 89–100%); however, the value of D-dimer levels to exclude chronic mesenteric ischaemia remains unclear.79,80 Based on these studies the expert panel concludes that normal lactate, lactate dehydrogenase and/or leucocyte levels do not exclude chronic mesenteric ischaemia.
GI endoscopy
Upper GI endoscopy is essential in the work-up of patients suspected of having chronic mesenteric ischaemia, mainly for exclusion of alternative diagnoses. Findings reported in chronic mesenteric ischaemia patients are oedema (35%), erythema (42%), atrophy of the duodenal mucosa, and gastric and duodenal ulcers that are not caused by Helicobacter pylori or non-steroidal anti-inflammatory drugs.14 A prospective study comparing endoscopic biopsies of 56 chronic mesenteric ischaemia patients and 26 patients without chronic mesenteric ischaemia could not demonstrate discriminative histological changes, and concluded that biopsies have no value in supporting the diagnosis of chronic mesenteric ischaemia.81 Based on the relatively low prevalence and often transient nature of upper GI endoscopic abnormalities in chronic mesenteric ischaemia patients, the expert panel states that a normal GI endoscopy does not exclude chronic mesenteric ischaemia.
| GRADE | Expert agreement | |
|---|---|---|
| Recommendation 15 | ||
| (a) Normal lactate, lactate dehydrogenase, and/or leucocytes levels do not exclude chronic mesenteric ischaemia.(b) Normal GI endoscopy does not exclude chronic mesenteric ischaemia. | 1C | 100% |
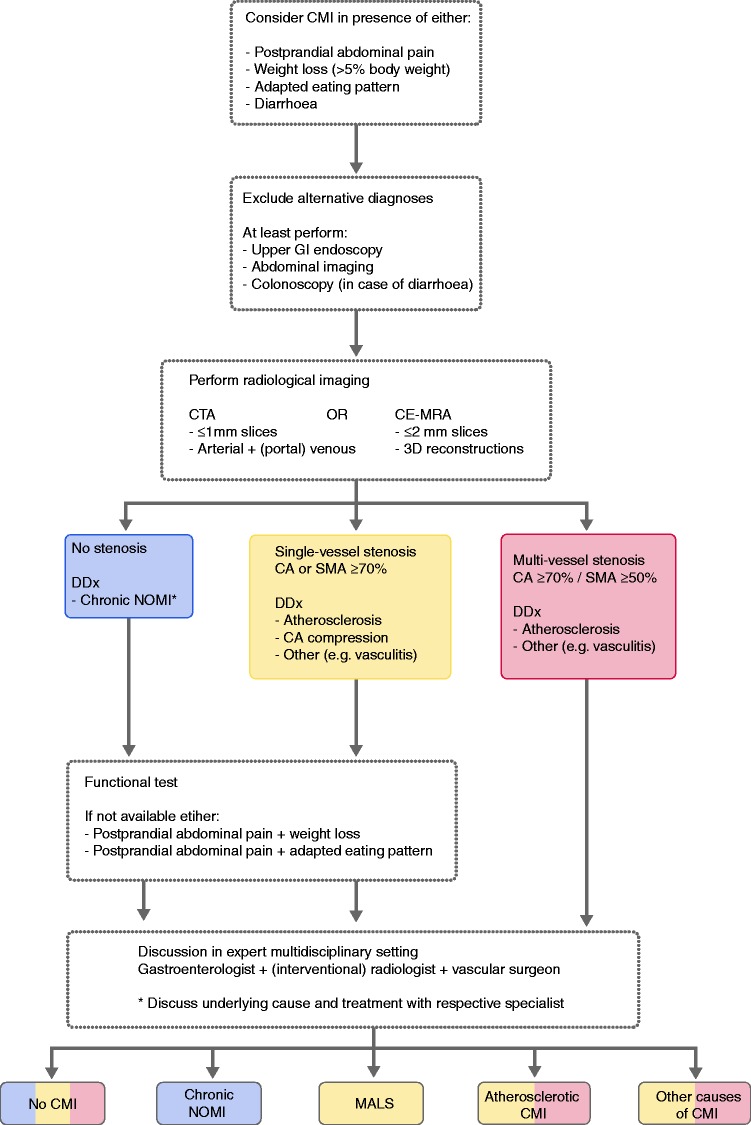
A flowchart containing an overview of the most important steps and criteria of the diagnostic work-up of patients with suspected chronic mesenteric ischaemia can be found in Figure 4.
Figure 4.
Flowchart of the diagnostic work-up of patients with suspected chronic mesenteric ischaemia (CMI).
CA: celiac artery; CE-MRA: contrast-enhanced magnetic resonance angiography; CTA: computed tomography angiography; DDx: differential diagnosis; GI: gastrointestinal; NOMI: non-occlusive mesenteric ischaemia; SMA: superior mesenteric artery.
Treatment
Over the last decades endovascular revascularization (ER) of the mesenteric arteries has replaced open surgical mesenteric artery revascularization (OSMAR). A meta-analysis of 100 studies reporting on OSMAR and/or ER found significantly more in-hospital complications and a trend towards a higher 30-day mortality after OSMAR (in-hospital complications relative risk (RR) 2.19 (95% confidence interval (CI) 1.84–2.60); 30-day mortality RR 1.57 (95% CI 0.84–2.93)).1 Nevertheless, long-term results appeared superior after OSMAR, with fewer symptom recurrences and a trend towards higher three-year survival (three-year symptom recurrence RR 0.47 (95% CI 0.34–1.57); three-year survival RR 0.96 (95% CI 0.86–1.07)).1 However, the quality of the evidence assembled in this meta-analysis is low, since the design and quality of the individual studies was flawed. The meta-analysis consisted of 18,762 patients, but few studies reported long-term outcomes.
Durability of revascularization is generally expressed by the patency of a vessel after revascularization. Primary patency is defined as the absence of a significant re-stenosis or occlusion after a primary revascularization. Secondary patency is defined as the absence of a significant re-stenosis or occlusion after a second revascularization of the target vessel. Two meta-analyses comparing patency rates of OSMAR and ER showed significantly higher primary patency rates for OSMAR (one-year primary patency OSMAR 91–94% vs ER 69–74%, five-year primary patency OSMAR 80–81% vs ER 51–52%).82,83 The numbers of patients included in the analyses of the one-year primary patency were 465 and 742 (meta-analyses of Gupta et al.83 and Pecoraro et al.,82 respectively); 481 and 679 patients were included in the analyses of the five-year primary patency. OSMAR also exhibited superior five-year secondary patency (OSMAR 96–98% vs ER 79–88%). The quality of the evidence provided by these meta-analyses is low, since the design and quality of the individual studies was flawed and the small sample sizes of the individual studies could have introduced publication bias. Furthermore, evidence on the cost-effectiveness and gain in quality of life of each treatment strategy was of low quality, and consisted of a single study showing no long-term differences between OSMAR and ER.84
The expert panel concluded that the reduction in short-term mortality and morbidity of ER outweighs the superior long-term results of OSMAR in this generally older population (mean age 69 years) with multiple comorbidities, especially since the long-term patency of repeated ERs is considered comparable to that of OSMAR.1 Therefore, the expert panel prefers ER and suggests mesenteric bypass procedures might be reserved for patients in whom ER is not suitable.
Nutritional status before mesenteric artery revascularization
Malnutrition is a common finding in patients with chronic mesenteric ischaemia. A small retrospective cohort study of chronic mesenteric ischaemia patients, treated by percutaneous mesenteric artery stenting (PMAS) or OSMAR, found an association between malnutrition and increased mortality (30-day mortality non-malnourished patients 0%, malnourished patients 26%).85 Although pre-operative nutrition is associated with improved surgical outcomes in patients without chronic mesenteric ischaemia (post-operative complications odds ratio (OR) 0.64 (95% CI 0.53–0.84)), pre-operative oral or enteral nutrition might worsen intestinal ischaemia in patients with chronic mesenteric ischaemia.86 Tonometry studies confirm that oral intake induces or worsens ischaemia in the stomach and jejunum of chronic mesenteric ischaemia patients.70 Additional circumstantial evidence can be found in case reports and a cohort study, which reported AMI in 2% of in-hospital patients receiving enteral tube feeding and even higher rates of AMI among intensive care unit (ICU) patients, since most ICU patients are haemodynamically unstable and unable to increase mesenteric blood flow.87–89 The latter did not use a control group (e.g. total parenteral nutrition (TPN)), and the haemodynamic situation in chronic mesenteric ischaemia patients differs from that of ICU patients, implying that these results should be interpreted with caution.
