Abstract

Benlysta is a new drug approved by the US Food and Drug Administration (US FDA) in 2019 for the treatment of systemic lupus erythematosus. In this study, we loaded the benlysta in the traditional sodium alginate (SA) hydrogel to investigate the potential application of the drug-loaded hydrogel for skin dressing or hypodermic drug. Live/dead staining images and the CCK-8 results showed that the benlysta-loaded hydrogel could promote the growth of human epidermal cells (HaCat), fibroblasts (L929), and endothelial cells while inhibiting the aggregation of inflammatory cells (macrophages). In addition, the hydrogel degradation and drug release are slow and controllable, and the gel time of drug-loaded hydrogel can be adjusted by adding sodium alginate ratios according to the requirement. In summary, we prepared a time-dependent drug-loaded hydrogel for potential application in the treatment of skin injury that may be caused by other diseases.
1. Introduction
As an important organ of human body, skin plays an important role in protecting the body from invasion and stimulation (temperature, hardness, humidity, pain, etc.) from the outside world. Therefore, skin damage brings a lot of discomfort and inconvenience to the human body. Especially, severe skin damage caused by external organic matter (scratch from a sharp weapon, burn, frostbite, etc.) and major diseases (skin disease, diabetes, lupus erythematosus, etc.) is not easy to heal. These skin injuries take a long time to heal, and it is an orderly and complex process that includes coagulation, inflammation regression, tissue regeneration, epidermal repair, etc.1,2 To solve these problems and accelerate wound healing, a series of hydrogel dressing have been developed.3−10 These hydrogel dressings greatly improve the microbial environment of skin wounds, effectively inhibit bacterial infection, and make up for the deficiency of antibacterial ability of skin wounds caused by major diseases.11−13 However, the repair of skin wounds needs to be considered in many aspects. In addition to antibacterial properties, the inhibition of inflammation (macrophage participation),14 the repair of connective tissue (fibroblasts participation),15 epidermis (epidermal cell participation), and capillaries (platelets, endothelial cells, and smooth muscle cell participation) should also be considered.16,17 For deeper skin wounds, where it is not enough to use hydrogels as dressings, they can also be injected.18 Because most of the current prescription drugs have multiple functions, it is a preferable choice to use hydrogel loaded with prescription drugs as biomaterials for skin injury treatment.
In the previous work, we have developed a series of injectable time-dependent sodium alginate (SA) hydrogels for stem cells and/or molecular loading.19,20 The advantage of sodium alginate is that it has huge storage capacity, and sodium alginate hydrogels can be formed under mild conditions when the molecule is combined with two valence cations. The sodium alginate hydrogels have been widely applied in tissue engineering due to their excellent biocompatibility, low toxicity, and immune response.21 Additionally, they are also used as carriers for an accurate delivery of drugs, cells, genes, or proteins to improve the therapeutic effect.22,23 Therefore, sodium alginate hydrogels are preferable materials for treating skin damage. British pharmaceutical giant GSK recently (April 2019) announced that the US Food and Drug Administration (US FDA) has approved the lupus drug benlysta for children aged 5 years and above with systemic lupus erythematosus (SLE) through priority review. SLE is the most common type of lupus, which will lead to serious long-term organ damage.24 In this contribution, we tried to load the new drug benlysta into sodium alginate hydrogels and systematically evaluated the effect of benlysta-loaded hydrogels on the growth of macrophages (inflammation evaluation), epidermal cells, fibroblasts, endothelial cells, etc. to obtain a more widely used biomaterial for the treatment of skin injury.
