Abstract
Mutations in the LMNA gene, encoding the nuclear envelope A-type lamins, are responsible for muscular dystrophies, the most severe form being the LMNA-related congenital muscular dystrophy (L-CMD), with severe defects in myonucleus integrity. We previously reported that L-CMD mutations compromise the ability of muscle stem cells to modulate the yes-associated protein (YAP), a pivotal factor in mechanotransduction and myogenesis. Here, we investigated the intrinsic mechanisms by which lamins influence YAP subcellular distribution, by analyzing different conditions affecting the balance between nuclear import and export of YAP. In contrast to wild type (WT) cells, LMNADK32 mutations failed to exclude YAP from the nucleus and to inactivate its transcriptional activity at high cell density, despite activation of the Hippo pathway. Inhibiting nuclear pore import abolished YAP nuclear accumulation in confluent mutant cells, thus showing persistent nuclear import of YAP at cell confluence. YAP deregulation was also present in congenital myopathy related to nesprin-1 KASH mutation, but not in cells expressing the LMNAH222P mutation, the adult form of lamin-related muscle dystrophy with reduced nuclear deformability. In conclusion, our data showed that L-CMD mutations increased YAP nuclear localization via an increased nuclear import and implicated YAP as a pathogenic contributor in muscle dystrophies caused by nuclear envelop defects.
Keywords: lamins, congenital myopathy, nucleo-cytoskeletal translocation, nuclear envelope
1. Introduction
The nuclear lamins A/C are type V intermediate filament proteins encoded by the LMNA gene. Lamins form complexes with other proteins of the nuclear membrane to influence mechanical cues and signaling pathways crucial for cellular proliferation and differentiation [1]. Mutations in the LMNA gene cause laminopathies, a highly heterogeneous group of disorders, including muscular dystrophies and cardiomyopathies [2,3]. The disease mechanisms underlying LMNA-related muscular dystrophy remains somewhat elusive.
There is clear evidence that A-type lamins and nuclear envelope proteins play a critical role in responding to mechanical cues from the extracellular matrix by adjusting the cytoskeleton and nuclear stiffness with the stiffness of the tissue microenvironments [4,5]. Structural changes in lamin A/C can also affect several signaling pathways, by altering either direct or indirect interactions of signaling molecules with A-type lamins [6,7]. Lamins A/C have already been shown to regulate the nuclear translocation and downstream signaling of the mechanosensitive transcription factor megakaryoblastic leukemia 1 (MKL1), a myocardin family member pivotal in cardiac development and function [8]. In muscle stem cells (MuSCs) from patients carrying A-type lamin mutations, we recently reported an impaired ability to sense matrix stiffness [9] and to withstand mechanical stretching of the extracellular matrix, causing aberrant regulation of the yes-associated protein (YAP) [10].
YAP is a transcriptional co-regulator that is modulated by diverse biomechanical signals and transduces them into cell-specific transcriptional responses, regulating cell proliferation and survival, organ growth, stem-cell renewal, and cell differentiation [11]. A major mechanism of YAP regulation occurs at the level of its subcellular localization, as YAP nuclear accumulation promotes target gene transcription and cell proliferation (reviewed in [11,12]). After phosphorylation by LATS1/2 kinase, YAP binds to 14–3–3 proteins, leading to its cytoplasmic retention and degradation (reviewed in [11,12]), and favoring skeletal muscle differentiation [12].
A-type lamins influence the localization and transcriptional activity of YAP [10]. It has been shown that lamin-A overexpression decreases both total YAP levels and nuclear localization in mesenchymal stem cells [5]. In contrast, increased YAP nuclear localization and activity in combination with reduced lamin levels is observed in cancers of many organ types (reviewed in [13]), as well as in LMNA mutant MuSCs cultured on soft matrices [10]. However, it remains unclear as to how mutant lamins cause defects in the YAP signaling pathway. Abnormal nuclear shape is observed in diseases where the A-type lamins are altered, including cancer [14,15] and laminopathies [16]. Altered nuclear morphology can in turn increase the rate of YAP import [17,18], by opening up nuclear pores [17]. One can hypothesize that A-type lamin mutations, responsible for severe skeletal muscle laminopathies, will cause an increase YAP nuclear localization because of an increased nuclear import.
To test this hypothesis, we investigated YAP subcellular distribution/activity in MuSCs with A-type lamin mutations responsible for severe congenital muscle dystrophy (L-CMD) in different conditions affecting the balance between nuclear import and export of YAP. Our study provides evidence that A-type lamin mutations impair YAP regulation by increasing the nuclear import of YAP. Intriguingly, we also found YAP nuclear accumulation in cells with nesprin-1 mutation responsible for a congenital myopathy and associated with defects in nuclear morphology [9,19], but not in cells carrying the LMNAH222P mutation responsible for a less severe form of the disease and much milder nuclear envelope structural defects. These findings support a causative role of nuclear envelope defects in abnormal YAP signaling and implicated YAP as a pathogenic contributor in the severity of muscle dystrophies caused by nuclear envelop mutations. Overall, our study gains insight into broader questions of how lamins and nuclear shape impact cellular function.
2. Materials and Methods
2.1. Human Cells and Cell Culture
We obtained muscle biopsies from the Bank of Tissues for Research (Myobank, a partner in the EU network EuroBioBank) in accordance with European recommendations and French legislation. All patients provided written informed consent and experimental protocols were approved by our institution (INSERM) (approval number AC-2013-1868, 28 May 2014 and AC-2019-3502, 2 Dec 2019). Experiments were performed using immortalized L-CMD human myoblasts carrying a heterozygous LMNAc.94_96delAAG, p.Lys32del (referred to as ΔK32), LMNA p.Arg249Trp (referred to as R249W), or LMNA p.Leu380Ser (referred to as L380S) mutation. Immortalized human myoblasts carrying SYNE-1 homozygous c.23560 G<T, p.E7854X leading to a stop codon in exon 133 and deletion of the carboxy-terminal KASH domain (referred to as nesprin-1ΔKASK) were also analyzed, given that this mutation alters the nuclear shape of MuScs [9,20]. Immortalized myoblasts, obtained from two healthy control subjects without muscular disorders, were used as controls (hereafter referred to wild-type, WT).
We also analyzed myogenic cells derived from fibroblasts obtained from a patient with classical form of EDMD and carrying the LMNA p.H222P mutation (LMNAH222P), and from a control patient [21]. Fibroblasts were obtained from skin biopsies and immortalized as previously described [22]. Doxycycline-inducible Myod1 lentivirus was used to induce myogenic conversion [23].
