Abstract

To better realize how CaO promoted the pyrolysis process of a corn stover, which was important for further development of its technology, various effects of calcium oxide (CaO) on the pyrolysis of the corn stover at different temperatures were studied. The pyrolysis of the corn stover mainly occurred at 90, 291, 335, and 385 °C, which were correspondent to the pyrolysis temperatures of water, hemicellulose, cellulose, and lignin, respectively. Moreover, CaO was found to absorb some CO2 and H2O produced during the pyrolysis, as well as promote the occurrence of pyrolysis, and reduce the activation energy required for the reaction. According to the calculation of the activation energy, the optimal addition ratio of CaO and the corn stover should be between 1:2 and 1:1. The analysis of the release of pyrolysis gas showed that CaO had a beneficial effect on deacidification and the production of hydrocarbons and aromatic compound gas. When the addition ratio was 1:1, the release amount of the acidic substance was the lowest. When the ratio of CaO and the corn stover was 1:2, the release amount of H2O, CO2, and aromatic rings was at the maximum. The change of content of tri-state products generated during pyrolysis at different final temperatures was also studied by the pyrolysis experiment. The changes of functional groups in char were observed by Fourier-transform infrared spectroscopy. The results showed that with the addition of CaO, the content of H2O in char, and the absorption of CO2 increased, which generated alkaline substances, while reacting with acidic substances, and the thermal decomposition of acidic substances in the corn stover was promoted, which caused the pyrolysis reaction of the corn stover to occur in the positive direction. With the increase of pyrolysis temperature, phenol and carboxylic acid became thermally resolved or neutralized. When the catalyst amount or temperature was gradually increased, the aliphatic group was steadily pyrolyzed while char increasingly became aromatized. Based on the comprehensive analysis of the above experimental results, it was believed that the optimal addition ratio of CaO and the corn stover should be between 1:2 and 1:1.
1. Introduction
About 80% of the world’s annual energy consumption is the energy produced from fossil fuels such as coal, oil, and natural gas. Researchers have predicted that fossil fuel around the world will be depleted in the next 40–50 years.1 Therefore, the development of renewable energy has become inevitable for economic and social development worldwide, and biomass has received wide attention as a renewable carbon source. There are many ways to produce energy from biomass, which include pyrolysis, gasification, combustion, carbonization, and fermentation. Pyrolysis is a key step in thermochemical conversion processes.2 Pindoria et al.3 have described the importance of biomass pyrolysis by analyzing and comparing the characteristics of pyrolysis and gasification of eucalyptus.
Biomass pyrolysis is a complex process because of its diverse components and reaction conditions.4 During pyrolysis, various reactions between major components of biomass, such as cellulose, hemicellulose, and lignin, can occur.5 For example, the interaction between hemicellulose and lignin promotes the production of lignin-derived phenol, therefore hindering the formation of hydrocarbons.6 Lignin can react with cellulose, which can hinder the formation of the l-glucose polymer from cellulose and thereby reduces the formation of biochar.7 The interaction of cellulose and hemicellulose has a lower effect on the formation and distribution of pyrolysis products. In addition to these reactions, there are a number of other parallel and series reactions that can take place during biomass pyrolysis, which include dehydration, depolymerization, isomerization, aromatization, decarboxylation, and charring.8,9 In general, biomass pyrolysis can be divided into three main stages: initial evaporation of free moisture; primary decomposition; and secondary reaction (oil cracking and repolymerization).10
Lappas et al.11 have described that mesoporous molecular sieve materials with uniform pore size distribution (MCM-41, MSU, and SBA-15), mixed microporous and mesoporous materials doped with noble and transition-metals and alkali metals are good catalyst candidates for biomass pyrolysis.
Nokkosmäki et al.12 and Fabbri et al.13 have reported that when ZnO is used as a catalyst, the liquid yield is slightly reduced, but the stability bio-oil is significantly improved. Lu et al.14 has utilized different metal oxide catalysts to study the changes of steam produced from biomass pyrolysis by using a Py-GC/MS technique. The study has observed that ZnO is a mild catalyst, and Fe2O3 catalysis can lead to the formation of various hydrocarbons. The CaO catalyst can significantly reduce the amount of phenol and anhydrous sugars and eliminate acid, thus increasing the formation of cyclopentanone, hydrocarbons, and some light compounds. In addition, a study using thermogravimetric analysis and Fourier transform infrared spectroscopy (FTIR) has shown that this catalyst is highly effective in reducing the content of acid and promoting the formation of hydrocarbons. D’Orazio et al.15 have used three different types of CaO-mixed catalysts as raw materials to study CO2 absorption in the reaction bed. Ding et al.16 have developed a CaO and HZSM-5 dual catalyst bed to convert acid in xylan pyrolysis products into hydrocarbons.
Chen et al.17 have applied CaO to the pyrolysis of a cotton stover to promote the formation of ketone, reduce the amount of acid, increase the concentrations of H2 and CH4, and decrease the concentration of CO2. Using chemical-looping gasification of biomass, Wu et al.18 have introduced steam and CaO into a fixed bed to produce syngas and observed that the addition of steam can promote the reforming and the water-gas shift reactions. Udomsirichakorn et al.19 and Wei et al.20 have also discussed the process and mechanism of CaO-catalyzed biomass gasification in a fixed bed.
Overall, CaO has many advantages in the catalytic pyrolysis of a corn stover, including nontoxicity and low cost. It is widely used in CO2 adsorption and catalytic cracking, the two processes in biomass pyrolysis.21 It can increase the calorific value of pyrolysis gas and the production of H2, CH4, and other gases, improve the quality of gas,22 catalyze the cracking of bio-oil, and reduce the production of bio-oil.14,23 It can also neutralize acidic substances, and promote the formation of hydrocarbons in char.24 Thus, CaO plays an important role in the adsorption of CO2 and catalytic biomass pyrolysis. Table 1 summarizes important reactions in biomass pyrolysis catalyzed by CaO.19,20
Table 1. Important Reactions Occurring during CaO Catalytic Biomass Pyrolysis.
| name of reaction | chemical equation | |
|---|---|---|
| pyrolysis | ||
| water gas (primary) | C + H2O → CO + H2 | |
| water gas (secondary) | C + 2H2O → CO2 + 2H2 | |
| tars reforming | ||
| carbonation | CaO + CO2 → CaCO3 |
At present, a lot of research work has been carried out on the pyrolysis process of the corn stover catalyzed by CaO. However, the mechanism of CaO on the pyrolysis of the corn stover and the optimal addition ratio of CaO are not clear. Therefore, it is still of great significance to carry out the research on the influence of CaO on the pyrolysis of the corn stover and its mechanism.