No evidence on TPN in chronic mesenteric ischaemia patients exists, but a study in patients without chronic mesenteric ischaemia has shown that TPN decreases mesenteric blood flow, an effect that could induce AMI in chronic mesenteric ischaemia patients.90 The increased risk of bloodstream infections associated with TPN is another disadvantage, especially since sepsis and subsequent haemodynamic instability will compromise an already threatened mesenteric circulation.91,92
The expert panel acknowledges that evidence on this topic is limited to non-existent, yet possible negative consequences of attempts to improve nutritional status are considered substantial. Therefore, increasing oral intake, starting enteral tube feeding or starting TPN before revascularization might be contra-indicated. Restoring mesenteric circulation – preferably by PMAS – has priority and should not be delayed by attempts to improve nutritional status.
| GRADE | Expert agreement | |
|---|---|---|
| Recommendation 16 | ||
| Mesenteric bypass procedures might be reserved for patients in whom endovascular revascularization is not suitable. | 2C | 100% |
| Recommendation 17 | ||
| In patients with chronic mesenteric ischaemia it might be disadvantageous to increase oral intake, start enteral tube feeding or start total parenteral nutrition before revascularization. | 2D | 96% |
PMAS
Two types of ER have been described in literature, percutaneous transluminal angioplasty (PTA) and PMAS. PMAS is the current standard approach and the rationale behind this strategy is based on technical success rates. Case series and expert experience suggest that the vast majority of mesenteric artery stenoses occur at the vessel’s origin and that these stenoses are often heavily calcified.93,94 Studies on revascularization of ostial renal artery stenosis have shown that elastic recoil is more likely to occur in ostial stenoses, causing recurrence of the stenosis when recoil is not prevented by stenting.95,96 Evidence comparing both strategies is of very low quality. A systematic review comparing mesenteric artery PTA and PMAS in 328 patients found a significantly higher technical success rate for PMAS (PMAS 95% vs PTA 83%), comparable symptom relief (PMAS 91% vs PTA 89%) and a higher restenosis rate (PMAS 35% vs PTA 21%).97 A more recent retrospective cohort study reported a lower reintervention rate after PTA compared to PMAS, although the difference was not statistically significant.98 Despite the very low quality of evidence, the expert panel strongly recommends PMAS over PTA alone in atherosclerotic mesenteric artery stenosis as technical success, and thus adequate revascularization, is considered the main objective.
For decades the preferred route of arterial access for endovascular mesenteric artery revascularization was via either the brachial or femoral artery. Some centres advocate use of (left) brachial artery access, since the caudal angulation of the mesenteric arteries makes this approach more convenient. Manipulation of guidewires, sheaths and catheters through the brachiocephalic artery and aortic arch is limited or avoided when using left brachial access instead of right brachial access. Left brachial access has been reported to decrease the risk of cerebral embolization due to dislodged aortic plaques or thrombus after advanced endovascular aortic repair with visceral artery stent grafting.99 However, reported complication risks of PMAS using brachial access (16–19%) appear to be higher than the complication risks of femoral access (7.5%).50,100 When using brachial access, ultrasound-guided cannulation of the brachial artery is advised to avoid nerve injury. Introduction of radial access provides a third possible access route for PMAS.101 Low rates of access-site complication have been reported in coronary artery angiographies; however, these results cannot be extrapolated to mesenteric artery interventions, considering that longer sheaths are often used for PMAS. The literature on radial access for PMAS is limited. A meta-analysis of radial and femoral access in infra-aortic interventions, using a maximum sheath size of 6–8.5 French, reported significantly fewer complications when radial access was used (OR 0.25, 95% CI 0.07–0.86).102 A meta-analysis of hepatic artery interventions in patients with primary or secondary liver malignancy reported no differences in access-site-related outcomes of radial and femoral access, but did show a patient preference for radial access.103 Based on extensive experience with femoral access, the still limited evidence supporting radial access, and the substantial complication rate of brachial access, the preferred entry site for PMAS is currently femoral, followed by (left) brachial or radial, but is primarily dependent on the expertise of the operator.
Retrograde open mesenteric stenting (ROMS) is a hybrid mesenteric artery revascularization strategy using midline laparotomy to gain direct retrograde vascular access to a mesenteric artery.104 This access technique can be used to perform stenting in patients with acute-on-chronic ischaemia undergoing a laparotomy for resection of necrotic bowel.104,105 ROMS might also be used to stent severely stenotic or occluded ostia of mesenteric arteries that cannot be cannulated through femoral, brachial or radial access.
Use of bare-metal stents (BMSs) for PMAS is standard practice, even though long-term primary patency is unsatisfactorily low (five-year primary patency: 51–52%) due to in-stent intimal hyperplasia.82,83 Some centres have used covered stents (CSs) in an attempt to reduce in-stent intimal hyperplasia. One retrospective study compared use of BMS and CS, suggesting a superior patency of CS over BMS (one-year primary patency CS 92% vs BMS 75%, three-year primary patency CS 92% vs BMS 52%).106 The CS also had superior freedom from symptom recurrence (one-year freedom from symptom recurrence CS 92% vs BMS 73%, three-year freedom from symptom recurrence CS 92% vs BMS 50%). Though promising, superiority of the CS remains debatable, since the quality of evidence is very low and results are biased by a low number of patients with a follow-up duration of >1 year after PMAS using a CS. Possible disadvantages of the CS are the higher costs than the BMS and unknown complications of the CS, e.g. stent thrombosis with subsequent AMI. Due to current uncertainties regarding superiority of the CS, the expert panel could not reach consensus on statements recommending use of the CS. The results of a randomised controlled trial (NCT02428582) comparing the CS and BMS are awaited.107 Comparative data on PMAS with a drug-eluting stent (DES) is not available in the literature.
In patients with occlusive disease of both CA and SMA, revascularization of one versus both arteries remains an ongoing debate. Evidence on partial vs complete revascularization is limited to low-quality cohort studies comparing partial and complete OSMAR, and cohort series showing high rates of clinical success after revascularization of the SMA alone.46,48,50,108–113 Sample sizes of comparative studies were too small to show a significant difference in five-year survival, but a trend towards superiority of complete revascularization was observed.48,108,110,113 Complete revascularization is associated with superior freedom from symptom recurrence at 3, 5 and 10 years after revascularization.48,50 The expert panel acknowledges current evidence should be interpreted with caution but does agree that complete revascularization seems beneficial in the long run. Accordingly, revascularization of both CA and SMA might be attempted when feasible. When single-vessel revascularization is performed, the SMA is considered the preferred target artery followed by the CA.
Antiplatelet therapy after PMAS
Initiation of dual antiplatelet therapy (DAPT) directly after PMAS is common practice. The duration of DAPT remains uncertain and there is always a trade-off between benefits (reduction of stent thrombosis and reintervention rates) and harm (increased risk of bleeding). Due to an absence of evidence on this topic the expert panel extrapolated evidence from stenting in the coronary arteries, since these are most similar to the mesenteric arteries with their tortuosity and small diameter. A one-month DAPT duration was common practice in the coronary BMS era. No studies have compared one-month DAPT with a longer duration of DAPT after coronary artery stenting using a BMS. A longer DAPT duration was introduced due to the increased risk of stent thrombosis associated with the DES. Current guidelines on DAPT in coronary artery disease recommend use of the DES and a consequent 12-month duration of DAPT.114 Meta-analyses of this antiplatelet strategy report a clear association between long DAPT duration and an increased risk of bleeding.115,116 In terms of patient preferences, a study questioning patients on preferences for long DAPT versus short DAPT reported that patients place high value on avoidance of the drawbacks associated with DAPT.117 Based on the extrapolated literature regarding DAPT after stenting in the coronary arteries, increased bleeding rates, patients’ preferences and use of BMS during PMAS, a one-month duration of DAPT is suggested after PMAS, followed by lifelong treatment with an antiplatelet monotherapy.
Combining antiplatelet agents with antithrombotic agents (e.g. direct oral anticoagulant (DOAC), vitamin K antagonist, low molecular weight heparin (LMWH)) increases the risk of bleeding events. A meta-analysis of patients after coronary artery stenting compared oral anticoagulation with the addition of either one (dual therapy) or two (triple therapy) antiplatelet agents. Risks of both major bleeding (OR 0.55, 95% CI 0.39–0.78) and minor bleeding (OR 0.43, 95% CI 0.33–0.56) were significantly lower when dual therapy was used.118 Risks of stent thrombosis, myocardial infarction and cardiovascular death did not differ significantly, though studies included in the meta-analysis were not powered for these outcomes. Studies comparing use of an antithrombotic agent alone and dual therapy, after coronary artery stenting, were not found. Based on the substantial risks of bleeding and questionable benefits of long duration antiplatelet therapy in patients treated with DOAC, vitamin K antagonists or LMWH, the expert panel suggests that the prescription of an antiplatelet agent be restricted to the first 4 weeks after PMAS.