Following muscular biopsy, MuSCs were immortalized and cultured in growth medium consisting of 1 vol 199 Medium /4 vol DMEM (Life Technologies, Carlsbad, CA, USA) supplemented with 20% fetal calf serum (Life Technologies), 5 ng/mL hEGF (Life Technologies), 0.5 ng/mL βFGF, 0.1 mg/mL Dexamethasone (Sigma-Aldrich, St. Louis, MO, USA), 50 µg/mL fetuin (Life Technologies), 5 µg/mL insulin (Life Technologies), and 50 mg/mL Gentamycin (GibcoTM, Life Technologies, Carlsbad, CA, USA). MyoD-transfected fibroblasts were cultured in a proliferation medium consisting of DMEM, supplemented with 10% fetal bovine serum (Life Technologies) and 0.1% gentamycin (Invitrogen, Carlsbad, CA, USA).
All cells were cultured on classic glass or plastic substrates. In addition, micro patterned glass slides with round islands of 700 µm2 (4D Cell, Montreuil, France) were coated with fibronectin and cells were seeded in a 200 µL drop at the center of the dish. After attachment, the wells containing the micro patterned slides were filled with proliferative medium for 24 h.
2.2. Drug Treatments
Importazole, a drug that blocks importin-β-dependent nuclear import [24], Leptomycin B, a drug that blocks CRM1-dependent nuclear export [25] or Dasatinib, Src-family kinase inhibitor drugs were diluted to final concentration of 40 µM, 100 nM, or 100 nM, respectively, for 24, 24, or 1 h. Latrunculin-A (Lat A, Sigma-Aldrich) or Cytochalasin D (Sigma-Aldrich) were diluted to final concentration of 2.0 and 1 µM, respectively, for 20 or 30 min. Vehicle control experiments using appropriate doses and time of dimethylsulfoxyde (DMSO) were used to assess the effects of specific drugs.
2.3. Luciferase Reporter Assays
MuSCs were transfected with Lipofectamine® 2000 (Invitrogen) reagents in growth media without antibiotics according to manufacturer’s instructions. TBS (Tead binding sequence: 14 times GGAATG)-Firefly Luciferase reporter constructs were used at a 1:5 ratio to the co-reporter vector for the weak constitutive expression of wild-type Renilla luciferase (pRL-TK, Promega GmbH, Mannhein, Germany). Transfected cells were seeded onto 24-, 48-, or 96-well plates and recovered overnight in growth medium. For the luciferase assay, cells were cultivated for 24 h after transfection under the stated conditions. The cells were lysed with passive lysis buffer (PJK GmbH, Kleinblittersdorf, Germany) and activity of the reporter was quantified by addition of firefly Luciferase substrate Beetle Juice (PJK GmbH). The activity of Renilla luciferase was quantified by addition of Renilla Juice (PJK GmbH) and measuring luciferase activity with Mithras LB940 Luminometer (Wildbad, Germany). Three separate experiments were performed per condition.
2.4. Immunocytochemistry and Image Analysis
MuSCs were fixed for 5 min with 4% formaldehyde, permeabilized with 0.1%Triton X100, and blocked with 10% bovine serum albumin (BSA) diluted in PBS. Cells were stained with Phalloidin-Alexa 568 to label F-actin (Interchim, Montluçon, France). The following primary antibodies were used for immunostaining: anti- Yes-associated protein (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ) (Santa-Cruz, Dallas, TX, USA, sc-10119s), anti-phosphorylated Ser127 YAP (pS127-YAP) (Cell Signaling, Danvers, MA, USA, cs-4911), and anti-phosphorylated Tyr357-YAP (pY357-YAP) (Abcam, Paris, France, ab62751). Secondary antibodies (Life Technologies, Saint-Aubin, France; 1/500) were Alexa Fluor 488 donkey anti-mouse IgG or Alexa Fluor 488 donkey anti-rabbit IgG. Nuclei were stained with Hoechst (ThermoFischer, Carlsbad, CA, USA) and Mowiol was used as mounting medium. Confocal images were taken with an Olympus FV 1200 (Olympus, Hamilton, Bermuda) and a laser-scanning microscopy Nikon Ti2 coupled to a Yokogawa CSU-W1 head (Nikon, Tokyo, Japan).
All image analyses were performed using Fiji software (NIH Image, version 1.51). For immunostained cells, Z-stacks of images were acquired for each channel, and the middle confocal slice was chosen from the images of the nucleus detected in the Hoechst channel. On the corresponding slice in the YAP channel, the average fluorescence intensity in the nucleus and just outside the nucleus (cytoplasm) was measured to determine the nuclear/cytoplasmic ratio.
2.5. SDS-PAGE and Protein Analysis
Cells were lysed in total protein extraction buffer (50 mM Tris-HCl, pH 7.5, 2% SDS, 250 mM sucrose, 75 mM urea, 1 mM DTT) with added protease inhibitors (25 μg/mL Aprotinin, 10 μg/mL Leupeptin, 1 mM 4-[2-aminoethyl]-benzene sulfonylfluoride hydrochloride, and 2 mM Na3VO4) or directly in 2× Laemmli buffer. Protein lysates were separated by SDS-PAGE and transferred on PVDF or nitrocellulose membranes. After blocking with bovine serum albumin, membranes were incubated with anti-YAP (Santa-Cruz, CA, USA, sc-10119), anti-pS127-YAP (Cell Signaling, Danvers, MA, USA, cs-4911), and anti-pY357-YAP (Abcam, Paris, France, ab62751) or anti-GAPDH (Cell Signaling, cs-2118). Goat anti-mouse, goat anti-rat, or donkey anti-goat HRP conjugates were used for HRP-based detection. Detection of adsorbed HRP-coupled secondary antibodies was performed by ECL reaction with Immobilon Western Chemiluminescent HRP Substrate (Millipore, Billerica, MA, USA). HRP signals were detected using a CCD-based detection system (Vilber Lourmat, Marne-La-Vallée, France) or a G-box system with GeneSnap software (Ozyme, Saint-Quentin, France). Membranes subjected to a second round of immunoblotting were stripped with stripping buffer (62.5 mM Tris-HCL pH 6.8, 2% sodium dodecyl sulfate (SDS), 100 mM β-mercaptoethanol) and incubated at 55 °C for 30 min with mild shaking before excessive washing with deionized water and re-blocking. Quantification was performed using ImageJ (NIH Image).
2.6. Quantification of Gene Expression
The mRNA was isolated from cell lysates using the RNeasy mini kit (Qiagen, Hilden, Germany) with the Proteinase K step, according to the manufacturer instructions. The complementary DNA (cDNA) was transcribed by SuperscriptIII (ThermoFischer, Carlsbad, CA, USA). Gene expression was quantified by using PerfeCTa-SYBR®Green SuperMix (Quanta, Biosciences, Gaithersburg, MD, USA) with the help of LightCycler 480 II (Roche Diagnostics GmbH, Mannheim, Germany). The primers were designed by Primer-BLAST (NCBI) and synthesized by Eurogentec (Liège, Belgium). Expression of all target genes was normalized to the expression of the reference gene RPLP0. Primer sequences are listed in Table 1.
Table 1.
