Abstract
Background
Cervical cancer is preventable through human papillomavirus vaccination and cervical cancer screening. However, possibly due to systemic, individual (e.g. low socio-economic staus) and socio-cultural barriers, it is likely that non-natives, especially non-westerns, are more prone to attend neither vaccination nor screening (combined non-attendance). This is disturbing as the non-native population in Denmark is predicted to rise to 21% by 2060. We aimed to investigate differences in combined non-attendance by nativity and region of origin, and to analyse the association between country of origin and combined non-attendance adjusted for socio-economic status.
Setting
1.6.2007–31.12.2016 Denmark.
Methods
Logistic regression was performed to estimate crude and adjusted odds ratios with 95% confidence intervals for combined non-attendance.
Results
170,158 women were included. Overall combined non-attendance was 11.8% [11.7–12.0]; 10.0% [9.8–10.1] for native women and 27.1% [26.4–27.7] for non-native women, with highest degrees among Middle-Eastern and North-Africans (30.1% [29.2–30.9]). Even when adjusted for socio-economics, women from Middle-East and North-Africa had substantially higher odds of combined non-attendance than natives (adj. OR = 7.5 [6.3–8.9] for Somali women).
Conclusion
Denmark has a relatively low degree of combined non-attendance. However, cervical cancer preventive programmes seem to be better tailored to the needs of native women and do not appear to cater sufficiently to the needs of the fast-growing non-native populations, particularly not to the needs of Middle-Eastern and North African women. In order to secure more just cervical cancer prevention, future studies are recommended to develop tailored intervention sensitive to the need of non-native women.
Keywords: Human papilloma virus, Vaccination, Screening, Non-participation, Non-attendance, Denmark, Socio-economic status, Nationality
1. Introduction
Cervical cancer is the most common human papillomavirus (HPV) induced cancer (Walboomers et al., 1999, Schiffman, 2007, Ostor, 1993) and is considered preventable through pre-exposed HPV vaccination (HPVV) and routine cervical cancer screening (CCS) (Giuliano et al., 2019, Gustafsson et al., 1997, Hall et al., 2019, Vaccarella et al., 1990, Vaccarella et al., 2014). In order to reach the best protection, high attendance in both programmes is required. In Denmark, the childhood HPVV programme currently has a 84% coverage among eligible girls (Girls born 2005) (Statens Serum, 2020), and the CCS programme has a 64% coverage among eligible women (Danish, 2017). However, HPVV and CCS coverages are considerably lower among non-native women, especially from non-western countries (Badre-Esfahani et al., 2019, Fernandez de Casadevante et al., 2016, Hertzum-Larsen et al., 2019, Victoria Fernández de, 2015, Harder et al., 2018).
Currently non-natives make up 14% of the Danish population, of which a approximately 60% originates from non-western countries such as Middle-East and North-Africa (MENA). It is predicted that the non-native population in Denmark will continue to rise, and will reach 21% by 2060 (Statistics Denmark). It is possible that non-western women face distinct attendance barriers on as well a systematic, individual, and socio-cultural level. On a systemic level, these individuals may be accustomed to non-western health care systems, which differ significantly from the health care systems in western countries (e.g. little or no availability to universal and free-of charge HPVV and CCS programmes) (Sancho-Garnier et al., 2013, Bradford and Goodman, 2013). On an individual level, they may have other preferences and expectations to the healthcare systems and healthcare professionals (Lokdam et al., 2016, Marlow et al., 2017). Non-westerns in Denmark are furthermore more often, refugees and have lower socioeconomic status (SES), than western non-natives, who most often have a work or study permit, in addition to having a higher SES (Statistics Denmark). Socio-culturally many non-westerns living in Denmark are often accustomed to diverse cultural and religious traditions (Johnson et al., 2020). All these factors could potentially prone non-western women to have high combined non-attendance (neither HPVV nor CCS). Although an increasing number of studies have investigated the HPVV and CCS attendance patterns among non-natives compared to natives, no studies have to our knowledge addressed differences in combined non-attendance patterns according to nativity and detailed region of origin, nor analysed this pattern among non-westerns compared to natives.
1.1. Aim
The aims of this study were thus to investigate differences in combined non-attendance by nativity and region of origin, and to analyse the association between country of origin and combined non-attendance adjusted for socio-economic status (SES).
2. Materials and methods
2.1. Setting
In Denmark, all citizens are registered with a personal ten-digit identification number, which is given at birth or to non-natives when they receive a residence permit. Free health care is provided to all citizens holding such personal identification number.
Denmark offers two programmes aiming to prevent cervical cancer; 1) a HPVV programme and 2) a CCS programme. The Danish Health Authority recommends that all eligible women attend both programmes (The Danish Health and Medicines, 2018). The Danish National CCS Programme invites women for their first screening when they are 23 years old. Until the age of 49 years, women are invited for screening every third year after their latest invitation or latest cervical cytology sample; women aged 50–64 years are invited every five years after the latest invitation or cervical sample. Women who do not respond to the screening invitation letter receive up to two reminders after three and six months, respectively (The Danish Health and Medicines, 2018). Since 2006, HPVV has been available through self-payment for all women regardless of their age. In 2009, HPVV was implemented in the existing childhood vaccination programme free of charge; at the time offering vaccination with the four-valent vaccine (Gardasil) to all girls aged 12–18 years (Statens Serum Institut, 2008). As a supplement to the routine childhood HPVV programme, temporary free-of-charge HPVV programmes have been launched throughout 2008–2015 for older girls born in 1985–1995 (Statens Serum, 2014). As of September 2019, the childhood HPVV programme also includes boys 12–18 years of age (Statens Serum Institut, 2019)
2.2. Study design
We conducted a nationwide register-based, closed cohort study with a study period from 1 June 2007 to 31 December 2016.
2.3. Study population
The study population encompassed women born from 1985 to 1993 who had lived permanently in Denmark during the entire study period, hence they have all been eligible for both free-of-charge HPVV and a first call for CCS.