| GRADE | Expert agreement | |
|---|---|---|
| Recommendation 18 | ||
| The preferred entry site for mesenteric artery revascularization is the femoral artery, followed by the left brachial or radial artery, and is dependent on expertise. | 1D | 87% |
| Recommendation 19 | ||
| In atherosclerotic mesenteric artery lesions, PTA and stenting is recommended over PTA alone. | 1D | 100% |
| Recommendation 20 | ||
| In patients with occlusive disease of both the CA and SMA, endovascular revascularization of both vessels might be attempted. The SMA is the preferred target artery followed by the CA. | 2D | 91% |
| Recommendation 21 | ||
| After endovascular mesenteric artery stenting, we suggest administering dual antiplatelet therapy for at least one month, followed by lifelong antiplatelet monotherapy. | 2D | 91% |
| Recommendation 22 | ||
| In patients treated with DOAC, vitamin K antagonists or LMWH, we suggest adding one antiplatelet agent for 4 weeks after endovascular mesenteric artery stenting. | 2D | 83% |
OSMAR
OSMAR is most frequently performed by constructing a mesenteric bypass. Bypass grafts can be positioned in an antegrade (origin proximal of stenotic artery) or retrograde (origin distal of stenotic artery) orientation. Studies comparing both strategies are of very low quality due to retrospective designs, low numbers of patients and the large variety in graft material. Four studies report no differences in survival or graft patency between antegrade and retrograde graft orientation.119–122 One study reported improved median survival when using an antegrade bypass (antegrade 5.7 years vs retrograde 4.0 years), but patients undergoing an antegrade bypass were younger (antegrade 65 years vs retrograde 75 years).110 Nowadays mesenteric bypass procedures are reserved for patients unsuitable for PMAS, resulting in a different patient population. Mesenteric bypass procedures are currently performed in older patients with more comorbidities and more extensive calcification, making supracoeliac anastomosis more challenging and increasing the risks of supracoeliac clamping and lengthy surgical procedures. The decision to use antegrade or retrograde graft orientation should be tailored to the patient’s local anatomy and comorbidities, while taking the surgeons’ experience into account.
A mesenteric bypass can be constructed using venous or prosthetic material, e.g. Dacron or polytetrafluoroethylene. Very low quality retrospective studies show similar patency rates for venous and prosthetic material.119–124 A disadvantage of prosthetic grafts is the potential risk of graft infection, especially in patients with acute-on-chronic ischaemia with spill of bowel content into the abdominal cavity. However, veins of sufficient quality are not always available and graft infection is rare, while patency is comparable to prosthetic grafts. There might not be a clear preference for venous or prosthetic grafts and the choice may depend on the type of procedure, patients’ comorbidities, and quality and availability of venous grafts.
| GRADE | Expert agreement | |
|---|---|---|
| Recommendation 23 | ||
| There might be no preference for an antegrade or retrograde approach when performing mesenteric bypass. | 2D | 81% |
| Recommendation 24 | ||
| There might be no preference for venous or prosthetic grafts when performing mesenteric bypass. | 2D | 71% |
Treatment of MALS
The existence of MALS is still questioned by many physicians who argue that available evidence is inconclusive and potentially affected by publication bias. Treatment of MALS is currently by surgical division of the MAL. A systematic review reported improvement of symptoms in 83% of 400 patients treated by CA release.125 Eighty per cent experienced sustained symptom relief, indicating that the placebo effect as an explanation of symptom relief is unlikely. Based on these substantial rates of symptom improvement, surgical CA release might be considered in patients with presumptive MALS.
Release of the CA can be performed by laparotomy, laparoscopy and retroperitoneal videoscopy. Evidence showing superiority of one technique over another is not available. The previously described systematic review included 279 patients with a CA release by laparotomy and 121 patients by laparoscopy.125 Sustained symptom improvement was present in 75% of patients after laparotomy and 90% after laparoscopy, suggesting laparoscopic CA release is feasible. Laparoscopic and retroperitoneal videoscopic CA release are less invasive, resulting in smaller scars, and have the advantage of lower rates of gastroesophageal reflux disease, since only one side of the crus is dissected. Not accessing the abdominal cavity is an additional advantage of retroperitoneal videoscopic CA release. This approach avoids the risk of female infertility due to adhesions in these often young patients. However, experience with endoscopic CA release is limited to only a few expert centres and little is known on the learning curve to safely perform these procedures. Due to the very low quality of evidence and uncertainty regarding the feasibility of implementation of laparoscopic and retroperitoneal videoscopic CA release in centres throughout Europe, the expert panel did not reach consensus on recommendations on this topic.
Evidence on the risks or benefits of PMAS as a primary treatment for MALS does not exist. However, according to expert experience, stenting of the CA without preceding CA release will likely result in a stent fracture, compromising blood flow and potentially resulting in life-threatening AMI. Due to this possible hazard the expert panel considers endovascular stenting of the CA contraindicated in patients with presumptive MALS and no preceding CA release.
| GRADE | Expert agreement | |
|---|---|---|
| Recommendation 25 | ||
| Patients with MALS might be considered for surgical coeliac artery release. | 2D | 96% |
| Recommendation 26 | ||
| In patients with MALS (and no preceding adequate coeliac artery release) endovascular stenting of the CA is contraindicated. | 1D | 100% |
Treatment of vasculitis
Vasculitis involving the mesenteric arteries is a rare cause of chronic mesenteric ischaemia, but a potentially life-threatening manifestation of systemic vasculitis. Literature on this topic consists of case reports and small case series. Still, the expert panel emphasises the importance of awareness of vasculitis as a potential cause of chronic mesenteric ischaemia, since a different clinical approach is needed. CTA seems to be a reasonable initial non-invasive screening method in suspected mesenteric vasculitis and can detect aneurysms in vessels as small as 3 mm.31 Nonetheless, angiography remains an important, and in some cases indispensable, imaging modality in patients with a high suspicion of small or medium vessel vasculitis, such as polyarteritis nodosa, and seems to be the preferred imaging modality when CTA is inconclusive or negative.31
A retrospective database study, including patients evaluated for vasculitis over a 24-year period, found symptoms of mesenteric ischaemia in 120 of 7514 (1.6%) patients.126 Only 15 of these patients required revascularization. Management with immunosuppressive medication was sufficient in the remaining patients. Though evidence is of very low quality, referral to an expert in the treatment of vasculitis is suggested before proceeding to ER in patients with symptoms and radiological features of vasculitis. An additional argument to promote early recognition and treatment is to prevent progression, which might otherwise result in a severe disease course such as kidney failure.
Asymptomatic mesenteric artery stenosis
Mesenteric artery stenosis is a frequent finding on abdominal imaging. Experts agree that revascularization is not needed in patients with asymptomatic single-vessel stenosis. Two prospective cohort studies, following asymptomatic patients for a mean duration of 2.6 and 6.5 years, reported no occurrences of AMI or chronic mesenteric ischaemia in patients with single-vessel disease.22,23 A decision not to intervene is less clear in asymptomatic patients with stenosis or occlusion of all three mesenteric arteries, since the ability to compensate and maintain adequate blood flow through collaterals is limited, creating a potential risk of AMI when atherosclerosis progresses or an acute occlusion (e.g. embolus, thrombosis) occurs. Little is known on the natural course of asymptomatic three-vessel mesenteric artery disease. The only study reporting on this matter dates from 1998 and included 15 patients with a mean follow-up duration of 2.6 years.22 Four out of 15 (27%) patients developed mesenteric ischaemia, one presented with AMI and the remaining three patients became symptomatic and were diagnosed with chronic mesenteric ischaemia. Given the low level of evidence, a tailor-made approach is suggested in all asymptomatic patients with stenosis or occlusion of all three mesenteric vessels. Risks and benefits of revascularization should be carefully assessed, taking age, comorbidities and patient preferences into account.
When planning major abdominal surgery in patients with known asymptomatic mesenteric artery stenosis, the surgeon needs to be aware of local anatomy and the collateral circulation. Especially in patients with stenosis or occlusion of two or more mesenteric arteries, ligation of collaterals could compromise the mesenteric circulation, resulting in AMI.127,128 ER might be considered, to prevent AMI, in patients with significant stenosis or occlusion of two or more mesenteric arteries undergoing major abdominal surgery with potential ligation of the collateral circulation.