2. Results and Discussion
2.1. Material Characterization of Benlysta-Loaded Hydrogels
Gelation is the most important feature and advantage of hydrogels.25Figure 1 shows that after the addition of two valence ions (calcium ions), the mixture of sodium alginate (SA) and benlysta rapidly gelled and adhered to the container wall, suggesting excellent gelation property of the benlysta-loaded SA hydrogel. At the skin wound, there are enough divalent ions, such as Ca2+, Mg2+, Fe2+, Zn2+, etc.,26,27 which can promote the rapid formation of the gelation. Figure 2 shows that the 2% SA ratio had a shorter gelation time (3 min) than the 1% SA ratio (7 min). As skin dressing, a shorter gelation time certainly has a better healing effect; however, as an injectable material, it needs to take more than 5 min to treat deeper skin wounds. Thus, this result indicated that the gelation time can be determined by regulating the SA ratios according to different treatment needs of the wounds. The water content of the normal skin tissue is in the rage of 25–70%; the deeper the tissue, the higher is the water content.28 The lack of water content will lead to dry, rough, and chapped skin tissue, leading to a series of skin problems.29Figure 3 displays that benlysta-loaded hydrogels with 1 and 2% SA ratios presented no significant difference: both samples had a water content that exceeded 95%, which can fully meet the needs of skin moisture content.
Figure 1.
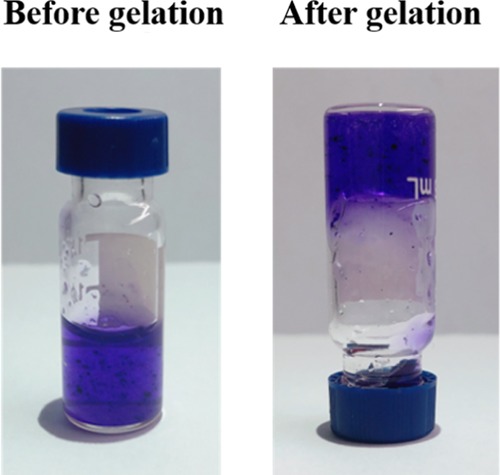
Pictorial diagram of the gelation of the benlysta-loaded SA hydrogel.
Figure 2.
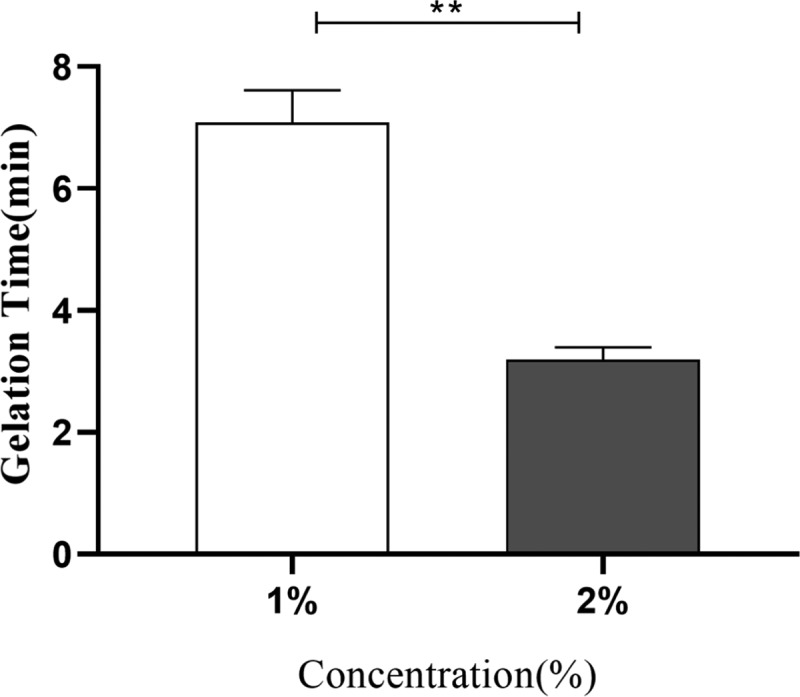
Gelation time of the benlysta-loaded hydrogels with 1 and 2% SA ratios (**p < 0.01, mean ± SD, n = 3).
Figure 3.