As it was not possible to distinguish between cervical cytologies collected for screening or medical reasons, all cytologies collected before the first call for CCS were excluded to ensure that the majority of cytologies included in the study were restricted to screening cytologies. Cytologies obtained between 22.5 and 23 years of age were, however, not excluded as they were obtained close to the first screening invitation. Furthermore, to ensure that vaccination data was restricted to free-of-charge HPVV, women, who have been HPV-vaccinated outside the HPVV programme (self-paid vaccination) were excluded as they could have displayed a different health-promoting behaviour than those who were vaccinated within the programme. Lastly, women with a history of surgical removal of cervix, either due to hysterectomy or trachelectomy, and women diagnosed with cervical cancer were excluded.
2.4. Data collection and definitions
The study population was defined in the Danish Civil Registration System (Pedersen, 2011) using the 10-digit personal identification number, allowing direct and individual linkage to other national registries.
Data on self-paid HPVV were collected through the Danish National Prescription Registry (Kildemoes et al., 2011), which holds information on all redeemed prescriptions from Danish pharmacies. We extracted the prescription codes for HPV vaccines Gardasil and Cervarix (J07BM01 and J07BM02), excluding women with at least one redeemed HPV vaccine prescription. Data on surgical removal of cervix were collected from the Danish National Patient Register (Lynge et al., 2011). Data on cervical cancer were collected from the Danish Cancer Registry (Gjerstorff, 2011).
Data on free-of-charge HPVV status were collected from the Danish National Health Service Register (Andersen et al., 2011), which holds information on all tax-funded public healthcare services provided in Denmark. We extracted all codes related to HPVV services for women born in 1985–1993. For the purpose of this study, women with at least one HPVV registration during the study period were considered “vaccinated”. All other women were considered “unvaccinated”.
Data on cervical cytology were collected from the Danish Pathology Register (Bjerregaard and Larsen, 2011). This register holds pathology data from all pathology departments classified according to the Systematized Nomenclature of Medicine (SNOMED) (Roger and Stanley Robboy, 1980). Cervical cytology samples were identified by the SNOMED codes T8X3* and material type 23. Women with at least one registered cytology sample between the age of 22.5 and 24 were considered “screened”. Those with no cervical cytology within this age range were defined as “unscreened”.
Based on HPVV and CCS attendance, four attendance combinations were defined:
(1) HPV-vaccinated and cervical cancer-screened women were categorized as having “Combined attendance”; (2) HPV-vaccinated and not cervical cancer-screened women were categorised as having “Partial attendance”; (3) Not HPV-vaccinated and cervical cancer-screened women were categorized as having “Partial attendance” and lastly, (4) Neither HPV-vaccinated nor cervical cancer–screened women were categorised as having “Combined non-attendance”. We considered women in the last category to be a particularly high-risk group as they had no preventive attendance at all compared with the other three groups.
2.5. Demographic and socio-economic variables
All demographic and socio-economic variables were collected from Statistics Denmark, which allows linkage of individual data from different health registers to demographic and socio-economic data (StatisticsDenmark, 2020).
Country of origin was used to determine native background and moreover used to categorize all countries of origin into six non-Danish regions of origin. Denmark was categorized separately to allow direct comparison. This categorisation into regions of origin was based on the WHO’s regional grouping of the countries (World Health Organization. Regional offices., 2020) and empirically modified to capture not only geographical cohesion between the region’s countries but also kinds of cultural and/or religious cohesion (original WHO regional classification is available in Appendix A)
For the purpose of this study native women were defined as those having Denmark as their country of origin and non-native as those not having Denmark as their country of origin, regardless of whether they were immigrants (defined as women born outside Denmark) or descendants (defined as women born in Denmark but with two immigrant parents).
Five variables were used as proxy measures for SES and used in the adjusted statistical analyses; (1) parental civil status, (2) highest completed education level by either parent, (3) family disposable income, (4) highest level of occupation by either parent and (5) degree of urbanisation. These socio-economic data were collected in 2013 when the participants were 20–27 years old. Some of these variables (e.g. civil status, highest education, income and occupation) may not be fully established during early adulthood. In contrast, parental SES is considered both stable and influential, even into young adulthood, and was therefore used in this study (Bruna et al., 2006).
2.6. Statistical analyses
Differences in sample characteristics between the four combined attendance groups were tabulated and reported with 95% confidence intervals (CI) for comparison. In logistic regression analyses, exposure was defined as having a MENA country as country of origin compared to having Denmark as country of origin. Outcome was defined as combined HPVV and CCS non-attendance compared with partial or combined attendance. The association between exposure and outcome was analysed using logistic regression, estimating the unadjusted and adjusted odds ratio (OR) with 95% CI. Adjustments were made using all five proxy measures for SES.
In order to test the robustness of our results, two sensitivity analyses were performed. As it is likely that descendants’ health-seeking behaviour is more similar to that of native women than to immigrants’ behaviour, we excluded all descendants from the population in our first sensitivity analysis. Furthermore, to test the robustness of our modified regional categorisation of the world, we used the original WHO categorisation in our second sensitivity analysis. All statistical analyses were conducted using STATA version 15 (STATA Corp., College Station, Texas, USA).
3. Results
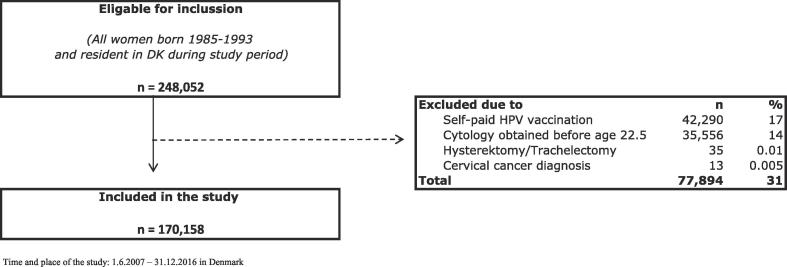
A total of 248,052 women were born in 1985–1993 and were resident in Denmark during the entire study period and thus eligible for inclusion. Of these, 42,290 (17%) were excluded due to self-paid HPVV. Another 35,556 (14%) were excluded due to cervical cytology obtained before the age of 22.5 years. Finally, 48 (0.02%) women were excluded due to a history of surgical removal of cervix and/or cervical cancer (Fig. 1). A total of 170,158 women remained in the study population of whom 136,264 (80% [79.8–80.2%]) were HPV-vaccinated during the study period and 83,880 (49% [49.0–49.5]) were screened (data not shown).