In this study, TG-FTIR was used to analyze the weight loss trend and weight loss rate of CaO-catalyzed corn stover pyrolysis. The effects of different CaO dosages on the activation energy of the pyrolysis process were discussed, and the optimal ratio of CaO addition amount was explored. The Lambert–Beer’s law was used to semi-quantitatively analyze the amount of catalyst on the volatile components of pyrolysis products. The optimal ratio of CaO addition for different gases was analyzed. In the pyrolysis experiments, the changes in the yields of pyrolysis gas, bio-oil, water, and char at different final temperatures after the addition of the catalyst were discussed. FTIR was used to analyze the changes of char’s functional groups at different final temperatures and different catalyst dosages.
Based on the experimental results, the effects of different catalyst dosages on the pyrolysis of the corn stover were analyzed from multiple perspectives. The optimal ratio of CaO was judged. The pyrolysis mechanism of the CaO-catalyzed corn stover was analyzed by analyzing the pyrolysis state at different temperatures. It provided a theoretical basis for further exploring the pyrolysis process of the corn stover.
2. Results and Discussion
2.1. Thermogravimetric Analysis
TG was employed as a key technique to analyze the relationship between the pyrolysis reaction temperature and sample weight loss. The DTG curve was the first derivative of the TG curve. Peak separation of the curve (DTG) was carried out using the Gaussian function. The fitting of the experimental curves and the calculation resulted in correlation coefficients (R2) of at least 0.998.
Figure 1 shows the distribution of TG-DTG peaks during the pyrolysis of the corn stover. The TG curve showed that the sample weight was continuously reduced throughout the pyrolysis process, and the highest weight loss occurred between 200 and 600 °C. When the final pyrolysis temperature reached 600 °C, the weight of the corn stover decreased to 35.17%. The DTG curve (Figure 1), a composite curve, shows a series of chemical reactions that took place during pyrolysis. The curve fitting of the four sub-curves can be used to identify the breakage of covalent bonds. Sub-curve 1 observed at about 90 °C is due to the decomposition of evaporation of water. Sub-curve 2 appeared near 291 °C is caused by the decomposition of hemicellulose, of which the rate is moderate. Sub-curve 3 at 300–370 °C is caused by the decomposition of cellulose, of which the rate is highest among all peaks. Sub-curve 4 representing lignin decomposition mainly appears at about 385 °C. The widest temperature range indicates that lignin takes the longest time, and has a slow pyrolysis rate, which indicate that the pyrolysis of lignin occurs slowly. These results indicate that the three components are successfully pyrolyzed, and there is no obvious temperature limit in the process.25−27
Figure 1.

TG/DTG and sub-curves of the corn stover.
Figure 2 shows TG/DTG curves of the six types of samples. With increasing the added amount of CaO, the TG curves showed that the percentage of remaining solids after pyrolysis increased, from 31.55% in the sample without CaO to 52.93% in the sample containing CaO at a 1:1 ratio. Additionally, the DTG curve showed that the temperature gradually shifted toward a higher region at 200–500 °C. The more the added CaO amount, the more prominent the offset effects are. This can be due to the following two reasons: (1) CaO may hinder heat conduction and absorbs part of the heat, thus delaying the pyrolysis process and (2) the CaO catalyst and the acidic substances generated during pyrolysis may cause a certain amount of water to be generated. During water evaporation, part of the heat is absorbed and the rate of increase of temperature in the sample is reduced. As a result, the DTG curve shifts toward the high-temperature region.
Figure 2.
TG/DTG curves of six types of samples: (a) TG; (b) DTG.
When the temperature was increased to 200–370 °C, the rate of weight loss (shown by the DTG curve) decreased with the increase of CaO amount. It is likely that with the addition of CaO, some water and CO2 gas are absorbed, and solids such as CaCO3, Ca(OH)2, and Ca(HCO3)2 are generated. As a result, the solid residue becomes dominant after the pyrolysis was completed, as indicated by the TG curve. Its chemical compositions mostly consist of CaO. At the same time, we found that the DTG peak at 291 °C disappeared after adding the CaO catalyst. Combining with the results of “Analysis of Pyrolysis Gas” in Section 2.3, it was found that this was because the acid gas generated by pyrolysis was neutralized by CaO and other basic substances [such as Ca(OH)2, etc.]. When the temperature was raised to 370–500 °C, the weight loss rate increased gradually as the amount of CaO increased. It is apparent that more H2O produced by the neutralization reaction of acidic substances has evaporated when the alkaline catalyst is presented in the pyrolysis. This observation is also in line with a study on bio-oil cracking, in which the catalytic effect of CaO on bio-oil cracking was observed.28 The study describes that in the absence of CaO, the decomposition of bio-oil becomes more difficult. Compared with those of the corn stover without the catalyst, the DTG curves of samples containing CaO showed that a significant weight loss takes place at 600–750 °C, and the weight loss increased with the increase of CaO amount. It is possible that the pyrolysis of CaCO3 and Ca(OH)2 occurs at these temperatures to produce CaO. This is also supported by the effect of CaO on CO2 absorption, in which when the CaO amount was increased, the absorption of CO2 became more prominent.
2.2. Pyrolysis Kinetics Analysis
In this study, the Coats Redfern kinetic method was used to analyze the biomass pyrolysis process. By calculating the results of thermogravimetric experiments, the change of the activation energy value of the pyrolysis process was analyzed, so as to determine the effect of the addition of the catalyst dose on the degree of pyrolysis. The reaction equation is as follows
| 1 |
Among them, A is the pre-exponential factor, E is the activation energy, T is the temperature, t is the time, and α is the conversion rate of the biomass. According to the approximate expression of Coats Redfern kinetic method, it can be derived as the following equation29
| 2 |
where R is the universal gas constant and β = dT/dt is the heating rate. Fit a straight line with the left side of the eq 2 as the ordinate and 1/T as the abscissa. Its slope is −E/R and its intercept is ln(AR/βE). Thus, the values of E and A can be derived. The results are shown in Table 2.
Table 2. Calculation Results of Coats–Redfern Model for Pyrolysis of the Corn Stover by CaO.
| sample | temperature (°C) | heating rate (°C·min–1) | E (kJ·mol–1) | R2 | ln(A) |
|---|---|---|---|---|---|
| non-catalyst | 220–335 | 10 | 61.48 | 0.9927 | 11.70 |
| CaO/stover = 1:20 | 60.41 | 0.9897 | 11.55 | ||
| CaO/stover = 1:10 | 58.88 | 0.9871 | 11.41 | ||
| CaO/stover = 1:5 | 56.51 | 0.9842 | 11.27 | ||
| CaO/stover = 1:2 | 50.56 | 0.9802 | 11.05 | ||
| CaO/stover = 1:1 | 48.85 | 0.9819 | 11.00 |
According to the TG curve, the calculated temperature of the kinetics is selected from 220 to 335 °C, and the weight loss effect at this time is more obvious. With the increased CaO dose, the pyrolysis activation energy of the corn stover significantly decreased from 61.48 kJ/mol (non-catalyst) to 48.85 kJ/mol (CaO/stover = 1:1). When the CaO/stover increased from 1:5 to 1:2, the activation energy decreased by 5.95 kJ/mol. When the CaO/stover increased from 1:2 to 1:1, the activation energy was only reduced by 1.71 kJ/mol. It is proved that the catalytic effect of CaO has decreased at this moment. Therefore, the most appropriate ratio of CaO and the corn stover should be between 1:1 and 1:2. The absorption of the pyrolysis gas by CaO and the neutralization of acidic substances promote the positive reaction of the corn stover pyrolysis reaction, which is the main reason for the decrease of activation energy of the pyrolysis reaction.
