Abstract
Crohn’s disease (CD) is a chronic inflammatory bowel disease that usually progresses to bowel damage, defined as strictures, fistulas and abscesses. These complications require intestinal resection and lead to further irreversible structural damage. Cross-sectional imaging, such as magnetic resonance imaging, computed tomography and ultrasound, are accurate in assessing intestinal damage at a definite time point and the progression of damage over time. Recently, an imaging-based index, the Lémann Index, has been proposed and developed in order to quantify bowel damage in CD patients; emerging data confirm that this Index can measure the structural damage with good sensitivity to change. One challenge remains to understand whether existing or future treatments might be able to stop bowel-damage progression or even reverse intestinal damage, improving the prognosis and changing the natural history of CD. We reviewed the current data available in the literature focused on the measure of structural damage in CD patients, mainly focusing on the impact on therapies in reversing bowel damage. We also explored some further perspectives on measuring and targeting intestinal damage in clinical research and in clinical practice as an ultimate therapeutic target.
Keywords: Ulcerative colitis, inflammatory bowel disease, IBD, Crohn’s disease, gastroenterology
Brief clinical case
Two 29-year-old patients were diagnosed with ileal Crohn’s disease (CD). Colonoscopy showed deep ulcers and passable stricture for 15 cm in the terminal ileum, and this was confirmed by magnetic resonance imaging (MRI) enterography. No obstructive symptoms were present. The two patients were treated for 24 weeks with anti-tumour necrosis factor (TNF). However, one patient achieved remission, and the other one had obstructive symptoms requiring ileal resection. After one year, both patients presented with clinical remission (Harvey–Bradshaw Index <4), biochemical remission (normal C-reactive protein (CRP)) and no endoscopic lesions. There was, however, one major difference: one of them had permanent CD-related structural damage due to ileal resection, whereas the other one had no active structural bowel damage.
How to diagnose structural damage in CD
As in rheumatoid arthritis,1 the concept of bowel damage has also been investigated in inflammatory bowel disease (IBD), in particular in CD. The advances in cross-sectional techniques, such as ultrasounds (US), computed tomography (CT) enterography and MRI, which are routinely used with the same accuracy to assess CD activity,2 have led to better evaluation of extra-intestinal complications (strictures, fistulas and abscesses), which can be considered as signs of bowel damage.3,4
There is no clear definition of bowel damage, since the presence of irreversible fibrosis or penetrating complications may coexist with active inflammation.5,6 Usually, in CD, a shift in disease behaviour according to the Montreal classification7 from non-stricturing non-penetrating behaviour to a stricturing or penetrating phenotype is considered as disease progression.8 Moreover, the presence of any complication, such as stricture, fistula or abscess, is widely accepted as bowel damage.3,8 Peyrin-Biroulet et al.8 defined bowel damage as the presence of any fistula (including perianal fistulas), abscess or stricture assessed by CT or MRI. Quantification of cumulative bowel damage is thus important to understand how the disease progresses and tp plan an effective therapeutic management that could prevent bowel-damage progression over time.
Quantifying structural bowel damage: available proposed scores
The assessment of bowel damage requires a full evaluation of the gastrointestinal tract. A combination of endoscopy9,10 together with imaging techniques, such as MRI, CT or US, allows the involvement of the bowel wall and extra-luminal complications (including fistulas and abscesses), as well as perianal disease,1,2,11–14 to be assessed and can give a precise overview of the damaged digestive tracts.
Two scores of intestinal damage based on imaging techniques have been proposed: the Lémann Index15,16 based on a combination of endoscopy, MRI or CT findings and surgical history, and the sonographic lesion index for CD (SLIC)17,18 based on the small intestine contrast ultrasonography (SICUS).17 While the validity of the Lémann Index has been widely investigated in observational cohort studies, the SLIC has yet to be validated in further studies, mainly because of the limitations and the poor reproducibility of the SICUS.