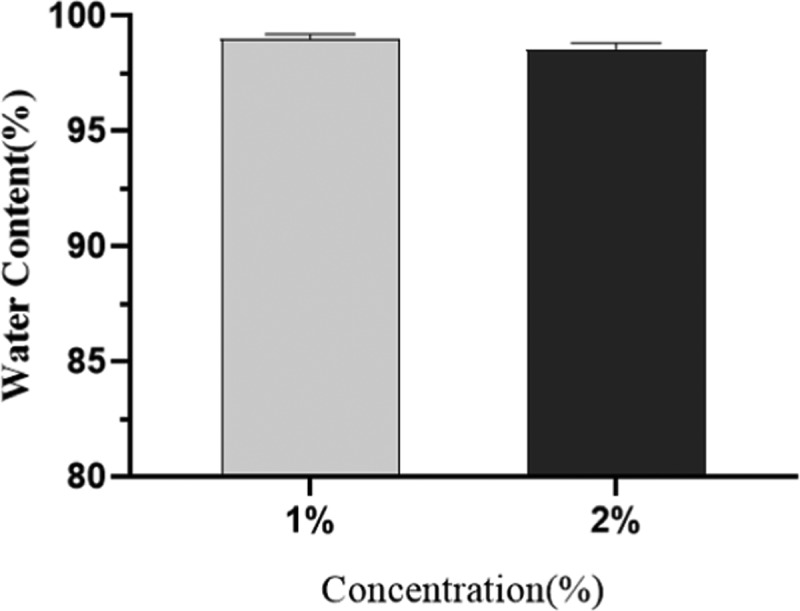
Water content of the benlysta-loaded hydrogels with 1 and 2% SA ratios (mean ± SD, n = 3).
It has been reported that the exudation of inflammatory fluid and tissue fluid often delays the healing of skin wounds, so it is helpful to absorb excessive surface fluid for wound healing.30 In skin dressing, the better swelling rate is beneficial to remove excess water from the wound. Figure 4 shows that the swelling rate of the benlysta-loaded SA hydrogel rapidly increased within 24 h and then remained at 150%. The drug release of the hydrogel is often affected by the water content and swelling rate, and Figure 5 shows that the benlysta-loaded SA hydrogel released about 50% drug in 72 h; the initial release of drugs in high dose is conducive to rapid hemostasis, sterilization, and inflammation inhibition of wounds. Then, the curve values were maintained at a sustained release level, which was beneficial to cell proliferation. In addition, the benlysta-loaded SA hydrogel also had a slow degradation property, sill keeping 95% weight within 72 h. In combination with the drug release result, the hydrogel has good stability in a certain period of time, as shown in Figure 6.
Figure 4.
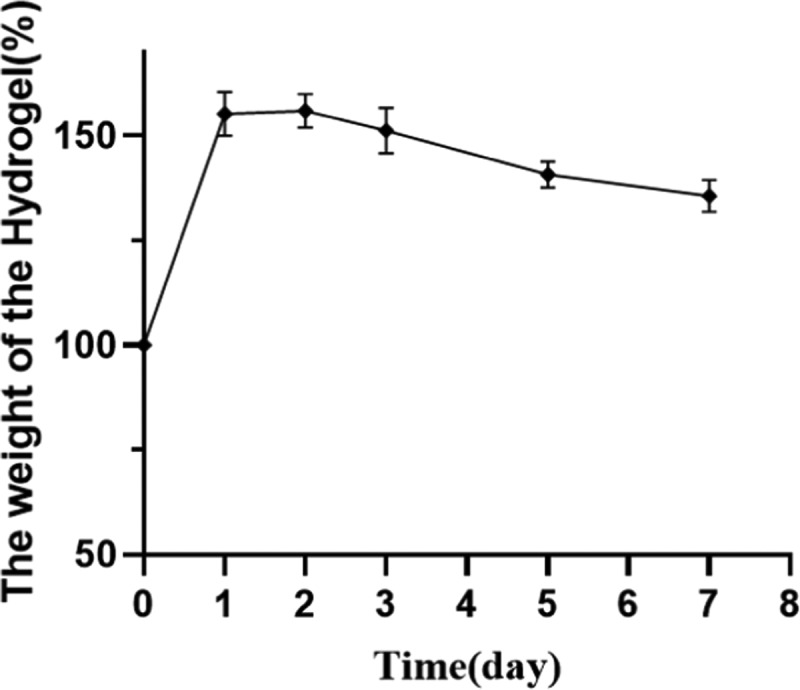
Swelling rate of the benlysta-loaded hydrogel (mean ± SD, n = 3).