2.3. Analysis of Pyrolysis Gas
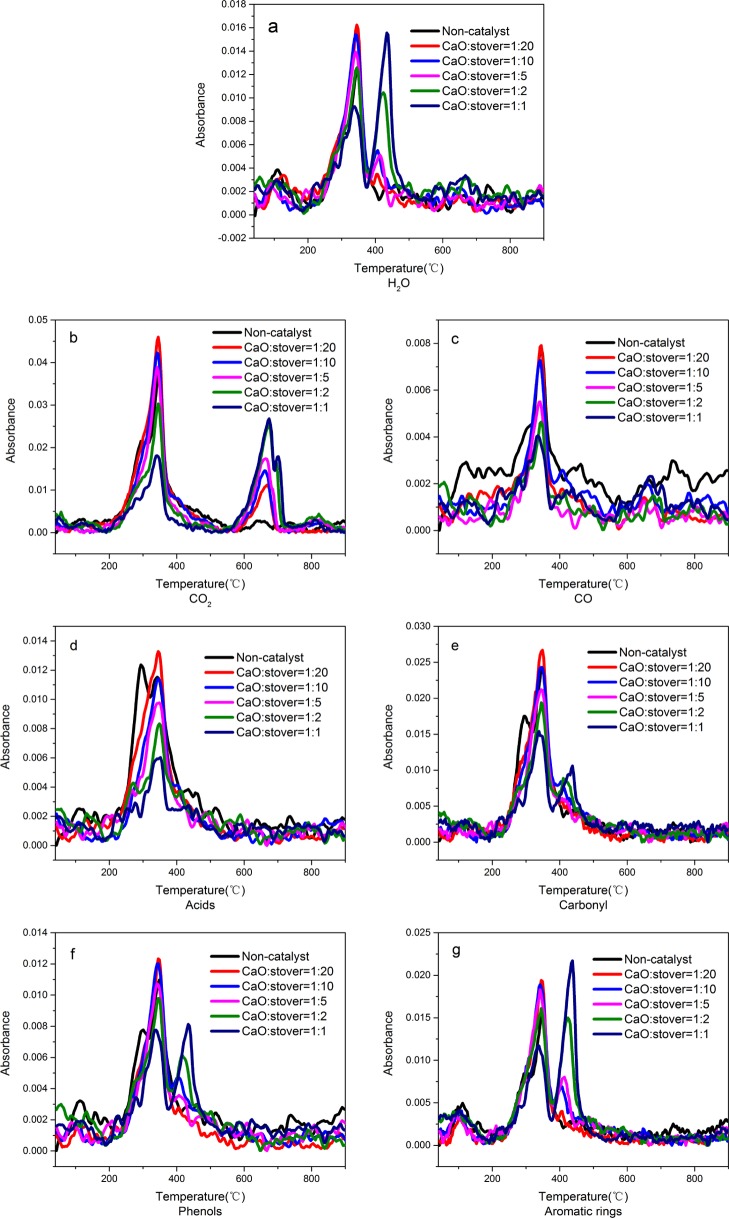
Pyrolysis gas produced during the pyrolysis of various samples was analyzed by FTIR. In the experiment, while light gases, such as CO2, CO, and so forth, can be easily detected, complex compounds, such as acid compounds, aromatic compounds, and so forth, which are mostly divided by class, are difficult to be distinguished. CO2 is formed by decarboxylation and carbonyl cleavage,30 and CO is formed by the cleavage of ether and C–O bonds.31 The characteristic wavenumbers of main pyrolysis gases and the temperature at which they were released at maximum amounts are summarized in Table 3.32−34 The infrared spectra of various pyrolysis gases are shown in Figure 3. In Figure 3, it is not difficult to find out the release amount of gases has more than one maximum value. Therefore, Table 3 also shows the two temperatures when the gas release reaches its maximum value.
Table 3. Characteristic Wavenumbers of Pyrolysis Gas and Temperature at Release Maximum.
| compounds | assignment | wavenumber (cm–1) | Tmax (°C) |
|---|---|---|---|
| H2O | O–H stretching | 3735 | 345/434 |
| CO2 | C=O stretching | 2315 | 345/674 |
| CO | C–O stretching | 2182 | 343 |
| acid | C–O stretching | 1170 | 293/347 |
| carbonyl compounds | C=O stretching | 1743 | 348/440 |
| phenol | O–H bending | 1364 | 346/434 |
| aromatic ring | C=C stretching | 1509 | 346/438 |
Figure 3.
Infrared spectrum of pyrolysis gas: (a) H2O; (b) CO2; (c) CO; (d) acids; (e) carbonyl; (f) phenols; and (g) aromatic rings.
Combined with the TG curve, the maximum rate of weight loss occurred when the release of pyrolysis gas reached a maximum value. As illustrated in I, during the catalytic process, a neutralization reaction with CaO converts acid to (RCOO)2Ca and H2O. (RCOO)2Ca is then decomposed to ketone substances and CaCO3 (II) while R–COOH is thermally cleaved by CaO (III) via a thermal cracking reaction and converted into hydrocarbonyl and CO2.24,35 CaO can also react with CO2 produced in the reaction to form CaCO3, as shown in IV. Therefore, the chemical equilibrium shifts in a direction favorable for removing acid and generating hydrocarbons.
| I |
| II |
| III |
| IV |
Acid undergoes three different types of neutralization reactions, thermal cracking, and catalytic cracking; its conversion is favorable for the formation of hydrocarbons. As shown in Figure 3, after CaO was added, the peak of the acid at about 291 °C disappeared. This was because the acid gas generated by pyrolysis was neutralized by CaO or other basic substances [such as Ca(OH)2, etc.]. At the same time, the peaks of carbonyl and phenols also decreased at this temperature. This was also due to the reduction in the amount of acid released. This also verified that the peak at 291 °C of the DTG curve in Figure 2 disappeared after adding CaO. However, the curves of hydrocarbons, including aromatic hydrocarbons, and those of phenol and water increased significantly at a temperature of up to 438 °C. This is likely due to the lesser extent of the catalytic cracking reaction of acid, which primarily occurs at a temperature below 430 °C. The comparison of DTG curves also showed that after CaO was added, the weight loss rate was significantly increased at temperatures between 430 and 440 °C, and the increase was more prominent at higher amounts of catalyst.
The infrared spectrum of CO2, as shown in Figure 3, demonstrates that with the presence of CaO, there was a considerable amount of gas released at temperatures between 600 and 700 °C. The infrared spectrum of H2O also showed that there was an amount of gas generated at temperatures between 400 and 500 °C. This may be caused by the decomposition of CaCO3 and Ca(OH)2 at high temperatures, which regenerates CaO.