Fig. 1.
Inclucsion and exclusion flow-chart.
Table 1 shows that 151,885 (89.3% [89.1–89.4]) were natives and 18,273 (10.7% [10.6–10.9]) non-natives of whom 11,115 (60.8% [60.1–61.5]) originated from the MENA region. A total of 20,131 (11.8% [11.7–12.0]) were combined non-attenders. Degree of combined non-attendance was 10.0% [9.8–10.1] for native women and 27.1% [26.4–27.7] for non-native women. Table 1 further shows that compared with all other regions of origin, women from MENA countries had the highest level of combined non-attendance (30.1% [29.2–30.9]).
Table 1.
Distribution of combined attendance in cervical cancer prevention of the total population, by nativety and Region of origin.
| Vaccinated | Vaccinated | Un-vaccinated | Un-vaccinated | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Screened |
un-screened |
Screened |
Un-screened |
|||||||||||||||||
| (n, %col) | n | % row | (95% CI) | n | % row | (95% CI) | n | % row | (95% CI) | n | % row | (95% CI) | ||||||||
| Total study population (170,158, 100%) | 70,117 | 41.2% | (41.0% | - | 41.4%) | 66,147 | 38.9% | (38.6% | - | 39.1%) | 13,763 | 8.1% | (8.0% | - | 8.2%) | 20,131 | 11.8% | (11.7% | - | 12.0%) |
| Native population (151,885, 89%) | 66,354 | 43.7% | (43.4% | – | 43.9%) | 57,798 | 38.1% | (37.8% | – | 38.3%) | 12,549 | 8.3% | (8.1% | – | 8.4%) | 15,184 | 10.0% | (9.8% | – | 10.1%) |
| Non-native population (18,273, 11%) | 3,763 | 20.6% | (20.0% | – | 21.2%) | 8,349 | 45.7% | (45.0% | – | 46.4%) | 1214 | 6.6% | (6.3% | – | 7.0%) | 4,947 | 27.1% | (26.4% | – | 27.7%) |
| Region of origin | ||||||||||||||||||||
| Middle-East North Africa (11,115, 61%) | 1,582 | 14.2% | (13.6% | – | 14.9%) | 5,558 | 50.0% | (49.1% | – | 50.9%) | 633 | 5.7% | (5.3% | – | 6.1%) | 3,342 | 30.1% | (29.2% | – | 30.9%) |
| Eastern Europe, Central Asia (3,283, 18%) | 969 | 29.5% | (28.0% | – | 31.1%) | 1,289 | 39.3% | (37.6% | – | 41.0%) | 301 | 9.2% | (8.2% | – | 10.2%) | 724 | 22.1% | (20.6% | – | 23.5%) |
| South East Asia (1,923, 11%) | 594 | 30.9% | (28.8% | – | 33.0%) | 849 | 44.1% | (41.9% | – | 46.4%) | 102 | 5.3% | (4.3% | – | 6.4%) | 378 | 19.7% | (17.9% | – | 21.5%) |
| Western countries (1,028, 6%) | 356 | 34.6% | (31.7% | – | 37.6%) | 335 | 32.6% | (29.7% | – | 35.5%) | 114 | 11.1% | (9.2% | – | 13.2%) | 223 | 21.7% | (19.2% | – | 24.3%) |
| Sub-Sahara (605, 3%) | 199 | 32.9% | (29.2% | – | 36.8%) | 233 | 38.5% | (34.6% | – | 42.5%) | 49 | 8.1% | (6.1% | – | 10.6%) | 124 | 20.5% | (17.3% | – | 23.9%) |
| Latin America (151, 1%) | 56 | 37.1% | (29.4% | – | 45.3%) | 61 | 40.4% | (32.5% | – | 48.7%) | 10 | 6.6% | (3.2% | – | 11.8%) | 24 | 15.9% | (10.5% | – | 22.7%) |
| Missing (168, 0.1%) | 7 | 4.2% | (1.7% | – | 8.4%) | 24 | 14.3% | (9.4% | – | 21.1%) | 5 | 3.0% | (1.0% | – | 6.8%) | 132 | 78.6% | (71.6% | – | 84.5%) |
Time and place of the study: 1.6.2007 – 31.12.2016 in Denmark
Table 2 shows the country-specific variation in the degree of combined-non-attendance in the MENA region. Degree of combined non-attendance ranged between 16.6% [13.6–20.0] among Iranian women to 52.0% [48.1–55.4] among Somali women.
Table 2.
Distribution of combined attendance in cervical cancer prevention according to country of origin.