Primer sequences.
| Gene name | Abbreviation | Forward/Reverse | Sequence |
|---|---|---|---|
| Human Yes-associated protein 1 |
hYAP1 | fw | GCTACAGTGTCCCTCGAACC |
| rev | CCGGTGCATGTGTCTCCTTA | ||
| Human Connective tissue growth factor |
h-CTGF | fw | ACCGACTGGAAGACACGTTTG |
| rev | CCAGGTCAGCTTCGCAAGG | ||
| Human Connective tissue growth factor |
h-RPLPO | fw | CTCCAAGCAGATGCAGCAGA |
| rev | ATAGCCTTGCGCATCATGGT | ||
| Human Glyceraldehyde 3-phosphate dehydrogenase |
h-GAPDH | fw | TGC-CAT-GTA-GAC-CCC-TTG-AA |
| rev | TGG-TTG-AGC-ACA-GGG-TAG-TT | ||
| Human Myosin light chain 9 |
h-Myl9 | fw | CGA-ATA-CCT-GGA-GGG-CAT-GAT |
| rev | AAA-CCT-GAG-GCT-TCC-TCG-TC |
2.7. Statistical Analysis
Graphpad Prism (Graphpad Software, La Jolla, CA, USA) was used to calculate and plot mean and standard error of the mean (SEM). Statistical significances were assessed by ANOVA followed by Bonferroni or two-tailed unpaired t-tests. Differences between conditions were considered significant at p < 0.05. Figures were plotted with Graphpad Prism.
3. Results
3.1. Impaired Yes-Associated Protein (YAP) Nuclear Exclusion in Confluent LMNA Mutant Muscle Stem Cells (MuSCs)
Wild-type (WT) MuSCs were plated either at low (10,000 cells/cm2) or high (40,000 cells/cm2) density and stained for YAP localization (Figure 1A,B). At low density, YAP was predominantly localized to the nucleus (Figure 1A,B). However, at high density conditions, WT cells showed predominantly cytoplasmic YAP, confirming previous reports for other cell types [26,27,28]. Similar YAP localization was observed in cells with the LMNAH222P mutation (Figure S1A,B) As expected, inhibition of CRM1-dependent nuclear export using Leptomycin B maintained preferential YAP nuclear distribution in confluent WT cells (Figure S2A).
Figure 1.
Modulation of yes-associated protein (YAP) localization in wild-type (WT) and mutant muscle stem cells (MuSCs). (A) Confocal images of YAP (green) in WT and LMNAΔK32 mutant MuSCs cultured in low and high density conditions. Nuclei are stained with Hoechst (blue). Scale bar: 20 µm. (B) Quantification of YAP nucleo-cytoplasmic (N/C) ratio in WT, LMNAΔK32, LMNAL380S, LMNAR249W, and nesprin-1 KASK mutant MuSCs. Pooled values of WT (WT1 and WT2) are presented. Values are expressed as mean ± SEM, n = 200 cells for each cell line. * p < 0.05 vs WT; $ < 0.05 vs. corresponding sparse condition. (C) Micro-patterning modulation of YAP. Confocal images of YAP (green) in WT and LMNAΔK32 cells cultured on small ECM substrate that limits cell spreading. Nuclei are stained with Hoechst (blue). Scale bar: 30 µm. (D) Quantification of YAP nucleo-cytoplasmic (N/C) ratio on small ECM substrates. Values are expressed as mean ± SEM, n ≥ 62 cells for each cell line. * p < 0.05 vs. WT.
Interestingly, LMNA mutant cells plated at high density showed impaired density-dependent YAP subcellular localization and failed to exclude YAP from the nucleus (Figure 1A,B and Figure S2B).
Lamin A/C’s are linked to the outer nuclear membrane protein nesprin-1 via SUN proteins in the lumen of the nuclear membrane. Nesprin-1 KASK mutation causes congenital myopathy and is also known to affect the nuclear shape [9,19]. Interestingly, nesprin-1 KASK cells displayed preferential nuclear YAP at high cell density (Figure 1B). Together, these finding revealed a striking correlation between the YAP mislocalization observed in vitro and the severity of the diseases.
Apart from cell–cell contacts, mechanical environments characterized by cell morphology and actin contractility regulate YAP nuclear localization [28]. Small cell surface adhesion is a known determinant for YAP nuclear exclusion [28]. Accordingly, WT cells on round micro-patterned surfaces of 700 µm2 displayed low nuclear staining of YAP (Figure 1C,D). In contrast, YAP was preferentially nuclear in LMNAΔK32 cells cultured on small ECM substrates (Figure 1C,D). However, at low density, treatment with LatA induced YAP exclusion from the nucleus both in LMNAΔK32 mutant and WT cells (Figure S2C), thus supporting a dominant regulation of YAP localization by actin polymerization.
3.2. Phosphorylated Ser127-YAP Accumulates in the Nucleus of LMNA Mutated Muscle Stem Cells (MuSCs)
YAP phosphorylation on Ser 127 residue by LATS1/2 allows interaction with 14–3–3 protein and thereby nuclear exclusion of YAP [29]. We thus asked whether persistent nuclear localization in LMNAΔK32 could be mediated by impaired LATS1/2 activity.
We found that under high density conditions LMNAΔK32 cells accumulated pS127-YAP in the nucleus, in contrast to WT cells (Figure 2A,B). Interestingly, cell treatment with cytochalasin D a drug known to activate the Hippo pathway, increased the intensity of p127YAP staining (Figure S3), thus supporting the specificity of the pS127-YAP staining.
Figure 2.
pS127-YAP accumulates in the nucleus of LMNAΔK32 MuSCs. (A) Confocal images of pS127-YAP (green), in WT and LMNAΔK32 mutant MuSCs cultured at low and high density conditions. Nuclei are stained with Hoechst (blue). Scale bar: 20 µm. (B) Quantification of pS127-YAP nucleo-cytoplasmic (N/C) ratio. Values are expressed as mean ± SEM, n = 150 cells for each cell line. * p < 0.005 compared with WT. (C) Representative Western-blot of YAP, pS127-YAP, and GAPDH in WT and LMNAΔK32 MuSCs plated at low and high cell density. (D) Quantification of pS127-YAP /YAP protein levels in low and high density conditions, expressed in arbitrary units (a.u.). GAPDH was used as a loading control. Pooled values of WT (WT1 and WT2) are presented. Values are mean ± SEM, n ≥ 4 per conditions. * p < 0.005 compared with WT.
At low density, the amount of pS127-YAP did not differ between WT and LMNAΔK32, and the level of pS127-YAP and the pS127-YAP/YAP ratio significantly increase with cell density in both cell lines (Figure 1C,D). This is in line with data reporting a cell density-dependent activation of the Hippo pathway [26].
3.3. Src-Dependent Tyr Phosphorylation of YAP is Activated in LMNAΔK32 MuSCs
Aside from pS127-YAP, tyrosine phosphorylation of YAP by Src-kinase family modulates the transcriptional activity of YAP and indirectly its localization [30]. In both WT and LMNAΔK32 cells, pY357-YAP was predominantly localized to the nucleus in low density conditions (Figure 3A,B).