Figure 5.
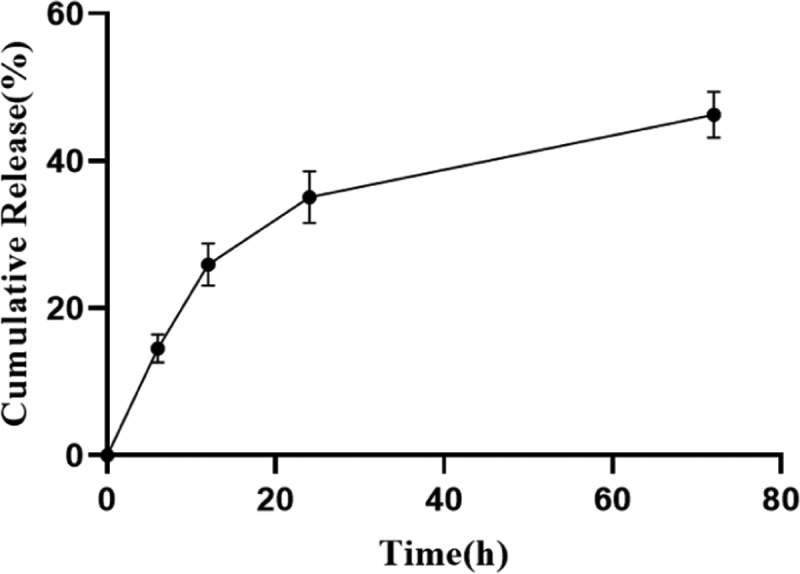
Benlysta release from the SA hydrogel (mean ± SD, n = 3).
Figure 6.
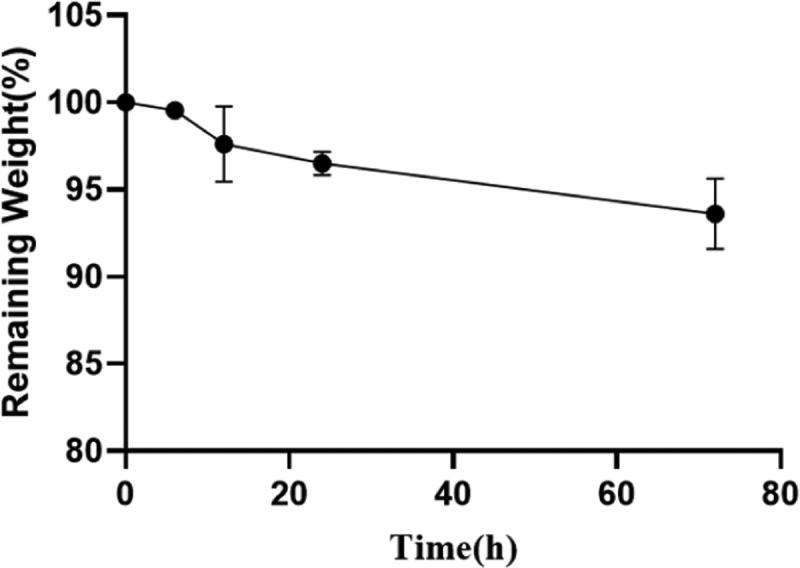
Degradation detection of the benlysta-loaded SA hydrogel (mean ± SD, n = 3).
2.2. Biocompatibility of Benlysta-Loaded Hydrogel
To investigate the anti-inflammation property of the benlysta-loaded SA hydrogel, the macrophage test was performed because macrophage is the most important participation of inflammation. Figure 7 shows that there are fewer macrophages in the benlysta-loaded SA hydrogel group compared with there are in the SA hydrogel group and the control group, suggesting the better anti-inflammation ability of the drug-loaded hydrogel.
Figure 7.