| GRADE | Expert agreement | |
|---|---|---|
| Recommendation 27 | ||
| In patients with symptoms and radiological features of vasculitis, referral to an expert in the treatment of vasculitis is indicated before proceeding to ER. | 1D | 83% |
| Recommendation 28 | ||
| Revascularization to prevent occurrence of AMI in asymptomatic patients with significant stenosis/occlusion of all three mesenteric vessels should only be performed after carefully weighing the risks and benefits of treatment, given the low level of evidence. | 2D | 83% |
| Recommendation 29 | ||
| In asymptomatic patients with significant stenosis/occlusion of 2 or more mesenteric vessels who need to undergo major abdominal surgery with potential ligation of collateral circulation, endovascular intervention may be considered to prevent occurrence of AMI. | 2D | 74% |
Secondary prevention
Secondary prevention is important when treating patients with atherosclerotic chronic mesenteric ischaemia, considering that atherosclerosis is a systemic disease and secondary prevention reduces the risk of all cardiovascular events. Evidence concerning secondary prevention in patients with atherosclerotic chronic mesenteric ischaemia is not currently available; therefore evidence and guidelines on secondary prevention in general have been extrapolated to chronic mesenteric ischaemia patients.129–131
To evaluate whether secondary prevention is indicated, an assessment of the cardiovascular risk profile is suggested in all symptomatic and asymptomatic patients with an atherosclerotic mesenteric artery stenosis, because all patients with proven atherosclerosis are prone to atherosclerosis and cardiovascular events in other vascular beds. A history of arterial revascularization (e.g. PMAS) is associated with a very high risk of cardiovascular events. Assessment of cardiovascular risk factors is essential when adjusting secondary prevention to an individual patient’s situation, especially since stricter treatment targets are recommended in patients with a very high risk profile.1,130 The expert panel suggests that secondary prevention should begin as soon as a diagnosis of atherosclerotic chronic mesenteric ischaemia is established. Current European Society of Cardiology guidelines provide recommendations on treatment target levels for low density lipid-cholesterol (LDL-C), and diastolic and systolic blood pressure targets.130,131 Recommendations regarding antiplatelet therapy are stated above.
| GRADE | Expert agreement | |
|---|---|---|
| Recommendation 30 | ||
| In patients with symptomatic atherosclerotic chronic mesenteric ischaemia, we suggest that cardiovascular secondary prevention should start as soon as the diagnosis is made. | 2C | 100% |
| Recommendation 31 | ||
| In patients with an asymptomatic atherosclerotic stenosis of the mesenteric arteries, we suggest that the cardiovascular risk profile be assessed. | 2D | 100% |
Follow-up after revascularization
Experts agree that clinical follow-up is important during the first year after mesenteric artery revascularization, especially considering the regularity of symptom recurrence due to in-stent restenosis and the potentially severe consequences of stent occlusion. A possible benefit of active surveillance is the ability to prevent AMI. By monitoring the development and progress of restenosis, revascularization can be performed before a total occlusion occurs and revascularization becomes more challenging. Possible drawbacks of active surveillance are the additional costs of imaging and risk of complications, issues that are especially relevant when performing re-interventions in asymptomatic patients.
Evidence on this topic is limited to a single prospective database of 145 patients, which showed 17% symptom recurrence and 39% in-stent restenosis on routine DUS imaging, with a mean follow-up of 12 months after mesenteric artery revascularization.132 Re-intervention was performed when symptoms recurred in the presence of a significant restenosis or when restenosis was severe while the collateral network was poor. Forty-seven percent of patients with in-stent restenosis remained free from re-intervention, suggesting restenosis alone does not necessitate a re-intervention. The expert panel weighed the evidence and advantages and disadvantages but could not reach agreement on recommendations regarding follow-up strategies.
Imaging is recommended to assess stent patency in patients with recurrence of symptoms. However, evidence is limited regarding the reliability of in-stent stenosis detection using available imaging techniques. No literature reports on CTA or MRA were found, but in clinical practice CTA is commonly used to detect in-stent stenosis. MRA would appear to be less suitable, since metal artefacts make assessment of stent patency challenging. Detection of in-stent stenosis by DUS is possible, but one should be aware of changes in haemodynamics after PMAS as several studies have shown higher PSV and EDV values in patients treated by PMAS.132–134 Two retrospective studies assessed the reliability of DUS after PMAS and both reported a low specificity (30–39%) when using a PSV cut-off of >200 cm/s in CA and >275 cm/s in SMA.98,135 A very low quality retrospective study of 30 patients after PMAS found 88–100% sensitivity and 89–100% specificity when using higher DUS cut-offs.135 A recent prospective study of 51 in-stent SMA stenoses assessed the performance of DUS, using a transstenotic mean arterial pressure gradient of ≥10 mm Hg as a reference.136 Receiver-operator characteristics showed an area under the curve of 0.75 for PSV and 0.80 for EDV. A combination of a PSV >300 cm/s and EDV >50 cm/s resulted in a sensitivity of 32% and a specificity of 100%. Based on the limitations of current imaging techniques and available evidence, the expert panel recommends the use of DUS and/or CTA to assess stent patency in patients with recurrence of symptoms.
In patients undergoing CA release to treat MALS, follow-up is indicated to assess symptom relief. A systematic review of CA release procedures reported that 17–26% of patients needed an additional arterial reconstruction or PTA to restore adequate blood flow after CA release.125 This outcome might be due to intraluminal narrowing and intimal hyperplasia caused by the sustained external pressure of the median arcuate ligament. In order to detect or exclude residual stenosis in patients without an improvement in symptoms after CA release, the expert panel recommends performing imaging as specified in recommendation 14.
| GRADE | Expert agreement | |
|---|---|---|
| Recommendation 32 | ||
| In a patient with recurrence of symptoms, DUS and/or CTA are the recommended diagnostic tools to assess in-stent stenosis. | 1D | 100% |
| Recommendation 33 | ||
| In patients without improvements in symptoms after coeliac artery release, a diagnostic test as specified in recommendation 14 should be performed. | 1D | 83% |
Future research
As the epidemiology of chronic mesenteric ischaemia is still poorly understood, the assessment of population-based incidence, prevalence and cause-specific mortality in chronic mesenteric ischaemia should be given priority. Further evidence is also required to fully understand the aetiology and pathophysiology of chronic mesenteric ischaemia. One starting point would be an international multicentre registry of patients with mesenteric artery disease, which would facilitate a better appreciation of the natural course of chronic mesenteric ischaemia. Examples of other research topics that could be addressed using this type of registry are: differences in symptomatology of early and end-stage chronic mesenteric ischaemia, the spectrum of radiological features associated with chronic mesenteric ischaemia, the influence of the presence or absence of collaterals on the probability of developing chronic mesenteric ischaemia, differences in the clinical manifestations of CA and SMA stenosis, factors associated with and predictive of acute-on-chronic mesenteric ischaemia, and the incidence and prevalence of acute-on-chronic ischaemia. Other topics that should be researched are the natural course of asymptomatic stenoses of one, two or all mesenteric arteries, and the need for prophylactic revascularization.
Detecting and characterising intestinal ischaemia is one of the major challenges when diagnosing chronic mesenteric ischaemia and hence a sensitive and specific (functional) test is needed to help detect ischaemia and identify chronic mesenteric ischaemia patients. A test of this type would help to prevent invasive treatments in patients misdiagnosed with chronic mesenteric ischaemia and facilitate the early diagnosis and appropriate treatment of chronic mesenteric ischaemia patients with atypical symptoms. The discriminative ability of diagnostic methods including preprandial and postprandial measurements of mesenteric blood flow using MRI, MRI-based detection and quantification of lactate in the portal vein, and endoscopic measurements of mucosal mitochondrial oxygen levels are currently being investigated. Development of biomarkers for the identification of patients with chronic mesenteric ischaemia or AMI would be of great clinical value and should be considered an important research topic.
As the existence of MALS is still questioned by many physicians, CA release remains a controversial procedure. A blinded, randomised controlled trial comparing a CA release with a sham operation would end this ongoing debate.
Patency rates of BMSs are known to be disappointingly low, yet evidence on the use of other stent types to improve patency of PMAS is still inconclusive. A randomised controlled trial comparing the BMS and CS is currently underway and results are expected in 2021.107 However, questions concerning the role of the DES in the mesenteric arteries and the optimal revascularization strategy in patients with an in-stent stenosis remain unanswered. Development of validated software tools for accurate grading of in-stent stenosis on CTA, using the transstenotic mean arterial pressure gradient under angiography as a reference, is another important research topic.
Supplemental Material
Supplemental material, UEG916681 Supplemental Material for European guidelines on chronic mesenteric ischaemia – joint United European Gastroenterology, European Association for Gastroenterology, Endoscopy and Nutrition, European Society of Gastrointestinal and Abdominal Radiology, Netherlands Association of Hepatogastroenterologists, Hellenic Society of Gastroenterology, Cardiovascular and Interventional Radiological Society of Europe, and Dutch Mesenteric Ischemia Study group clinical guidelines on the diagnosis and treatment of patients with chronic mesenteric ischaemia by Luke G Terlouw, Adriaan Moelker, Jan Abrahamsen, Stefan Acosta, Olaf J Bakker, Iris Baumgartner, Louis Boyer, Olivier Corcos, Louisa JD van Dijk, Mansur Duran, Robert H Geelkerken, Giulio Illuminati, Ralph W Jackson, Jussi M Kärkkäinen, Jeroen J Kolkman, Lars Lönn, Maria A Mazzei, Alexandre Nuzzo, Felice Pecoraro, Jan Raupach, Hence JM Verhagen, Christoph J Zech, Desirée van Noord and Marco J Bruno in United European Gastroenterology Journal
Acknowledgements
The authors thank the information specialist of the Erasmus MC medical library, Wichor Bramer, for his assistance in defining the literature search for this guideline.