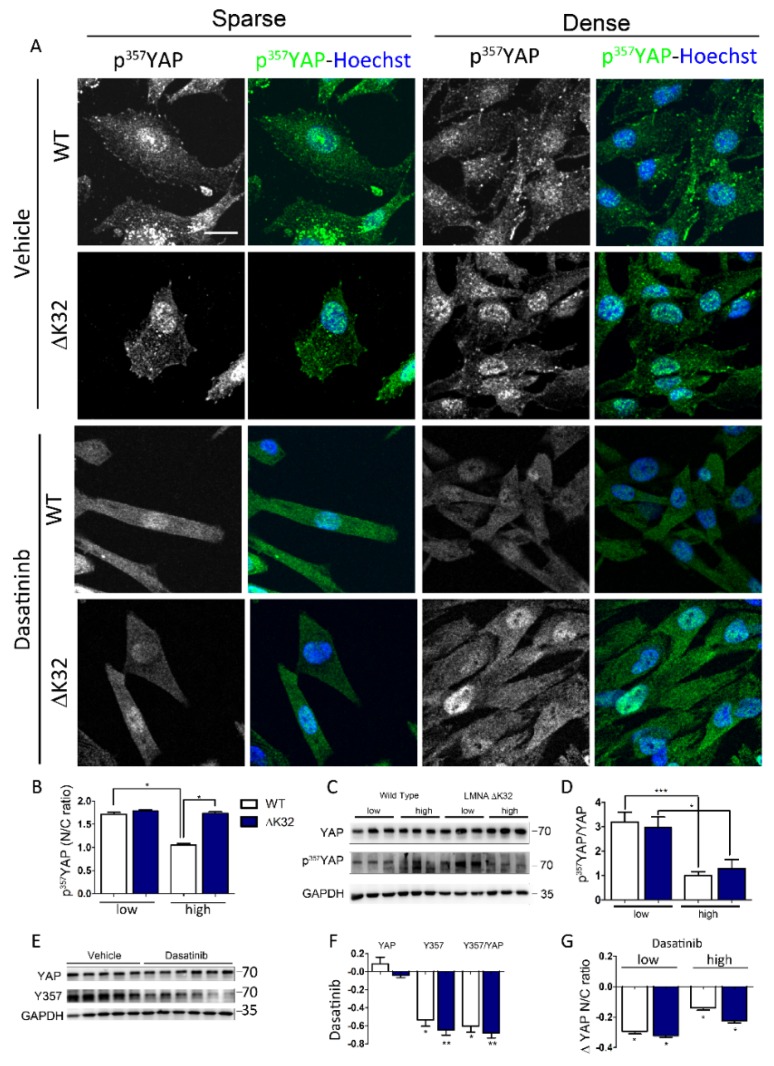
Figure 3.
Y357 phosphorylation of YAP in WT and LMNA mutated MuSCs. (A) Confocal images of pY357-YAP (green) in WT and LMNAΔK32 mutant MuSCs cultured in low and high density conditions, at baseline and after treatment with Dasatinib. Nuclei are stained with Hoechst (blue). Scale bar: 20 µm. (B) Quantification of pY357-YAP nucleo-cytoplasmic (N/C) ratio. Values are expressed as mean ± SEM, n = 150 cells for each cell line. (C) Representative Western-blot of YAP, pY357-YAP, and GAPDH in WT and LMNAΔK32 MuSCs plated at low and high cell density. (D) Quantification of pY357-YAP/YAP protein levels in low and high density conditions, expressed in arbitrary units (a.u.). GAPDH was used as a loading control. Pooled values of WT (WT1 and WT2) are presented. Values are mean ± SEM, n ≥ 4 per conditions. * p < 0.005, ** p < 0.01, *** p < 0.001 compared with WT. (E) Representative Western-blot of YAP, pY357-YAP, and GAPDH in WT and LMNAΔK32 MuSCs treated with vehicle or Dasatinib. (F) Quantification of YAP, pY357-YAP, and pY357-YAP /YAP protein levels after treatment with Dasatinib. Values are expressed as percent change of values obtained without treatment. GAPDH was used as a loading control. Pooled values of WT (WT1 and WT2) are presented. Values are mean ± SEM, n ≥ 4 per conditions. * p < 0.005, ** p < 0.01, compared with WT. (G) Quantification of pY357-YAP nucleo-cytoplasmic (N/C) ratio after Dasatinib treatment expressed as a fraction of control value obtained in sparse or dense conditions before treatment. Values are expressed as mean ± SEM, n ≥ 110 cells for each cell line.
The nucleo-cytoplasmic ratio of pY357-YAP significantly decreased at high densities in WT but not in LMNAΔK32 mutant cells (Figure 3A,B). However, at the protein level, the pY357-YAP/YAP ratio did not differ between cell lines and decreased with cell density in both cell lines (Figure 3C,D). Dasatinib, an Abl and Src-family kinase inhibitor, significantly reduced the amount of pY357-YAP phosphorylation and the pY357-YAP/YAP ratio (Figure 3F,G) as well as the nucleo-cytoplasmic ratio of pY357-YAP (Figure 3A,H).
3.4. Blockade of Nuclear Import Inhibits Nuclear Accumulation of YAP in Confluent LMNAΔK32 MuSCs
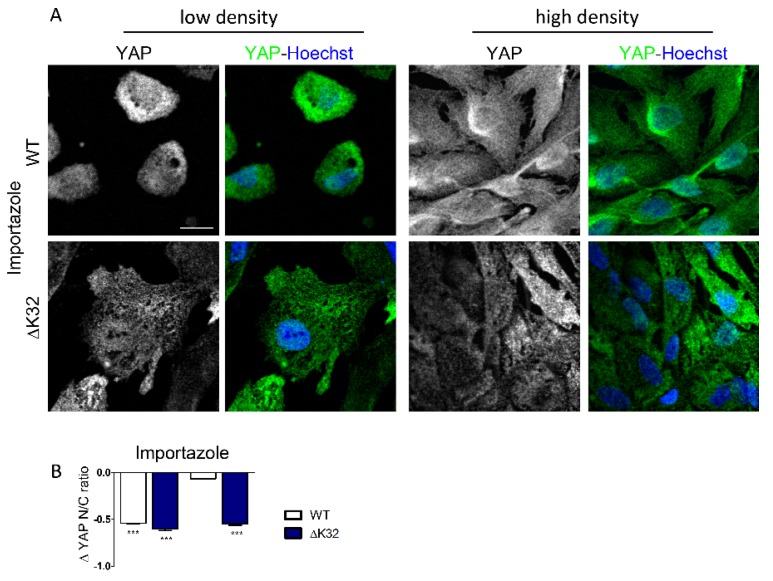
It is clearly established that YAP localization depends on its dynamic shuttling between the cytoplasm and the nucleus, and is maintained at a steady state by a balance between nuclear export and import rates. To exclude the possibility of nonspecific permeabilization of the nuclear envelope due to the lamin mutation, and generally to determine the exact pathway of nuclear import of YAP, we used importazole, an importin β-specific inhibitor [24]. In low density cultures, importazole inhibited YAP nuclear localization in both WT and LMNAΔK32 cells, with no significant difference in YAP nucleo-cytoplasmic ratio between cell lines (Figure 4; each p < 0.001). In high density cultures, importazole significantly reduced nuclear localization of YAP in LMNAΔK32 cells (Figure 4A,B, each p < 0.001), but not in WT cells, thus attesting to a persistent nuclear import of YAP in high density LMNAΔK32 cell cultures.