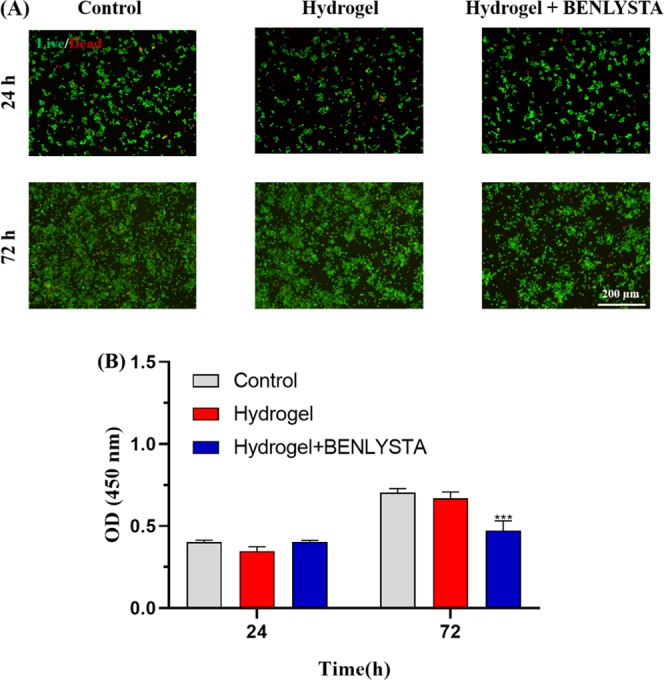
(A) Acridine orange and ethidium bromide (AO/EB) staining images and (B) CCK-8 detection of macrophages in the benlysta-loaded SA hydrogel group and controls (***p < 0.001 compared with other samples, mean ± SD, n = 3).
Fibroblasts are the main components of deep connective tissue of the skin.31Figure 8 shows that both SA hydrogel and benlysta-loaded SA hydrogel improved the fibroblast (L929 cell line) proliferation on the third day, wherein the benlysta-loaded SA hydrogel possessed a higher number of L929 compared with the single SA hydrogel, which indicated that the drug benlysta had a strong role in improving the fibroblast growth. Epidermal cells are the important outermost barrier of the skin.32Figure 9 shows that the SA hydrogel and the benlysta-loaded SA hydrogel also improve the epidermal cell (HaCat cell line) proliferation on the third day. It is notable that the benlysta-loaded SA hydrogel as early as the first day began to improve the Faster HaCat growth, suggesting a better function in promoting skin epidermal healing.
Figure 8.
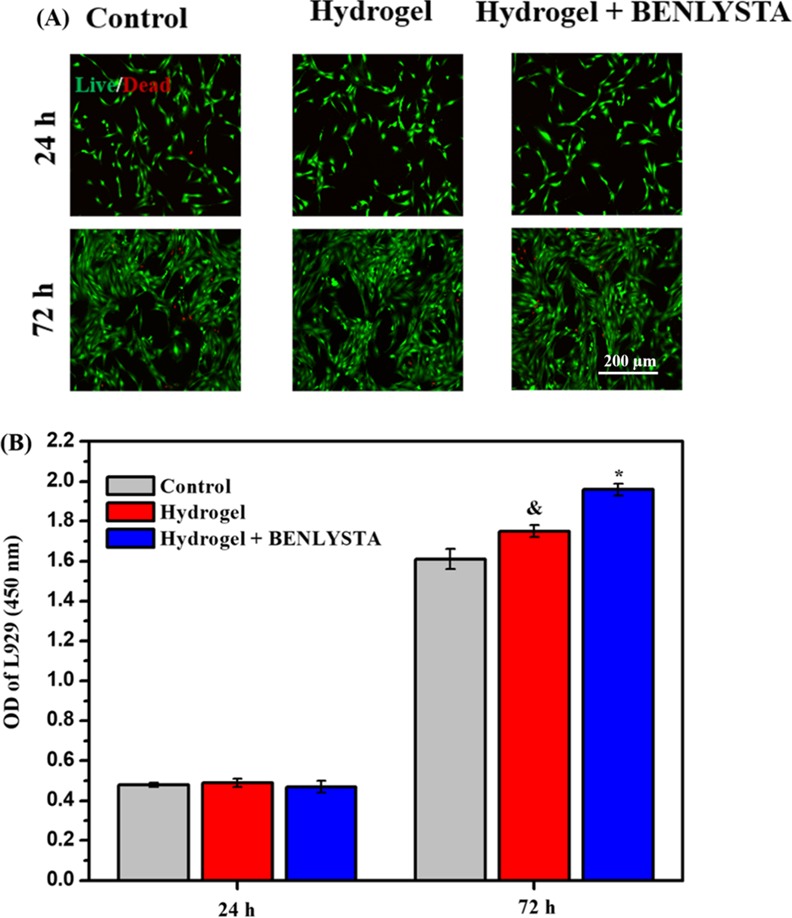
(A) AO/EB staining images and (B) CCK-8 detection of L929 cell line in the benlysta-loaded SA hydrogel group and controls (*p < 0.05 compared with other samples, &p < 0.05 compared with the control group, mean ± SD, n = 3).
