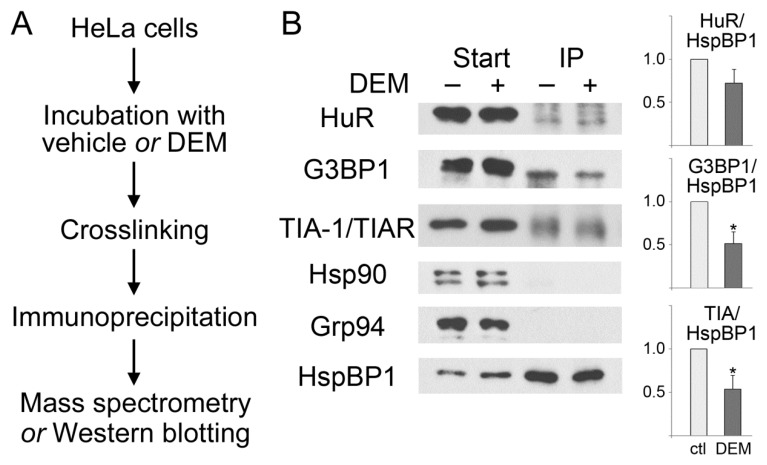
Figure 2.
HspBP1 associates with SG proteins. Protein complexes were immunopurified with antibodies against HspBP1 from control and DEM-treated cells. (A) Workflow of the experiment. (B) Western blotting. Samples were incubated with vehicle (−) or DEM (+) as specified. Crude extracts (Start) and immunoprecipitates (IP) were analyzed with antibodies against HuR, G3BP1, TIA-1/TIAR, hsp90 or Grp94 as indicated. Filters were stripped and reprobed with antibodies against HspBP1. ECL signals were quantified to determine the stress-dependent changes in the association of HspBP1 with different binding partners. The ratio of interacting protein/HspBP1 was normalized to control conditions. Means +SEM are shown for three independent experiments. The student’s t-test identified significant differences; * p < 0.05. No binding of HspBP1 to hsp90 or Grp94 was detected under these conditions, demonstrating the specificity of our assay. For each protein, all lanes are from the same blot.