Figure 4.
Importazole inhibits nuclear localization of YAP in WT and mutant MuSCs. (A) Confocal images of YAP (green) in WT and LMNAΔK32 mutant MuSCs cultured in low and high density conditions. Nuclei are stained with Hoechst (blue). Scale bar: 20 µm. (B) Quantification of YAP nucleo-cytoplasmic (N/C) ratio after importazole treatment. Values are expressed as mean ± SEM, as a fraction of value obtained before importazole treatment, n ≥ 60 cell in each cell line.
3.5. Functional Consequences of YAP Nuclear Accumulation in High Density LMNAΔK32 MuSCs
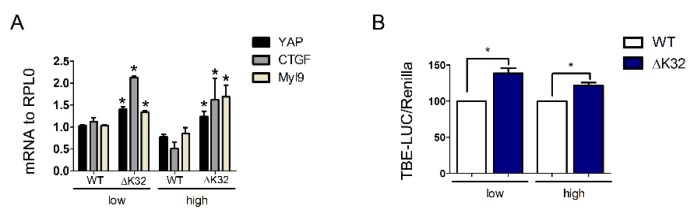
To characterize the consequences of altered YAP translocation, we assessed expression of select YAP target genes and TEAD-dependent transcriptional activity using a luciferase reporter. Confluent LMNAΔK32 cells had an increased expression of YAP and YAP target genes CTGF and MYL9 (Figure 5A). Moreover, LMNAΔK32 cells show increased TEAD-dependent luciferase reporter activity when compared to WT cells (Figure 5B), thus confirming elevated YAP transcriptional activity. Taken together, these data indicate that the LMNAΔK32 mutation affects YAP localization and density-dependent inactivation of YAP.
Figure 5.
Transcriptional activity of YAP. (A) Histogram represents mRNA transcripts of YAP, CTGF, and Myl9 normalized to RPLP0 and expressed as fold-changes. Values are ± SEM, n = 3 separate experiments. * p < 0.05 compared with WT. (B) Quantification of YAP activity using the TBE-Luciferase (LUC)/Renilla reporter. Values are ± SEM, n = 3 separate experiments. * p < 0.05 compared with WT cells.
4. Discussion
In this study, we showed that A-type lamin mutations which are responsible for congenital muscle disorders impacted the yes-associated protein (YAP) signaling pathway by increasing the nuclear import of YAP through the nuclear pore complexes. More importantly, YAP was transcriptionally active despite activation of the Hippo pathway, and thus may contribute to the impaired muscle differentiation in congenital muscle dystrophies. In addition, our data revealed a striking correlation between YAP deregulation, nuclear envelope defects, and disease severity, thus supporting a critical role of nuclear morphology in regulating YAP nuclear import.
4.1. Canonical Regulation of YAP Nucleo-Cytoplasmic Localization Via the Hippo Pathway
In skeletal muscle, YAP/transcriptional coactivator with PDZ-binding motif (TAZ) are powerful co-transcription factors which regulate muscle cell proliferation and differentiation [31,32] and play critical roles in controlling muscle growth [32,33]. The nuclear presence and the transcriptional activity of YAP/TAZ can be modulated by mechanical cues, such as substrate stiffness, cell spreading, and stretching (review in [34]) as well as by non-mechanical cues [26,35]. In previous work [10], we have shown that mutations in the LMNA gene associated to congenital muscle dystrophy cause a loss of environmental mechanosensing with elevated YAP signaling despite the soft environment, thus suggesting that A-type lamins modulate the mechanical regulation of YAP. Consistent with an abnormal mechanical regulation of YAP, we found that reducing cell spreading was ineffective to induce cytoplasmic relocalization of YAP in LMNAΔK32 mutant cells (Figure 1C,D). In addition, here we also showed that cell–cell contact failed to inhibit YAP nuclear localization and activity in LMNA mutant cells from LMNA-related congenital muscular dystrophy (L-CMD), contrary to what was observed in our wild-type (WT) (Figure 1A,B) and LMNAH222P (Figure S1) cells and in other non-cancer cells [26,27,28]. Taken as a whole, these data showed that defective nucleo-cytoplasmic shuttling of YAP was a hallmark of the most severe muscle dystrophies related to nuclear envelope mutations. Moreover, abnormal YAP regulation in LMNA mutant cells from L-CMD involved both mechanical and non-mechanical regulations of YAP nucleo-cytoplasmic shuttling. While our study mainly focused on YAP, whether mutations in nuclear envelope proteins also affect the nucleo-cytoplasmic shuttling of TAZ remain to be precisely determined.
The mechanisms by which cell density modulates YAP activation and nucleo/cytoskeletal shuttling have been extensively studied during the last decade [11,36,37]. The Hippo signaling pathway critically regulates cell–cell contact-mediated YAP cytoplasmic translocation [38,39,40]. In cells grown at low density, YAP is primarily localized to the nucleus where it promotes target gene transcription and proliferation. When cells reach a critical density, YAP translocates to the cytoplasm [26,27,28], thus underlying the classical paradigm of the contact inhibition of proliferation [26]. The Hippo signaling pathway functions as a highly conserved canonical upstream regulator of YAP activity and localization [41]. At the core of the Hippo pathway, LAT1/2 mediates serine phosphorylation on several serine residues, including serine 127, thus mediating YAP nuclear export and subsequent cytoplasmic association with 14–3–3 proteins [40,42,43,44]. In confluent cultures, loss of Ser127 phosphorylation and LATS1/2 activity with persistent nuclear localization of YAP is a hallmark of cancer cells [30]. In contrast, the high density cultures of LMNA mutated cells exhibited high protein levels of pS127-YAP and persistent nuclear localization of pS127-YAP (Figure 2), thus indicating activation of the Hippo pathway signaling. This nuclear accumulation of YAP and pS127-YAP in LMNAΔK32 cells suggests that YAP nuclear export is insufficient to counterbalance YAP nuclear entry. Accordingly, it is known that the presence of pS122-YAP is a prerequisite, but is not sufficient for nuclear exclusion of YAP [45,46]. Although the role of exportin1 in YAP nuclear export has been clearly identified [29,47,48], however, regulation of YAP nuclear export remains largely unknown.