Figure 9.
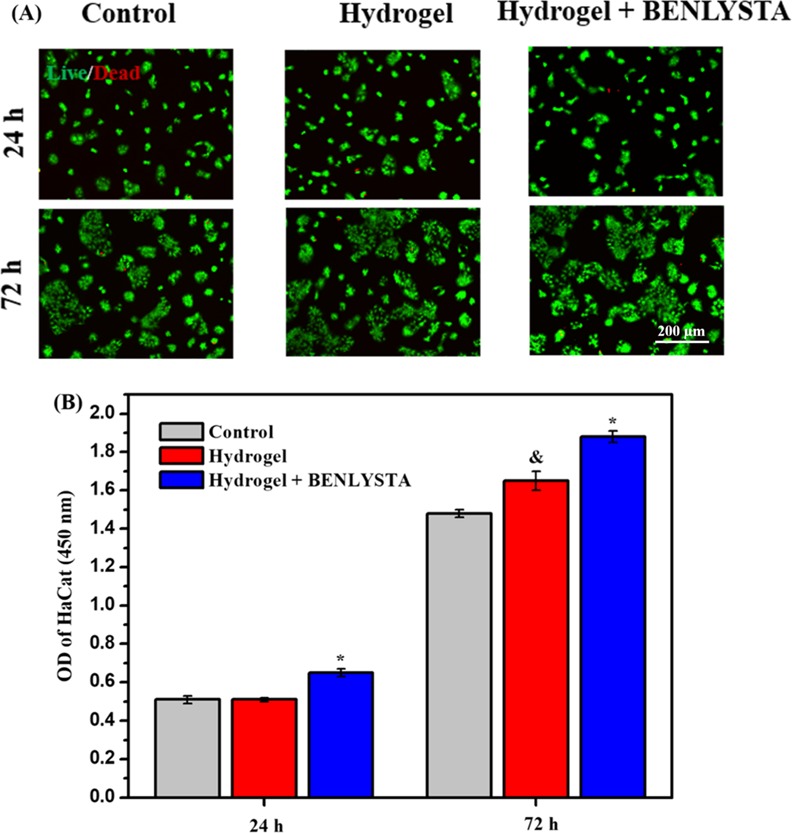
(A) AO/EB staining images and (B) CCK-8 detection of HaCat cell line in the benlysta-loaded SA hydrogel group and controls (*p < 0.05 compared with other samples, &p < 0.05 compared with the control group, mean ± SD, n = 3).