The Lémann Index
During the last decade, the IPNIC group has developed the Crohn’s Disease Digestive Damage Score (CD3S) – namely, the Lémann Index.19 This index aims to measure cumulative digestive tissue damage based on a comprehensive assessment of structural bowel damage, including stricturing lesions and penetrating lesions (fistulas and abscesses) together with surgical resection history, in different CD settings. The Index divides the entire gastrointestinal tract into four organs (upper tract, small bowel, colon/rectum and anus) and then divides each organ into different segments, scoring stricturing and penetrating lesions on a four-degree scale (0–3), according to the severity of the lesions (Table 1). Surgical resection is also considered and is scored as the maximum grade of bowel damage at the segment level (grade 3). Multivariate analysis defined coefficients for both severity (from grade 0 to grade 3) and location (upper tract, small bowel, colon and anus; Table 1).16 In this development phase, cut-off values for neither the presence of bowel damage nor sensitivity to change were defined and assessed. The full validation of this index is yet to be completed.
Table 1.
|
Severity scale based on complications and surgical
history
15
| |||||
|---|---|---|---|---|---|
| Severity | Definitions |
||||
| Stricturing lesions | Penetrating lesions | Surgical procedures | |||
| Grade 0 | Normal | Normal | None | ||
| Grade 1 | Wall thickening <3 mm and/or segmental enhancement without prestenotic dilatation | – | – | ||
| Grade 2 | Wall thickening ≥3 mm and/or mural stratification without prestenotic dilatation | Deep transmural ulceration | Bypass diversion or stricturoplasty | ||
| Grade 3 | Stricture with prestenotic dilatation | Abscess or any type of fistula | Resection | ||
| Coefficients for the Lémann Index calculation 16 | |||||
| Organ |
Complication |
Grade of complication |
Complication coefficient |
Organ coefficient |
Notes |
| Upper tract (oesophagus, stomach, duodenum) | Stricture | 2 | 3.5 | 2.0 | Grade 1 strictures, and grade 1 and grade 2 penetrating are not included in the evaluation |
| Stricture | 3 | 3.5 | |||
| Penetrating | 3 | 2.0 | |||
| Small bowel (20 segments of 20 cm) | Stricture | 1 | 1.0 | 5.0 | Grade 1 penetrating complications are not included in the evaluation |
| Stricture | 2 | 2.5 | |||
| Stricture | 3 | 5.0 | |||
| Penetrating | 2 | 1.5 | |||
| Penetrating | 3 | 4.0 | |||
| Colon and rectum | Stricture | 2 | 2.0 | 3.5 | Grade 1 strictures are not included in the evaluation |
| Stricture | 3 | 5.5 | |||
| Penetrating | 1 | 1.0 | |||
| Penetrating | 2 | 2.5 | |||
| Penetrating | 3 | 4.5 | |||
| Anus | Stricture | 2 | 1.5 | 3.5 | Grade 1 strictures, and grade 1 and grade 2 penetrating are not included in the evaluation |
| Stricture | 3 | 3.5 | |||
| Penetrating | 2 | 2.5 | |||
| Penetrating | 3 | 3.5 | |||
Lémann calculation: (number of segments with each complication × complication coefficient × organ coefficient). As an example, a patient with one frank ileal 15-cm-long stricture (stricturing grade 3) with deep transmural ulceration in the same tract (penetrating grade 2) will have a Lémann score of (1 × 5 × 5 + 1 × 1.5 × 5) = 32.5.
Natural history of bowel-damage progression in CD
IBD, including CD and ulcerative colitis (UC), are chronic, destructive, progressive and disabling diseases. CD involves the entire gastrointestinal tract, mainly the ileum and the proximal colonic tract,20 and can affect the entire bowel wall, even with extra-mural complications, whereas UC affects only the colonic tract and is limited at the mucosal level.21 In CD, the chronic inflammatory pattern activates tissue-repairing mechanisms which lead to irreversible fibrosis, or deep ulcers may go through the entire bowel wall, resulting in fistulas and abscesses.22 All these complications cause permanent bowel damage, since they usually require surgery.23 In UC, these complications are rare. However, in long-standing disease, there could be an infiltration of neutrophils and activation of fibroblasts, resulting in colonic damage and loss of function.24
Up to 60% of patients who present with a non-stricturing non-penetrating CD phenotype at diagnosis evolve to stricturing or penetrating disease.25,26 About 40% of CD patients present with bowel damage at the time of diagnosis.4 Cumulative CD-related bowel damage can compromise the intestinal function, with important consequences for patients’ quality of life, including disability,27 although no studies have directly investigated this association. Whether prevention of organ damage by early effective therapy or treatment to reverse bowel damage in IBD can be considered achievable goals remains unclear.
