Abstract
Allopregnanolone, a GABAergic neurosteroid and progesterone derivative, was recently approved by the Food and Drug Administration for the treatment of postpartum depression (PPD). Several mechanisms appear to be involved in the pathogenesis of PPD, including neuroendocrine dysfunction, neuroinflammation, neurotransmitter alterations, genetic and epigenetic modifications. Recent evidence highlights the higher risk for incidence of PPD in mothers exposed to unhealthy diets that negatively impact the microbiome composition and increase inflammation, all effects that are strongly correlated with mood disorders. Conversely, healthy diets have consistently been reported to decrease the risk of peripartum depression and to protect the body and brain against low-grade systemic chronic inflammation. Several bioactive micronutrients found in the so-called functional foods have been shown to play a relevant role in preventing neuroinflammation and depression, such as vitamins, minerals, omega-3 fatty acids and flavonoids. An intriguing molecular substrate linking functional foods with improvement of mood disorders may be represented by the peroxisome-proliferator activated receptor (PPAR) pathway, which can regulate allopregnanolone biosynthesis and brain-derived neurotropic factor (BDNF) and thereby may reduce inflammation and elevate mood.
Herein, we discuss the potential connection between functional foods and PPAR and their role in preventing neuroinflammation and symptoms of PPD through neurosteroid regulation. We suggest that healthy diets by targeting the PPAR-neurosteroid axis and thereby decreasing inflammation may offer a suitable functional strategy to prevent and safely alleviate mood symptoms during the perinatal period.
Keywords: Postpartum depression, Brexanolone, Neurosteroids, Functional foods, Allopregnanolone, PPAR
1. Introduction
In the pathogenesis of mood disorders, including major depressive disorder (MDD) and postpartum depression (PPD), both neuroinflammation and glutamate-mediated excitotoxicity mechanisms (neuronal death through glutamate-based over-activated stimulation) have been suggested to play a key role (Gerhard et al., 2016; Haroon and Miller, 2017; Leonard, 2018).
Neurosteroids, and specifically allopregnanolone and its isomer pregnanolone, act as endogenous potent, positive, allosteric modulators of the action of γ-aminobutyric acid (GABA) at GABA type A (GABAA) receptors (Puia et al., 1990; Majewska et al., 1986; Pinna et al., 2000). In addition, sulfated forms of these neurosteroids (e.g., pregnanolone sulfate) influence glutamatergic activity by inhibiting tonic N-methyl-D-aspartate (NMDA) receptor-mediated neurotransmission, thereby providing neuroprotection (Vyklicky et al., 2016; Tuem and Atey, 2017). Alterations in GABAergic/glutamatergic system may play a pivotal role in the molecular mechanisms underlying PPD (Walton and Maguire, 2019). Interestingly, allopregnanolone reduces calcium influx through the activation of GABAA receptors expressed on cerebrocortical nerve terminals leading to decreased glutamate release and glial activation, supporting neuroprotective effects (Chang et al., 2019; Noorbakhsh et al., 2014; Lee et al., 2011). Moreover, evidence showed allopregnanolone inhibits the L-type calcium channel activation-evoked glutamate release in the medial prefrontal cortex (Hu et al., 2007).
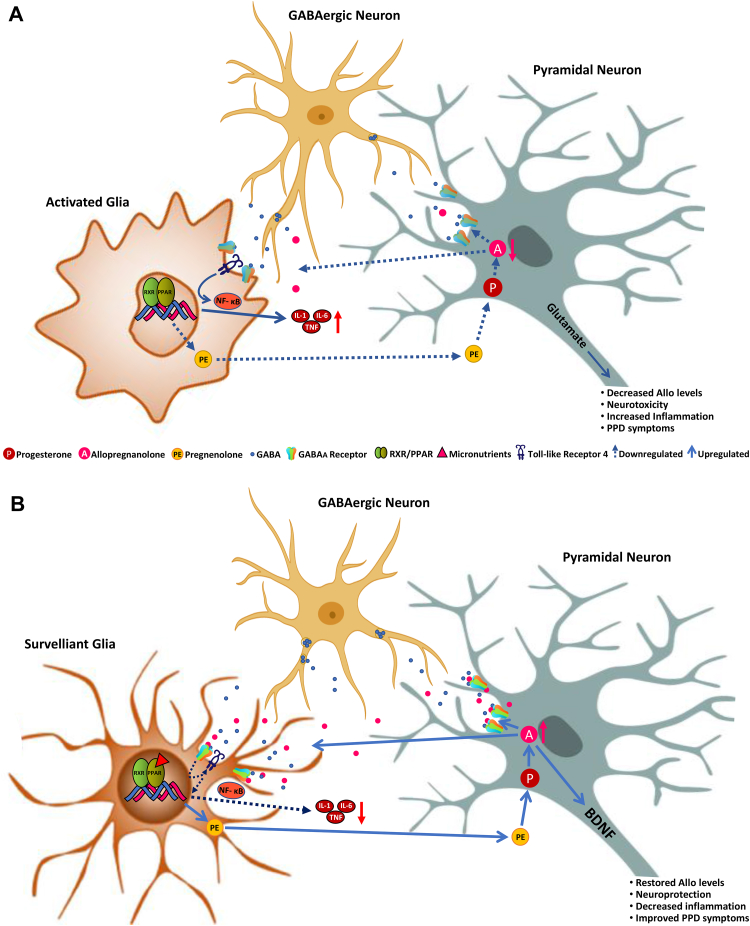
Neuroactive steroids can be synthesized peripherally from steroid hormone precursors, such as progesterone, or can be synthesized de novo in the brain starting from the conversion of cholesterol to pregnenolone in glial cells (Fig. 1). Pregnenolone, which is taken up by neurons can be further metabolized to progesterone and by the rate-limiting step enzymes, 5α-reductase Type I (5α-RI) and 3α-hydroxysteroid dehydrogenase (3α-HSD) to allopregnanolone (Agís-Balboa et al., 2007, Agís-Balboa et al., 2006; Melcangi et al., 2011; reviewed in Pinna et al., 2008). Locally produced allopregnanolone is responsible for the fine-tuning of GABAA receptors in corticolimbic glutamatergic neurons (Pinna et al., 2000), a mechanism that has been linked with improvement of behavioral dysfunction (Agis-Balboa et al., 2007; Pinna et al., 2008). At this level, allopregnanolone may conceivably dampen neuroinflammatory processes following activation of glial-type GABAA receptors. Recent evidence shows allopregnanolone, following activation of α2-containing GABAA receptors and subsequent inhibition of toll-like receptor 4, can regulate the immune response by inhibiting proinflammatory processes (Fig. 1) (Noorbakhsh et al., 2014; Li et al., 2016; Balan et al., 2019).
Fig. 1.
A. Schematic representation of the relationship between the GABAergic neurosteroid, allopregnanolone, microglial activation and PPAR-α stimulation in postpartum depression (PPD). Immediately after delivery and during the first weeks of the postpartum period, the dramatic drop in circulating progesterone leads to decreased levels of allopregnanolone, which is synthesized from peripherally-derived progesterone by glutamatergic neurons, including pyramidal neurons of the frontal cortex, granular cells of the dentate gyrus and CA1-3 pyramidal neurons in the hippocampus, and pyramidal-like neurons of the basolateral amygdala both in rodent and human brain (Agís-Balboa et al., 2006, Agís-Balboa et al., 2014; reviewed in Pinna et al., 2008). Allopregnanolone plays a central neuromodulatory role in facilitating the action of GABA at GABAA receptors (Majewska et al., 1986) and endogenously produced allopregnanolone plays a neurophysiological role in the fine-tuning of the GABAA receptors to GABAmimetics, positive allosteric modulators, and GABA agonists (Pinna et al., 2000). By this mechanism, allopregnanolone also regulates emotional behavior and stress-responses. Prolonged stress in animal models results in decreased corticolimbic allopregnanolone levels, which is associated with behavioral dysfunction, including elevated aggressiveness, anxiety-like and depressive-like behavior, and exaggerated fear responses and impaired contextual fear extinction (Pinna et al., 2003, 2008; Pibiri et al., 2008; Locci and Pinna, 2017, 2019). Low levels of allopregnanolone and symptoms of depression and PTSD have been observed in several clinical studies (Uzunova et al., 1998; Romeo et al., 1998; Rasmusson et al., 2006, 2019; Agis-Balboa et al., 2014; Kim et al., 2020). These neurosteroid deficits result in alterations of GABA and glutamate neurotransmission and in changes in GABAA receptor sensitivity (reviewed by Pinna, 2018) causing a GABAergic/glutamatergic imbalance. Moreover, decreased allopregnanolone levels in the postpartum period is associated with increased inflammation likely by activated microglia, which releases pro-inflammatory biomarkers, such as IL-1, IL-6 and TNF-α via the NFκB pathway that is also regulated by PPAR. Another mechanism involves the toll-like receptor 4 (TLR4), which, once activated by different triggers such as lipopolysaccharide (LPS), pathogen-associated molecular patterns (PAMPs), alcohol, stress or decreased levels of pregnenolone, forms a complex with intracellular co-activators, such as TIR Domain-Containing Adaptor Protein (TIRAP) and TRIF-related Adaptor Molecule (TRAM) to initiate a pro-inflammatory cascade that leads to NFκB activation and pro-inflammatory cytokines release (Li et al., 2016). Low levels of allopregnanolone lead to increased calcium channel activity in activated nerve terminals and increased release of glutamate that facilitates excitotoxicity mechanisms (Hu et al., 2007). Unhealthy diets, including high fatty diets or alcohol abuse play deleterious effects on PPAR function that fails to regulate pro-inflammatory processes and greatly contribute to the neuroinflammation mechanisms underlying the pathogenesis of major depression and, possibly, PPD (Henriques et al., 2018; Orio et al., 2019). Furthermore, neuroinflammation-associated release of glutamate from activated microglia worsens the neurodegenerative process found in mood disorders. B. A schematic representation of the regulatory effects of PPARs following its activation by micronutrients found in functional food that show the ability to bind to PPAR-α. PPAR-α activation by its endogenous modulator, PEA results in enhancement of allopregnanolone biosynthesis, by upregulating the expression of neurosteroidogenic enzymes and proteins, including StAR, P450ssc, that facilitate the conversion of cholesterol into pregnenolone, the precursor of all neurosteroids. Pregnenolone is then taken up by glutamatergic neurons and further converted into progesterone and allopregnanolone by the rate-limiting step enzymes, 5α-reductase type I (5α-RI) and 3α-hydroxysteroid dehydrogenase (3α-HSD) in several corticolimbic areas (Locci and Pinna, 2019). In this scenario, restored allopregnanolone binding at GABAA receptors that are expressed in microglia (Agís-Balboa et al., 2007, Agís-Balboa et al., 2006; Lee et al., 2011), dampens inflammatory processes by an effect mediated through inhibition of TLR4 that results in the subsequent repression of NFκB signaling cascade (Singh et al., 2012; Lee et al., 2011; Noorbakhsh et al., 2014). Altogether, these actions lead to downregulation in the release of pro-inflammatory cytokines, such as TNF-α and IL-6 and attenuate neurotoxicity. Similar results are observed by administering a diet with functional foods rich in bioactive micronutrients, such as fatty acids, flavonoids, minerals, and vitamins, which represent a non-pharmacological strategy to potentiate PPAR expression and function. PPAR-activation in glia engages neuronal allopregnanolone biosynthesis to regulate inhibition of inflammatory mechanisms, which are ultimately mediated by potentiation of glia GABAergic neurotransmission. At the same time, increased allopregnanolone levels in neurons may stimulate brain derived neurotropic factor (BDNF) and exert important neuroprotective functions (Nin et al., 2011; Almeida et al., 2019). We suggest that healthy diets enriched in micronutrients that are PPAR-agonists by enhancing the PPAR-allopregnanolone axis and decreasing inflammation may offer an alternative strategy to pharmacological treatments to prevent and safely treat mood disorders, including PPD.