According to the Lambert–Beer’s law (eq 3), the absorbance at a certain wavenumber has a linear relationship with gas concentration. In eq 3, As is the absorbance at a specific wavenumber; Cs is the instantaneous concentration (mol·L–1) of the species of interest at a particular wavelength; b is the optical path length (cm); and εs is the molar absorption coefficient (mol–1·cm–1) the species being measured. Derivation of eq 3 resulted in eqs II–IV.36
| 3 |
| 4 |
| 5 |
 |
6 |
where: Vs is the formation rate (mol·s–1) of the species of interest; q is the flow rate of carrier gas (L·s–1); and ns is the amount (mol) of the species of interest.
The equation can be described as the infrared curve is first integrated, and the integrated value of the sample with the catalyst is then divided by that of the sample without the catalyst. The obtained value can be used to indicate the degree of change of the amount of gas, which reflects the extent of the effect of the catalyst on gas. The results are shown in Figure 4.
Figure 4.

Relative molarity of major volatiles produced by pyrolysis of the corn stover under different doses of the catalyst.
The results depicted in Figure 4 show that during the pyrolysis catalyzed by CaO, the composition of pyrolysis gas changed significantly. Compared to that without the catalyst, the process with the catalyst at a mass ratio of 1:20 reduced the molarity of acid by 22.81%. In addition, as the amount of the catalyst was increased, more acid was neutralized so that its molarity was gradually reduced. When the catalyst having a mass ratio of 1:1 was used, the molarity of acid was reduced by 45.97%. It was not difficult to find out from Figure 4 that when the addition ratio of CaO and the corn stover was 1:1, the effect of acid absorption was the best. The molarity of aromatics decreased by 20.31% when the catalyst with a mass ratio of 1:20 was used. By contrast, the molarity of the aromatics gradually increased with the increase of the catalyst amount, reaching its maximum value (an increase by 15.68%) when the catalyst with a mass ratio of 1:2 was used. The results show that CaO has a significant effect on deacidification and can increase the production of hydrocarbon gases, as indicated by the calorific value of the gas.
On the other hand, the molarity of the phenolic material produced in the process catalyzed by CaO was lower than that produced in the noncatalytic process. The molarity was decreased by 34.13% when the catalyst having a mass ratio of 1:20 was used; however, such a value increased when the amount of the catalyst was increased. When the catalyst at a high mass ratio of 1:1 was used, the molarity of phenolic substances decreased by 14.81%. There are two possible reasons that can describe the decrease of phenolic substances: (1) CaO can react with acidic functional groups in phenol and (2) CaO can catalyze the decomposition of phenol to produce CO.37 However, the production of CO was significantly reduced (Figure 4) because of the water-gas shift reaction between a large amount of H2O produced and CO.38 In addition, the molarity of carbonyl compounds also decreased slightly by about 8%. The removal of acid may be the main cause of such a decrease.
It is worth noting that the molarity of H2O increased significantly after CaO was applied. Most O elements in the corn stover were released in the form of H2O. When the mass ratio of catalyst to corn stover was increased to 1:1, the molarity of H2O increased by 33.73% because of the neutralization reaction of CaO with acid and the decomposition of Ca(OH)2 at high temperatures. From Figure 4, when the addition ratio of CaO and the corn stover was 1:2, the release amount of CO2 and H2O is at the maximum.
2.4. Analysis of Pyrolysis Yield
In the pyrolysis experiment, char was collected, and the changes of yield of pyrolytic tri-state products at different final temperatures were determined. The results are shown in Table 4 and Figure 5.
Table 4. Yield of Tri-state Products in Both Catalytic and Noncatalyzed States at Different Final Pyrolysis Temperatures.
| sample | temperature (°C) | oil yield (%) | char yield (%) | water yield (%) | gas yield (%) |
|---|---|---|---|---|---|
| non-catalyst | 291 | 9.00 | 55.80 | 22.00 | 13.20 |
| CaO/stover = 1:20 | 8.10 | 59.31 | 19.29 | 13.30 | |
| non-catalyst | 335 | 13.33 | 50.25 | 22.00 | 14.42 |
| CaO/stover = 1:20 | 8.19 | 56.52 | 20.24 | 15.05 | |
| non-catalyst | 520 | 14.03 | 39.75 | 22.75 | 23.47 |
| CaO/stover = 1:20 | 13.55 | 43.12 | 23.33 | 20.00 |
Figure 5.

Yield of tri-state products in both catalytic and noncatalyzed states at different final pyrolysis temperatures.
According to the oil yield, it is clear that after the catalyst was added, the yield of bio-oil was significantly decreased in all temperature ranges. Among them was 335 °C, at which the effect was most obvious. It appears that CaO can promote bio-oil cracking while absorbing CO2.39−41 Studies have shown that CaO can accelerate the cracking of the ring structure and the active center of CaO has a spatially diffused electron cloud. O2– formed can cause instability of the electron cloud of a bio-oil compound by destabilizing its aromatic ring, which leads to ring splitting.42 Additionally, CaO also promotes the shedding of methyl groups and the precipitation of carbon. Because of the negatively charged cyclic π– electron of aromatic hydrocarbons, they can be adsorbed on the positively charged Ca2+ of CaO. A hydrogen atom in the fat side chain can be transferred to the oxygen atom on CaO, and thus can change the dehydrogenation property of fat. Because the side chain of fat can remove protons while repelling the negatively charged cyclic π– electron, the bond energy of the bond between the aromatic ring and the fat is further weakened so that the aliphatic chain is more strongly adsorbed onto Ca2+ of CaO. Because the aromatic ring has higher affinity for protons than for alkyl groups and metal ions, it can bind to the proton located on the surface of CaO and finally depart from the ring. The long side chain of the aromatic ring can be decomposed to a low-molecular weight olefin RCH=CH2 and alkane R′CH3 or separated from the ring. In addition, hydrogen radicals can be removed to form olefin R″CH=CH2 and further combine with each other to form hydrogen gas.43
The analysis of gas yield demonstrated that the decomposition of the aromatic ring in bio-oil led to the increase of CO. However, because CaO could absorb CO2 produced during the pyrolysis, the gas yield was thus reduced. Because the water–gas shift reaction (H2O + CO = CO2 + H2) is exothermic, it is favorable at low temperatures. Therefore, at low temperatures, CO produced by the decomposition of the aromatic ring in bio-oil reacts with H2O to form CO2 and H2. CaO can also react with H2O to form Ca(OH)2, which causes the water yield to reduce. When the temperature was increased, the acidic substances produced during pyrolysis are neutralized by the catalyst, and H2O is regenerated.
When CaO was used as a catalyst, the yield of char remarkably increased mainly because CO2 can be absorbed by CaO to form CaCO3.