Is bowel damage reversible in IBD?
There have been a number of studies conducted recently assessing whether the Lémann Index is able to measure structural damage, and whether it is sensitive to changes over time28–32 (Table 2). Gilletta et al. retrospectively evaluated 221 CD subjects, and they found that >50% of patients had bowel damage (defined as a Lémann Index score of >2.0) after 2–10 years since diagnosis. A high Lémann Index score at first evaluation, duration of clinical activity and intestinal resection were associated with bowel damage over time.28 Duveau et al. found that the Lémann Index score increased in >30% of patients during a median follow-up period of 23 months in a small population of 30 subjects.
Table 2.
Measures of structural bowel damage in clinical studies.
| Study | Study design | Study population | Measure of structural damage | Main outcomes | Main results |
|---|---|---|---|---|---|
| Gilletta et al.30 | Retrospective cohort analysis | 221 CD subjects up to 10 years FU | LI | LI cut-off for damage | A LI score >2.0 is associated with bowel damage A high LI score at baseline, chronically active disease and intestinal resection were associated to BD progression over time |
| Duveau et al.32 | Retrospective cohort analysis | 30 CD subjects | LI | LI evolution over time (median time 23 months) | 1/3 of subjects have BD progression over time |
| Fiorino et al.31 | Prospective cohort | 30 CD subjects starting anti-TNF (median FU 32.5 months) | LI | Efficacy of anti-TNF on BD progression | Anti-TNFs block BD in 83% of subjects BD progression is associated with a higher risk for surgery Cut-off for BD: 4.8 Cut off for BD progression: >0.3 |
| Bodini et al.34 | Retrospective cohort analysis | 88 CD subjects treated with anti-TNF, IMM, or 5-ASA median FU 26 months | LI | Efficacy of different therapies on LI reduction | Anti-TNFs were more effective than IMM and 5-ASA at stopping bowel damage (p = 0.004) |
| Fiorino et al.12 | Retrospective cohort analysis | 142 consecutive CD patients (Milan, Nancy) at the time of diagnosis | LI | Prevalence of bowel damage at CD diagnosis Prognostic value of the LI in the follow-up (median time 4.9 years) | LI and presence of bowel damage were associated with a higher risk of surgery (HR = 1.1 and 3.2, respectively; p < 0.001) and hospitalisation (HR = 1.08, p = 0.002, and HR = 1.88, p < 0.001, respectively) in the follow-up period |
| Amitai et al.33 | Prospective cohort study | 61 patients with quiescent small bowel CD since at least three months undergoing MRI and VCE | LI | Progression of bowel damage over time | LI remained stable in the follow-up time (14.8 ± 2.5 months) |
| Ribaldone et al.35 | Retrospective cohort analysis | 91 CD patients were enrolled, 31 (34.1%) treated with adalimumab and 60 (65.9%) with azathioprine (FU time 12 months) | LI | Efficacy of adalimumab and azathioprine in reducing bowel damage | Adalimumab decreased the mean LI score after 12 months (from 9.9 to 8.8), while azathioprine increased it (from 7.7 to 8.8) |
| Rispo et al.37 | Prospective cohort study | 71 patients undergoing MRI and US | LI | Correlation between MRI-based and US-based LI | High correlation between MRI-based and US-based LI score (r = 0.90; p < 0.001) |
| Zorzi et al.28 | Prospective cohort study | 29 patients with ileal or ileo-colonic CD treated with anti-TNF | SLIC | Sensitivity to change of the SLIC | Patients responding to anti-TNFs showed a significant decrease in the SLIC compared to non-responders |
CD: Crohn’s disease; FU: follow-up; TNF: tumour necrosis factor; BD: bowel damage; LI: Lémann Index; IMM: immunomodulators; 5-ASA: 5-aminosalycilic acid; POR: postoperative recurrence; HR: hazard ratio; VCE: video capsule endoscopy; SLIC: sonographic lesion index for CD; US: bowel ultrasound; MRI: magnetic resonance imaging.