The recent US Food and Drug Administration (FDA)-approval of the allopregnanolone-based compound, brexanolone, commercially called Zulresso™ for PPD treatment represents an exciting breakthrough for PPD management and opens the field of translational psychiatry to a new generation of neurosteroid-based therapeutics (Leader et al., 2019). These novel agents are characterized by a rapid and long-lasting pharmacological effect after a short-course treatment (reviewed in Zorumski et al., 2019; Meltzer-Brody and Kanes, 2020). Before brexanolone approval, PPD was mainly treated with psychotherapy for mild-to-moderate cases and selective serotonin reuptake inhibitor (SSRI) antidepressants for the management of severe cases, which are associated with low efficacy and significant side effects (reviewed in Anderson and Maes, 2013, and Pinna, 2015; Howard et al., 2017; Sie et al., 2012). Most importantly, the pharmacological effects of SSRIs only appear after several weeks, which leaves few options for the management of severe PPD. Often, mothers who present suicidal ideations and negative thoughts against self and the baby need to be hospitalized during the lag of time between the start of SSRI treatment and the onset of the beneficial pharmacological effects. This often results in high hospitalization costs and management problems for babies and families (De Crescenzo et al., 2014).
Indeed, PPD, whose prevalence is estimated between 10% and 15%, is a mild-to-severe psychiatric condition characterized by the presence of depressed mood, anxiety, sleep disorder, cognitive dysfunction and emotional liability that may begin during pregnancy and/or after birth and lasting for several weeks or months after delivery. According to a Center for Disease Control (CDC) study, 1 in 9 women experience symptoms of PPD and prevalence can be as high as 1 in 5 women (Ko et al., 2017; Shorey et al., 2018). Risk factors include history of depressive symptoms, neurotic personality traits, lower social support, lower socioeconomic status, obstetric complications, and major life negative events, trauma and/or stressors during pregnancy (Hirst and Moutier, 2010; Rasmussen et al., 2017). If left untreated, PPD may lead to serious health consequences, including child psychological development dysfunction and suicidal behaviors. In addition, women with PPD have higher risk for alcohol abuse compared with women who were neither pregnant nor postpartum (Chapman and Wu, 2013) and alcohol intake has also been associated with peripheral inflammation and neuroinflammation (Orio et al., 2019). In addition, recent evidence suggests that an unhealthy dietary pattern increases the risk of systemic low-grade inflammation and neuroinflammation associated with PPD (Ellsworth-Bowers and Corwin, 2012; Osborne and Monk, 2013; van Bussel et al., 2013; Brites and Fernandes, 2015; Serati et al., 2016; Spencer et al., 2017; Sparling et al., 2017; Melo et al., 2019; Samodien et al., 2019; Popa-Wagner et al., 2020).
Brexanolone, despite its proven high efficacy, showed adverse effects such as headache, dizziness, somnolence, and, in some cases, excessive sedation, which may complicate the safety of its use (Leader et al., 2019). Developing alternative neurosteroid-based interventions may improve PDD management more safely.
In this review, we will focus on the role of allopregnanolone in the inflammatory response that may play a relevant role for the onset of depression, as well as the role of functional food-containing bioactive compounds, that show anti-inflammatory effects and may potentially benefit the management of perinatal depression.
New findings show that peroxisome-proliferator activated receptor (PPAR)-α, a ligand-activated transcription factor, which is widely distributed in the mammalian central nervous system (CNS), exhibits anti-inflammatory effects (reviewed by Bougarne et al., 2018). Together with PPAR-γ, PPAR-α is deeply involved in several physiological and pathological conditions, including regulation of mitochondrial and proteasomal function, neuroinflammation, oxidative stress and neurodegeneration, which are considered key pathogenetic mechanisms involved in stress-related disorders, including anxiety and depression (discussed in Locci and Pinna, 2019; Esmaeili et al., 2016; Agarwal et al., 2017; D'Orio et al., 2018). Intriguingly, PPAR mediates anti-inflammatory responses under several pathophysiological conditions and can stimulate biosynthesis of neurosteroids, such as allopregnanolone, with documented anti-inflammatory actions and role in improving mood symptoms, which suggests that the PPAR-neurosteroid axis may have a pivotal function in the modulation of mood by regulating inflammatory processes. Hence, a specific focus of this article is devoted on the role of PPAR-α in inflammation while we discuss whether its regulation by functional food-containing active micronutrients, by enhancing allopregnanolone biosynthesis, may benefit prevention and treatment of psychiatric disorders, and specifically PPD.
2. PPAR and inflammation: A role for neurosteroids
Nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) activation and production of proinflammatory cytokines, such as TNF-α, IL-1β and IL-6, prostaglandins, nitric oxide (NO) and reactive oxygen species (ROS) is regarded as a leading mechanism for local and systemic inflammation. PPAR-α and PPAR-γ are widely distributed in several mammalian organs and tissues and in several areas of the CNS. They mediate several physiological and pathological processes, such as neuroinflammation, regulation of mitochondrial and proteasomal dysfunction, oxidative stress, neurodegeneration, and neuronal differentiation (Moreno et al., 2004; Fidaleo et al., 2014).
PPAR-γ is highly expressed in adipocytes and its synthetic agonists have been initially studied as insulin-sensitizing drugs for type-2 diabetes and metabolic syndrome treatment (Botta et al., 2018; Mirza et al., 2019). PPAR-γ is also expressed in microglia and astrocytes and plays a major anti-inflammatory role and it is investigated as molecular target for different neurodegenerative diseases, such as brain damage, cerebral ischemia, Parkinson's and Alzheimer's diseases (Combs et al., 2000; Carta and Pisanu, 2013). Its activation, induced by the natural ligand 15-deoxy-d12,14-prostaglandin J2 (15dPGJ2) or synthetic agonists (i.e., pioglitazone), results in a decrease in proinflammatory cytokine production as well as cyclooxygenase-2 (COX-2) through the inhibition of NFκB signaling pathway (Kaundal and Sharma, 2010; Du et al., 2011; Cheng et al., 2019). In brain, PPAR-γ is involved in the regulation of inflammatory-related gene expression on activated microglia, through which it mitigates neuroinflammatory processes under neuronal insults (Villapol, 2018). Thus, PPAR-γ, together with PPAR-α, is a suitable pharmacological target to treat inflammatory-derived neuropsychiatric conditions, including MDD and PPD.
Intriguingly, PPAR-α activation stimulates the biosynthesis of allopregnanolone that in addition to elevating mood, has also been associated with an anti-inflammatory effect. Indeed, allopregnanolone binds at GABAA receptors expressed both on microglia and astrocytes and in glutamatergic pyramidal neurons (Agís-Balboa et al., 2006, Agís-Balboa et al., 2007; Lee et al., 2011), and it mediates anti-inflmmatory effects through blocking toll-like receptor 4 (Balan et al., 2019; Singh et al., 2012; Lee et al., 2011). This results in NFκB inhibition (reviewed in Tufano and Pinna, 2020 and depicted in Fig. 1). Allopregnanolone's binding at GABAA receptors on monocytoid cells leads to diminished production of inflammatory mediators by these cells (Noorbakhsh et al., 2014). Further, GABA suppresses astrocytes and microglia inflammatory responses to lipopolysaccharide (LPS) and INF-γ by inhibiting the NFκB activation pathway and P38 MAP kinase (Lee et al., 2011). This process leads to a decreased release of pro-inflammatory cytokines, such as TNF-α and IL-6 and results in an attenuation of neurotoxicity in vitro (Lee et al., 2011). Interesting, similar anti-inflammatory effects were observed following the administration of the GABAA receptor agonist, muscimol and the GABAB receptor agonist, baclofen, suggesting the direct role of both types of GABA receptors in reducing neuroinflammation. Interestingly, GABAA receptors are also expressed in macrophages and lymphocytes T cells and their activation produces anti-inflammatory effects (Reyes-Garcia et al., 2007; Bhat et al., 2010). In addition, neuroinflammation-associated release of glutamate from activated microglia has been implicated in the progression of neurodegenerative diseases, including Alzheimer's and Parkinson's disease and recent studies have shown that PPARs can modulate neurotoxicity by inhibiting glutamate release in LPS-activated microglia (Lee et al., 2018).
This evidence collectively supports the hypothesis of a GABAergic/glutamatergic neurotransmission dysregulation as potential molecular mechanism underlying neuroinflammatory processes, whereby the PPAR family and neurosteroid biosynthesis may play a pivotal role (see Fig. 1). Indeed, recent evidence suggests that neurosteroids regulate neurodegeneration and neuroinflammation supporting neuronal survival either through a direct effect on neurons and/or by decreasing neuroinflammatory responses of microglia and astrocytes (Hong et al., 2018; Arcuri et al., 2017). In vitro and in vivo studies show that 17β-estradiol, progesterone, and allopregnanolone reduce microglial-mediated inflammation (Yilmaz et al., 2019).
Hence, an intriguing molecular player that might link inflammation and neurosteroids is represented by PPAR that has been implicated in the pathology of numerous diseases, such as diabetes, stroke, cancer, obesity and even mood disorders (Stienstra et al., 2007; Liu et al., 2018; Cheng et al., 2019; Tufano and Pinna, 2020).
3. PPAR role in psychiatric disorders and behavioral regulation
PPAR-α and PPAR-γ exert functions, including glucose and lipid metabolism regulation, anti-inflammatory and oxidative stress inhibition, via a direct inhibition of NFκB signaling (Kauppinen et al., 2013; Sakamoto et al., 2016; Marion-Letellier et al., 2016). Moreover, PPAR-γ mediates intestinal anti-inflammatory effects, decreases oxidative stress in brain and increases insulin sensitization (Marion-Letellier et al., 2016). PPARs are key molecular regulators of cell metabolism, energy homeostasis, cellular development, and differentiation; thus, their ligands find several clinical applications, such as hyperlipidemia and hypertriglyceridemia in combination with statins, type-2 diabetes mellitus, metabolic syndrome and non-alcoholic fatty liver disease (Pawlak et al., 2015; Botta et al., 2018; Cheng et al., 2019).