| Vaccinated | Vaccinated | Un-vaccinated | Un-vaccinated | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Screened |
Un-screened |
Screened |
Un-screened |
|||||||||||||||||
| (n; %col) | n | % row | (95% CI) | n | % row | (95% CI) | n | % row | (95% CI) | n | % row | (95% CI) | ||||||||
| Denmark (151,885) | 66,354 | 43.7% | (43.4% | - | 43.9%) | 57,798 | 38.1% | (37.8% | - | 38.3%) | 12,549 | 8.3% | (8.1% | - | 8.4%) | 15,184 | 10.0% | (9.8% | - | 10.1%) |
| MENA countries* (11,115) | ||||||||||||||||||||
| Turkey (3,849; 35%) | 557 | 14.5% | (13.4% | – | 15.6%) | 2,067 | 53.7% | (52.1% | – | 55.3%) | 184 | 4.8% | (4.1% | – | 5.5%) | 1,041 | 27.0% | (25.6% | – | 28.5%) |
| Iraq (1,566; 35%) | 240 | 15.3% | (13.6% | – | 17.2%) | 709 | 45.3% | (42.8% | – | 47.8%) | 115 | 7.3% | (6.1% | – | 8.7%) | 502 | 32.1% | (29.7% | – | 34.4%) |
| Lebanon(1,312; 12%) | 215 | 16.4% | (14.4% | – | 18.5%) | 670 | 51.1% | (48.3% | – | 53.8%) | 91 | 6.9% | (5.6% | – | 8.4%) | 336 | 25.6% | (23.3% | – | 28.1%) |
| Pakistan(1,086; 10%) | 101 | 9.3% | (7.6% | – | 11.9%) | 559 | 51.5% | (48.5% | – | 54.5%) | 38 | 3.5% | (2.5% | – | 4.8%) | 388 | 35.7% | (32.9% | – | 38.7%) |
| Afghanistan (956; 9%) | 114 | 11.9% | (9.9% | – | 14.1%) | 557 | 58.3% | (55.1% | – | 61.4%) | 39 | 4.1% | (2.9% | – | 5.5%) | 246 | 25.7% | (23.0% | – | 28.6%) |
| Somalia (734; 7%) | 52 | 7.1% | (5.3% | – | 9.2%) | 250 | 34.1% | (30.6% | – | 37.6%) | 52 | 7.1% | (5.4% | – | 9.2%) | 380 | 52.0% | (48.1% | – | 55.4%) |
| Iran (560; 5%) | 162 | 28.9% | (25.2% | – | 32.9%) | 265 | 47.3% | (43.1% | – | 51.6%) | 40 | 7.1% | (5.2% | – | 9.6%) | 93 | 16.6% | (13.6% | – | 20.0%) |
| Other MENA (574; 5%) | 79 | 13.8% | (11.1% | – | 16.9%) | 276 | 48.1% | (43.9% | – | 52.3%) | 47 | 8.2% | (6.1% | – | 10.7%) | 172 | 30.0% | (26.2% | – | 33.9%) |
| Morocco (478; 4%) | 62 | 13.0% | (10.1% | – | 16.3%) | 205 | 42.9% | (38.4% | – | 47.5%) | 27 | 5.6% | (3.8% | – | 8.1%) | 184 | 38.5% | (34.1% | – | 43.0%) |
*MENA countries with more than 450 observations are reported individually, while the remaining countries reported as “other MENA“
Time and place of the study: 1.6.2007 – 31.12.2016 in Denmark
Table 3 shows that compared to native women, women from all MENA countries had higher odds of being “combined non-attenders” than being either “partially or combined attenders”. This tendency decreased slightly after adjusting for demographics and SES, but remained both statistically and clinically significant for all countries. The adjusted OR of combined non-attendance ranged from 1.6 [1.3–2.1] for Iranian women to 7.5 [6.3–8.9] for Somali women.
Table 3.
Odds Ratios (OR) for combined non-attendance among women from MENA countries compared to women from Denmark.
| Unadjusted OR |
(95% CI) |
Adjusted OR* |
(95% CI) |
|||||
|---|---|---|---|---|---|---|---|---|
| Denmark | 1 | Ref. | 1 | Ref. | ||||
| Turkey | 3.3 | (3.1 | - | 3.6) | 3.0 | (2.8 | - | 3.3) |
| Iraq | 4.2 | (3.8 | – | 4.7) | 3.3 | (2.9 | – | 3.7) |
| Lebanon | 3.1 | (2.7 | – | 3.5) | 2.7 | (2.3 | – | 3.1) |
| Pakistan | 5.0 | (4.4 | – | 5.7) | 4.5 | (3.9 | – | 5.1) |
| Afghanistan | 3.1 | (2.7 | – | 3.6) | 2.6 | (2.2 | – | 3.1) |
| Somalia | 9.7 | (8.4 | – | 11.2) | 7.5 | (6.3 | – | 8.9) |
| Iran | 1.8 | (1.4 | – | 2.2) | 1.6 | (1.3 | – | 2.1) |
| Other MENA | 3.9 | (3.2 | – | 4.6) | 3.5 | (2.8 | – | 4.2) |
| Morocco | 5.6 | (4.7 | – | 6.8) | 4.6 | (3.7 | – | 5.7) |
* Adj. for highest achieved parental education, parental civil status, highest parental occupation disposable household income and individual degree of urbanization
Time and place of the study: 1.6.2007 – 31.12.2016 in Denmark.
Our first sensitivity analysis using the original WHO categorisation of world regions did not alter our conclusions regarding differences in combined non-attendance by regions of origin (Appendix B). Our second sensitivity analyses excluding descendants from the non-native population resulted in a higher combined non-attendance across all regions of origin (Appendix C), as well as higher adj. OR of combined non-attendance among women from most MENA countries compared to native (Appendix D).
4. Discussion
4.1. Main findings
This nationwide register-based cohort study showed that 10.0% of native women were combined non-attenders, while 27.1% of non-native women were combined non-attenders. Among all six regions of origin, women from the MENA region had the highest level of combined non-attendance (30.1%). Even after adjusting for SES, women from MENA countries had considerably higher odds of combined non-attendance than native women. However, large differences were observed within the MENA region. Thus, compared to native women, the odds for combined non-attendance was 7.5 times higher for Somali women and 1.5 times higher for Iranian women.
4.2. Strengths and limitations
The greatest strength of the present study is its use of individually linked nationwide register based data. This allowed us to report actual attendance in organized HPVV and CCS programmes, while limiting bias due to selection and information errors. Furthermore, we adjusted for potential confounding by adjusting for both parental and individual SES. Another embedded strength is the free-of-charge nature of the Danish HPVV and CCS programmes, which reduced any financial barrier that could have barred women with low income (such as non-natives) from attending the programmes.