Fiorino et al.29 investigated bowel damage variations in 30 subjects treated with anti-TNF and followed up prospectively for a median of 32.5 months. A Lémann Index score of 4.8 was found to be the best cut-off value for bowel damage, and an increase of >0.3 in the Lémann Index score was associated with bowel-damage progression. Anti-TNFs were effective in stopping bowel-damage progression in 83% of subjects. Bowel-damage progression was associated with the risk of major surgery in the follow-up period (hazard ratio (HR) = 0.19, p = 0.005).29 Bodini et al.32 evaluated 104 CD patients divided in three study groups according to treatments received – biological drugs (n = 40; 38.4%), azathioprine (n = 19; 18.3%) and mesalazine (n = 45; 43.3%) – for a median time of 29.5 months. The median Lémann Index score did not change significantly in the biological group (p = 0.543), whereas it increased in the azathioprine group (p = 0.0006) and in the mesalazine group (p < 0.0001), suggesting that resolution of inflammation may be associated with the blockade of disease and damage progression in CD patients. These results are also confirmed by a retrospective analysis by Ribaldone et al.33 who found a decrease in the Lémann Index score after 12 months (from 9.9 to 8.8) in patients with CD treated with adalimumab compared to CD patients treated with azathioprine, in whom the mean Lémann Index score increased (from 7.7 to 8.8). In this study, adalimumab was associated with a significantly higher bowel-damage regression/blockade than in patients treated with azathioprine (67% vs. 28%, respectively; p = 0.006). Magro et al. also showed that disease progression was lower for both monotherapy with azathioprine (HR = 0.15, p < 0.001) or combination therapy with anti-TNF-α (HR = 0.33, p < 0.001), whereas upper gastrointestinal-tract involvement, male sex and steroid use were associated with an early progression of phenotype from B1 to B2 or B3 (p < 0.001).34
A prospective study31 of 61 CD patients with known quiescent small-bowel CD, followed up with magnetic resonance enterographies and video capsule endoscopies for a median of 14 months, showed that significant structural bowel damage was present in 21.4% at baseline, defined as a Lémann Index score >4.8. However, structural bowel-damage progression, defined as an increase in the Lémann Index score of >0.3, was negligible in the follow-up time.
All these data suggest that the blockade of disease progression as assessed by the Lémann Index might be a relevant goal to assess the long-term effectiveness of therapeutic management in CD. Early treatment may be effective in preventing bowel-damage progression in CD. A retrospective analysis of 88 patients followed up at the Mount Sinai Hospital in New York showed that damage progressed in 29 (50%), regressed in 20 (34.5%) and stabilised in 9 (15.5%) among the 58 early-treated cases. There was a trend in favour of earlier introduction to slow the rate of progression (ρ = 0.241; p = 0.069). However, further prospective large studies are needed to confirm these preliminary findings.
The Lémann Index has been developed by using MRI or CT as a standard cross-sectional imaging technique to assess structural bowel damage. Recent advances in knowledge in regards of bowel US suggest that bowel damage can be assessed by this technique, translating the Lémann Index calculation by using US findings. Rispo et al.35 developed a US-based Lémann Index and compared this index to the Lémann Index assessed by MRI. They prospectively enrolled 71 consecutive patients with CD. Seventy-three per cent of patients had complications (strictures and/or fistulas). Median MRI Lémann Index and US Lémann Index scores were 6.62 (95% confidence interval (CI) 4.2–9.7) and 6.04 (95% CI 3.6–9.2), respectively (ρ = 0.90; p < 0.001). There was no significant correlation between the Lémann Index and the Harvey–Bradshaw index (p = 0.9), while a significant correlation was found between both scores (US and MRI based) and CD duration (p = 0.01).