In summary, the participation of CaO in the catalytic pyrolysis process is the synergism of physical adsorption, chemical reaction, and catalytic action. Physical adsorption, that is, the mixing of powdery CaO and corn stover, increases the contact area with the surface of the corn stover, and promotes the occurrence of chemical reactions and catalysis. In the process of pyrolysis, CaO can chemically react with CO2, H2O, and acid gases produced by pyrolysis, thereby reducing the concentration of pyrolysis products and promoting the pyrolysis reaction to occur in a forward direction. At the same time, it promotes the production of bio-oil, and CaO can also catalyze the cracking of bio-oil through catalytic action. When the temperature rises, CaCO3 and other substances undergo chemical reactions and are decomposed to form CaO. In the whole process, CaO promotes pyrolysis and is finally regenerated, so it can be regarded as a catalytic process. However, looking at the whole actual process, the reaction of CaO to catalyze the pyrolysis of the corn stover is the synergy of physical adsorption, chemical reaction, and catalysis action.
2.5. Analysis of Biomass Char
Char produced in the pyrolysis experiments, carried out with different catalyst amounts at different final temperatures, was collected. Its chemical composition were analyzed by FTIR, and the results are shown in Figures 6 and 7.
Figure 6.
Infrared spectra of char at different final temperatures (a) 291; (b) 335; and (c) 520 °C.
Figure 7.
Infrared spectra of uncatalyzed char and catalyzed char (a) noncatalyst; (b) CaO/stover = 1:20; (c) CaO/stover = 1:10; (d) CaO/stover = 1:5; (e) CaO/stover = 1:2; and (f) CaO/stover = 1:1.
The characteristic peaks of functional groups of corn stover char are summarized in Table 5.44,45 Comparing Figures 6 and 7, with the presence of the CaO catalyst, a sharp and distinct peak associated with the stretching vibration of O–H appeared at 3642 cm–1, and the peak height increased with increasing CaO amount. This may be caused by the fact that acidic substances produced by pyrolysis are neutralized to produce a large amount of H2O. A broad peak corresponding to the stretching vibration of intermolecular hydrogen bonds appeared at 3200–3600 cm–1 and is caused by O–H bond-containing compounds, such as H2O, phenol, and carboxylic acid. The peak gradually became flat when the temperature was gradually increased. This indicates that phenol and carboxylic acid in the corn stover are gradually decomposed or neutralized as the temperature gradually increases.
Table 5. Characteristic Wavenumbers of Various Functional Groups in Char.
| functional group assignment | wavenumber (cm–1) |
|---|---|
| free hydroxyl group O–H stretching (sharp) | 3642 |
| intermolecular hydrogen bond O–H stretching (wide) | 3200–3600 |
| CH3CH2CH symmetric and antisymmetric stretching | 3000–2800 |
| carboxyl C=O stretching | 1750–1690 |
| aromatic ring C=O stretching | 1600 |
| aromatic ring C=O stretching | 1500 |
| CO32– | 1430 |
| polysaccharide | 1300–900 |
| Si–O | 1032 |
| alcohol ether ester C–O–C | 1170, 1090 |
| CO32– | 870 |
| bending vibration of aromatic ring C–H | 793 |
A small peak observed at around 2800–3000 cm–1 is due to the symmetric and antisymmetric stretching vibrations of methyl, methylene, and methine, indicating that the corn stover is composed of an aliphatic structure. The peak became flat when the amount of the catalyst or the temperature was increased, suggesting that while the aliphatic group is gradually pyrolyzed, the char is gradually aromatized.46,47
A peak at 1750–1650 cm–1 is caused by the stretching vibrations of carbonyl groups in ketones, aldehydes, carboxylic acid, or esters. With the increase of temperature, the peak height slowly decreased, indicating that gases produced from acidic substances are slowly released during the pyrolysis process. The peak height was significantly reduced after the catalyst CaO was added. It is clear that the neutralization reaction occurs between the catalyst and the acidic substances. As a result, the thermal decomposition of acidic substances in the corn stover is promoted, and the effect is remarkable.
Peaks at 1600 and 1500 cm–1 are associated with the vibration of C=C in the aromatic ring, and a peak at 793 cm–1 is correspondent to the bending vibration of C–H outside of the ring. After the catalyst was added, the heights of these peaks were markedly decreased at high temperatures compared to those at elevated temperatures. Combined with the data obtained from the gas phase infrared, we can conclude that the catalyst CaO can effectively increase the release of aromatic substances during the pyrolysis of the corn stover.
Peaks corresponding CO32– were observed at 1430 and 870 cm–1, indicating that CaCO3 is generated as a result of CO2 absorption by CaO.
A peak at 1300–900 cm–1 is derived mainly from functional groups such as alcohols, ethers, and esters found in a polysaccharide, whereas a peak at 1032 cm–1 is due to the vibration of Si–O. When the catalyst was added, the peak height decreased slightly. Most sugars in the biomass are from cellulose. When the temperature was increased, the sugar content decreased, forming a precipitate called bio-oil. Additionally, the content of dehydrated carbohydrates (mainly l-glucose) gradually decreased when the temperature was significantly increased. When the chemical bonds in the cellulose are broken, l-glucose is generated. However, because l-glucose has low thermal stability, it can be easily degraded at high temperatures.26
3. Conclusions
In this paper, pyrolysis of the corn stover was carried out in the presence of a catalyst CaO, and TG/DTG, TG-FTIR, and FTIR analyses were employed to analyze its products, by means of changes of weight loss, gas release, tri-state product yield, and chemical structure of char. CaO had a significant effect on the pyrolysis process of the corn stover. It reacted with CO2 to form CaCO3, reacted with water to form an alkaline substance, and neutralized the acidic substance produced by pyrolysis. This greatly reduced the concentration of the product. Thus, the pyrolysis reaction of the corn stover was greatly promoted to the positive direction. Other substances or functional groups might have many additional reactions because of changes in concentration. Therefore, some experimental results were not a simple linear trend. At the same time, based on a comprehensive analysis of the experimental results, it was believed that the effect of the addition of CaO and corn stover between 1:2 and 1:1 on the pyrolysis effect was the best. The specific conclusions are as follows:
-
(1)
The DTG curve was fitted by four sub-curves. The fitted sub-curves indicated that the pyrolysis of water, hemicellulose, cellulose, and lignin occurred at 90, 291, 335, and 385 °C, respectively. After the CaO catalyst was added, the DTG curve shifted toward a high-temperature region, and the rate of weight loss was decreased. The presence of CaO was also found to delay the pyrolysis, absorb CO2 and H2O produced during the process, and catalyzed the cracking process of bio-oil. When the temperature was raised to 600–750 °C, the generated CaCO3 and Ca(OH)2 were decomposed, by high temperature, to CaO. At the same time, with the increased CaO dose, the pyrolysis activation energy of the corn stover was significantly reduced. However, when the ratio of CaO to the corn stover increased from 1:2 to 1:1, we found that the reduction in activation energy did not reach the expected value. Therefore, the optimal addition ratio of CaO and corn stover should be between 1:2 and 1:1.