A number of limitations must be acknowledged. First, due the nature of our closed cohort study, those who arrived in Denmark later than 2007 were excluded. Furthermore, women without residence permit were not eligible for preventive health care in Denmark (e.g. HPVV or CCV) and thus naturally not included in the study. Consequently, the most newly arrived immigrants were not represented in our results. As we expect that a longer residence has a positive effect on utilizing health care services such as HPVV and CCS (Kreusch et al., 2018), we believe that this limitation merely biased our result toward the null. This was also underlined in our sensitivity analyses excluding descendants. As cytology registrations in Denmark are not labelled according to uptake indication, we were unable to distinguish between cytologies collected for screening or medical purposes. However, by excluding women with cytologies obtained prior to their first screening invitation (<22.5 yrs.), we believed to have excluded most samples collected for other reason beside screening (e.g. for medical purposes) and thereby minimising the risk of information bias in our outcome. Furthermore, we had no data on whether non-native women had attended HPVV or CCS in their country of origin. Many western countries currently provide HPVV and CCS programmes. These women’s combined non-attendance could therefore actually be lower than reported in our study. In contrast, it is hardly likely that non-western women attend vaccination or/and screening in their home countries, as organized screenings programmes are insufficiently introduced in the MENA region (Sancho-Garnier et al., 2013). Thus, the results regarding these women would therefore be accurate despite the lack of this information. The absence of a valid and culturally sensitive categorisation of regions of the world required us to modify existing categorisations. We analysed our descriptive results using the original WHO grouping to test the robustness of this modification and to challenge our findings regarding MENA countries having the highest combined non-attendance. This sensitivity analysis did not alter our conclusions. It is likely that descendants have a health behaviour more adapted to the Danish ways compared to immigrants. However, due to lack of power, we were unable to stratify non-native women according to birth country. This would have been favourable, suggested by our sensitivity analyses. However, due to the relatively few birth cohorts offered HPV vaccination has reached screenings age, it was not possible for us to increase our dataset and achieve power to do so. Finally, due to the relatively short follow-up period (18 months), some participants may have attended CCS at a later time and may therefore have been misclassified as “un-screened”. CCS participation is generally lowest among the youngest (<30 yrs.) group of women and more pronounced among non-native women than native women (Hertzum-Larsen et al., 2019). Therefore, it might be possible that more women, particularly non-natives, would have attended if we had included a second screening round. Besides, data on CCS participation were collected between 22.5 and 23 years of age, and some women could have postponed their screening participation due to pregnancy, as screening during pregnancy is not recommended in Denmark. Thus, some women would falsely be defined as unscreened when in fact they did participate in CCS at a later time. However, relatively few women become pregnant at 23 years (Statistics, 2017) in Denmark where the mean age at first pregnancy is 29.2 years (Statistics, 2017). Nonetheless, young non-native women from non-western countries have a slightly higher fertility rate (1958, 1854 pr. 1000 immigrant and descendant women) than native women (1762 pr. 1000 women) (Statistics, 2017). This could potentially have slightly affected our results, as more non-native than native women could have postponed CCS. In future studies, it is therefore recommended to include information of pregnancy in the interpretation of CCS attendance.
4.3. Findings in relation to other studies
Our main findings were in line with other national and international studies showing generally lower HPVV and CCS attendance among non-native women, especially among women from MENA region (Hertzum-Larsen et al., 2019, Fernandez de Casadevante et al., 2016, Moller et al., 2016, Moller et al., 2018, Fernandez de Casadevante and Gil Cuesta, 2015, Widgren et al., 2011, Slattelid Schreiber et al., 2015, Hertzum-Larsen et al., 2019, Aminisani et al., 2012, Lofters et al., 2010, Lofters et al., 2017), compared to native women. Our results add to this existing literature by illustrating that across non-native populations, combined attendance patterns are diverse and particularly non-favourable for women from the MENA region. This overrepresentation of combined non-attendance among women from MENA countries was present even when adjusting for parental and individual SES.
Even though sharing many similar cultural and religious beliefs, the MENA region covers several countries with diverse language, history, traditions and healthcare systems, potentially giving rise to the observed differences in attendance within the MENA region. One possible explanation for the excessive CCS non-attendance rates observed among Somali women compared to women from other MENA countries, is the traditional female circumcision which have been practiced intensely previously in Somali (Abdullahi et al., 2009). However, it is not likely that female circumcision explains Somali women’s excessive HPVV non-attendance. Contrary, we observed that Iranian women had a level of combined non-attendance closer to that of native women. It is likely that Iranian women differ from women from other MENA countries in reasons of emigration. As Iranians dominantly emigrate from Iran due to political persecution and resistance to the religious regime, and not due to war, natural catastrophes or hunger as is the main reason for most other refugees in the world. Hence, Iranian women may be less inhibited by religious and traditional barriers than women from other Middle Eastern countries. Common for non-western immigrants whether they flee due to war, poverty, religious or political persecution is a more traumatising migration process than experienced by western immigrants which may have affected negatively on the women’s health preventive behaviour due to mental issues (Mangrio et al., 2018, Hudson et al., 2016). Our study design did, however not allow investigation of such subjective underlying barriers.
Previous qualitative studies of either HPVV or CCS have identified the following barriers for HPVV and CCS for women from MENA countries; language barriers, lack of knowledge and awareness regarding cervical cancer and its prevention, low perceived risk as well as fear and embarrassment regarding screening uptake, stigmatization of cancer especially in female genitals, fatalistic beliefs, fear of promiscuous behaviour after HPVV, and lack of trust in the health care system (Chan and So, 2017, Ferdous et al., 2018, Ferrer et al., 2014, Gele et al., 2017, Johnson et al., 2008, Marlow et al., 2015, Wilson et al., 2018, Zeraiq et al., 2015, Islam, 2017, Salad et al., 2015).