Measuring structural damage in IBD: future challenges
The concept of bowel damage is an emerging goal for the general management of CD, and it is strongly connected with disease severity. A Delphi consensus by 14 IBD specialists under the umbrella of the International Organization for IBD has agreed on the relevance of accumulating bowel damage (in particular, complications and major surgery with intestinal resection) as associated with overall CD severity.36 However, the best way to define, quantify and monitor bowel damage over time is still debated.
The Lémann Index is accurate for assessing bowel damage at a definite time point. However, there are some issues regarding this score in measuring bowel damage that are still to be clarified. First, data from Fiorino et al.29 showed that structural bowel damage as measured by the Lémann Index can be reduced by effective therapies (Figure 1), in contrast with the definition of damage, which should be irreversible by a definition.6 The presence of parameters of inflammation, such as deep ulcers, is the main reason for such a discrepancy. The persistence of bowel damage measured by the Lémann Index when inflammation is absent, and the absence of a correlation with the current endoscopic and radiological scores, such as the Simplified Endoscopic index of Severity or the MaRIA score,29,37 indicate, however, that the Lémann Index may be influenced by but is not a score of inflammation. In the same study, the authors suggest the evaluation of ‘residual bowel damage’, that is, the damage assessed by the Lémann Index after successful treatment of inflammation, as a pure parameter of the structural damage not related to active inflammation, and therefore the difference in the Lémann Index score at two different time points (i.e. before and after adequate time of therapy) would give clearer and more valid information on the disease course than a single measure at one definite time point. This aspect may raise some concerns as to the validity of a single measurement of the Lémann Index in patients at high risk of progression, since endoscopic activity and the assessment of mucosal healing are key aspects for CD prognosis.29
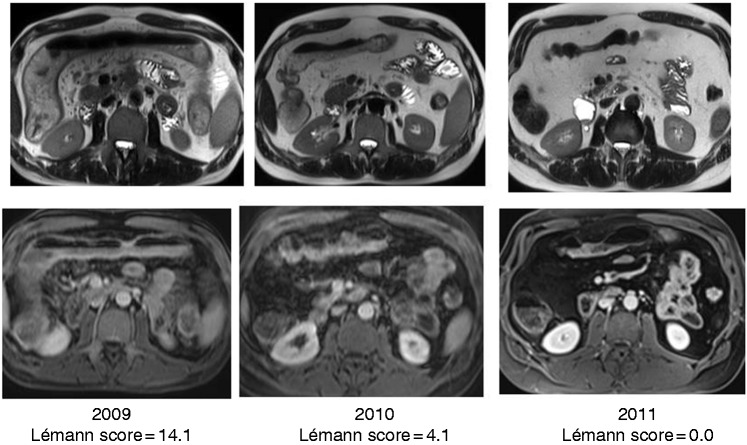
Figure 1.
Resolution of bowel damage after effective therapy on an extensive colonic Crohn’s disease treated by anti-tumour necrosis factor. After two years, there was a complete resolution of bowel damage in the affected colon.
Several studies have shown that blockade of bowel damage progression measured by the Lémann Index could be associated with positive outcomes in the long term,29,31,32 although further research is needed. There are no validated cut-offs to discriminate the presence of bowel damage and clinically relevant changes over time. Gilletta et al. found that a score >2.0 was related to the presence of bowel damage, although this cut-off was calculated on patients undergoing surgery for complications.28 Fiorino et al. propose a cut-off of 4.8, based on a blinded independent clinical evaluation by a gastroenterologist.29 The same cut-off was used in further observational studies with similar results.31,32,35 Recently, Fiorino et al.4 found that any unit of increase of the Lémann Index at diagnosis may be associated with negative course of disease, in particular with a significantly higher risk of hospitalisation and surgery, weakly confirming that probably the increase of the Lémann Index at two definite time points rather than a clear cut-off may be more useful in clinical practice and in further clinical trials.