-
(2)
The release of H2O, CO2 and CO gases, acid, and carbonyl, phenol, and aromatic ring compounds was analyzed by TG-FTIR. The quantitative analysis of the release was achieved by calculating the integral ratio of the experimental results. The analysis showed that in the pyrolysis catalyzed by the CaO catalyst, the content of H2O significantly increased, whereas that of CO2 was slightly changed compared with that without the catalyst. Additionally, with the increase of the catalyst amount, the content of the aromatic ring compounds first decreased and thereafter increased; and whereas the content of carbonyl and phenolic compounds partially decreased, that of CO and acid were significantly decreased. This observation indicates that CaO has an obvious effect on deacidification, the production of hydrocarbons. It can also increase in the production of the aromatic compound gas. When the ratio of CaO to the corn stover was 1:2, the release amount of H2O, CO2, and aromatic rings was at the maximum. When the addition ratio was 1:1, the release amount of the acidic substance was the lowest. Therefore, considering the release of the gas and the reaction process, the optimal addition ratio should be between 1:2 and 1:1.
-
(3)
The change of amount of tri-state products produced during the pyrolysis catalyzed by CaO at a mass ratio of 1:20 at different final temperatures was analyzed. The absorption of CO2 by CaO, the catalysis of bio-oil cracking, the effect of CaO on the neutralization of acid, and the occurrence of the water–gas shift reaction were verified.
-
(4)
The FTIR analysis showed that with the increase of the CaO amount, the content of H2O in char and the absorption of CO2 increased, and the thermal decomposition of acid in the corn stover was promoted, causing the deacidification effect to become more significant. The analysis also showed that the presence of CaO could promote the decomposition of bio-oil polysaccharides and the release of aromatic compounds. At a high pyrolysis temperature, the phenol and carboxylic acid were resolved or neutralized. With increasing the catalyst amount or temperature, the aliphatic group was gradually pyrolyzed while char was gradually aromatized.
After experimental research, the process of CaO catalyzed pyrolysis of the corn stover was discussed. The analysis of bio-oil produced during the pyrolysis of the corn stover by CaO and the mechanism of CaO promoting bio-oil cracking need further experimental research and exploration.
4. Materials and Methods
4.1. Materials
The corn stover used in this research was obtained from Changshan Town, Songyuan, Jilin, China. The corn stover was ground to sizes of below 0.2 mm and dried to a constant mass in a vacuum oven at 105 °C for 24 h. The low heating value analyzed using a bomb calorimeter and the results of proximate and ultimate analyses are shown in Table 6. Among them, the proximate analysis used a SDLA718 proximate analyzer (Sundy Company of Hunan, China). The ultimate analysis was performed using an EA3000 automatic elemental analyzer (Euro Vector, Italy) and SDS350 infrared sulfur analyzer (Sundy Company of Hunan, China). Superscript “ad” stands for air dried basis. Six samples were prepared by mixing CaO (analytical grade) with the corn stover at different mass ratios: 0:1; 1:20; 1:10; 1:5; 1:2; and 1:1.
Table 6. Low-Heating Value, Proximate Analysis, and Ultimate Analysis Results.
| proximate
analysis (wt. %) |
ultimate
analysis (wt. %) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| LHV (J/g) | Mad | Aad | Vad | FCad | Cad | Had | Oad | Nad | Sad |
| 12,537.47 | 3.53 | 30.37 | 53.80 | 12.30 | 40.21 | 5.22 | 20.01 | 0.35 | 0.31 |
4.2. Thermogravimetry-FTIR
TG-FTIR analysis was carried out using a thermogravimeter (TGA/DSC; METTLER-TOLEDO, Switzerland) in conjunction with a Fourier transform infrared spectrometer (NICOLET iS10, Thermo Fisher Scientific, USA) to simultaneously study the weight loss of the sample, the rate of mass loss, and the formation of typical gas products. In a TG analysis, 20 ± 0.02 mg of the sample was placed in a crucible and then heated from 40 to 900 °C at a heating rate of 10 °C·min–1. A high-purity nitrogen stream (99.999%) at a flow rate of 50 ml·min–1 was used as a purge and shielding gas to avoid the effects of atmospheric water vapor and CO2 on the FTIR spectrum. The volatile gas products generated as a result of heating the sample were swept into a FTIR chamber, where the changes of gas products with respect to increasing temperature were monitored. Prior to the experiment, the transfer line and FTIR chamber were preheated to 185 °C to avoid the condensation of volatiles, which can block the line. The FTIR spectrum was recorded at a frequency range of 400–4000 cm–1 and a resolution of 4 cm–1.25,34
4.3. Pyrolysis Experiments
Pyrolysis experiments were performed in a reactor as shown in Figure 8. In each experiment, 40 g of the sample was mixed with the corresponding proportion of the catalyst into the reactor. By adjusting the heating device, the temperature was raised to 291, 335, and 520 °C, respectively. After completion of the temperature increase, the temperature was maintained for 20 min to ensure that the reaction was completed. The generated gas was released from the reactor into the conical flask. The temperature was cooled by an ice-water bath, and the condensable gas was liquefied in the conical flask. After the experiment, the product stuck to the inner wall of the connecting tube and the conical flask was washed with xylene into the conical flask. The water content in the liquid was measured by distillation (ASTM D95). All experiments were repeated at least 2 times to ensure the accuracy of the results. The error was within an average value of ±1%. The yields of bio-oil, pyrolysis water, char, and gas produced from the pyrolysis were calculated by eqs 3–6.
| 7 |
| 8 |
| 9 |
| 10 |
where: Ychar, Ytar, Yw, and Ygas are the yields of char, bio-oil, pyrolysis water, and gas products after pyrolysis, respectively; Wo is the initial mass of the material subjected to pyrolysis, which is 40 g; Wchar is the mass of char obtained as a result of pyrolysis; W1 is the total mass of the collected liquid; Vw is the volume of water in the liquid generated during pyrolysis; and ρw is the density of water at 298 K and 0.1 MPa.
Figure 8.
Pyrolysis experimental device.
4.4. Fourier Transform Infrared Spectroscopy
Infrared spectroscopic measurements were performed on a Spectrum Two portable Fourier transform infrared spectrometer. The char sample at a mass of 1 ± 0.01 mg obtained from pyrolysis at different final temperatures of 291, 335, and 520 °C were thoroughly mixed and ground with 150 mg of potassium bromide in an agate mortar. After that, 40 mg of the mixture was pressed into a transparent sheet with a thickness of 0.1–1.0 mm under a pressure of 10 MPa, and thereafter dried under nitrogen at 60 °C for 6 h. Subsequently, the sample was placed in the sample chamber for FTIR analysis, in which it was scanned 32 times at a frequency range of 400 to 4000 cm–1.44,45
Acknowledgments
This work was supported by the National Key R&D Program of China (no. 2018YFB1501405).
The authors declare no competing financial interest.