Qualitative studies exploring common barriers towards HPVV and CCS attendance are, however, very few. Allen et al. interviewed 31 health care-insured Somali women living in the USA about barriers and facilitators for combined attendance, reporting a generally high level of knowledge of cervical cancer and CCS, but relatively low levels of knowledge of HPV and HPVV. In an attempt to improve combined attendance, they suggested that direct and community-based educational interventions be implemented (Allen et al., 2019). Grandahl et al investigated combined non-attendance among 50 newly arrived non-natives living in Sweden. Although the interviewees were positive towards cervical cancer prevention, they disclosed several barriers, mainly low language skills and limited knowledge regarding HPV and cervical cancer. Grandahl et al recommended that health information be given in native language, and that health care providers be more aware of non-native women’s culturally distinct barriers(Grandahl et al., 2015). Based on the above studies, it seems that tailored interventions targeting both HPVV and CCS are needed in order to increase combined attendance among non-native women, particularly from MENA countries. However, no such studies have to our knowledge yet been conducted.
5. Conclusion
In general, Denmark has a relatively low degree of combined non-attendance. However, cervical cancer preventive programmes seem to be better tailored to the needs of native women and do not appear to cater sufficiently to the needs of the fast-growing non-native populations, particularly not to the needs of Middle-Eastern and North African women. In order to secure more just cervical cancer prevention, future studies are recommended to develop tailored intervention sensitive to the need of non-native women.
Acknowledgments
Acknowledgments
The author group would like to thank data manager Bo Søborg from at the Department of Public Health Programmes, Randers Regional Hospital for assisting the data cleaning and initial analyses. As well as thanking research Secretary Marianne Rævsbæk Pedersen from at the Department of Public Health Programmes, Randers Regional Hospital for the final revision of the manuscript.
Ethical approval
According to Danish legislation and the Central Denmark Region Committees on Biomedical Research Ethics, the study did not require ethical approval because it was based on register data. The same institutions waived patient consent for use of register data. In accordance with Danish law and the EU's General Data Protection Regulation, the project was listed at the Central Denmark Region internal list of research projects (J. No.: 1-16-02-400-16).
Disclosure
Andersen B has received HPV test kits and HPV self-sampling devices from Roche and Axlab for other studies. Pedersen LK has received speaker’s fee from Merck and Sanofi Pasteur in relation to lectures on HPV vaccines. The authors report no conflicts of interest in this work.
Funding
This study was funded by the Family Hede Nielsen’s Foundation and Helsefonden. The sponsors had no influence on the scientific process.
Author contributions
All authors made substantial contributions to both design, achievement of data, analysis, and interpretation of data.
The first author drafted the manuscript as well as conducted the initial analysis. The other authors took part in revising the manuscript, figure and tables critically, gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2020.101106.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Walboomers J.M., Jacobs M.V., Manos M.M. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999;189(1):12. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Schiffman MC, Philip E; Jeronimo, Jose; Rodriguez, Ana C; Wacholder, Sholom. Human papillomavirus and cervical cancer. The Lancet. 2007; 370, 9590; ProQuestpg. 890. [DOI] [PubMed]
- Ostor A.G. Natural history of cervical intraepithelial neoplasia: a critical review. Int. J. Gynecol. Pathol.: official journal of the International Society of Gynecological Pathologists. 1993;12(2):186. [PubMed] [Google Scholar]
- Gustafsson L., Ponten J., Zack M., Adami H.O. International incidence rates of invasive cervical cancer after introduction of cytological screening. Cancer Causes Control. 1997;8(5):755–763. doi: 10.1023/a:1018435522475. [DOI] [PubMed] [Google Scholar]
- Vaccarella S, Lortet-Tieulent J, Plummer M, Franceschi S, Bray F. Worldwide trends in cervical cancer incidence: impact of screening against changes in disease risk factors. European journal of cancer (Oxford, England : 1990). 2013;49(15):3262-3273. [DOI] [PubMed]
- Vaccarella S., Franceschi S., Engholm G., Lonnberg S., Khan S., Bray F. 50 years of screening in the Nordic countries: quantifying the effects on cervical cancer incidence. Br J Cancer. 2014;111(5):965–969. doi: 10.1038/bjc.2014.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano A.R., Joura E.A., Garland S.M. Nine-valent HPV vaccine efficacy against related diseases and definitive therapy: comparison with historic placebo population. Gynecol Oncol. 2019;154(1):110–117. doi: 10.1016/j.ygyno.2019.03.253. [DOI] [PubMed] [Google Scholar]
- Hall M.T., Simms K.T., Lew J.B. The projected timeframe until cervical cancer elimination in Australia: a modelling study. Lancet Public Health. 2019;4(1):e19–e27. doi: 10.1016/S2468-2667(18)30183-X. [DOI] [PubMed] [Google Scholar]
- Harder, Juul, Jensen Factors associated with non-participation in cervical cancer screening – a nationwide study of nearly half a million women in Denmark. Prev Med. 2018 doi: 10.1016/j.ypmed.2018.02.035. [DOI] [PubMed] [Google Scholar]
- Hertzum-Larsen R., Kjaer S.K., Frederiksen K., Thomsen L.T. Participation in cervical cancer screening among immigrants and Danish-born women in Denmark. Prev. Med. 2019;123:55–64. doi: 10.1016/j.ypmed.2019.02.023. [DOI] [PubMed] [Google Scholar]
- Fernandez de Casadevante V., Cantarero-Arevalo L., Cuesta J.G., Valentiner-Branth P. Ethnic background and human papillomavirus vaccine uptake in Denmark: A countrywide retrospective cohort study including 274,154 women aged 19–28 years. Papillomavirus Res. 2016;2:78–84. doi: 10.1016/j.pvr.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victoria Fernández de Casadevante iGCaiC. Determinants in the uptake of the human papillomavirus vaccine: a systematic review based on. European studies. 2015 doi: 10.3389/fonc.2015.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badre-Esfahani S., Larsen M.B., Seibaek L. Non-Adherence To Childhood HPV Vaccination Is Associated With Non-Participation In Cervical Cancer Screening - A Nationwide Danish Register-Based Cohort Study. Clinical epidemiology. 2019;11:969–980. doi: 10.2147/CLEP.S203023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danish cervical cancer screening quality database [Dansk Kvalitetsdatabase for Livmoderhalskræftscreening]. Annual report 2017. Accessed 13-02-2020.