Regulation of YAP localization is also modulated by other kinases, including NLK [35] and Src-family kinases [47,49]. Whereas YAP phosphorylation on Ser residues are well-known negative regulators of YAP stability, Src-mediated phosphorylation of tyrosine 357 has been correlated with nuclear localization and increased YAP transcriptional activity [18,47,49]. Higher pY357-YAP levels in LMNADK32 cells may thus contribute to the nuclear retention by promoting binding between YAP and TEAD transcription factors. Whether increased nuclear pY357-YAP at both low and high cell densities could explain the increased transcriptional activity of YAP observed in LMNAΔK32 cells (Figure 5) remains to be determined.
4.2. Nuclear Import of YAP in LMNAΔK32 Mutant Cells
YAP nuclear import is mediated by active translocation involving importins through nuclear pores [50]. To interfere with active nuclear import, we used importazole, a drug known to inhibit importin-dependent nuclear translocation [24] and YAP nuclear localization [18]. In low density conditions, blocking nuclear entry considerably reduced nuclear localization of YAP in both WT and LMNA mutant cells (Figure 4). Thus, in low density conditions, YAP nuclear entry was mediated by an importin-dependent nuclear import in both WT and LMNAΔK32 mutated MuSCs. Passive diffusion across the damaged nuclear envelope [51,52], if any, was not a main contributor of YAP nuclear import in LMNAΔK32 mutant MuSCs. Moreover, importazole inhibited YAP nuclear localization in high density LMNAΔK32 but not in WT cells. Therefore, in WT cells, at high cell density active nuclear import of YAP was inhibited and no further inhibition was observed after treatment with importazole. In contrast, active nuclear import of YAP persisted in LMNAΔK32 cells, a finding consistent with a dominant active nuclear import of YAP at cell confluence (Figure S4). MuSCs carrying a mutation in the gene encoding the nuclear envelope protein nesprin1, also failed to regulate YAP localization, thus suggesting a nuclear envelope-related dysfunction.
Nuclear deformability is specific to LMNA and nesprin mutations rather than to muscular dystrophy and specifically affected the most severe forms of muscle disorders related to nuclear envelope defects [16,52]. Recent studies have reported that force-induced nuclear deformations increase YAP nuclear translocation through the nuclear pore complex [17,18]. It is thus conceivable that nuclear deformations per se drive nuclear translocation of YAP in LMNA and nesprin-1ΔKASH mutant MuSCs (Figure S4), regardless of how nuclear deformation can be caused [53].
4.3. Functional Consequence of YAP Deregulation in Myogenesis
Nuclear localization of the transcriptional co-activator YAP and activation of the TEAD family transcription factors are required to promote proliferation but prevent differentiation of human stem cells [54,55]. In myogenic cell precursors cultured in vitro, high YAP expression and activity promotes proliferation of myogenic cell precursors whilst preventing their differentiation [31]. Therefore, one can speculate that persistent activation of YAP in LMNA mutant MuSCs has additive negative effects on skeletal muscle differentiation. Further studies are needed to precisely determine their contribution in the physiopathology of lamin-related muscle dystrophy.
Acknowledgments
We thank the IRIS-platform (Sorbonne University) for imaging facility. We also thank Petra Knaus for providing constructs for the luciferase reporter assays.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4409/9/4/816/s1, Figure S1: (A) Confocal images of YAP (green) in confluent WT and LMNAH222P myogenic cells cultured in low and high density conditions. Nuclei are stained with Hoechst (blue). Scale bar: 50 µm. (B) Quantification of YAP nucleo-cytoplasmic (N/C) ratio. Values are expressed as mean ± SEM, n ≥ 50 cells for each cell line, Figure S2: (A) Confocal images of YAP (green) and nuclei (blue) in confluent WT MuSCs treated with Leptomycin B (LB). Scale bar = 20 µm; (B) Confocal images of YAP (green) and nuclei (blue) in WT and LMNAΔK32 mutant MuSCs cultured in low and high density conditions. Nuclei are stained with Hoechst (blue). Scale bar: 20 µm. (C) Confocal images of YAP (green) and nuclei (blue) in confluent WT MuSCs treated with latrunculin A (LatA). Scale bar = 20 µm; (D) Quantification of YAP nucleo-cytoplasmic (N/C) ratio after Lat A treatment expressed as a fraction of control value obtained in sparse conditions. Values are expressed as mean ± SEM, n ≥ 60 cells for each cell line, *** p < 0.001 versus value obtained before LatA treatment, Figure S3: Confocal images of p127-YAP (green) and nuclei (blue) in WT MuSCs treated with vehicle (DMSO) or Cytochalasin D (cyto D). Scale bar = 50 µm, Figure S4: Proposed model of the mechanisms by which lamins influence YAP subcellular distribution at high cell density. (A) In WT MuSCs plated at high cell density, the Hippo pathway is activated, and p127-YAP was excluded from the nucleus. YAP nuclear entry was lower than YAP nuclear export. (B) In L-CMD and nesprin-1DKASH mutations, YAP nuclear export is insufficient to counterbalance YAP nuclear entry despite activation of the Hippo pathway. This results in persistent nuclear localization of pS127-YAP nuclear import. Potential mechanisms imply increased nuclear deformability and/or dysfunctional LINC complex.
Author Contributions
Conceptualization, C.C.; methodology, C.C., M.F. and D.J.O.; validation, C.C.; formal analysis, C.C., D.J.O., and M.F.; investigation, C.C., M.F., S.M., S.J.; resources, K.M.; writing—original draft preparation, C.C.; writing—review and editing, M.F., D.O., G.B.-B.; supervision, C.C.; project administration, C.C.; funding acquisition, C.C. All authors have read and agree to the published version of the manuscript.