PPAR-α endogenous modulators include palmitoylethanolamide (PEA), oleoylethanolamine (OEA) and stearoylethanolamide (SEA). Specifically, PEA, which was discovered by the Italian Nobel Prize laureate Rita Levi Montalcini in the 90s, is an endogenously synthesized lipid with well described neuroprotective and anti-inflammatory properties (Levi-Montalcini et al., 1996). PEA may play a relevant role in stress-related disorders, including depression and post-traumatic stress disorder (PTSD) (Hillard, 2018). Indeed, the levels of PEA, OEA and SEA are decreased in PTSD patients (Wilker et al., 2016). Both synthetic agonists of PPAR-α and PPAR-γ have been investigated in clinical trials for their ability to improve depression symptoms (reviewed by Tufano and Pinna, 2020; Cheng et al., 2019). A recent clinical trial investigated the role of PEA as add-on therapy for depression showing symptoms improvement in patients with major depressive disorder treated with citalopram (Ghazizadeh-Hashemi et al., 2018). Selective PPAR-γ agonists, rosiglitazone and pioglitazone were originally approved by the FDA for diabetes treatment. These compounds also reduce the response to chronic stress and have been studied in a number of clinical trials that evaluated their efficacy in improving depression symptoms (reviewed by Colle et al., 2017; Tufano and Pinna, 2020). A combined therapy of pioglitazone with the SSRI antidepressant, citalopram, showed a higher pharmacological response and remission rate, and rapid onset compared to citalopram alone (Sepanjnia et al., 2012). In another study, a 12-week pioglitazone administration induced antidepressant effects in patients with comorbid insulin resistance, supporting a link between depression and metabolic dysregulation (Lin et al., 2015).
This evidence suggests that for both PPAR-α and γ, in addition to their anti-cholesterol and anti-diabetic effects, an unforeseen potential therapeutic profile is emerging in the treatment of mood disorders.
4. Functional foods: role in inflammation and PPD
Functional food is defined as “natural or processed food that contain known or unknown biologically-active compounds; which, in defined, effective, and non-toxic amounts, provide a clinically proven and documented health benefit for the prevention, management, or treatment of chronic disease” (Dhiman et al., 2014; Martirosyan and Singh, 2015). A growing body of evidence suggests that many functional foods can modulate inflammation both acutely and chronically through their bioactive compounds and ultimately improve stress-related mood disorders (Alkhatib et al., 2017; Granado-Lorencio and Hernández-Alvarez, 2016; Spagnuolo et al., 2018). In fact, diet represents a major modifiable risk factor that modulate the immune system reactivity and a suitable strategy to fight against systemic low-grade inflammation, which is now strongly considered to be involved in the pathogenesis of several chronic diseases, including cardiovascular disease, obesity, diabetes, autoimmune diseases, neurodegenerative diseases, and mood disorders (Libby, 2007; Berk et al., 2013; Cox et al., 2015).
Bioactive compounds, such as flavonoids, essential fatty acids, minerals, vitamins, and phytonutrients are essential for overall health and to prevent, manage or treat chronic diseases, including metabolic disorders, diabetes, cardiovascular diseases and hypertension, or pathologies affecting the CNS, including mood disorders (Simopoulos, 2008; Zhang et al., 2015; Pérez-Cano and Castell, 2016; Mozaffarian and Wu, 2018). Increasing evidence indicates that neuroinflammation represents a key biological component in the pathogenesis of psychiatric disorders (Brites and Fernandes, 2015; Benatti et al., 2016; Maeng and Hong, 2019). Based on the role of inflammation in depressive disorders, including PPD (Payne and Maguire, 2019), as well as the finding from recent clinical studies that have evaluated the efficacy of changes in diet in improving depressive symptoms (Molendijk et al., 2018), we propose that integrating PPD management with functional foods rich in anti-inflammatory micronutrients might represent an innovative strategy for improving the overall clinical outcome.
PPD shares the same diagnostic criteria as MDD, although the cause of PPD remains more obscure. As in MDD, a combination of environmental and biological factors may occur, including family history, stressful events, unhealthy diet and genetic risk factors (Payne and Maguire, 2019). Moreover, in PPD, a rapid drop in progesterone levels after delivery is considered one of the major risk factors (Schiller et al., 2015). Attenuating this dramatic hormonal change might be helpful to avoid severe clinical consequences. Nutrition plays a biological role in depression, especially during pregnancy and lactation during which micronutrients, such as vitamins, minerals, essential fatty acids, and antioxidants become depleted and may play a role in the development of perinatal depression. Some of these nutrients are essential fatty acids, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), vitamin D, B-vitamins (specifically B6, B9, B12), and trace minerals (like zinc, iron, and selenium) (Amini et al., 2019; Sauer and Grabrucker, 2019).
Omega-3 fatty acids have well-known anti-inflammatory and anti-allergic activity, predominantly through the inhibition of excessive immune responses. EPA- and DHA-derived resolvins and protectins actively ameliorate a pro-inflammatory condition. Polyunsaturated fatty acids (PUFAs) are highly concentrated in neural phospholipids and are important components of the neuronal cell membrane and play a pivotal role in enhancing synaptic plasticity and neuronal communication (Pérez et al., 2017). Resolvins and protectins belong to the class of PUFA metabolites, which exert anti-inflammatory activity that regulate the synthesis and release of pro-inflammatory mediators (Joffre et al., 2019). DHA and EPA exert different functions, such as maintaining cell-membrane fluidity, inhibiting inflammatory processes by decreasing secretion of proinflammatory cytokines. Foods rich in omega-3 PUFAs are walnuts, sunflower seeds, flax seeds and oil and fatty fish, such as salmon. A recent meta-analysis conducted by Lin et al. (2017) suggests a potential role of PUFAs for perinatal depression treatment (Lin et al., 2017). Previous studies have reported controversial results on the beneficial effects of omega-3 fatty acids for perinatal depression treatment in part due to flaws in study design (Freeman et al., 2008; Rees et al., 2008). However, a randomized, double-blind, placebo-controlled trial showed that omega-3 improved depressive symptoms (Su et al., 2008).
The main anti-inflammatory properties of flavonoids include suppressing release of cytokines, such as IL-1β and TNF-α, from activated microglia (Fig. 1). They may also affect inducible NO synthase regulation and inhibit the activation of NADPH oxidase, and down-regulate the activity of pro-inflammatory transcription factors, such as NFκB, that play a key role in the intestinal inflammatory response (Atreya et al., 2008). Green tea, broccoli, onions, and berries are enriched in phytonutrients, such as carotenoids, ellagic acid, flavonoids, resveratrol, glucosinolates, and phytoestrogens that help fighting oxidation and inflammation. Naringenin, quercetin, hydrocaffeic acid, procyanidins, and anthocyanidins (Table 1) belong to the class of flavonoids and exert anti-inflammatory effects in vivo and in vitro by inhibiting the expression of iNOS, ICAM-1, MCP-1, COX-2, TNF-α, IL-1β and IL-6 expression (Chen et al., 2018; Gil-Cardoso et al., 2016; discussed in Matrisciano and Pinna, 2019).
Table 1.
Functional Foods rich in micronutrients that activate PPAR-α and PPAR-γ and induce pharmacological effects.
| Phytochemical | Bioactive Compound | Food Source | Physiological effects | References |
|---|---|---|---|---|
| Flavonoids | ||||
| Flavonols | Kaempferol, Myricetin, Quercetin | Berries, kale, grapes, spinach, bell peppers, cocoa, broccoli, sweet potatoes, tomatoes, capers | Anti-carcinogenic, anti-inflammatory. antioxidant and antiviral activities. mitigation of microglia-mediated neuroinflammation | Wang et al. (2014); Botta et al. (2018); Mozaffarian et al. (2018); Ward et al. (2018). |
| Flavanones | Hesperetin, Naringenin | Citrus fruits (lemons and oranges), grapes | Antioxidant, anti-inflammatory | |
| Flavones | Apigenin, Luteolin | Celery, fresh parsley, olives, oregano, peppers and rosemary | Suppression of oxidative stress via anti-inflammatory effects on NF-kB, brain support, protection and memory increase | |
| Flavanols | Epicatechin-gallates, Procyanidins, Catechin | Tea, grapes, lentils, cocoa, apples with peel on, apricots, cherries, peaches, blackberries, black grapes, strawberries, blueberries and raspberries | Antioxidant, free radicals scavenging properties. Decrease of the hypothalamic inflammation and microglia overactivation. Improve cognition | |
| Isoflavones | Daidzein | Grape seeds, soy products | Improve in adipose inflammation, and insulin resistance. Improve in cognitive function | |
| Flavans | Genistein | Soybeans | Antioxidant and neuroprotective activities. Improve in glucose metabolism, and cognitive function. | |
| Phytocannabinoids | Cannabidiol | Cannabis sativa plant/supplements | attenuates oxidative stress, anti-inflammatory effects | Vallée et al. (2017); Esposito et al. (2011). |
| flavonoid glycoside | Rutin | Buckwheat, apples with skin, asparagus (specially the bottom part), grapefruit, lemons, orange juice, oranges | Anti-inflammatory, antioxidant, neuroprotective, nephroprotective, hepatoprotective effects. | Nkpaa et al. (2019) |
| Palmitoylethanolamide (PEA) |
Fatty acid amide N-acylethanolamine family |
Egg yolk, soy oil, peanut oil, and corn, peas and beans, tomatoes and potatoes | Antioxidant Properties, anti-inflammatory, microglia inhibition, neuroprotective effects | Peritore et al. (2019). |
| Phenolic acids and Polyphenols | Mallik et al. (2016). | |||
| Phenolic acids | Caffeic acid, Ferulic acid | Apples, coffee beans, blueberries, oranges, peaches, potatoes, pears | Antioxidant and anti-inflammatory properties. Improve in cognition and neurodegeneration | |
| Hydroxy-benzoioc acids | Gallic acids, Oleanolic acid | Grape and raspberry grape juice, longan seeds, strawberries, olive oil | NF-κB inhibitors, anti-inflammatory properties. |
Liu et al. (2020); Abdel-Moneim et al. (2018); Georgiadis et al. (2015). |
| Trihydroxy-stilbenes | Resveratrol | Grape skin, peanuts, red wine, cranberries | Anti-aging, chemo-preventive, anti-carcinogenic, anti-inflammatory and antioxidant effects | Barone et al. (2019); Qi et al. (2018). |
| Tannins/Proanthocyanidins | Catechin, Tannic acids | Coffee, cocoa, lentils, peas, walnuts, berries, olives, plums, tea, chickpeas, herbs and spices | Antioxidant properties, neuroprotective effects |
Ide et al. (2018). D'Orio et al. (2018). |
| Diferuloylmethane | Curcumin | Turmeric plants | Anti-inflammatory and antioxidant activities | Yin et al. (2018). |
Another bioactive compound relevant for brain functioning is vitamin D. Vitamin D belongs to the class of fat-soluble vitamins along with vitamin A, E and K. It is considered a steroid hormone, mostly known for its role in calcium metabolism and its ability to increase the absorption of calcium and phosphorus from the intestine (Cui et al., 2017). However, vitamin D exerts many other biological effects including processes involved in brain development and neuronal activity (Eyles et al., 2011). Deficiency in vitamin D is considered a risk factor for neuropsychiatric disorders, including PPD, MDD and schizophrenia (Amini et al., 2020; Szpunar, 2019; Zhu et al., 2019; McGrath et al., 2010). It causes alterations in brain structure and in dopamine and glutamate signaling. Major dietary sources of vitamin D are salmon, shrimp, herring and sardines, egg yolks and mushrooms (Matrisciano and Pinna, 2019). Zinc is an essential trace mineral required for all physiological systems, including neural functioning and proper cellular function, such as DNA replication, transcription, protein synthesis, maintenance of cell membranes, cellular transport, as well as endocrine, immunological and neuronal systems. Zinc is found mainly in red meat, poultry, fish, and dairy. Dysregulation of zinc is associated with reduced immunological functioning, alterations in cognitive performance, gastrointestinal complaints (Bonaventura et al., 2015). Lower zinc levels can be a consequence of inflammation or nutritional deficiencies and it helps to modulate the hyper-glutamatergic state associated with depression (Wang et al., 2018). Approximately 20% of dietary zinc intake is used by intestinal bacteria supporting the major role of a healthy microbiome in brain function (Sauer and Grabrucker, 2019). In addition, zinc modulates PPAR-γ signaling, which is impaired in zinc deficiency (Meerarani et al., 2003).