- Sancho-Garnier H., Khazraji Y.C., Cherif M.H. Overview of cervical cancer screening practices in the extended Middle East and North Africa countries. Vaccine. 2013;31(Suppl 6):G51. doi: 10.1016/j.vaccine.2012.06.046. [DOI] [PubMed] [Google Scholar]
- Bradford L., Goodman A. Cervical cancer screening and prevention in low-resource settings. Clin Obstet Gynecol. 2013;56(1):76–87. doi: 10.1097/GRF.0b013e31828237ac. [DOI] [PubMed] [Google Scholar]
- Lokdam N., Kristiansen M., Handlos L.N., Norredam M. Use of healthcare services in the region of origin among patients with an immigrant background in Denmark: a qualitative study of the motives. BMC Health Serv Res. 2016;16:99. doi: 10.1186/s12913-016-1346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlow L.A., Meisel S.F., Wardle J. Ethnic minority women prefer strong recommendations to be screened for cancer. BMC Public Health. 2017;17(1):164. doi: 10.1186/s12889-017-4093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistic Denmark. Immigrants in Denmark annual report 2019 [Indvandrere i Danmark 2019]. 2019. Accessed 13-02-2020.
- The Danish Health and Medicines Authority. National recommendations for cervical cancer screening [Screening for livmoderhalskræft – anbefalinger]. 2018. Accessed 13-02-2020 Link: https://www.sst.dk/da/udgivelser/2018/screening-for-livmoderhalskraeft.
- Statens Serum Institut. Newsletter regarding implementing HPV vaccine in childhood vaccination programme (Overvågning og forebyggelse af smitsomme sygdomme. Human Papillomavirus vaccine i børnevaccinationsprogrammet. Nyhedsbrev uge 35. 2008). Link: https://www.ssi.dk.
- Statens Serum Institut. Newsletter regarding implementing cathc up HPV vaccination [HPVvaccination i børnevaccinationsprogrammet og til kvinder født i 19931997 De midlertidige vaccineskift i børnevaccinationsprogrammet.] 2014. Link: https://www.ssi.dk.
- Statens Serum Institut. Newsletter implementing childhood HPV vaccination for boys (Uge 24/25 - 2019. 2020). Accessed 10-02-2020. Link: https://www.ssi.dk/aktuelt/nyheder/2019/hpv-vaccination-af-drenge-udskydes-til-1-september-2019.
- Pedersen C.B. The Danish Civil Registration System. Scand J Public Health. 2011;39(7 Suppl):22–25. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- Johnson, Todd M., Grim . In: World Religion Database. Brian J., editor. Brill; Leiden/Boston: 2020. [Google Scholar]
- Kildemoes H.W., Sorensen H.T., Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39(7 Suppl):38–41. doi: 10.1177/1403494810394717. [DOI] [PubMed] [Google Scholar]
- Lynge E., Sandegaard J.L., Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(7):30–33. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- Gjerstorff M.L. The Danish Cancer Registry. Scand J Public Health. 2011;39(7):42–45. doi: 10.1177/1403494810393562. [DOI] [PubMed] [Google Scholar]
- Andersen J.S., Olivarius Nde F., Krasnik A. The Danish National Health Service Register. Scand J Public Health. 2011;39(7 Suppl):34–37. doi: 10.1177/1403494810394718. [DOI] [PubMed] [Google Scholar]
- Bjerregaard B., Larsen O.B. The Danish Pathology Register. Scand J Public Health. 2011;39(7 Suppl):72–74. doi: 10.1177/1403494810393563. [DOI] [PubMed] [Google Scholar]
- Roger A. MD M, Stanley Robboy, MD. Progress in Medical Information Management Systematized Nomenclature of Medicine (SNOMED). JAMA. 1980;1980;243(8):756-762. [DOI] [PubMed]
- StatisticsDenmark. 2020 [Main page in English]. Accessed 13-02-2020. Link: https://www.dst.dk/en.
- World Health Organization. Regional offices. 2020. Accessed 13-02-2020 Link https://www.who.int/about/who-we-are/regional-offices.
- Bruna G., Shaw M., Lawlor D.A., Lynch J.W. Indicators of socioeconomic position (part 1) J Epidemiol Community Health. 2006;60(1):7–12. doi: 10.1136/jech.2004.023531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreusch T., Wang J., Sparen P., Sundstrom K. Opportunistic HPV vaccination at age 16–23 and cervical screening attendance in Sweden: a national register-based cohort study. BMJ Open. 2018;8(10) doi: 10.1136/bmjopen-2018-024477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistics Denmark. Maen age at first child delivery. 2017. Link: https://www.dst.dk/da. Accessed 13-02-2020.
- Statistics Denmark. Mothers age at firts child delivery. 2017. Link: https://www.dst.dk/da. Accessed 13-02-2020.
- Statistics Denmark. Population [Befolkning], FERT1. Link: https://www.dst.dk/da. 2017 Accessed 13-02-2020.