Ethics approval
Not applicable.
Declaration of conflicting interests
The following authors disclose conflicts of interest for the submitted work: OC received grant support from MSDAvenir. The following authors disclose conflicts of interests outside the submitted work: LB received grant support from Guerbet and Boston Scientific; MB received grant support from Boston Scientific, Cook Medical, Pentax Medical, and 3M, honoraria for consulting from Boston Scientific, Cook Medical, Pentax Medical, 3M, Mylan, MediRisk, and Medicom, as well as speaker fees from Boston Scientific, Cook Medical, Pentax Medical, 3M, and GastroUpdate; OC received grant support from Shire; RJ received grant support from Abbott Vascular, as well as speaker fees from Gore Medical; AN received grant support from Fondation de lávenir, Sociéte’Française de Gastro-enterologie, and MSDAvenir; HV received personal fees from Medtronic, WL Gore, Endologix, Arsenal, Abbott, and Philips; CZ received honoraria for consulting and speaker fees from Bayer AG. The remaining authors declare no conflicts of interests.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This guideline was developed with the support of a UEG Activity Grant.
Informed consent
Not applicable.
ORCID iDs
Luke G Terlouw https://orcid.org/0000-0002-4983-5741
Louisa JD van Dijk https://orcid.org/0000-0002-6507-5666
Robert H Geelkerken https://orcid.org/0000-0003-4640-8725
Christoph J Zech https://orcid.org/0000-0002-4707-4563
Supplemental material
Supplemental material for this article is available online.
References
- 1.Alahdab F, Arwani R, Pasha AK, et al. A systematic review and meta-analysis of endovascular versus open surgical revascularization for chronic mesenteric ischemia. J Vasc Surg 2018; 67: 1598–1605. [DOI] [PubMed] [Google Scholar]
- 2.Bjornsson S, Resch T, Acosta S. Symptomatic mesenteric atherosclerotic disease–lessons learned from the diagnostic workup. J Gastrointest Surg 2013; 17: 973–980. [DOI] [PubMed] [Google Scholar]
- 3.ter Steege RW, Sloterdijk HS, Geelkerken RH, et al. Splanchnic artery stenosis and abdominal complaints: Clinical history is of limited value in detection of gastrointestinal ischemia. World J Surg 2012; 36: 793–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ginsburg M, Obara P, Lambert DL, et al. ACR appropriateness criteria imaging of mesenteric ischemia. J Am Coll Radiol 2018; 15: S332–S340. [DOI] [PubMed] [Google Scholar]
- 5.Pillai AK, Kalva SP, Hsu SL, et al. Quality improvement guidelines for mesenteric angioplasty and stent placement for the treatment of chronic mesenteric ischemia. J Vasc Interv Radiol 2018; 29: 642–647. [DOI] [PubMed] [Google Scholar]
- 6.Björck M, Koelemay M, Acosta S, et al. Editor’s choice – management of the diseases of mesenteric arteries and veins: Clinical practice guidelines of the European Society of Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2017; 53: 460–510. [DOI] [PubMed] [Google Scholar]
- 7.Schünemann H, Brożek J, Guyatt G, et al. (eds) GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013 The GRADE Working Group, guidelinedevelopment. https://gdt.gradepro.org/app/handbook/handbook.html#h.fueh5iz0cor4 (October 2013, accessed 14 January 2018)
- 8.Hsu C, Sandford BA. (2007). The Delphi Technique: Making Sense of Consensus. Practical Assessment Research & Evaluation, 12(10). Available online: http://pareonline.net/getvn.asp?v=12&n=10
- 9.Dorman SL, Shelton JA, Stevenson RA, et al. Management of medial humeral epicondyle fractures in children: A structured review protocol for a systematic review of the literature and identification of a core outcome set using a Delphi survey. Trials 2018; 19: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drake RL, Vogl AW, Mitchell AWM, et al. Gray’s anatomy for students. 2nd ed. London, UK: Churchill Livingstone, 2009. [Google Scholar]
- 11.Jaster A, Choudhery S, Ahn R, et al. Anatomic and radiologic review of chronic mesenteric ischemia and its treatment. Clin Imaging 2016; 40: 961–969. [DOI] [PubMed] [Google Scholar]
- 12.Douard R, Chevallier JM, Delmas V, et al. Clinical interest of digestive arterial trunk anastomoses. Surg Radiol Anat 2006; 28: 219–227. [DOI] [PubMed] [Google Scholar]
- 13.Lange JF, Komen N, Akkerman G, et al. Riolan’s arch: Confusing, misnomer, and obsolete. A literature survey of the connection(s) between the superior and inferior mesenteric arteries. Am J Surg 2007; 193: 742–748. [DOI] [PubMed] [Google Scholar]
- 14.Mensink PB, Moons LM, Kuipers EJ. Chronic gastrointestinal ischaemia: Shifting paradigms. Gut 2011; 60: 722–737. [DOI] [PubMed] [Google Scholar]
- 15.Croft RJ, Menon GP, Marston A. Does ‘intestinal angina’ exist? A critical study of obstructed visceral arteries. Br J Surg 1981; 68: 316–318. [DOI] [PubMed] [Google Scholar]
- 16.Derrick JR, Pollard HS, Moore RM. The pattern of arteriosclerotic narrowing of the celiac and superior mesenteric arteries. Ann Surg 1959; 149: 684–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glockner JF. Incidental findings on renal MR angiography. AJR Am J Roentgenol 2007; 189: 693–700. [DOI] [PubMed] [Google Scholar]
- 18.Hansen KJ, Wilson DB, Craven TE, et al. Mesenteric artery disease in the elderly. J Vasc Surg 2004; 40: 45–52. [DOI] [PubMed] [Google Scholar]
- 19.Jarvinen O, Laurikka J, Sisto T, et al. Atherosclerosis of the visceral arteries. Vasa 1995; 24: 9–14. [PubMed] [Google Scholar]
- 20.Park CM, Chung JW, Kim HB, et al. Celiac axis stenosis: Incidence and etiologies in asymptomatic individuals. Korean J Radiol 2001; 2: 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roobottom CA, Dubbins PA. Significant disease of the celiac and superior mesenteric arteries in asymptomatic patients: Predictive value of Doppler sonography. AJR Am J Roentgenol 1993; 161: 985–988. [DOI] [PubMed] [Google Scholar]
- 22.Thomas JH, Blake K, Pierce GE, et al. The clinical course of asymptomatic mesenteric arterial stenosis. J Vasc Surg 1998; 27: 840–844. [DOI] [PubMed] [Google Scholar]
- 23.Wilson DB, Mostafavi K, Craven TE, et al. Clinical course of mesenteric artery stenosis in elderly americans. Arch Intern Med 2006; 166: 2095–2100. [DOI] [PubMed] [Google Scholar]
- 24.Harki J, Vergouwe Y, Spoor JA, et al. Diagnostic accuracy of the combination of clinical symptoms and CT or MR angiography in patients with chronic gastrointestinal ischemia. J Clin Gastroenterol 2017; 51: e39–e47. [DOI] [PubMed] [Google Scholar]
- 25.Veenstra RP, Ter Steege RWF, Geelkerken RH, et al. The cardiovascular risk profile of atherosclerotic gastrointestinal ischemia is different from other vascular beds. Am J Med 2012; 125: 394–398. [DOI] [PubMed] [Google Scholar]
- 26.Moawad J, Gewertz BL. Chronic mesenteric ischemia: Clinical presentation and diagnosis. Surg Clin North Am 1997; 77: 357–369. [DOI] [PubMed] [Google Scholar]
- 27.Petnys A, Puech-Leão P, Zerati AE, et al. Prevalence of signs of celiac axis compression by the median arcuate ligament on computed tomography angiography in asymptomatic patients. J Vasc Surg 2018; 68: 1782–1787. [DOI] [PubMed] [Google Scholar]
- 28.Kazan V, Qu W, Al-Natour M, Abbas J, et al. Celiac artery compression syndrome: A radiological finding without clinical symptoms? Vascular 2013; 21: 293–299. [DOI] [PubMed] [Google Scholar]
- 29.Soman S, Sudhakar SV, Keshava SN. Celiac axis compression by median arcuate ligament on computed tomography among asymptomatic persons. Indian J Gastroenterol 2010; 29: 121–123. [DOI] [PubMed] [Google Scholar]
- 30.Kim EN, Lamb K, Relles D, et al. Median arcuate ligament syndrome – review of this rare disease. JAMA Surg 2016; 151: 471–477. [DOI] [PubMed] [Google Scholar]