5. A natural strategy to potentiate the PPAR-neurosteroid axis and improve mood
Dietary fatty acids have been implicated in immune and inflammatory processes by regulating NFκB and PPAR-α and γ transcription factor pathways (Calder, 2013). In rodents, for instance, high-fat diets alter PPAR pathway causing abnormalities in the microbiome that can be reversed by rosiglitazone, a PPAR-γ agonist (Tomas et al., 2016). Several natural bioactive compounds act on PPARs, including the tea plant, soybeans, palm oil, ginger, grapes and wine as well as a number of culinary herbs and spices (e.g. Origanum vulgare, Rosmarinus officinalis, Salvia officinalis, Thymus vulgaris) (reviewed by Wang et al., 2014). Curcumin has also shown anti-inflammatory and antioxidant effects by increasing PPAR-γ activity (Li et al., 2017). OEA, which is a natural metabolite of oleic acid, and an endogenous PPAR-α modulator, shows anti-inflammatory activity (Yang et al., 2016). Then, foods rich in oleic acid, such as olive oil, avocado and almond oil can be used as part of the anti-inflammatory dietary patterns. In addition, omega-3 (or n-3) PUFAs and their metabolites are natural ligands for PPAR-γ (Table 1). PUFAs affect the neuroendocrine-immune axis of depression through their activity on inflammatory responses (Bhathena, 2006; Marion-Letellier et al., 2015). EPA and DHA supplementation have been shown to decrease levels of key inflammatory cytokines TNF-α, IL-1β, IL-6, and IL-8 (Zhao et al., 2004). Recently, a major role in improving symptoms of depression and anxiety in patients or in elevating mood in healthy individuals was shown in populations that were fed with a Mediterranean-based diet, which is rich in fruits, vegetables, olive oil, and legumes and low in saturated fatty acids and processed foods (Sánchez-Villegas et al., 2013). Dietary flavonoids found in fruits and vegetables exert anti-inflammatory effects, as well by suppressing microglia activation through the PPAR-γ mediated pathway (Feng et al., 2016; Dang et al., 2003).
Resveratrol, for example, is considered as a natural PPAR ligand (Nakata et al., 2012), showed beneficial effects on depression and anxiety treatment by suppression of inflammatory processes exerted by inhibiting the activation of NLRP3 and NFκB in hippocampus (de Oliveira et al., 2018). Resveratrol, which is present at high levels in red grapes, nuts, and pomegranates, exerts metabolic, antioxidant, and anti-inflammatory activities, as well as neuroprotective effects through PPAR-activation (Lagouge et al., 2006). Similarly, quercetin induced antidepressant-like effect in the unpredictable chronic mild stress animal model of depression and induces antioxidant, anti-inflammatory activities, reduces excitotoxicity and augments 5-HT levels (Khan et al., 2019), linking the role of inflammation to depression. Foods rich in quercetin are capers, goji berries, onions, asparagus, spinach and red grapes. Resveratrol and quercetin are polyphenolic compounds that improve metabolic syndrome by altering PPAR expression (Castrejón-Tellez et al., 2016). PEA shows antidepressant effects by binding at its main target, PPAR-α (De Gregorio et al., 2019), and may increase endogenous levels of the endocannabinoids, anandamide (AEA) and 2-arachinoylglycerol (2-AG) and exert anti-inflammatory, analgesic, and neuroprotective properties (Peritore et al., 2019). PEA-rich foods are egg yolk, soy oil, peanut oil, corn seeds, legumes, such as peas and beans, and vegetables, such as tomatoes and potatoes (summarized in Table 1).
Altogether, food rich in micronutrients that have the ability to stimulate PPAR, in addition to exert important anti-inflammatory actions may also induce significant mood-elevating properties, although the underlying mechanisms are not fully understood. We propose that PPAR might work in synergism with stimulation of neurosteroid biosynthesis to exert their beneficial effects by decreasing inflammation and relieving mood symptoms. Intriguingly, PEA-induced PPAR-α activation engages allopregnanolone levels in frontal cortex, hippocampus and amygdala to improve behavioral abnormalities in an animal model of stress-induced mood disorders (Locci and Pinna, 2019; Pinna, 2019). Previously, Sasso and colleagues (2010, 2012) showed that PEA-induced activation of PPAR-α increases allopregnanolone levels in the rodent spinal cord (Sasso et al., 2010, 2012). Moreover, allopregnanolone is also involved in BDNF expression and neurogenesis. In socially isolated mice reduced levels of allopregnanolone in corticolimbic areas are associated with BDNF deficiency, reduced neurogenesis and depressive- and anxiety-like behavior, supporting a multifunctional role of allopregnanolone for depression and anxiety prevention (Nin et al., 2011; Evans et al., 2012; Bali and Jaggi, 2014; Almeida et al., 2019). Intriguingly, studies have suggested that PPAR-α activation by administering synthetic PPAR-α agonists, including fenofibrate, is associated with stimulation of BDNF signaling cascade and improvement of behavioral dysfunction (Jiang et al., 2016). These findings suggest that PPAR-α regulation may represent a suitable target for developing new strategies for the treatment of neuropsychiatric disorders characterized by deficiency in neurosteroidogenesis, including MDD, PTSD and PPD (Uzunova et al., 1998; Romeo et al., 1998; Rasmusson et al., 2006, 2019; Pineles et al., 2018; Pinna et al., 2006; Meltzer-Brody and Kanes, 2020). Moreover, FDA-approved synthetic PPAR-α agonists, including the fibrates (e.g., fenofibrate, clofibrate), prescribed for the treatment of hypercholesterolemia, could be repurposed to treat mood disorders by targeting the PPAR-allopregnanolone axis (discussed in Pinna, 2019).
These summaries are in support of a PPAR role both as a mediator of neuroinflammation during neuropathophysiological conditions and as a suitable pharmacological target to develop novel therapeutic approaches to treat mood disorders by designing functional diets that mediate upregulation of neurosteroid biosynthesis.
6. Conclusion
The nuclear receptors, PPAR-α and γ appear as novel fascinating targets for the treatment of mood disorders caused by stressful conditions, such as during mood instability and low-grade inflammatory states that occur during pregnancy or after birth. This review focuses on the role of neuroinflammation as a molecular mechanism underlying mood disorders, including perinatal depression and highlights the potential impact of PPAR regulation in these processes. We addressed the possibility that by downregulating inflammatory processes, following activation of PPAR with a healthy diet rich in PPAR-bioactive micronutrients found in functional foods, may help preventing, managing and treating mood disorders. These ligands by stimulating anti-inflammatory mechanisms and, possibly, by enhancing neurosteroid biosynthesis, may provide a future, more natural and safe approach to prevent and alleviate pathophysiological processes that lead to perinatal depression and other mood disorders.
CRediT authorship contribution statement
Francesco Matrisciano: Writing - original draft. Graziano Pinna: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Visualization, Writing - original draft, Writing - review & editing.
Acknowledgments
This study was supported by the United States Department of Defense Grant W81XWH-15-1-0521 to GP.