References
- Saidur R.; Abdelaziz E. A.; Demirbas A.; Hossain M. S.; Mekhilef S. A review on biomass as a fuel for boilers. Renewable Sustainable Energy Rev. 2011, 15, 2262–2289. 10.1016/j.rser.2011.02.015. [DOI] [Google Scholar]
- Wang S.; Dai G.; Yang H.; Luo Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. 10.1016/j.pecs.2017.05.004. [DOI] [Google Scholar]
- Pindoria R. V.; Megaritis A.; Messenböck R. C.; Dugwell D. R.; Kandiyoti R. Comparison of the pyrolysis and gasification of biomass: effect of reacting gas atmosphere and pressure on eucalyptus wood. Fuel 1998, 77, 1247–1251. 10.1016/S0016-2361(98)00018-0. [DOI] [Google Scholar]
- Kan T.; Strezov V.; Evans T. J. Lignocellulosic biomass pyrolysis: a review of product properties and effects of pyrolysis parameters. Renewable Sustainable Energy Rev. 2016, 57, 1126–1140. 10.1016/j.rser.2015.12.185. [DOI] [Google Scholar]
- Caballero J. A.; Conesa J. A.; Font R.; Marcilla A. Pyrolysis kinetics of almond shells and olive stones considering their organic fractions. J. Anal. Appl. Pyrolysis 1997, 42, 159–175. 10.1016/S0165-2370(97)00015-6. [DOI] [Google Scholar]
- Wang S.; Guo X.; Wang K.; Luo Z. Influence of the interaction of components on the pyrolysis behavior of biomass. J. Anal. Appl. Pyrolysis 2011, 91, 183–189. 10.1016/j.jaap.2011.02.006. [DOI] [Google Scholar]
- Hosoya T.; Kawamoto H.; Saka S. Pyrolysis behaviors of wood and its constituent polymers at gasification temperature. J. Anal. Appl. Pyrolysis 2007, 78, 328–336. 10.1016/j.jaap.2006.08.008. [DOI] [Google Scholar]
- Vamvuka D. Bio-oil, solid and gaseous biofuels from biomass pyrolysis processes-an overview. Int. J. Energy Res. 2011, 35, 835–862. 10.1002/er.1804. [DOI] [Google Scholar]
- Collard F.-X.; Blin J. A review on pyrolysis of biomass constituents: mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renewable Sustainable Energy Rev. 2014, 38, 594–608. 10.1016/j.rser.2014.06.013. [DOI] [Google Scholar]
- White J. E.; Catallo W. J.; Legendre B. L. Biomass pyrolysis kinetics: a comparative critical review with relevant agricultural residue case studies. J. Anal. Appl. Pyrolysis 2011, 91, 1–33. 10.1016/j.jaap.2011.01.004. [DOI] [Google Scholar]
- Lappas A. A.; Kalogiannis K. G.; Iliopoulou E. F.; Triantafyllidis K. S.; Stefanidis S. D. Catalytic pyrolysis of biomass for transportation fuels. Wiley Interdiscip. Rev.: Energy Environ. 2012, 1, 285–297. 10.1002/wene.16. [DOI] [Google Scholar]
- Nokkosmäki M. I.; Kuoppala E. T.; Leppämäki E. A.; Krause A. O. I. Catalytic conversion of biomass pyrolysis vapours with zinc oxide. J. Anal. Appl. Pyrolysis 2000, 55, 119–131. 10.1016/S0165-2370(99)00071-6. [DOI] [Google Scholar]
- Fabbri D.; Torri C.; Baravelli V. Effect of zeolites and nanopowder metal oxides on the distribution of chiral anhydrosugars evolved from pyrolysis of cellulose: an analytical study. J. Anal. Appl. Pyrolysis 2007, 80, 24–29. 10.1016/j.jaap.2006.12.025. [DOI] [Google Scholar]
- Lu Q.; Zhang Z.-F.; Dong C.-Q.; Zhu X.-F. Catalytic upgrading of biomass fast pyrolysis vapors with nano metal oxides: an analytical py-gc/ms study. Energies 2010, 3, 1805–1820. 10.3390/en3111805. [DOI] [Google Scholar]
- D’Orazio A.; Di Carlo A.; Dionisi N.; Dell’Era A.; Orecchini F. Toluene steam reforming properties of cao based synthetic sorbents for biomass gasification process. Int. J. Hydrogen Energy 2013, 38, 13282–13292. 10.1016/j.ijhydene.2013.07.075. [DOI] [Google Scholar]
- Ding K.; Zhong Z.; Wang J.; Zhang B.; Fan L.; Liu S.; Wang Y.; Liu Y.; Zhong D.; Chen P.; Ruan R. Improving hydrocarbon yield from catalytic fast co-pyrolysis of hemicellulose and plastic in the dual-catalyst bed of cao and hzsm-5. Bioresour. Technol. 2018, 261, 86–92. 10.1016/j.biortech.2018.03.138. [DOI] [PubMed] [Google Scholar]
- Chen X.; Chen Y.; Yang H.; Chen W.; Wang X.; Chen H. Fast pyrolysis of cotton stalk biomass using calcium oxide. Bioresour. Technol. 2017, 233, 15–20. 10.1016/j.biortech.2017.02.070. [DOI] [PubMed] [Google Scholar]
- Wu Y.; Liao Y.; Liu G.; Ma X. Syngas production by chemical looping gasification of biomass with steam and cao additive. Int. J. Hydrogen Energy 2018, 43, 19375–19383. 10.1016/j.ijhydene.2018.08.197. [DOI] [Google Scholar]
- Udomsirichakorn J.; Basu P.; Salam P. A.; Acharya B. Effect of cao on tar reforming to hydrogen-enriched gas with in-process co2 capture in a bubbling fluidized bed biomass steam gasifier. Int. J. Hydrogen Energy 2013, 38, 14495–14504. 10.1016/j.ijhydene.2013.09.055. [DOI] [Google Scholar]
- Wei L.; Yang H.; Li B.; Wei X.; Chen L.; Shao J.; Chen H. Absorption-enhanced steam gasification of biomass for hydrogen production: effect of calcium oxide addition on steam gasification of pyrolytic volatiles. Int. J. Hydrogen Energy 2014, 39, 15416–15423. 10.1016/j.ijhydene.2014.07.064. [DOI] [Google Scholar]
- Constantinou D. A.; Fierro J. L. G.; Efstathiou A. M. The phenol steam reforming reaction towards H2 production on natural calcite. Appl. Catal., B 2009, 90, 347–359. 10.1016/j.apcatb.2009.03.018. [DOI] [Google Scholar]
- Zhang X.; Sun L.; Chen L.; Xie X.; Zhao B.; Si H.; Meng G. Comparison of catalytic upgrading of biomass fast pyrolysis vapors over cao and fe(iii)/cao catalysts. J. Anal. Appl. Pyrolysis 2014, 108, 35–40. 10.1016/j.jaap.2014.05.020. [DOI] [Google Scholar]