- 31.Koster MJ, Warrington KJ. Vasculitis of the mesenteric circulation. Best Pract Res Clin Gastroenterol 2017; 31: 85–96. [DOI] [PubMed] [Google Scholar]
- 32.Rits Y, Oderich GS, Bower TC, et al. Interventions for mesenteric vasculitis. J Vasc Surg 2010; 51: 392–400.e2. [DOI] [PubMed] [Google Scholar]
- 33.Ha HK, Lee SH, Rha SE, et al. Radiologic features of vasculitis involving the gastrointestinal tract. Radiographics 2000; 20: 779–794. [DOI] [PubMed] [Google Scholar]
- 34.Harnik IG, Brandt LJ. Mesenteric venous thrombosis. Vasc Med 2010; 15: 407–418. [DOI] [PubMed] [Google Scholar]
- 35.Orr DW, Harrison PM, Devlin J, et al. Chronic mesenteric venous thrombosis: Evaluation and determinants of survival during long-term follow-up. Clin Gastroenterol Hepatol 2007; 5: 80–86. [DOI] [PubMed] [Google Scholar]
- 36.Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic therapy and prevention of thrombosis, 9th ed.: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2012; 141: e419S–e496S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilcox MG, Howard TJ, Plaskon LA, et al. Current theories of pathogenesis and treatment of nonocclusive mesenteric ischemia. Dig Dis Sci 1995; 40: 709–716. [DOI] [PubMed] [Google Scholar]
- 38.Corcos O, Nuzzo A. Gastro-intestinal vascular emergencies. Best Pract Res Clin Gastroenterol 2013; 27: 709–725. [DOI] [PubMed] [Google Scholar]
- 39.Mensink PB, van Petersen AS, Geelkerken RH, et al. Clinical significance of splanchnic artery stenosis. Br J Surg 2006; 93: 1377–1382. [DOI] [PubMed] [Google Scholar]
- 40.Sana A, Vergouwe Y, van Noord D, et al. Radiological imaging and gastrointestinal tonometry add value in diagnosis of chronic gastrointestinal ischemia. Clin Gastroenterol Hepatol 2011; 9: 234–241. [DOI] [PubMed] [Google Scholar]
- 41.Dominguez-Munoz JE, Drewes AM, Lindkvist B, et al. Recommendations from the United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis. Pancreatology 2018; 18: 847–854. [DOI] [PubMed] [Google Scholar]
- 42.van Dijk LJD, Moons LMG, van Noord D, et al. Persistent symptom relief after revascularization in patients with single-artery chronic mesenteric ischemia. J Vasc Surg 2018; 68: 779–785. [DOI] [PubMed] [Google Scholar]
- 43.Silva JA, White CJ, Collins TJ, et al. Endovascular therapy for chronic mesenteric ischemia. J Am Coll Cardiol 2006; 47: 944–950. [DOI] [PubMed] [Google Scholar]
- 44.Char D, Hines G. Chronic mesenteric ischemia: Diagnosis and treatment. Heart Dis 2001; 3: 231–235. [DOI] [PubMed] [Google Scholar]
- 45.Cademartiri F, Palumbo A, Maffei E, et al. Noninvasive evaluation of the celiac trunk and superior mesenteric artery with multislice CT in patients with chronic mesenteric ischaemia. Radiol Med 2008; 113: 1135–1142. [DOI] [PubMed] [Google Scholar]
- 46.Mateo RB, O’Hara PJ, Hertzer NR, et al. Elective surgical treatment of symptomatic chronic mesenteric occlusive disease: Early results and late outcomes. J Vasc Surg 1999; 29: 821–832. [DOI] [PubMed] [Google Scholar]
- 47.Zwolak RM, Fillinger MF, Walsh DB, et al. Mesenteric and celiac duplex scanning: A validation study. J Vasc Surg 1998; 27: 1078–1087; discussion: 1088. [DOI] [PubMed] [Google Scholar]
- 48.Lejay A, Georg Y, Tartaglia E, et al. Chronic mesenteric ischemia: 20 Year experience of open surgical treatment. Eur J Vasc Endovasc Surg 2015; 49: 587–592. [DOI] [PubMed] [Google Scholar]
- 49.Moneta GL, Lee RW, Yeager RA, et al. Mesenteric duplex scanning: A blinded prospective study. J Vasc Surg 1993; 17: 79–84; discussion: 85–86. [DOI] [PubMed] [Google Scholar]
- 50.Peck MA, Conrad MF, Kwolek CJ, et al. Intermediate-term outcomes of endovascular treatment for symptomatic chronic mesenteric ischemia. J Vasc Surg 2010; 51: 140–7.e2. [DOI] [PubMed] [Google Scholar]
- 51.Kolkman JJ, Geelkerken RH. Diagnosis and treatment of chronic mesenteric ischemia: An update. Best Pract Res Clin Gastroenterol 2017; 31: 49–57. [DOI] [PubMed] [Google Scholar]
- 52.Schaefer PJ, Pfarr J, Trentmann J, et al. Comparison of noninvasive imaging modalities for stenosis grading in mesenteric arteries. Rofo 2013; 185: 628–634. [DOI] [PubMed] [Google Scholar]
- 53.Lehtimaki TT, Karkkainen JM, Saari P, et al. Detecting acute mesenteric ischemia in CT of the acute abdomen is dependent on clinical suspicion: Review of 95 consecutive patients. Eur J Radiol 2015; 84: 2444–2453. [DOI] [PubMed] [Google Scholar]
- 54.Lourenco PDM, Rawski R, Mohammed MF, et al. Dual-energy CT iodine mapping and 40-keV monoenergetic applications in the diagnosis of acute bowel ischemia. AJR Am J Roentgenol 2018; 211: 564–570. [DOI] [PubMed] [Google Scholar]
- 55.Wasser MN, Geelkerken RH, Kouwenhoven M, et al. Systolically gated 3D phase contrast MRA of mesenteric arteries in suspected mesenteric ischemia. J Comput Assist Tomogr 1996; 20: 262–268. [DOI] [PubMed] [Google Scholar]
- 56.Holland GA, Dougherty L, Carpenter JP, et al. Breath-hold ultrafast three-dimensional gadolinium-enhanced MR angiography of the aorta and the renal and other visceral abdominal arteries. AJR Am J Roentgenol 1996; 166: 971–981. [DOI] [PubMed] [Google Scholar]
- 57.Meaney JF, Prince MR, Nostrant TT, Stanley JC. Gadolinium-enhanced MR angiography of visceral arteries in patients with suspected chronic mesenteric ischemia. J Magn Reson Imaging 1997; 7: 171–176. [DOI] [PubMed] [Google Scholar]
- 58.Carlos RC, Stanley JC, Stafford-Johnson D, et al. Interobserver variability in the evaluation of chronic mesenteric ischemia with gadolinium-enhanced MR angiography. Acad Radiol 2001; 8: 879–887. [DOI] [PubMed] [Google Scholar]
- 59.Hany TF, Schmidt M, Schoenenberger AW, et al. Contrast-enhanced three-dimensional magnetic resonance angiography of the splanchnic vasculature before and after caloric stimulation. Original investigation. Invest Radiol 1998; 33: 682–686. [DOI] [PubMed] [Google Scholar]
- 60.van Petersen AS, Meerwaldt R, Kolkman JJ, et al. The influence of respiration on criteria for transabdominal duplex examination of the splanchnic arteries in patients with suspected chronic splanchnic ischemia. J Vasc Surg 2013; 57: 1603–1611. [DOI] [PubMed] [Google Scholar]
- 61.Bowersox JC, Zwolak RM, Walsh DB, et al. Duplex ultrasonography in the diagnosis of celiac and mesenteric artery occlusive disease. J Vasc Surg 1991; 14: 780–786; discussion 786–788. [DOI] [PubMed] [Google Scholar]
- 62.Perko MJ, Just S, Schroeder TV. Importance of diastolic velocities in the detection of celiac and mesenteric artery disease by duplex ultrasound. J Vasc Surg 1997; 26: 288–293. [DOI] [PubMed] [Google Scholar]
- 63.AbuRahma AF, Stone PA, Srivastava M, et al. Mesenteric/celiac duplex ultrasound interpretation criteria revisited. J Vasc Surg 2012; 55: 428–436. [DOI] [PubMed] [Google Scholar]
- 64.van Dijk LJ, van Petersen AS, Moelker A. Vascular imaging of the mesenteric vasculature. Best Pract Res Clin Gastroenterol 2017; 31: 3–14. [DOI] [PubMed] [Google Scholar]
- 65.Osiecki M, Zukowski P, Brzozowski K, et al. Angiographic evaluation of celiac trunk functional stenosis – own experiences. Chir Pol 2003; 5: 229–234. [Google Scholar]
- 66.Patten RM, Coldwell DM, Ben-Menachem Y. Ligamentous compression of the celiac axis: CT findings in five patients. AJR Am J Roentgenol 1991; 156: 1101–1103. [DOI] [PubMed] [Google Scholar]
- 67.Al-Zoubi NA, Yaghan RJ, Hijazi EM, et al. Prevalence of incidental celiac artery compression caused by median arcuate ligament among patients with peripheral arterial disease Ital J Vasc Endovasc Surg 2017; 24: 47–49. [Google Scholar]