References
- Abdel-Moneim A., El-Twab S.M.A., Yousef A.I., Reheim E.S.A., Ashour M.B. Modulation of hyperglycemia and dyslipidemia in experimental type 2 diabetes by gallic acid and P-coumaric acid: the role of adipocytokines and PPARγ. Biomed. Pharmacother. 2018;105:1091–1097. doi: 10.1016/j.biopha.2018.06.096. [DOI] [PubMed] [Google Scholar]
- Agarwal S., Yadav A., Chaturvedi R.K. Peroxisome proliferator-activated receptors (PPARs) as therapeutic target in neurodegenerative disorders. Biochem. Biophys. Res. Commun. 2017;483(4):1166–1177. doi: 10.1016/j.bbrc.2016.08.043. [DOI] [PubMed] [Google Scholar]
- Agís-Balboa R.C., Pinna G., Zhubi A., Maloku E., Veldic M., Costa E. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc Natl Acad Sci U S A. 2006;103:14602–14607. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agís-Balboa R.C., Pinna G., Pibiri F., Kadriu B., Costa E., Guidotti A. Down-regulation of neurosteroid biosynthesis in corticolimbic circuits mediates social isolation-induced behavior in mice. Proc Natl Acad Sci U S A. 2007;104:18736–18741. doi: 10.1073/pnas.0709419104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agís-Balboa R.C., Guidotti A., Pinna G. 5α-reductase type I expression is downregulated in the prefrontal cortex/brodmann's area 9 (BA9) of depressed patients. Psychopharmacology. 2014;231(17):3569–3580. doi: 10.1007/s00213-014-3567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhatib A., Tsang C., Tiss A., Bahorun T., Arefanian H., Barake R. Functional foods and lifestyle approaches for diabetes prevention and management. Nutrients. 2017;9(12) doi: 10.3390/nu9121310. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida F.B., Gomez R., Tannhauser Barros H.M., Nin M.S. Hemisphere-dependent changes in mRNA expression of GABA A receptor subunits and BDNF after intra-prefrontal cortex allopregnanolone infusion in rats. Neuroscience. 2019;397:56–66. doi: 10.1016/j.neuroscience.2018.11.029. [DOI] [PubMed] [Google Scholar]
- Amini S., Jafarirad S., Amani R. Postpartum depression and vitamin D: a systematic review. Crit. Rev. Food Sci. Nutr. 2019;59:1514–1520. doi: 10.1080/10408398.2017.1423276. [DOI] [PubMed] [Google Scholar]
- Amini S., Amani R., Jafarirad S., Cheraghian B., Sayyah M., Hemmati A.A. The effect of vitamin D and calcium supplementation on inflammatory biomarkers, estradiol levels and severity of symptoms in women with postpartum depression: a randomized double-blind clinical trial. Nutr. Neurosci. 2020;3:1–11. doi: 10.1080/1028415X.2019.1707396. [DOI] [PubMed] [Google Scholar]
- Anderson G., Maes M. Postpartum depression: psychoneuroimmunological underpinnings and treatment. Neuropsychiatric Dis. Treat. 2013;9:277–287. doi: 10.2147/NDT.S25320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcuri C., Mecca C., Bianchi R., Giambanco I., Donato R. The pathophysiological role of microglia in dynamic surveillance, phagocytosis and structural remodeling of the developing CNS. Front. Mol. Neurosci. 2017;10:191. doi: 10.3389/fnmol.2017.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atreya I., Atreya R., Neurath M.F. NF-kappaB in inflammatory bowel disease. J. Intern. Med. 2008;263:591–596. doi: 10.1111/j.1365-2796.2008.01953.x. [DOI] [PubMed] [Google Scholar]
- Balan I., Beattie M.C., O'Buckley T.K., Aurelian L., Morrow A.L. Endogenous neurosteroid (3α,5α)3-hydroxypregnan-20-one inhibits toll-like-4 receptor activation and pro-inflammatory signaling in macrophages and brain. Sci. Rep. 2019;9(1):1220. doi: 10.1038/s41598-018-37409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bali A., Jaggi A.S. Multifunctional aspects of allopregnanolone in stress and related disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2014;48:64–78. doi: 10.1016/j.pnpbp.2013.09.005. [DOI] [PubMed] [Google Scholar]
- Barone R., Rizzo R., Tabbì G., Malaguarnera M., Frye R.E., Bastin J. Nuclear peroxisome proliferator-activated receptors (PPARs) as therapeutic targets of resveratrol for autism spectrum disorder. Int. J. Mol. Sci. 2019;20(8) doi: 10.3390/ijms20081878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benatti C., Blom J.M., Rigillo G., Alboni S., Zizzi F., Torta R. Disease-induced neuroinflammation and depression. CNS Neurol. Disord. - Drug Targets. 2016;15:414–433. doi: 10.2174/1871527315666160321104749. [DOI] [PubMed] [Google Scholar]
- Berk M., Williams J., Jacka F.N., O'Neil A., Pasco J.A., Moylan S. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11:200. doi: 10.1186/1741-7015-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R., Axtell R., Mitra A., Miranda M., Lock C., Tsien R.W. Inhibitory role for GABA in autoimmune inflammation. Proc. Natl. Acad. Sci. U.S.A. 2010;107:2580–2585. doi: 10.1073/pnas.0915139107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhathena S.J. Relationship between fatty acids and the endocrine and neuroendocrine system. Nutr. Neurosci. 2006;9(1–2):1–10. doi: 10.1080/10284150600627128. [DOI] [PubMed] [Google Scholar]
- Bonaventura P., Benedetti G., Albarède F., Miossec P. Zinc and its role in immunity and inflammation. Autoimmun. Rev. 2015;14:277–285. doi: 10.1016/j.autrev.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Botta M., Audano M., Sahebkar A., Sirtori C.R., Mitro N., Ruscica M. PPAR agonists and metabolic syndrome: an established role? Int. J. Mol. Sci. 2018;19(4) doi: 10.3390/ijms19041197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bougarne N., Weyers B., Desmet S.J., Deckers J., Ray D.W., Staels B. Molecular actions of PPARα in lipid metabolism and inflammation. Endocr. Rev. 2018;39:760–802. doi: 10.1210/er.2018-00064. [DOI] [PubMed] [Google Scholar]
- Brites D., Fernandes A. Neuroinflammation and depression: microglia activation, extracellular microvesicles and microRNA dysregulation. Front. Cell. Neurosci. 2015;17:9–476. doi: 10.3389/fncel.2015.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder P.C. Long chain fatty acids and gene expression in inflammation and immunity. Curr. Opin. Clin. Nutr. Metab. Care. 2013;16:425–433. doi: 10.1097/MCO.0b013e3283620616. [DOI] [PubMed] [Google Scholar]
- Carta A.R., Pisanu A. Modulating microglia activity with PPAR-c agonists: a promising therapy for Parkinson's disease. Neurotox. Res. 2013;23:112–123. doi: 10.1007/s12640-012-9342-7. [DOI] [PubMed] [Google Scholar]
- Castrejón-Tellez V., Rodríguez-Pérez J.M., Pérez-Torres I., Pérez-Hernández N., Cruz-Lagunas A., Guarner-Lans V. The effect of resveratrol and quercetin treatment on PPAR mediated uncoupling protein (UCP-) 1, 2, and 3 expression in visceral white adipose tissue from metabolic syndrome rats. Int. J. Mol. Sci. 2016;17(7):E1069. doi: 10.3390/ijms17071069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y., Hsieh H.L., Huang S.K., Wang S.J. Neurosteroid allopregnanolone inhibits glutamate release from rat cerebrocortical nerve terminals. Synapse. 2019;73 doi: 10.1002/syn.22076. [DOI] [PubMed] [Google Scholar]
- Chapman S.L.C., Wu L.T. Postpartum substance use and depressive symptoms: a review. Women Health. 2013;53(5):479–503. doi: 10.1080/03630242.2013.804025. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Teng H., Jia Z., Battino M., Miron A., Yu Z. Intracellular signaling pathways of inflammation modulated by dietary flavonoids: the most recent evidence. Crit. Rev. Food Sci. Nutr. 2018;58:2908–2924. doi: 10.1080/10408398.2017.1345853. [DOI] [PubMed] [Google Scholar]
- Cheng H.S., Tan W.R., Low Z.S., Marvalim C., Lee J.Y.H., Tan N.S. Exploration and development of PPAR modulators in health and disease: an update of clinical evidence. Int. J. Mol. Sci. 2019;20(20):E5055. doi: 10.3390/ijms20205055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colle R., de Larminat D., Rotenberg S., Hozer F., Hardy P., Verstuyft C. PPAR-γ agonists for the treatment of major depression: a review. Pharmacopsychiatry. 2017;50(2):49–55. doi: 10.1055/s-0042-120120. [DOI] [PubMed] [Google Scholar]
- Combs C.K., Johnson D.E., Karlo J.C., Cannady S.B., Landreth G.E. Inflammatory mechanisms in Alzheimer's disease: inhibition of b-amyloid-stimulated proinflammatory responses and neurotoxicity by PPARc agonists. J. Neurosci. 2000;20(2):558–567. doi: 10.1523/JNEUROSCI.20-02-00558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox A.J., West N.P., Cripps A.W. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015;3:207–215. doi: 10.1016/S2213-8587(14)70134-2. [DOI] [PubMed] [Google Scholar]
- Cui X., Gooch H., Petty A., McGrath J.J., Eyles D. Vitamin D and the brain: genomic and non-genomic actions. Mol. Cell. Endocrinol. 2017;453:131–143. doi: 10.1016/j.mce.2017.05.035. [DOI] [PubMed] [Google Scholar]
- D'Orio B., Fracassi A., Ceru M.P., Moreno S. Targeting PPARalpha in alzheimer's disease. Curr. Alzheimer Res. 2018;15(4):345–354. doi: 10.2174/1567205014666170505094549. 2018 Feb 22. [DOI] [PubMed] [Google Scholar]
- Dang Z.C., Audinot V., Papapoulos S.E., Boutin J.A., Löwik C.W. Peroxisome proliferator-activated receptor gamma (PPARgamma ) as a molecular target for the soy phytoestrogen genistein. J. Biol. Chem. 2003;278:962–967. doi: 10.1074/jbc.M209483200. [DOI] [PubMed] [Google Scholar]
- De Crescenzo F., Perelli F., Armando M., Vicari S. Selective serotonin reuptake inhibitors (SSRIs) for post-partum depression (PPD): a systematic review of randomized clinical trials. J. Affect. Disord. 2014;152–154:39–44. doi: 10.1016/j.jad.2013.09.019. [DOI] [PubMed] [Google Scholar]
- De Gregorio D., Manchia M., Carpiniello B., Valtorta F., Nobile M., Gobbi G. Role of palmitoylethanolamide (PEA) in depression: translational evidence: special section on "translational and neuroscience studies in affective disorders. J. Affect. Disord. 2019;255 doi: 10.1016/j.jad.2018.10.117. pii: S0165-0327(18)31599-4. [DOI] [PubMed] [Google Scholar]
- de Oliveira M.R., Chenet A.L., Duarte A.R., Scaini G., Quevedo J. Molecular mechanisms underlying the anti-depressant effects of resveratrol: a review. Mol. Neurobiol. 2018;55:4543–4559. doi: 10.1007/s12035-017-0680-6. [DOI] [PubMed] [Google Scholar]
- Dhiman Anju, Walia Vaibhav, Nanda Arun. second ed. vol. 172. Prepared Foods; Richardson, TX: 2014. "Introduction to the functional foods." introduction to functional food science: textbook; pp. 89–92. (Functional Food Center, O'Donnell, C.D. 2003. Ten Trends in Nutraceutical Ingredients). [Google Scholar]