Measuring bowel damage by the proposed indexes may be quite complex to be used routinely in clinical practice. The Lémann Index requires a combination of MRI (abdominal MRI, and pelvic MRI in the case of perianal disease) with endoscopy and surgical history, careful measurement of all the involved segments and a complex calculation of the final score. However, the recent study by Fiorino et al.4 demonstrated that a simpler assessment of bowel damage, defined as the presence of any strictures, fistulas or abscesses, as previously proposed by the Paris Consensus on early CD definition,38 predicts hospitalisation and surgery in newly diagnosed CD patients. This simpler approach may be more useful in clinical practice, limiting the measure and the quantification by the Lémann Index in selected patients where the accurate quantification of bowel damage may be crucial for therapeutic management, as well as a valid outcome measure in clinical trials.
There are several other gaps in the current evidence that should be addressed in further research. First, all studies cited in this review used the Lémann Index, even though it has not been validated. Second, the observation period in these studies was between 12 and 36 months, which can be considered quite a short period of time to see significant bowel-damage progression and the actual impact on the natural history of the disease. Third, the majority of the studies are retrospective and do not take into account the potential impact of the time of exposure to medical therapy on bowel-damage evolution. Finally, the definition of significant bowel damage probably needs some clarifications and adjustments. As an example, the LYRIC study39 showed that limited ileocaecal resection in CD patients who failed conventional therapies has potentially the same benefits on patients’ overall quality of life than infliximab. In this case, limited structural bowel damage does not result in a negative course of disease and can be considered fully acceptable.
In conclusion, assessing and addressing structural bowel damage as a primary outcome may be the ultimate goal for the therapeutic management of IBD, at least in CD, together with quality of life and disability.37 Further data are strongly needed to set clear outcome measures and to understand the impact of medications on bowel damage, and how this goal may impact on the natural history of disease. Moreover, further investigation is needed in patients with UC, especially with long-standing disease.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: G.F. received consultancy fees from Ferring, MSD, AbbVie, Takeda, Janssen, Amgen, Sandoz, Samsung Bioepis, Roche, Celltrion, Mylan. S.D. has served as a speaker, consultant and advisory board member for Schering-Plough, Abbott (AbbVie) Laboratories, Merck and Co, UCB Pharma, Ferring, Cellerix, Millenium Takeda, Nycomed, Pharmacosmos, Actelion, Alfa Wasserman, Genentech, Grunenthal, Pfizer, Astra Zeneca, Novo Nordisk, Cosmo Pharmaceuticals, Vifor, and Johnson and Johnson. The other authors have no conflicts to disclose.
Funding
The authors received no financial support for the research, authorship and/or publication of this article.
References
- 1.Fiorino G, Peyrin-Biroulet L, Danese S. Bowel damage assessment in Crohn’s disease by magnetic resonance imaging. Curr Drug Targets 2012; 13: 1300–1307. [DOI] [PubMed] [Google Scholar]
- 2.Panes J, Bouzas R, Chaparro M, et al. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn’s disease. Aliment Pharmacol Ther 2011; 34: 125–145. [DOI] [PubMed] [Google Scholar]
- 3.Simon M, Cosnes J, Gornet JM, et al. Endoscopic detection of small bowel dysplasia and adenocarcinoma in Crohn’s disease: a prospective cohort-study in high-risk patients. J Crohns Colitis 2017; 11: 47–52. [DOI] [PubMed] [Google Scholar]