Funding
This research was funded by ANR Sorbonne-University grant (ANR-11-IDEX-0004-02).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Osmanagic-Myers S., Dechat T., Foisner R. Lamins at the crossroads of mechanosignaling. Genes Dev. 2015;29:225–237. doi: 10.1101/gad.255968.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Worman H.J., Bonne G. “Laminopathies”: A wide spectrum of human diseases. Exp. Cell Res. 2007;313:2121–2133. doi: 10.1016/j.yexcr.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertrand A.T., Chikhaoui K., Yaou R.B., Bonne G. Clinical and genetic heterogeneity in laminopathies. Biochem. Soc. Trans. 2011;39:1687–1692. doi: 10.1042/BST20110670. [DOI] [PubMed] [Google Scholar]
- 4.Guilluy C., Osborne L.D., Van Landeghem L., Sharek L., Superfine R., Garcia-Mata R., Burridge K. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat. Cell Biol. 2014;16:376–381. doi: 10.1038/ncb2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swift J., Ivanovska I.L., Buxboim A., Harada T., Dingal P.C., Pinter J., Pajerowski J.D., Spinler K.R., Shin J.W., Tewari M., et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaughan A., Alvarez-Reyes M., Bridger J.M., Broers J.L., Ramaekers F.C., Wehnert M., Morris G.E., Whitfield W.G.F., Hutchison C.J. Both emerin and lamin C depend on lamin A for localization at the nuclear envelope. J. Cell Sci. 2001;114:2577–2590. doi: 10.1242/jcs.114.14.2577. [DOI] [PubMed] [Google Scholar]
- 7.Markiewicz E., Tilgner K., Barker N., van de Wetering M., Clevers H., Dorobek M., Hausmanowa-Petrusewicz I., Ramaekers F.C., Broers J.L., Blankesteijn W.M., et al. The inner nuclear membrane protein emerin regulates beta-catenin activity by restricting its accumulation in the nucleus. EMBO J. 2006;25:3275–3285. doi: 10.1038/sj.emboj.7601230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho C.Y., Jaalouk D.E., Vartiainen M.K., Lammerding J. Lamin A/C and emerin regulate MKL1-SRF activity by modulating actin dynamics. Nature. 2013;497:507–511. doi: 10.1038/nature12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz C., Fischer M., Mamchaoui K., Bigot A., Lok T., Verdier C., Duperray A., Michel R., Holt I., Voit T., et al. Lamins and nesprin-1 mediate inside-out mechanical coupling in muscle cell precursors through FHOD1. Sci. Rep. 2017;7:1253. doi: 10.1038/s41598-017-01324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertrand A.T., Ziaei S., Ehret C., Duchemin H., Mamchaoui K., Bigot A., Mayer M., Quijano-Roy S., Desguerre I., Laine J., et al. Cellular microenvironments reveal defective mechanosensing responses and elevated YAP signaling in LMNA-mutated muscle precursors. J. Cell Sci. 2014;127:2873–2884. doi: 10.1242/jcs.144907. [DOI] [PubMed] [Google Scholar]
- 11.Panciera T., Azzolin L., Cordenonsi M., Piccolo S. Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017;18:758–770. doi: 10.1038/nrm.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fischer M., Rikeit P., Knaus P., Coirault C. YAP-Mediated Mechanotransduction in Skeletal Muscle. Front. Physiol. 2016;7:41. doi: 10.3389/fphys.2016.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irianto J., Pfeifer C.R., Ivanovska I.L., Swift J., Discher D.E. Nuclear lamins in cancer. Cell. Mol. Bioeng. 2016;9:258–267. doi: 10.1007/s12195-016-0437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chow K.H., Factor R.E., Ullman K.S. The nuclear envelope environment and its cancer connections. Nat. Rev. Cancer. 2012;12:196–209. doi: 10.1038/nrc3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zink D., Fischer A.H., Nickerson J.A. Nuclear structure in cancer cells. Nat. Rev. Cancer. 2004;4:677–687. doi: 10.1038/nrc1430. [DOI] [PubMed] [Google Scholar]
- 16.Dahl K.N., Ribeiro A.J., Lammerding J. Nuclear shape, mechanics, and mechanotransduction. Circ. Res. 2008;102:1307–1318. doi: 10.1161/CIRCRESAHA.108.173989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elosegui-Artola A., Trepat X., Roca-Cusachs P. Control of Mechanotransduction by Molecular Clutch Dynamics. Trends Cell Biol. 2018;28:356–367. doi: 10.1016/j.tcb.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Aureille J., Buffiere-Ribot V., Harvey B.E., Boyault C., Pernet L., Andersen T., Bacola G., Balland M., Fraboulet S., Van Landeghem L., et al. Nuclear envelope deformation controls cell cycle progression in response to mechanical force. EMBO Rep. 2019;20:e48084. doi: 10.15252/embr.201948084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou C., Rao L., Shanahan C.M., Zhang Q. Nesprin-1/2: Roles in nuclear envelope organisation, myogenesis and muscle disease. Biochem. Soc. Trans. 2018;46:311–320. doi: 10.1042/BST20170149. [DOI] [PubMed] [Google Scholar]
- 20.Puckelwartz M.J., Kessler E.J., Kim G., Dewitt M.M., Zhang Y., Earley J.U., Depreux F.F., Holaska J., Mewborn S.K., Pytel P., et al. Nesprin-1 mutations in human and murine cardiomyopathy. J. Mol. Cell. Cardiol. 2010;48:600–608. doi: 10.1016/j.yjmcc.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perovanovic J., Dell’Orso S., Gnochi V.F., Jaiswal J.K., Sartorelli V., Vigouroux C., Mamchaoui K., Mouly V., Bonne G., Hoffman E.P. Laminopathies disrupt epigenomic developmental programs and cell fate. Sci. Transl. Med. 2016;8:335ra358. doi: 10.1126/scitranslmed.aad4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aure K., Mamchaoui K., Frachon P., Butler-Browne G.S., Lombes A., Mouly V. Impact on oxidative phosphorylation of immortalization with the telomerase gene. Neuromuscul. Disord. 2007;17:368–375. doi: 10.1016/j.nmd.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 23.Chaouch S., Mouly V., Goyenvalle A., Vulin A., Mamchaoui K., Negroni E., Di Santo J., Butler-Browne G., Torrente Y., Garcia L., et al. Immortalized skin fibroblasts expressing conditional MyoD as a renewable and reliable source of converted human muscle cells to assess therapeutic strategies for muscular dystrophies: Validation of an exon-skipping approach to restore dystrophin in Duchenne muscular dystrophy cells. Hum. Gene Ther. 2009;20:784–790. doi: 10.1089/hum.2008.163. [DOI] [PubMed] [Google Scholar]
- 24.Soderholm J.F., Bird S.L., Kalab P., Sampathkumar Y., Hasegawa K., Uehara-Bingen M., Weis K., Heald R. Importazole, a small molecule inhibitor of the transport receptor importin-beta. ACS Chem. Biol. 2011;6:700–708. doi: 10.1021/cb2000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kudo N., Wolff B., Sekimoto T., Schreiner E.P., Yoneda Y., Yanagida M., Horinouchi S., Yoshida M. Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1. Exp. Cell Res. 1998;242:540–547. doi: 10.1006/excr.1998.4136. [DOI] [PubMed] [Google Scholar]