- 68.Wang XM, Hua XP, Zheng GL. Celiac artery compression syndrome evaluated with 3-D contrast-enhanced ultrasonography: A new approach. Ultrasound Med Biol 2018; 44: 243–250. [DOI] [PubMed] [Google Scholar]
- 69.Gruber H, Loizides A, Peer S, et al. Ultrasound of the median arcuate ligament syndrome: A new approach to diagnosis. Med Ultrason 2012; 14: 5–9. [PubMed] [Google Scholar]
- 70.van Noord D, Kolkman JJ. Functional testing in the diagnosis of chronic mesenteric ischemia. Best Pract Res Clin Gastroenterol 2017; 31: 59–68. [DOI] [PubMed] [Google Scholar]
- 71.Mensink PBF, van Petersen AS, Kolkman JJ, et al. Gastric exercise tonometry: The key investigation in patients with suspected celiac artery compression syndrome. J Vasc Surg 2006; 44: 277–281. [DOI] [PubMed] [Google Scholar]
- 72.Otte JA, Geelkerken RH, Oostveen E, et al. Clinical impact of gastric exercise tonometry on diagnosis and management of chronic gastrointestinal ischemia. Clin Gastroenterol Hepatol 2005; 3: 660–666. [DOI] [PubMed] [Google Scholar]
- 73.Van Noord D, Sana A, Benaron DA, et al. Endoscopic visible light spectroscopy: A new, minimally invasive technique to diagnose chronic GI ischemia. Gastrointest Endosc 2011; 73: 291–298. [DOI] [PubMed] [Google Scholar]
- 74.van Dijk LJD, van der Wel T, van Noord D, et al. Intraobserver and interobserver reliability of visible light spectroscopy during upper gastrointestinal endoscopy. Expert Rev Med Devices 2018; 15: 605–610. [DOI] [PubMed] [Google Scholar]
- 75.van Dijk LJD, Harki J, van Noord D, et al. Detection of mesenteric ischemia by means of endoscopic visible light spectroscopy after luminal feeding. Gastrointest Endosc 2019; 89: 94–102. [DOI] [PubMed] [Google Scholar]
- 76.Ubbink R, van Dijk LJD, van Noord D, et al. Evaluation of endoscopic visible light spectroscopy: Comparison with microvascular oxygen tension measurements in a porcine model. J Transl Med 2019; 17: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Noord D, Mensink PB, de Knegt RJ, et al. Serum markers and intestinal mucosal injury in chronic gastrointestinal ischemia. Dig Dis Sci 2011; 56: 506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mensink PB, Hol L, Borghuis-Koertshuis N, et al. Transient postprandial ischemia is associated with increased intestinal fatty acid binding protein in patients with chronic gastrointestinal ischemia. Eur J Gastroenterol Hepatol 2009; 21: 278–282. [DOI] [PubMed] [Google Scholar]
- 79.Derikx JP, Schellekens DH, Acosta S. Serological markers for human intestinal ischemia: A systematic review. Best Pract Res Clin Gastroenterol 2017; 31: 69–74. [DOI] [PubMed] [Google Scholar]
- 80.Acosta S, Nilsson T. Current status on plasma biomarkers for acute mesenteric ischemia. J Thromb Thrombolysis 2012; 33: 355–361. [DOI] [PubMed] [Google Scholar]
- 81.Van Noord D, Biermann K, Moons LM, et al. Histological changes in patients with chronic upper gastrointestinal ischaemia. Histopathology 2010; 57: 615–621. [DOI] [PubMed] [Google Scholar]
- 82.Pecoraro F, Rancic Z, Lachat M, et al. Chronic mesenteric ischemia: Critical review and guidelines for management. Ann Vasc Surg 2013; 27: 113–122. [DOI] [PubMed] [Google Scholar]
- 83.Gupta PK, Horan SM, Turaga KK, et al. Chronic mesenteric ischemia: Endovascular versus open revascularization. J Endovasc Ther 2010; 17: 540–549. [DOI] [PubMed] [Google Scholar]
- 84.Hogendoorn W, Hunink MG, Schlosser FJ, et al. A comparison of open and endovascular revascularization for chronic mesenteric ischemia in a clinical decision model. J Vasc Surg 2014; 60: 715–25.e2. [DOI] [PubMed] [Google Scholar]
- 85.Allain C, Besch G, Guelle N, et al. Prevalence and impact of malnutrition in patients surgically treated for chronic mesenteric ischemia. Ann Vasc Surg 2019; 58: 24–31. [DOI] [PubMed] [Google Scholar]
- 86.Burden S, Todd C, Hill J, et al. Pre-operative nutrition support in patients undergoing gastrointestinal surgery. Cochrane Database Syst Rev 2012; 11: CD008879. [DOI] [PubMed] [Google Scholar]
- 87.Sarap AN, Sarap MD, Childers J. Small bowel necrosis in association with jejunal tube feeding. JAAPA 2010; 23: 28, 30–32. [DOI] [PubMed] [Google Scholar]
- 88.Melis M, Fichera A, Ferguson MK. Bowel necrosis associated with early jejunal tube feeding: A complication of postoperative enteral nutrition. Arch Surg 2006; 141: 701–704. [DOI] [PubMed] [Google Scholar]
- 89.Wang K, McIlroy K, Plank LD, et al. , Outcomes, and management of enteral tube feeding intolerance: A retrospective cohort study in a tertiary center. JPEN J Parenter Enteral Nutr 2017; 41: 959–967. [DOI] [PubMed] [Google Scholar]
- 90.Gatt M, MacFie J, Anderson AD, et al. Changes in superior mesenteric artery blood flow after oral, enteral, and parenteral feeding in humans. Crit Care Med 2009; 37: 171–176. [DOI] [PubMed] [Google Scholar]
- 91.Dissanaike S, Shelton M, Warner K, et al. The risk for bloodstream infections is associated with increased parenteral caloric intake in patients receiving parenteral nutrition. Crit Care 2007; 11: R114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mueller C, Borriello R, Perlov-Antzis L. Parenteral nutrition support of a patient with chronic mesenteric artery occlusive disease. Nutr Clin Pract 1993; 8: 73–77. [DOI] [PubMed] [Google Scholar]
- 93.Landis MS, Rajan DK, Simons ME, et al. Percutaneous management of chronic mesenteric ischemia: Outcomes after intervention. J Vasc Interv Radiol 2005; 16: 1319–1325. [DOI] [PubMed] [Google Scholar]
- 94.Sheeran SR, Murphy TP, Khwaja A, et al. Stent placement for treatment of mesenteric artery stenoses or occlusions. J Vasc Interv Radiol 1999; 10: 861–867. [DOI] [PubMed] [Google Scholar]
- 95.van de Ven PJ, Kaatee R, Beutler JJ, et al. Arterial stenting and balloon angioplasty in ostial atherosclerotic renovascular disease: A randomised trial. Lancet 1999; 353: 282–286. [DOI] [PubMed] [Google Scholar]
- 96.Dorros G, Prince C, Mathiak L. Stenting of a renal artery stenosis achieves better relief of the obstructive lesion than balloon angioplasty. Cathet Cardiovasc Diagn 1993; 29: 191–198. [DOI] [PubMed] [Google Scholar]
- 97.Kougias P, El Sayed HF, Zhou W, et al. Management of chronic mesenteric ischemia. The role of endovascular therapy. J Endovasc Ther 2007; 14: 395–405. [DOI] [PubMed] [Google Scholar]
- 98.Schoch DM, LeSar CJ, Joels CS, et al. Management of chronic mesenteric vascular insufficiency: An endovascular approach. J Am Coll Surg 2011; 212: 668–675; discussion 675–677. [DOI] [PubMed] [Google Scholar]
- 99.Mirza AK, Oderich GS, Sandri GA, et al. Outcomes of upper extremity access during fenestrated-branched endovascular aortic repair. J Vasc Surg 2019; 69: 635–643. [DOI] [PubMed] [Google Scholar]
- 100.Oderich GS, Tallarita T, Gloviczki P, et al. Mesenteric artery complications during angioplasty and stent placement for atherosclerotic chronic mesenteric ischemia. J Vasc Surg 2012; 55: 1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 2019; 40: 87–165. [DOI] [PubMed] [Google Scholar]
- 102.Meertens MM, Ng E, Loh SEK, et al. Transradial approach for aortoiliac and femoropopliteal interventions: A systematic review and meta-analysis. J Endovasc Ther 2018; 25: 599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen YY, Liu P, Wu YS, et al. Transradial vs transfemoral access in patients with hepatic malignancy and undergoing hepatic interventions: A systematic review and meta-analysis. Medicine (Baltimore ) 2018; 97: e13926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oderich GS, Macedo R, Stone DH, et al. Multicenter study of retrograde open mesenteric artery stenting through laparotomy for treatment of acute and chronic mesenteric ischemia. J Vasc Surg 2018; 68: 470–80.e1. [DOI] [PubMed] [Google Scholar]