- 4.Fiorino G, Morin M, Bonovas S, et al. Prevalence of bowel damage assessed by cross-sectional imaging in early Crohn’s disease and its impact on disease outcome. J Crohns Colitis 2017; 11: 274–280. [DOI] [PubMed] [Google Scholar]
- 5.Fiorino G, Bonifacio C, Allocca M, et al. Bowel damage as assessed by the Lémann Index is reversible on anti-TNF therapy for Crohn’s disease. J Crohns Colitis 2015; 9: 633–639. [DOI] [PubMed] [Google Scholar]
- 6.Vuitton L, Marteau P, Sandborn WJ, et al. IOIBD technical review on endoscopic indices for Crohn’s disease clinical trials. Gut 2016; 65: 1447–1455. [DOI] [PubMed] [Google Scholar]
- 7.Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005; 19: 5A–36A. [DOI] [PubMed] [Google Scholar]
- 8.Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, et al. Early Crohn disease: a proposed definition for use in disease-modification trials. Gut 2010; 59: 141–147. [DOI] [PubMed] [Google Scholar]
- 9.Daperno M, D’Haens G, Van Assche G, et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc 2004; 60: 505–512. [DOI] [PubMed] [Google Scholar]
- 10.Mary JY, Modigliani R. Development and validation of an endoscopic index of the severity for Crohn’s disease: a prospective multicentre study. Groupe d’Etudes Thérapeutiques des Affections Inflammatoires du Tube Digestif (GETAID). Gut 1989; 30: 983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pariente B, Peyrin-Biroulet L, Cohen L, et al. Gastroenterology review and perspective: the role of cross-sectional imaging in evaluating bowel damage in Crohn disease. AJR Am J Roentgenol 2011; 197: 42–49. [DOI] [PubMed] [Google Scholar]
- 12.Fiorino G, Bonifacio C, Peyrin-Biroulet L, et al. Prospective comparison of computed tomography enterography and magnetic resonance enterography for assessment of disease activity and complications in ileocolonic Crohn’s disease. Inflamm Bowel Dis 2011; 17: 1073–1080. [DOI] [PubMed] [Google Scholar]
- 13.Horsthuis K, Bipat S, Bennink RJ, et al. Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: meta-analysis of prospective studies. Radiology 2008; 247: 64–79. [DOI] [PubMed] [Google Scholar]
- 14.Jauregui-Amezaga A, Rimola J, Ordas I, et al. Value of endoscopy and MRI for predicting intestinal surgery in patients with Crohn’s disease in the era of biologics. Gut 2014; 64: 1397–1402. [DOI] [PubMed] [Google Scholar]
- 15.Pariente B, Cosnes J, Danese S, et al. Development of the Crohn’s disease digestive damage score, the Lémann score. Inflamm Bowel Dis 2011; 17: 1415–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pariente B, Mary JY, Danese S, et al. Development of the Lémann index to assess digestive tract damage in patients with Crohn’s disease. Gastroenterology 2015; 148: 52–63.e3. [DOI] [PubMed] [Google Scholar]
- 17.Calabrese E, Zorzi F, Zuzzi S, et al. Development of a numerical index quantitating small bowel damage as detected by ultrasonography in Crohn’s disease. J Crohns Colitis 2012; 6: 852–860. [DOI] [PubMed] [Google Scholar]
- 18.Zorzi F, Stasi E, Bevivino G, et al. A sonographic lesion index for Crohn’s disease helps monitor changes in transmural bowel damage during therapy. Clin Gastroenterol Hepatol 2014; 12: 2071–2077. [DOI] [PubMed] [Google Scholar]
- 19.Pariente B, Cosnes J, Danese S, et al. Development of the Crohn’s disease digestive damage score, the Lemann score. Inflamm Bowel Dis 2011; 17: 1415–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freeman HJ. Natural history and clinical behavior of Crohn’s disease extending beyond two decades. J Clin Gastroenterol 2003; 37: 216–219. [DOI] [PubMed] [Google Scholar]
- 21.Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med 2011; 365: 1713–1725. [DOI] [PubMed] [Google Scholar]
- 22.Szabò H, Fiorino G, Spinelli A, et al. Review article: anti-fibrotic agents for the treatment of Crohn’s disease – lessons learnt from other diseases. Aliment Pharmacol Ther 2010; 31: 189–201. [DOI] [PubMed] [Google Scholar]