- Du H., Chen X., Zhang J., Chen C. Inhibition of COX-2 expression by endocannabinoid 2-arachidonoylglycerol is mediated via PPAR-γ. Br. J. Pharmacol. 2011;163(7):1533–1549. doi: 10.1111/j.1476-5381.2011.01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellsworth-Bowers E.R., Corwin E.J. Nutrition and the psychoneuroimmunology of postpartum depression. Nutr. Res. Rev. 2012;25(1):180–192. doi: 10.1017/S0954422412000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaeili M.A., Yadav S., Gupta R.K., Waggoner G.R., Deloach A., Calingasan N.Y. Preferential PPAR-α activation reduces neuroinflammation, and blocks neurodegeneration in vivo. Hum. Mol. Genet. 2016;25(2):317–327. doi: 10.1093/hmg/ddv477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G., Scuderi C., Valenza M., Togna G.I., Latina V., De Filippis D. Cannabidiol reduces aβ-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PloS One. 2011;6(12) doi: 10.1371/journal.pone.0028668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J., Sun Y., McGregor A., Connor B. Allopregnanolone regulates neurogenesis and depressive/anxiety-like behaviour in a social isolation rodent model of chronic stress. Neuropharmacology. 2012;63(8):1315–1326. doi: 10.1016/j.neuropharm.2012.08.012. [DOI] [PubMed] [Google Scholar]
- Eyles D., Burne T., McGrath J. Vitamin D in fetal brain development. Semin. Cell Dev. Biol. 2011;22(6):629–636. doi: 10.1016/j.semcdb.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Feng X., Weng D., Zhou F., Owen Y.D., Qin H., Zhao J. Activation of PPARγ by a natural flavonoid modulator, apigenin ameliorates obesity-related inflammation via regulation of macrophage polarization. EBioMedicine. 2016;9:61–76. doi: 10.1016/j.ebiom.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidaleo M., Fanelli F., Ceru M.P., Moreno S. Neuroprotective properties of peroxisome proliferator-activated receptor alpha (PPARα) and its lipid ligands. Curr. Med. Chem. 2014;21:2803–2821. doi: 10.2174/0929867321666140303143455. [DOI] [PubMed] [Google Scholar]
- Freeman M.P., Davis M., Sinha P., Wisner K.L., Hibbeln J.R., Gelenberg A.J. Omega-3 fatty acids and supportive psychotherapy for perinatal depression: a randomized placebo-controlled study. J. Affect. Disord. 2008;110:142–148. doi: 10.1016/j.jad.2007.12.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiadis I., Karatzas T., Korou L.M., Katsilambros N., Perrea D. Beneficial health effects of chios gum mastic and peroxisome proliferator-activated receptors: indications of common mechanism. J. Med. Food. 2015;18(1):1–10. doi: 10.1089/jmf.2014.0021. [DOI] [PubMed] [Google Scholar]
- Gerhard D.M., Wohleb E.S., Duman R.S. Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discov. Today. 2016;21(3):454–464. doi: 10.1016/j.drudis.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazizadeh-Hashemi M., Ghajar A., Shalbafan M.R., Ghazizadeh-Hashemi F., Afarideh M. Palmitoylethanolamide as adjunctive therapy in major depressive disorder: a double-blind, randomized and placebo-controlled trial. J. Affect. Disord. 2018;232:127–133. doi: 10.1016/j.jad.2018.02.057. [DOI] [PubMed] [Google Scholar]
- Gil-Cardoso K., Ginés I., Pinent M., Ardévol A., Blay M., Terra X. Effects of flavonoids on intestinal inflammation, barrier integrity and changes in gut microbiota during diet-induced obesity. Nutr. Res. Rev. 2016;29:234–248. doi: 10.1017/S0954422416000159. [DOI] [PubMed] [Google Scholar]
- Granado-Lorencio F., Hernández-Alvarez E. Functional foods and health effects: a nutritional biochemistry perspective. Curr. Med. Chem. 2016;23(26):2929–2957. doi: 10.2174/0929867323666160615105746. (Review. [DOI] [PubMed] [Google Scholar]
- Haroon E., Miller A.H. Inflammation effects on glutamate as a pathway to neuroprogression in mood disorders. Mod Trends Pharmacopsychiatry. 2017;31:37–55. doi: 10.1159/000470805. [DOI] [PubMed] [Google Scholar]
- Henriques J.F., Portugal C.C., Canedo T., Relvas J.B., Summavielle T., Socodato R. Microglia and alcohol meet at the crossroads: microglia as critical modulators of alcohol neurotoxicity. Toxicol. Lett. 2018;283:21–31. doi: 10.1016/j.toxlet.2017.11.002. [DOI] [PubMed] [Google Scholar]
- Hillard C.J. Circulating endocannabinoids: from whence do they come and where are they going? Neuropsychopharmacology. 2018;43:155–172. doi: 10.1038/npp.2017.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst K.P., Moutier C.Y. Postpartum major depression. Am. Fam. Physician. 2010;82:926–933. [PubMed] [Google Scholar]
- Hong Y., Liu Y., Zhang G., Wu H., Hou Y. Progesterone suppresses Aβ42-induced neuroinflammation by enhancing autophagy in astrocytes. Int. Immunopharm. 2018;54:336–343. doi: 10.1016/j.intimp.2017.11.044. [DOI] [PubMed] [Google Scholar]
- Howard M.M., Mehta N.D., Powrie R. Peripartum depression: early recognition improves outcomes. Cleve. Clin. J. Med. 2017;84(5):388–396. doi: 10.3949/ccjm.84a.14060. (Review. [DOI] [PubMed] [Google Scholar]
- Hu A.Q., Wang Z.M., Lan D.M., Fu Y.M., Zhu Y.H., Dong Y. Inhibition of evoked glutamate release by neurosteroid allopregnanolone via inhibition of L-type calcium channels in rat medial prefrontal cortex. Neuropsychopharmacology. 2007;32:1477–1489. doi: 10.1038/sj.npp.1301261. [DOI] [PubMed] [Google Scholar]
- Ide K., Matsuoka N., Yamada H., Furushima D., Kawakami K. Effects of tea catechins on alzheimer's disease: recent updates and perspectives. Molecules. 2018;23(9) doi: 10.3390/molecules23092357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B., Wang Y.-J., Wang H., Song L., Huang C., Zhu Q. Antidepressant-like effectsof fenofibrate in mice via the hippocampal brain-derived neurotrophic factor signalling pathway. Br. J. Pharmacol. 2016;174:177–194. doi: 10.1111/bph.13668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffre C., Rey C., Layé S. N-3 polyunsaturated fatty acids and the resolution of neuroinflammation. Front. Pharmacol. 2019;10 doi: 10.3389/fphar.2019.01022. 022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaundal R.K., Sharma S.S. Peroxisome proliferator-activated receptor-c agonists as neuroprotective agents. Drug News Perspect. 2010;23(4):241–256. doi: 10.1358/dnp.2010.23.4.1437710. [DOI] [PubMed] [Google Scholar]
- Kauppinen A., Suuronen T., Ojala J., Kaarniranta K., Salminen A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell. Signal. 2013;25(10):1939–1948. doi: 10.1016/j.cellsig.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Khan K., Najmi A.K., Akhtar M. A natural phenolic compound quercetin showed the usefulness by targeting inflammatory, oxidative stress markers and augment 5-HT levels in one of the animal models of depression in mice. Drug Res. 2019;69:392–400. doi: 10.1055/a-0748-5518. [DOI] [PubMed] [Google Scholar]
- Kim B.K., Fonda J.R., Hauger R.L., Pinna G., Anderson G.M., Valovski I.T., Rasmusson A.M. Composite contributions of cerebrospinal fluid GABAergic neurosteroids, neuropeptide Y and interleukin-6 to PTSD symptom severity in men with PTSD. Neurobiol. Stress. 2020;12:100220. doi: 10.1016/j.ynstr.2020.100220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J.Y., Rockhill K.M., Tong V.T., Morrow B., Farr S.L. Trends in postpartum depressive symptoms - 27 states, 2004, 2008, and 2012. MMWR Morb. Mortal. Wkly. Rep. 2017;66:153–158. doi: 10.15585/mmwr.mm6606a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1 alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Leader L.D., O'Connell M., VandenBerg A. Brexanolone for postpartum depression: clinical evidence and practical considerations. Pharmacotherapy. 2019;39:1105–1112. doi: 10.1002/phar.2331. [DOI] [PubMed] [Google Scholar]
- Lee M., Schwab C., McGeer P.L. Astrocytes are GABAergic cells that modulate microglial activity. Glia. 2011;59:152–165. doi: 10.1002/glia.21087. [DOI] [PubMed] [Google Scholar]
- Lee E.J., Park J.S., Lee Y.Y., Kim D.Y., Kang J.L., Kim H.S. Anti-inflammatory and anti-oxidant mechanisms of an MMP-8 inhibitor in lipoteichoic acid-stimulated rat primary astrocytes: involvement of NF-κB, Nrf 2, and PPAR-γ signaling pathways. J. Neuroinflammation. 2018;15:326. doi: 10.1186/s12974-018-1363-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard B.E. Inflammation and depression: a causal or coincidental link to the pathophysiology? Acta Neuropsychiatr. 2018;30:1–16. doi: 10.1017/neu.2016.69. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R., Skaper S.D., Dal Toso R., Petrelli L., Leon A. Nerve growth factor: from neurotrophin to neurokine. Trends Neurosci. 1996;19:514–520. doi: 10.1016/S0166-2236(96)10058-8. [DOI] [PubMed] [Google Scholar]
- Li J., Adam Csakai A., Jin J., Zhang F., Yin H. Therapeutic developments targeting toll-like receptor 4 mediated neuroinflammation. ChemMedChem. 2016;11(2):154–165. doi: 10.1002/cmdc.201500188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.Y., Yang M., Li Z., Meng Z. Curcumin inhibits angiotensin II-induced inflammation and proliferation of rat vascular smooth muscle cells by elevating PPAR-γ activity and reducing oxidative stress. Int. J. Mol. Med. 2017;39:1307–1316. doi: 10.3892/ijmm.2017.2924. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammatory mechanisms: the molecular basis of inflammation and disease. Nutr. Rev. 2007;65(12 Pt 2):S140–S146. doi: 10.1111/j.1753-4887.2007.tb00352.x. [DOI] [PubMed] [Google Scholar]
- Lin K.W., Wroolie T.E., Robakis T., Rasgon N.L. Adjuvant pioglitazone for unremitted depression: clinical correlates of treatment response. Psychiatr. Res. 2015;230:846–852. doi: 10.1016/j.psychres.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P.Y., Chang C.H., Chong M.F., Chen H., Su K.P. Polyunsaturated fatty acids in perinatal depression: a systematic review and meta-analysis. Biol. Psychiatr. 2017;82(8):560–569. doi: 10.1016/j.biopsych.2017.02.1182. [DOI] [PubMed] [Google Scholar]
- Liu Y., Colby J.K., Zuo X., Jaoude J., Wei D., Shureiqi I. The role of PPAR-δ in metabolism, inflammation, and cancer: many characters of a critical transcription factor. Int. J. Mol. Sci. 2018;19(11) doi: 10.3390/ijms19113339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.L., Hsu C.C., Huang H.J., Chang C.H., Sun S.H., Lin A.M.Y. Gallic acid attenuated LPS-induced neuroinflammation: protein aggregation and necroptosis. Mol. Neurobiol. 2020;57(1):96–104. doi: 10.1007/s12035-019-01759-7. [DOI] [PubMed] [Google Scholar]
- Locci A., Pinna G. Neurosteroid biosynthesis down-regulation and changes in GABAA receptor subunit composition: a biomarker axis in stress-induced cognitive and emotional impairment. Br. J. Pharmacol. 2017;174:3226–3241. doi: 10.1111/bph.13843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locci A., Pinna G. Stimulation of peroxisome proliferator-activated receptor-α by N-palmitoylethanolamine engages allopregnanolone biosynthesis to modulate emotional behavior. Biol. Psychiatr. 2019;85:1036–1045. doi: 10.1016/j.biopsych.2019.02.006. [DOI] [PubMed] [Google Scholar]