- Lu Q.; Zhang Y.; Tang Z.; Li W.-z.; Zhu X.-f. Catalytic upgrading of biomass fast pyrolysis vapors with titania and zirconia/titania based catalysts. Fuel 2010, 89, 2096–2103. 10.1016/j.fuel.2010.02.030. [DOI] [Google Scholar]
- Ding L.; Rahimi P.; Hawkins R.; Bhatt S.; Shi Y. Naphthenic acid removal from heavy oils on alkaline earth-metal oxides and zno catalysts. Appl. Catal., A 2009, 371, 121–130. 10.1016/j.apcata.2009.09.040. [DOI] [Google Scholar]
- Biagini E.; Barontini F.; Tognotti L. Devolatilization of biomass fuels and biomass components studied by tg/ftir technique. Ind. Eng. Chem. Res. 2006, 45, 4486–4493. 10.1021/ie0514049. [DOI] [Google Scholar]
- Zhao C.; Jiang E.; Chen A. Volatile production from pyrolysis of cellulose, hemicellulose and lignin. J. Energy Inst. 2017, 90, 902–913. 10.1016/j.joei.2016.08.004. [DOI] [Google Scholar]
- Nakamura T.; Kawamoto H.; Saka S. Pyrolysis behavior of japanese cedar wood lignin studied with various model dimers. J. Anal. Appl. Pyrolysis 2008, 81, 173–182. 10.1016/j.jaap.2007.11.002. [DOI] [Google Scholar]
- Sutton D.; Kelleher B.; Ross J. R. H. Review of literature on catalysts for biomass gasification. Fuel Process. Technol. 2001, 73, 155–173. 10.1016/S0378-3820(01)00208-9. [DOI] [Google Scholar]
- Chen D.; Zhou J.; Zhang Q. Effects of Torrefaction on the Pyrolysis Behavior and Bio-Oil Properties of Rice Husk by Using TG-FTIR and Py-GC/MS. Energy Fuels 2014, 28, 5857–5863. 10.1021/ef501189p. [DOI] [Google Scholar]
- Fu P.; Hu S.; Xiang J.; Sun L.; Su S.; An S. Study on the gas evolution and char structural change during pyrolysis of cotton stalk. J. Anal. Appl. Pyrolysis 2012, 97, 130–136. 10.1016/j.jaap.2012.05.012. [DOI] [Google Scholar]
- Granada E.; Eguía P.; Vilan J. A.; Comesaña J. A.; Comesaña R. Ftir quantitative analysis technique for gases. Application in a biomass thermochemical process. Renewable Energy 2012, 41, 416–421. 10.1016/j.renene.2011.11.020. [DOI] [Google Scholar]
- Luo Z.; Wang S.; Guo X. Selective pyrolysis of organosolv lignin over zeolites with product analysis by tg-ftir. J. Anal. Appl. Pyrolysis 2012, 95, 112–117. 10.1016/j.jaap.2012.01.014. [DOI] [Google Scholar]
- Si Z.; Wang C.; Bi K.; Zhang X.; Yu C.; Dong R.; Ma L.; Pang C. Py-gc/ms study of lignin pyrolysis and effect of catalysts on product distribution. Int. J. Agric. Biol. Eng. 2017, 10, 214–225. 10.25165/j.ijabe.20171005.2852. [DOI] [Google Scholar]
- Yang J.; Chen H.; Zhao W.; Zhou J. Tg-ftir-ms study of pyrolysis products evolving from peat. J. Anal. Appl. Pyrolysis 2016, 117, 296–309. 10.1016/j.jaap.2015.11.002. [DOI] [Google Scholar]
- Zhang A.; Ma Q.; Wang K.; Liu X.; Shuler P.; Tang Y. Naphthenic acid removal from crude oil through catalytic decarboxylation on magnesium oxide. Appl. Catal., A 2006, 303, 103–109. 10.1016/j.apcata.2006.01.038. [DOI] [Google Scholar]
- Wang D.; Xiao R.; Zhang H.; He G. Comparison of catalytic pyrolysis of biomass with mcm-41 and cao catalysts by using tga-ftir analysis. J. Anal. Appl. Pyrolysis 2010, 89, 171–177. 10.1016/j.jaap.2010.07.008. [DOI] [Google Scholar]
- Franklin H. D.; Peters W. A.; Howard J. B. Mineral matter effects on the rapid pyrolysis and hydropyrolysis of a bituminous coal. Fuel 1982, 61, 1213–1217. 10.1016/0016-2361(82)90022-9. [DOI] [Google Scholar]
- Yongbin J.; Jiejie H.; Yang W. Effects of calcium oxide on the cracking of coal tar in the freeboard of a fluidized bed. Energy Fuels 2004, 18, 1625–1632. 10.1021/ef034077v. [DOI] [Google Scholar]
- Jordan C. A.; Akay G. Effect of cao on tar production and dew point depression during gasification of fuel cane bagasse in a novel downdraft gasifier. Fuel Process. Technol. 2013, 106, 654–660. 10.1016/j.fuproc.2012.09.061. [DOI] [Google Scholar]
- Dalai A. K.; Sasaoka E.; Hikita H.; Ferdous D. Catalytic gasification of sawdust derived from various biomass. Energy Fuels 2003, 17, 1456–1463. 10.1021/ef030037f. [DOI] [Google Scholar]
- Yu Q.-Z.; Brage C.; Nordgreen T.; Sjöström K. Effects of chinese dolomites on tar cracking in gasification of birch. Fuel 2009, 88, 1922–1926. 10.1016/j.fuel.2009.04.020. [DOI] [Google Scholar]
- Torres W.; Pansare S. S.; Goodwin J. G. Hot gas removal of tars, ammonia, and hydrogen sulfide from biomass gasification gas. Catal. Rev. 2007, 49, 407–456. 10.1080/01614940701375134. [DOI] [Google Scholar]
- Hongwei C.; Jinquan W.; Yongmei P.; Riguang W. Experimental study of catalytic pyrolysis of corn stalk. Renew. Energy Resour. 2007, 25, 19–22. [Google Scholar]
- Pandey K. K. A study of chemical structure of soft and hardwood and wood polymers by ftir spectroscopy. J. Appl. Polym. Sci. 2015, 71, 1969–1975. . [DOI] [Google Scholar]
- Xu F.; Yu J.; Tesso T.; Dowell F.; Wang D. Qualitative and quantitative analysis of lignocellulosic biomass using infrared techniques: a mini-review. Appl. Energy 2013, 104, 801–809. 10.1016/j.apenergy.2012.12.019. [DOI] [Google Scholar]
- Fu P.; Yi W.; Li Z.; Bai X.; Wang L. Evolution of char structural features during fast pyrolysis of corn straw with solid heat carriers in a novel v-shaped down tube reactor. Energy Convers. Manage. 2017, 149, 570–578. 10.1016/j.enconman.2017.07.068. [DOI] [Google Scholar]
- Wang K.; Kim K. H.; Brown R. C. Catalytic pyrolysis of individual components of lignocellulosic biomass. Green Chem. 2014, 16, 727–735. 10.1039/c3gc41288a. [DOI] [Google Scholar]