- 23.Van Assche G, Dignass A, Panes J, et al. The second European evidence-based consensus on the diagnosis and management of Crohn’s disease: definitions and diagnosis. J Crohns Colitis 2010; 4: 7–27. [DOI] [PubMed] [Google Scholar]
- 24.Gouveia C, Torres J. A step forward in the understanding of structural and functional bowel damage in patients with ulcerative colitis. GE Port J Gastroenterol 2018; 25: 59–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Louis E, Collard A, Oger AF, et al. Behaviour of Crohn’s disease according to the Vienna classification: changing pattern over the course of the disease. Gut 2001; 49: 777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis 2002; 8: 244–250. [DOI] [PubMed] [Google Scholar]
- 27.Peyrin-Biroulet L, Cieza A, Sandborn WJ, et al. Development of the first disability index for inflammatory bowel disease based on the international classification of functioning, disability and health. Gut 2012; 61: 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilletta C, Lewin M, Bourrier A, et al. Changes in the Lémann Index values during the first years of Crohn’s disease. Clin Gastroenterol Hepatol 2015; 13: 1633–1640.e3. [DOI] [PubMed] [Google Scholar]
- 29.De Cruz P, Kamm MA, Prideaux L, et al. Mucosal healing in Crohn’s disease: a systematic review. Inflamm Bowel Dis 2013; 19: 429–444. [DOI] [PubMed] [Google Scholar]
- 30.Duveau N, Azahaf M, Panchal H, et al. Evolution of the Lemann Index in Crohn’s disease: a retrospective study. J Crohns Colitis 2015; 9: S57–S57. [Google Scholar]
- 31.Amitai MM, Zarchin M, Lahat A, et al. Structural bowel damage in quiescent Crohn’s disease. Dig Liver Dis 2017; 49: 490–494. [DOI] [PubMed] [Google Scholar]
- 32.Bodini G, Giannini EG, De Maria C, et al. Anti-TNF therapy is able to stabilize bowel damage progression in patients with Crohn’s disease. A study performed using the Lémann Index. Dig Liver Dis 2017; 49: 175–180. [DOI] [PubMed] [Google Scholar]
- 33.Ribaldone DG, Caviglia GP, Pellicano R, et al. Adalimumab versus azathioprine to halt the progression of bowel damage in Crohn’s disease: application of Lémann Index. Scand J Gastroenterol 2019; 54: 1339–1345. [DOI] [PubMed] [Google Scholar]
- 34.Magro F, Rodrigues-Pinto E, Coelho R, et al. Is it possible to change phenotype progression in Crohn’s disease in the era of immunomodulators? Predictive factors of phenotype progression. Am J Gastroenterol 2014; 109: 1026–1036. [DOI] [PubMed] [Google Scholar]
- 35.Rispo A, Imperatore N, Testa A, et al. Bowel damage in Crohn’s disease: direct comparison of ultrasonography-based and magnetic resonance-based Lemann Index. Inflamm Bowel Dis 2017; 23: 143–151. [DOI] [PubMed] [Google Scholar]
- 36.Siegel CA, Whitman CB, Spiegel BM, et al. Development of an index to define overall disease severity in IBD. Gut 2018; 67: 244–254. [DOI] [PubMed] [Google Scholar]
- 37.Straksyte V, Kiudelis G, Gineikiene I, et al. Lemann Index for assessment of Crohn’s disease: correlation with the quality of life, endoscopic disease activity, magnetic resonance index of activity and C-reactive protein. Open Med (Wars) 2019; 14: 785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peyrin-Biroulet L, Billioud V, D’Haens G, et al. Development of the Paris definition of early Crohn’s disease for disease-modification trials: results of an international expert opinion process. Am J Gastroenterol 2012; 107: 1770–1776. [DOI] [PubMed] [Google Scholar]
- 39.Ponsioen CY, De Groof EJ, Eshuis EJ, et al. Laparoscopic ileocaecal resection versus infliximab for terminal ileitis in Crohn’s disease: a randomised controlled, open-label, multicentre trial. Lancet Gastroenterol Hepatol 2017; 2: 785–792. [DOI] [PubMed] [Google Scholar]