- Maeng S.H., Hong H. Inflammation as the potential basis in depression. Int Neurourol J. 2019;23(Suppl. 2):S63–71. doi: 10.5213/inj.1938226.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska M.D., Harrison N.L., Schwartz R.D., Barker J.L., Paul S.M. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232(4753):1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Mallik S.B., Mudgal J., Nampoothiri M., Hall S., Anoopkumar- Dukie S., Grant G. Caffeic acid attenuates lipopolysaccharide-induced sickness behaviour and neuroinflammation in mice. Neurosci. Lett. 2016;632:218–223. doi: 10.1016/j.neulet.2016.08.044. [DOI] [PubMed] [Google Scholar]
- Marion-Letellier R., Savoye G., Ghosh S. Polyunsaturated fatty acids and inflammation. IUBMB Life. 2015;67(9):659–667. doi: 10.1002/iub.1428. [DOI] [PubMed] [Google Scholar]
- Marion-Letellier R., Savoye G., Ghosh S. Fatty acids, eicosanoids and PPAR gamma. Eur. J. Pharmacol. 2016;785:44–49. doi: 10.1016/j.ejphar.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Martirosyan, Singh . Functional Food Center; Dallas, Texas: 2015. A New Definition for Functional Food by FFC: Creating Functional Food Products Using New Definition.http://functionalfoodscenter.net Web. 3 Apr. 2015. [Google Scholar]
- Matrisciano F., Pinna G. Book: Functional Foods and Mental Health Publisher. Food Science Publisher; 2019. Functional food and nutrition for the management of autism spectrum disorders and schizophrenia. [Google Scholar]
- McGrath J.J., Eyles D.W., Pedersen C.B., Anderson C., Ko P., Burne T.H. Neonatal vitamin D status and risk of schizophrenia: a population-based case-control study. Arch. Gen. Psychiatr. 2010;67:889–894. doi: 10.1001/archgenpsychiatry.2010.110. [DOI] [PubMed] [Google Scholar]
- Meerarani P., Reiterer G., Toborek M., Hennig B. Zinc modulates PPARgamma signaling and activation of porcine endothelial cells. J. Nutr. 2003;133:3058–3064. doi: 10.1093/jn/133.10.3058. [DOI] [PubMed] [Google Scholar]
- Melcangi R.C., Panzica G., Garcia-Segura L.M. Neuroactive steroids: focus on human brain. Neuroscience. 2011;191:1–5. doi: 10.1016/j.neuroscience.2011.06.024. [DOI] [PubMed] [Google Scholar]
- Melo H.M., Santos L.E., Ferreira S.T. Diet-derived fatty acids, brain inflammation, and mental health. Front. Neurosci. 2019;13:265. doi: 10.3389/fnins.2019.00265. (Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer-Brody S., Kanes S.J. Allopregnanolone in postpartum depression: role in pathophysiology and treatment. Neurobiology of Stress. 2020;12 doi: 10.1016/j.ynstr.2020.100212. May 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza A.Z., Althagafi, Shamshad H. Role of PPAR receptor in different diseases and their ligands: physiological importance and clinical implications. Eur. J. Med. Chem. 2019;166:502–513. doi: 10.1016/j.ejmech.2019.01.067. [DOI] [PubMed] [Google Scholar]
- Molendijk M., Molero P., Ortuño Sánchez-Pedreño F., Van der Does W. Angel Martínez-González M4. Diet quality and depression risk: a systematic review and dose-response meta-analysis of prospective studies. J. Affect. Disord. 2018;226:346–354. doi: 10.1016/j.jad.2017.09.022. [DOI] [PubMed] [Google Scholar]
- Moreno S., Farioli-Vecchioli S., Cerù M.P. Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience. 2004;23:131–145. doi: 10.1016/j.neuroscience.2003.08.064. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D., Wu J.H.Y. Flavonoids, dairy foods, and cardiovascular and metabolic health: a review of emerging biologic pathways. Circ. Res. 2018;122(2):369–384. doi: 10.1161/CIRCRESAHA.117.309008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata R., Takahashi S., Inoue H. Recent advances in the study on resveratrol. Biol. Pharm. Bull. 2012;35:273–279. doi: 10.1248/bpb.35.273. [DOI] [PubMed] [Google Scholar]
- Nin M.S., Martinez L.A., Pibiri F., Nelson M., Pinna G. Neurosteroids reduce social isolation-induced behavioral deficits: a proposed link with neurosteroid-mediated upregulation of BDNF expression. Front. Endocrinol. 2011;2:73. doi: 10.3389/fendo.2011.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkpaa K.W., Onyeso G.I., Kponee K.Z. Rutin abrogates manganese-induced striatal and hippocampal toxicity via Inhibition of iron depletion, oxidative stress, inflammation and suppressing the NF-κB signaling pathway. J. Trace Elem. Med. Biol. 2019;53:8–15. doi: 10.1016/j.jtemb.2019.01.014. [DOI] [PubMed] [Google Scholar]
- Noorbakhsh F., Baker G.B., Power C. Allopregnanolone and neuroinflammation: a focus on multiple sclerosis. Front. Cell. Neurosci. 2014;8:134. doi: 10.3389/fncel.2014.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orio L., Alen F., Pavón F.J., Serrano A., García-Bueno B. Oleoylethanolamide, neuroinflammation, and alcohol abuse. Front. Mol. Neurosci. 2019;11:490. doi: 10.3389/fnmol.2018.00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne L.M., Monk C. Perinatal depression--the fourth inflammatory morbidity of pregnancy?: theory and literature review. Psychoneuroendocrinology. 2013;38(10):1929–1952. doi: 10.1016/j.psyneuen.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlak M., Lefebvre P., Staels B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J. Hepatol. 2015;62(3):720–733. doi: 10.1016/j.jhep.2014.10.039. [DOI] [PubMed] [Google Scholar]
- Payne J.L., Maguire J. Pathophysiological mechanisms implicated in postpartum depression. Front. Neuroendocrinol. 2019;52:165–180. doi: 10.1016/j.yfrne.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez M.A., Peñaloza-Sancho V., Ahumada J., Fuenzalida M., Dagnino-Subiabre A. n-3 polyunsaturated fatty acid supplementation restored impaired memory and GABAergic synaptic efficacy in the Hippocampus of stressed rats. Nutr. Neurosci. 2017;21(8):556–569. doi: 10.1080/1028415X.2017.1323609. [DOI] [PubMed] [Google Scholar]
- Pérez-Cano F.J., Castell M. Flavonoids, inflammation and immune system. Nutrients. 2016;8(10) doi: 10.3390/nu8100659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peritore A.F., Siracusa R., Crupi R., Cuzzocrea S. Therapeutic efficacy of palmitoylethanolamide and its new formulations in synergy with different antioxidant molecules present in diets. Nutrients. 2019;11(9) doi: 10.3390/nu11092175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pibiri F, Nelson M, Costa E, Guidotti A, Pinna G. Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: A model relevant for posttraumatic stress disorder. Proc. Natl. Acad. Sci. USA. 2008;105:5567–5572. doi: 10.1073/pnas.0801853105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineles S.L., Yi Nillni, Pinna G., Irvine J., Webb A., Arditte Hall K.A. PTSD in women is associated with a block in conversion of progesterone to the GABAergic neurosteroids allopregnanolone and pregnanolone measured in plasma. Psychoneuroendocrinology. 2018;93:133–141. doi: 10.1016/j.psyneuen.2018.04.024. [DOI] [PubMed] [Google Scholar]
- Pinna G. Nova Science Publishers, Inc; 2015. Fluoxetine: Pharmacology, Mechanisms of Action and Potential Side Effects. (ISBN(Print): 9781634820776, 9781634820769) [Google Scholar]
- Pinna G. Biomarkers for PTSD at the interface of the endocannabinoid and neurosteroid Axis. Front. Neurosci. 2018;12:482. doi: 10.3389/fnins.2018.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G. Animal models of PTSD: the socially isolated mouse and the biomarker role of allopregnanolone. Front. Behav. Neurosci. 2019;13:114. doi: 10.3389/fnbeh.2019.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G., Uznuova V., Matsumoto K., Puia G., Mienville J.M., Costa E. Brain allopregnanolone regulates the potency of the GABA(A) receptor agonist muscimol. Neuropharmacology. 2000;39:440–448. doi: 10.1016/s0028-3908(99)00149-5. [DOI] [PubMed] [Google Scholar]
- Pinna G, Costa E, Guidotti A. Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses that are inactive on 5-HT reuptake. Psychopharmacology (Berl) 2006;186:362–372. doi: 10.1007/s00213-005-0213-2. [DOI] [PubMed] [Google Scholar]
- Pinna G., Dong E., Matsumoto K., Costa E., Guidotti A. In socially isolated mice, the reversal of brain allopregnanolone down-regulation mediates the anti-aggressive action of fluoxetine. Proc Natl Acad Sci U S A. 2003;100(4):2035–2040. doi: 10.1073/pnas.0337642100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna G., Agis-Balboa R.C., Pibiri F., Nelson M., Guidotti A., Costa E. Neurosteroid biosynthesis regulates sexually dimorphic fear and aggressive behavior in mice. Neurochem. Res. 2008;33(10):1990–2007. doi: 10.1007/s11064-008-9718-5. [DOI] [PubMed] [Google Scholar]
- Popa-Wagner A., Dumitrascu D.I., Capitanescu B., Petcu E.B., Surugiu R., Fang W.H. Dietary habits, lifestyle factors and neurodegenerative diseases. Neural Regen Res. 2020;15(3):394–400. doi: 10.4103/1673-5374.266045. (Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puia G., Santi M.R., Vicini S., Pritchett D.B., Purdy R.H., Paul S.M. Neurosteroids act on recombinant human GABAA receptors. Neuron. 1990;4(5):759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- Qi B., Shi C., Meng J., Xu S., Liu J. Resveratrol alleviates ethanol-induced neuroinflammation in vivo and in vitro: involvement of TLR2-MyD88-NF-κb pathway. Int. J. Biochem. Cell Biol. 2018;103:56–64. doi: 10.1016/j.biocel.2018.07.007. [DOI] [PubMed] [Google Scholar]
- Rasmussen M.L.H., Strom M., Wohlfahrt J., Videbech P., Melbye M. Risk, treatment duration, and recurrence risk of postpartum affective disorder in women with no prior psychiatric history: a population-based cohort study. PLoS Med. 2017;14(9) doi: 10.1371/journal.pmed.1002392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson A.M., Pinna G., Paliwal P., Weisman D., Gottschalk C., Charney D. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biol. Psychiatr. 2006;60(7):704–713. doi: 10.1016/j.biopsych.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Rasmusson A.M., King M.W., Valovski I., Gregor K., Scioli-Salter E., Pineles S.L. Relationships between cerebrospinal fluid GABAergic neurosteroid levels and symptom severity in men with PTSD. Psychoneuroendocrinology. 2019;102:95–104. doi: 10.1016/j.psyneuen.2018.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees A.-M., Austin M.-P., Parker G.B. Omega-3 fatty acids as a treatment for perinatal depression: randomized double-blind placebo-controlled trial. Aust. N. Z. J. Psychiatr. 2008;42:199–205. doi: 10.1080/00048670701827267. [DOI] [PubMed] [Google Scholar]