- 26.Kim N.G., Koh E., Chen X., Gumbiner B.M. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc. Natl. Acad. Sci. USA. 2011;108:11930–11935. doi: 10.1073/pnas.1103345108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao B., Wei X., Li W., Udan R.S., Yang Q., Kim J., Xie J., Ikenoue T., Yu J., Li L., et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aragona M., Panciera T., Manfrin A., Giulitti S., Michielin F., Elvassore N., Dupont S., Piccolo S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 29.Dupont S., Morsut L., Aragona M., Enzo E., Giulitti S., Cordenonsi M., Zanconato F., Le Digabel J., Forcato M., Bicciato S., et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 30.Calvo F., Ege N., Grande-Garcia A., Hooper S., Jenkins R.P., Chaudhry S.I., Harrington K., Williamson P., Moeendarbary E., Charras G., et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol. 2013;15:637–646. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Judson R.N., Tremblay A.M., Knopp P., White R.B., Urcia R., De Bari C., Zammit P.S., Camargo F.D., Wackerhage H. The Hippo pathway member Yap plays a key role in influencing fate decisions in muscle satellite cells. J. Cell Sci. 2012;125:6009–6019. doi: 10.1242/jcs.109546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wackerhage H., Del Re D.P., Judson R.N., Sudol M., Sadoshima J. The Hippo signal transduction network in skeletal and cardiac muscle. Sci. Signal. 2014;7:re4. doi: 10.1126/scisignal.2005096. [DOI] [PubMed] [Google Scholar]
- 33.Goodman C.A., Dietz J.M., Jacobs B.L., McNally R.M., You J.S., Hornberger T.A. Yes-Associated Protein is up-regulated by mechanical overload and is sufficient to induce skeletal muscle hypertrophy. FEBS Lett. 2015;589:1491–1497. doi: 10.1016/j.febslet.2015.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dupont S. Role of YAP/TAZ in cell-matrix adhesion-mediated signalling and mechanotransduction. Exp. Cell Res. 2016;343:42–53. doi: 10.1016/j.yexcr.2015.10.034. [DOI] [PubMed] [Google Scholar]
- 35.Moon S., Kim W., Kim S., Kim Y., Song Y., Bilousov O., Kim J., Lee T., Cha B., Kim M., et al. Phosphorylation by NLK inhibits YAP-14-3-3-interactions and induces its nuclear localization. EMBO Rep. 2017;18:61–71. doi: 10.15252/embr.201642683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zanconato F., Cordenonsi M., Piccolo S. YAP/TAZ at the Roots of Cancer. Cancer Cell. 2016;29:783–803. doi: 10.1016/j.ccell.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu F.X., Zhao B., Guan K.L. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell. 2015;163:811–828. doi: 10.1016/j.cell.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oh H., Irvine K.D. In vivo regulation of Yorkie phosphorylation and localization. Development. 2008;135:1081–1088. doi: 10.1242/dev.015255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao B., Lei Q.Y., Guan K.L. The Hippo-YAP pathway: New connections between regulation of organ size and cancer. Curr. Opin. Cell Biol. 2008;20:638–646. doi: 10.1016/j.ceb.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan E.H., Nousiainen M., Chalamalasetty R.B., Schafer A., Nigg E.A., Sillje H.H. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005;24:2076–2086. doi: 10.1038/sj.onc.1208445. [DOI] [PubMed] [Google Scholar]
- 41.Mo J.S., Park H.W., Guan K.L. The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep. 2014;15:642–656. doi: 10.15252/embr.201438638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hao Y., Chun A., Cheung K., Rashidi B., Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J. Biol. Chem. 2008;283:5496–5509. doi: 10.1074/jbc.M709037200. [DOI] [PubMed] [Google Scholar]
- 43.Lei Q.Y., Zhang H., Zhao B., Zha Z.Y., Bai F., Pei X.H., Zhao S., Xiong Y., Guan K.L. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol. Cell. Biol. 2008;28:2426–2436. doi: 10.1128/MCB.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oka T., Mazack V., Sudol M. Mst2 and Lats kinases regulate apoptotic function of Yes kinase-associated protein (YAP) J. Biol. Chem. 2008;283:27534–27546. doi: 10.1074/jbc.M804380200. [DOI] [PubMed] [Google Scholar]
- 45.Wada K., Itoga K., Okano T., Yonemura S., Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138:3907–3914. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- 46.Das A., Fischer R.S., Pan D., Waterman C.M. YAP Nuclear Localization in the Absence of Cell-Cell Contact Is Mediated by a Filamentous Actin-dependent, Myosin II- and Phospho-YAP-independent Pathway during Extracellular Matrix Mechanosensing. J. Biol. Chem. 2016;291:6096–6110. doi: 10.1074/jbc.M115.708313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ege N., Dowbaj A.M., Jiang M., Howell M., Hooper S., Foster C., Jenkins R.P., Sahai E. Quantitative Analysis Reveals that Actin and Src-Family Kinases Regulate Nuclear YAP1 and Its Export. Cell Syst. 2018;6:692.e13–708.e13. doi: 10.1016/j.cels.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kofler M., Speight P., Little D., Di Ciano-Oliveira C., Szaszi K., Kapus A. Mediated nuclear import and export of TAZ and the underlying molecular requirements. Nat. Commun. 2018;9:4966. doi: 10.1038/s41467-018-07450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levy D., Adamovich Y., Reuven N., Shaul Y. Yap1 phosphorylation by c-Abl is a critical step in selective activation of proapoptotic genes in response to DNA damage. Mol. Cell. 2008;29:350–361. doi: 10.1016/j.molcel.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 50.Wang S., Li H., Wang G., Zhang T., Fu B., Ma M., Quan Z., Chen G. Yes-associated protein (YAP) expression is involved in epithelial-mesenchymal transition in hepatocellular carcinoma. Clin. Transl. Oncol. 2016;18:172–177. doi: 10.1007/s12094-015-1353-4. [DOI] [PubMed] [Google Scholar]
- 51.Zwerger M., Jaalouk D.E., Lombardi M.L., Isermann P., Mauermann M., Dialynas G., Herrmann H., Wallrath L.L., Lammerding J. Myopathic lamin mutations impair nuclear stability in cells and tissue and disrupt nucleo-cytoskeletal coupling. Hum. Mol. Genet. 2013;22:2335–2349. doi: 10.1093/hmg/ddt079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Earle A.J., Kirby T.J., Fedorchak G.R., Isermann P., Patel J., Iruvanti S., Moore S.A., Bonne G., Wallrath L.L., Lammerding J. Mutant lamins cause nuclear envelope rupture and DNA damage in skeletal muscle cells. Nat. Mater. 2019 doi: 10.1038/s41563-019-0563-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jahed Z., Mofrad M.R. The nucleus feels the force, LINCed in or not! Curr. Opin. Cell Biol. 2019;58:114–119. doi: 10.1016/j.ceb.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 54.Ohgushi M., Minaguchi M., Sasai Y. Rho-Signaling-Directed YAP/TAZ Activity Underlies the Long-Term Survival and Expansion of Human Embryonic Stem Cells. Cell Stem Cell. 2015;17:448–461. doi: 10.1016/j.stem.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 55.Musah S., Wrighton P.J., Zaltsman Y., Zhong X., Zorn S., Parlato M.B., Hsiao C., Palecek S.P., Chang Q., Murphy W.L., et al. Substratum-induced differentiation of human pluripotent stem cells reveals the coactivator YAP is a potent regulator of neuronal specification. Proc. Natl. Acad. Sci. USA. 2014;111:13805–13810. doi: 10.1073/pnas.1415330111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.