- 105.Blauw JT, Meerwaldt R, Brusse-Keizer M, et al. Retrograde open mesenteric stenting for acute mesenteric ischemia. J Vasc Surg 2014; 60: 726–734. [DOI] [PubMed] [Google Scholar]
- 106.Oderich GS, Erdoes LS, Lesar C, et al. Comparison of covered stents versus bare metal stents for treatment of chronic atherosclerotic mesenteric arterial disease. J Vasc Surg 2013; 58: 1316–1323. [DOI] [PubMed] [Google Scholar]
- 107.van Dijk LJD, Harki J, van Noord D, et al. Covered stents versus bare-metal stents in chronic atherosclerotic gastrointestinal ischemia (CoBaGI): Study protocol for a randomized controlled trial. Trials 2019; 20: 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wagenhäuser MU, Meyer-Janiszewski YK, Dueppers P, et al. Chronic mesenteric ischemia: Patient outcomes using open surgical revascularization. Dig Surg 2017; 34: 340–349. [DOI] [PubMed] [Google Scholar]
- 109.Flis V, Mrdža B, Štirn B, et al. Revascularization of the superior mesenteric artery alone for treatment of chronic mesenteric ischemia. Wien Klin Wochenschr 2016; 128: 109–113. [DOI] [PubMed] [Google Scholar]
- 110.Park WM, Cherry KJ, Jr, Chua HK, et al. Current results of open revascularization for chronic mesenteric ischemia: A standard for comparison. J Vasc Surg 2002; 35: 853–858. [DOI] [PubMed] [Google Scholar]
- 111.Foley MI, Moneta GL, Abou-Zamzam Jr AM, et al. Revascularization of the superior mesenteric artery alone for treatment of intestinal ischemia. J Vasc Surg 2000; 32: 37–47. [DOI] [PubMed] [Google Scholar]
- 112.Gentile AT, Moneta GL, Taylor LM, Jr, et al. Isolated bypass to the superior mesenteric artery for intestinal ischemia. Arch Surg 1994; 129: 926–932. [DOI] [PubMed] [Google Scholar]
- 113.McAfee MK, Cherry KJ, Jr, Naessens JM, et al. Influence of complete revascularization on chronic mesenteric ischemia. Am J Surg 1992; 164: 220–224. [DOI] [PubMed] [Google Scholar]
- 114.Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for Dual Antiplatelet Therapy in Coronary Artery Disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018; 39: 213–260. [DOI] [PubMed] [Google Scholar]
- 115.Bittl JA, Baber U, Bradley SM, et al. Duration of dual antiplatelet therapy: A systematic review for the 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016; 68: 1116–1139. [DOI] [PubMed] [Google Scholar]
- 116.Giustino G, Baber U, Sartori S, et al. Duration of dual antiplatelet therapy after drug-eluting stent implantation: A systematic review and meta-analysis of randomized controlled trials. J Am Coll Cardiol 2015; 65: 1298–1310. [DOI] [PubMed] [Google Scholar]
- 117.Qintar M, Chhatriwalla AK, Arnold SV, et al. Beyond restenosis: Patients’ preference for drug eluting or bare metal stents. Catheter Cardiovasc Interv 2017; 90: 357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cavallari I, Patti G. Meta-analysis comparing the safety and efficacy of dual versus triple antithrombotic therapy in patients with atrial fibrillation undergoing percutaneous coronary intervention. Am J Cardiol 2018; 121: 718–724. [DOI] [PubMed] [Google Scholar]
- 119.Cho JS, Carr JA, Jacobsen G, et al. Long-term outcome after mesenteric artery reconstruction: A 37-year experience. J Vasc Surg 2002; 35: 453–460. [DOI] [PubMed] [Google Scholar]
- 120.Cormier JM, Fichelle JM, Vennin J, et al. Atherosclerotic occlusive disease of the superior mesenteric artery: Late results of reconstructive surgery. Ann Vasc Surg 1991; 5: 510–518. [DOI] [PubMed] [Google Scholar]
- 121.McMillan WD, McCarthy WJ, Bresticker MR, et al. Mesenteric artery bypass: Objective patency determination. J Vasc Surg 1995; 21: 729–741. [DOI] [PubMed] [Google Scholar]
- 122.Rheudasil JM, Stewart MT, Schellack JV, et al. Surgical treatment of chronic mesenteric arterial insufficiency. J Vasc Surg 1988; 8: 495–500. [PubMed] [Google Scholar]
- 123.Kihara TK, Blebea J, Anderson KM, et al. Risk factors and outcomes following revascularization for chronic mesenteric ischemia. Ann Vasc Surg 1999; 13: 37–44. [DOI] [PubMed] [Google Scholar]
- 124.Geelkerken RH, van Bockel JH, de Roos WK, et al. Chronic mesenteric vascular syndrome. Results of reconstructive surgery. Arch Surg 1991; 126: 1101–1106. [DOI] [PubMed] [Google Scholar]
- 125.Jimenez JC, Harlander-Locke M, Dutson EP. Open and laparoscopic treatment of median arcuate ligament syndrome. J Vasc Surg 2012; 56: 869–873. [DOI] [PubMed] [Google Scholar]
- 126.Rits Y, Oderich GS, Bower TC, et al. Interventions for mesenteric vasculitis. J Vasc Surg 2010; 51: 392–400.e2. [DOI] [PubMed] [Google Scholar]
- 127.Gaujoux S, Sauvanet A, Vullierme MP, et al. Ischemic complications after pancreaticoduodenectomy: Incidence, prevention, and management. Ann Surg 2009; 249: 111–117. [DOI] [PubMed] [Google Scholar]
- 128.Machado MA, Herman P, Montagnini AL, et al. A new test to avoid arterial complications during pancreaticoduodenectomy. Hepatogastroenterology 2004; 51: 1671–1673. [PubMed] [Google Scholar]
- 129.Aboyans V, Ricco JB, Bartelink MEL, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: The European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J 2018; 39: 763–816. [DOI] [PubMed] [Google Scholar]
- 130.Piepoli MF, Hoes AW, Agewall S, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention and Rehabilitation (EACPR). Eur Heart J 2016; 37: 2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J 2018; 39: 3021–3104. [DOI] [PubMed] [Google Scholar]
- 132.Tallarita T, Oderich GS, Macedo TA, et al. Reinterventions for stent restenosis in patients treated for atherosclerotic mesenteric artery disease. J Vasc Surg 2011; 54: 1422–9.e1. [DOI] [PubMed] [Google Scholar]
- 133.Baker AC, Chew V, Li CS, et al. Application of duplex ultrasound imaging in determining in-stent stenosis during surveillance after mesenteric artery revascularization. J Vasc Surg 2012; 56: 1364–1371; discussion: 1371. [DOI] [PubMed] [Google Scholar]
- 134.Mitchell EL, Chang EY, Landry GJ, et al. Duplex criteria for native superior mesenteric artery stenosis overestimate stenosis in stented superior mesenteric arteries. J Vasc Surg 2009; 50: 335–340. [DOI] [PubMed] [Google Scholar]
- 135.Aburahma AF, Mousa AY, Stone PA, et al. Velocity criteria for native celiac/superior mesenteric artery stenosis vs in-stent stenosis. J Vasc Surg 2012; 55: 730–738. [DOI] [PubMed] [Google Scholar]
- 136.Acosta S, Bjorgell O, Ekberg O. Prospective study on diagnostic performance of color doppler ultrasound using trans-stenotic mean arterial pressure gradient as a reference in stented superior mesenteric artery. Ann Vasc Surg 2019; 56: 294–302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, UEG916681 Supplemental Material for European guidelines on chronic mesenteric ischaemia – joint United European Gastroenterology, European Association for Gastroenterology, Endoscopy and Nutrition, European Society of Gastrointestinal and Abdominal Radiology, Netherlands Association of Hepatogastroenterologists, Hellenic Society of Gastroenterology, Cardiovascular and Interventional Radiological Society of Europe, and Dutch Mesenteric Ischemia Study group clinical guidelines on the diagnosis and treatment of patients with chronic mesenteric ischaemia by Luke G Terlouw, Adriaan Moelker, Jan Abrahamsen, Stefan Acosta, Olaf J Bakker, Iris Baumgartner, Louis Boyer, Olivier Corcos, Louisa JD van Dijk, Mansur Duran, Robert H Geelkerken, Giulio Illuminati, Ralph W Jackson, Jussi M Kärkkäinen, Jeroen J Kolkman, Lars Lönn, Maria A Mazzei, Alexandre Nuzzo, Felice Pecoraro, Jan Raupach, Hence JM Verhagen, Christoph J Zech, Desirée van Noord and Marco J Bruno in United European Gastroenterology Journal