- Moller S.P., Hjern A., Andersen A.M., Norredam M. Differences in uptake of immunisations and health examinations among refugee children compared to Danish-born children: a cohort study. Eur. J. Pediatr. 2016;175(4):539–549. doi: 10.1007/s00431-015-2663-9. [DOI] [PubMed] [Google Scholar]
- Moller S.P., Kristiansen M., Norredam M. Human papillomavirus immunization uptake among girls with a refugee background compared with Danish-born girls: a national register-based cohort study. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation (ECP). 2018;27(1):42–45. doi: 10.1097/CEJ.0000000000000274. [DOI] [PubMed] [Google Scholar]
- Fernandez de Casadevante V, Gil Cuesta J, Cantarero-Arevalo L. Determinants in the Uptake of the Human Papillomavirus Vaccine: A Systematic Review Based on European Studies. Frontiers in oncology. 2015;5:141. [DOI] [PMC free article] [PubMed]
- Widgren K., Simonsen J., Valentiner-Branth P., Molbak K. Uptake of the human papillomavirus-vaccination within the free-of-charge childhood vaccination programme in Denmark. Vaccine. 2011;29(52):9663. doi: 10.1016/j.vaccine.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Slattelid Schreiber S.M., Juul K.E., Dehlendorff C., Kjaer S.K. Socioeconomic predictors of human papillomavirus vaccination among girls in the Danish childhood immunization program. J Adolesc Health. 2015;56(4):402–407. doi: 10.1016/j.jadohealth.2014.12.008. [DOI] [PubMed] [Google Scholar]
- Hertzum-Larsen R., Thomsen L.T., Frederiksen K., Kjaer S.K. Human papillomavirus vaccination in immigrants and descendants of immigrants in Denmark. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation (ECP) 2019 doi: 10.1097/CEJ.0000000000000524. [DOI] [PubMed] [Google Scholar]
- Aminisani N., Armstrong B.K., Canfell K. Cervical cancer screening in Middle Eastern and Asian migrants to Australia: a record linkage study. Cancer epidemiology. 2012;36(6):e394–e400. doi: 10.1016/j.canep.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Lofters AK, Hwang SW, Moineddin R, Glazier RH. Cervical cancer screening among urban immigrants by region of origin: a population-based cohort study. Preventive medicine. 2010;51(6):509-516. [DOI] [PubMed]
- Lofters AK, Vahabi M, Kim E, Ellison L, Graves E, Glazier RH. Cervical Cancer Screening among Women from Muslim-Majority Countries in Ontario, Canada. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2017;26(10):1493-1499. [DOI] [PubMed]
- Mangrio E., Zdravkovic S., Carlson E. A qualitative study of refugee families' experiences of the escape and travel from Syria to Sweden. BMC research notes. 2018;11(1) doi: 10.1186/s13104-018-3702-1. 594–018-3702-3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson C.C., Adams S., Lauderdale J. Cultural Expressions of Intergenerational Trauma and Mental Health Nursing Implications for U.S. Health Care Delivery Following Refugee Resettlement: An Integrative Review of the Literature. J. Transcult. Nurs. 2016;27(3):286–301. doi: 10.1177/1043659615587591. [DOI] [PubMed] [Google Scholar]
- Abdullahi A., Copping J., Kessel A., Luck M., Bonell C. Cervical screening: Perceptions and barriers to uptake among Somali women in Camden. Public Health. 2009;123(10):680–685. doi: 10.1016/j.puhe.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Marlow L.A., Waller J., Wardle J. Barriers to cervical cancer screening among ethnic minority women: a qualitative study. J Family Plan. Reproduct. Health Care. 2015;41(4):248–254. doi: 10.1136/jfprhc-2014-101082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gele A.A., Qureshi S.A., Kour P., Kumar B., Diaz E. Barriers and facilitators to cervical cancer screening among Pakistani and Somali immigrant women in Oslo: a qualitative study. Int. J. Women's Health. 2017;9:487–496. doi: 10.2147/IJWH.S139160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdous M., Lee S., Goopy S. Barriers to cervical cancer screening faced by immigrant women in Canada: a systematic scoping review. BMC Women's Health. 2018;18(1) doi: 10.1186/s12905-018-0654-5. 165–018-0654-0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C.E., Mues K.E., Mayne S.L., Kiblawi A.N. Cervical cancer screening among immigrants and ethnic minorities: a systematic review using the Health Belief Model. J. Lower Genital Tract Dis. 2008;12(3):232–241. doi: 10.1097/LGT.0b013e31815d8d88. [DOI] [PubMed] [Google Scholar]
- Chan D.N.S., So W.K.W. A Systematic Review of the Factors Influencing Ethnic Minority Women's Cervical Cancer Screening Behavior: From Intrapersonal to Policy Level. Cancer Nurs. 2017;40(6):E1–E30. doi: 10.1097/NCC.0000000000000436. [DOI] [PubMed] [Google Scholar]
- Zeraiq L., Nielsen D., Sodemann M. Attitudes towards human papillomavirus vaccination among Arab ethnic minority in Denmark: A qualitative study. Scand J Public Health. 2015;43(4):408–414. doi: 10.1177/1403494815569105. [DOI] [PubMed] [Google Scholar]
- Ferrer H.B., Trotter C., Hickman M., Audrey S. Barriers and facilitators to HPV vaccination of young women in high-income countries: a qualitative systematic review and evidence synthesis. BMC public Health. 2014;14 doi: 10.1186/1471-2458-14-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L., Rubens-Augustson T., Murphy M. Barriers to immunization among newcomers: A systematic review. Vaccine. 2018;36(8):1055–1062. doi: 10.1016/j.vaccine.2018.01.025. [DOI] [PubMed] [Google Scholar]
- Statens Serum Institut. HPV1 coverage girls born 2005 Denmark. 2020. Accessed 13-02-2020.
- Islam, N. Understanding barriers and facilitators to breast and cervical cancer screening among muslim women in New York City- Perspectives from Key Informants. SM J Community Med. 2017;3(1). pii: 1022. Epub 2017 Feb 23. [PMC free article] [PubMed]
- Salad J, Verdonk P, de Boer F, Abma TA. “A Somali girl is Muslim and does not have premarital sex. Is vaccination really necessary?” A qualitative study into the perceptions of Somali women in the Netherlands about the prevention of cervical cancer. Int. J. Equity Health. 2015;14:68-015-0198-0193. [DOI] [PMC free article] [PubMed]
- Allen E.M., Lee H.Y., Pratt R. Facilitators and Barriers of Cervical Cancer Screening and Human Papilloma Virus Vaccination Among Somali Refugee Women in the United States: A Qualitative Analysis. J Transcult Nurs. 2019;30(1):55–63. doi: 10.1177/1043659618796909. [DOI] [PubMed] [Google Scholar]
- Grandahl M., Tyden T., Gottvall M., Westerling R., Oscarsson M. Immigrant women's experiences and views on the prevention of cervical cancer: a qualitative study. Health Expect. 2015;18(3):344–354. doi: 10.1111/hex.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.