- Reyes-Garcia M.G., Hernandez-Hernandez F., Hernandez-Tellez B., GarciaTamayo F. GABA (A) receptor subunits RNA expression in mice peritoneal macrophages modulate their IL-6/IL-12 production. J. Neuroimmunol. 2007;188:64–68. doi: 10.1016/j.jneuroim.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Romeo E., Ströhle A., Spalletta G., di Michele F., Hermann B., Holsboer F. Effects of antidepressant treatment on neuroactive steroids in major depression. Am. J. Psychiatr. 1998;155(7):910–913. doi: 10.1176/ajp.155.7.910. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y., Kanatsu J., Toh M., Naka A., Kondo K., Iida K. The dietary isoflavone daidzein reduces expression of pro-inflammatory genes through PPARα/γ and JNK pathways in adipocyte and macrophage Co-cultures. PloS One. 2016;11(2) doi: 10.1371/journal.pone.0149676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samodien E., Johnson R., Pheiffer C., Mabasa L., Erasmus M., Louw J. Diet-induced hypothalamic dysfunction and metabolic disease, and the therapeutic potential of polyphenols. Mol Metab. 2019;27:1–10. doi: 10.1016/j.molmet.2019.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Villegas A., Martínez-González M.A., Estruch R., Salas-Salvadó J., Corella D., Covas M.I., Arós Fet. Mediterranean dietary pattern and depression: the PREDIMED randomized trial. BMC Med. 2013;11:208. doi: 10.1186/1741-7015-11-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasso O., La Rana G., Vitiello S., Russo R., D'Agostino G., Iacono A. Palmitoylethanolamide modulates pentobarbital-evoked hypnotic effect in mice: involvement of allopregnanolone biosynthesis. Eur. Neuropsychopharmacol. 2010;20(3):195–206. doi: 10.1016/j.euroneuro.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Sasso O., Russo R., Vitiello S., Raso G.M., D'Agostino G., Iacono A. Implication of allopregnanolone in the antinociceptive effect of N-palmitoylethanolamide in acute or persistent pain. Pain. 2012;153(1):33–34. doi: 10.1016/j.pain.2011.08.010. 2012 Jan. [DOI] [PubMed] [Google Scholar]
- Sauer A.K., Grabrucker A.M. Zinc deficiency during pregnancy leads to altered microbiome and elevated inflammatory markers in mice. Front. Neurosci. 2019;13:1295. doi: 10.3389/fnins.2019.01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller C.E., Meltzer-BrodyS, David R., Rubinow D.R. The role of reproductive hormones in postpartum depression. CNS Spectr. 2015;20(1):48–59. doi: 10.1017/S1092852914000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepanjnia K., Modabbernia A., Ashrafi M., Modabbernia M.J., Akhondzadeh S. Pioglitazone adjunctive therapy for moderate-to-severe major depressive disorder: randomized double-blind placebo-controlled trial. Neuropsychopharmacology. 2012;37:2093–2100. doi: 10.1038/npp.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serati M., Redaelli M., Buoli M M., Altamura Ac A.C. Perinatal major depression biomarkers: a systematic review. J. Affect. Disord. 2016;193:391–404. doi: 10.1016/j.jad.2016.01.027. [DOI] [PubMed] [Google Scholar]
- Shorey S., Chee C.Y.I., Ng E.D., Chan Y.H., Tam W.W.S., Chong Y.S. Prevalence and incidence of postpartum depression among healthy mothers: a systematic review and meta-analysis. J. Psychiatr. Res. 2018;104:235–248. doi: 10.1016/j.jpsychires.2018.08.001. [DOI] [PubMed] [Google Scholar]
- Sie S.D., Wennink J.M.B., van Driel J.J., te Winkel A.G.W., Boer K., Casteelen G. Maternal use of SSRIs, SNRIs and NaSSAs: practical recommendations during pregnancy and lactation. Arch. Dis. Child. Fetal Neonatal Ed. 2012;97(6):F472–F476. doi: 10.1136/archdischild-2011-214239. [DOI] [PubMed] [Google Scholar]
- Simopoulos A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008;233(6):674–688. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- Singh C., Liu L., Wang J.M., Irwin R.W., Yao J., Chen S. Allopregnanolone restores hippocampal-dependent learning and memory and neural progenitor survival in aging 3xTgAD and nonTg mice. Neurobiol. Aging. 2012;33:1493–1506. doi: 10.1016/j.neurobiolaging.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnuolo C., Moccia S., Russo G.L. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur. J. Med. Chem. 2018;153:105–115. doi: 10.1016/j.ejmech.2017.09.001. [DOI] [PubMed] [Google Scholar]
- Sparling T.M., Henschke N., Nesbitt R.C., Gabrysch S. The role of diet and nutritional supplementation in perinatal depression: a systematic review. Matern. Child Nutr. 2017;13(1) doi: 10.1111/mcn.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer S.J., D'Angelo H., Soch A., Watkins L.R., Maier S.F., Barrientos R.M. High-fat diet and aging interact to produce neuroinflammation and impair hippocampal- and amygdalar-dependent memory. Neurobiol. Aging. 2017;58:88–101. doi: 10.1016/j.neurobiolaging.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stienstra R., Duval C., Müller M., Kersten S. PPARs, obesity, and inflammation. PPAR Res. 2007;2007 doi: 10.1155/2007/95974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su K.P., Huang S.Y., Chiu T.H., Huang K.C., Huang C.L., Chang H.C. Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind placebo-controlled trial. J. Clin. Psychiatr. 2008;69(4):644–651. doi: 10.4088/jcp.v69n0418. [DOI] [PubMed] [Google Scholar]
- Szpunar M.J. Association of antepartum vitamin D deficiency with postpartum depression: a clinical perspective. Publ. Health Nutr. 2019:1–6. doi: 10.1017/S136898001800366X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas J., Mulet C., Saffarian A., Cavin J.B., Ducroc R., Regnault B. High-fat diet modifies the PPAR-γ pathway leading to disruption of microbial and physiological ecosystem in murine small intestine. Proc Natl Acad Sci U S A. 2016;113:E5934–E5943. doi: 10.1073/pnas.1612559113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuem K.B., Atey T.M. Neuroactive steroids: receptor interactions and responses. Front. Neurol. 2017;8:442. doi: 10.3389/fneur.2017.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufano M., Pinna G. Is there a future for PPARs in the treatment of neuropsychiatric disorders? Review. Molecules. 2020;25:1062. doi: 10.3390/molecules25051062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzunova V., Sheline Y., Davis J.M., Rasmusson A., Uzunov D.P., Costa E. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci U S A. 1998;95(6):3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallée A., Lecarpentier Y., Guillevin R., Jean-Noël Vallée J.N. Effects of cannabidiol interactions with Wnt/β-catenin pathway and PPARγ on oxidative stress and neuroinflammation in alzheimer's disease. Acta Biochim. Biophys. Sin. 2017;49(10):853–866. doi: 10.1093/abbs/gmx073. [DOI] [PubMed] [Google Scholar]
- van Bussel B.C., Soedamah-Muthu S.S., Henry R.M., Schalkwijk C.G., Ferreira I., Chaturvedi N., EURODIAB Prospective Complications Study Group Unhealthy dietary patterns associated with inflammation and endothelial dysfunction in type 1 diabetes: the EURODIAB study. Nutr. Metabol. Cardiovasc. Dis. 2013;23(8):758–764. doi: 10.1016/j.numecd.2012.04.005. 2013. [DOI] [PubMed] [Google Scholar]
- Villapol S. Roles of peroxisome proliferator-activated receptor gamma on brain and peripheral inflammation. Cell. Mol. Neurobiol. 2018;38(1):121–132. doi: 10.1007/s10571-017-0554-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyklicky V., Smejkalova T., Krausova B., Balik A., Korinek M., Borovska J. Preferential inhibition of tonically over phasically activated NMDA receptors by pregnane derivatives. J. Neurosci. 2016;36:2161–2175. doi: 10.1523/JNEUROSCI.3181-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton N., Maguire J. Allopregnanolone-based treatments for postpartum depression: Why/how do they work? Neurobiol Stress. 2019;11:100198. doi: 10.1016/j.ynstr.2019.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Waltenberger B., Pferschy-Wenzig E.M., Blunder M., Liu X., Malainer C. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): a review. Biochem. Pharmacol. 2014;92:73–89. doi: 10.1016/j.bcp.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Um P., Dickerman B.A., Liu J. Zinc, magnesium, selenium and depression: a review of the evidence, potential mechanisms and implications. Nutrients. 2018;10(5) doi: 10.3390/nu10050584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward A.B., Mir H., Kapur N., Gales D.N., Carriere P.P., Singh S. Quercetin inhibits prostate cancer by attenuating cell survival and inhibiting anti-apoptotic pathways. World J. Surg. Oncol. 2018;16(1):108. doi: 10.1186/s12957-018-1400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilker S., Pfeiffer A., Elbert T., Ovuga E., Karabatsiakis A., Krumbholz A. Endocannabinoid concentrations in hair are associated with PTSD symptom severity. Psychoneuroendocrinology. 2016;67:198–206. doi: 10.1016/j.psyneuen.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Yang L., Guo H., Li Y., Meng X., Yan L., Zhang D. Oleoylethanolamide exerts anti-inflammatory effects on LPS-induced THP-1 cells by enhancing PPARα signaling and inhibiting the NF-κB and ERK1/2/AP-1/STAT3 pathways. Sci. Rep. 2016;6 doi: 10.1038/srep34611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz C., Karali K., Fodelianaki G., Gravanis A., Chavakis T., Charalampopoulos I. Neurosteroids as regulators of neuroinflammation. Front. Neuroendocrinol. 2019;55:100788. doi: 10.1016/j.yfrne.2019.100788. [DOI] [PubMed] [Google Scholar]
- Yin H., Guo Q., Li X., Tang T., Cuiling Li C., Wang H. Curcumin suppresses IL-1β secretion and prevents inflammation through inhibition of the NLRP3 inflammasome. J. Immunol. 2018;200(8):2835–2846. doi: 10.4049/jimmunol.1701495. [DOI] [PubMed] [Google Scholar]
- Zhang Y.J., Gan R.Y., Li S., Zhou Y., Li A.N., Xu D.P. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules. 2015;20(12):21138–21156. doi: 10.3390/molecules201219753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Joshi-Barve S., Barve S. Eicosapentaenoic acid prevents LPS-induced TNF-α expression by preventing NF-κB activation. J. Am. Coll. Nutr. 2004;23:71–78. doi: 10.1080/07315724.2004.10719345. [DOI] [PubMed] [Google Scholar]
- Zhu D.M., Zhao W., Zhang B., Zhang Y., Yang Y., Zhang C. The relationship between serum concentration of vitamin D, total intracranial volume, and severity of depressive symptoms in patients with major depressive disorder. Front. Psychiatr. 2019;10:322. doi: 10.3389/fpsyt.2019.00322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski C.F., Paul S.M., Covey D.F., Mennerick S. Neurosteroids as novel antidepressants and anxiolytics: GABA-A receptors and beyond. Neurobiol Stress. 2019;11:100196. doi: 10.1016/j.ynstr.2019.100196. [DOI] [PMC free article] [PubMed] [Google Scholar]