Many healing defects of skin damage caused by major diseases are due to the lack of blood vessels in the damaged tissue, which cannot provide enough nutrition, related factors, cells, etc. Thus, the vascularization of the focus is very important for the repair of damaged skin. Endothelial cells are the key components of blood vessels and play an important role in the process of tissue engineering vascularization.33 In the present work, endothelial cells were seeded on the surface of the benlysta-loaded SA hydrogel, the single SA hydrogel, and the control group to evaluate the vascularization ability of each group. The fluorescence images (Figure 10A) and counting results (Figure 10B) showed that both the benlysta-loaded SA hydrogel and the single SA hydrogel promote endothelial cells growth, wherein the benlysta-loaded SA hydrogel showed higher number of endothelial cells. In addition, the endothelial cells on the benlysta-loaded SA hydrogel had a higher spreading area compared to the cells on the single SA hydrogel and the control group. All these results indicated that the benlysta-loaded SA hydrogel had a better ability to improve tissue engineering vascularization.
Figure 10.
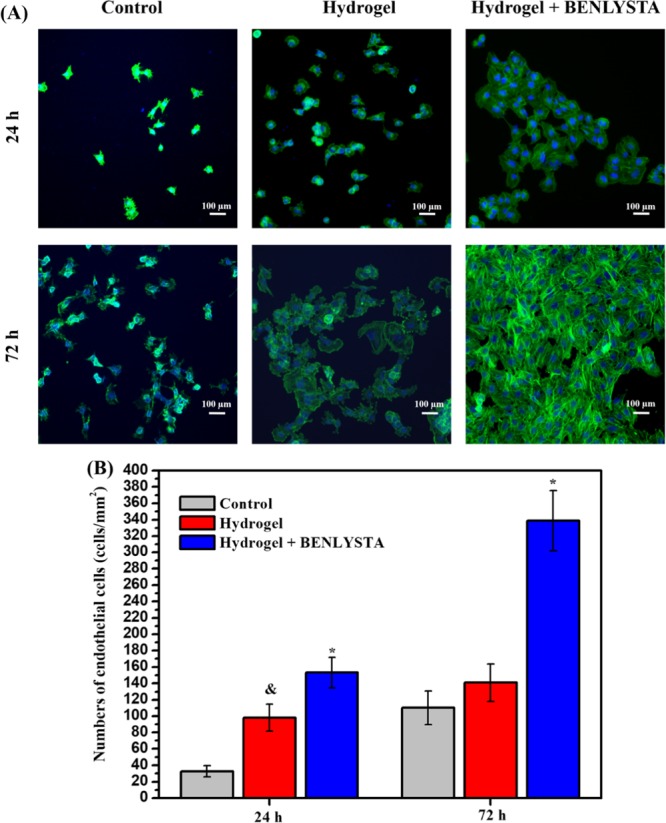
(A) Phalloidin (cytoskeleton) and 4,6-diamino-2-phenyl indole (DAPI) (nucleus) staining images and (B) counting results of endothelial cells in the benlysta-loaded SA hydrogel group and controls (*p < 0.05 compared with other samples, &p < 0.05 compared with the control group, mean ± SD, n = 3).
3. Conclusions
In this work, we prepared a benlysta-loaded SA hydrogel for anti-inflammation and promoting the growth of skin cells. The results of gelation experiment indicated that the benlysta-loaded SA hydrogel was time-dependent, and the gelation time could be controlled by the SA ratio, which promised potential application both as skin dressing and injectable subcutaneous material. The systematic material characterization also showed that the benlysta-loaded SA hydrogel had good water content, swelling rate, drug release function, and stability. Cell experiments showed that the benlysta-loaded SA hydrogel could inhibit the growth of macrophages and promote the growth of fibroblasts and skin epidermal cells. We hope this benlysta-loaded SA hydrogel may provide new ideas for treating skin injury that may be caused by external organic matter and/or major diseases.
4. Experimental Section
4.1. Preparation and Evaluation of Benlysta-Loaded SA Hydrogels
The preparation procedure of the SA hydrogels was the same as described in detail in the previous work.19,34 In that study, benlysta was introduced into a SA hydrogel solution with a concentration of 320 mg/L before adding Ca2+ to trigger gelation. The gelation-forming process and the physical map of the benlysta-loaded SA hydrogels before and after the gelation were photographed using a HUAWEI MATE 10PRO mobile phone camera.21 Material properties such as gelation time, water content, swelling rate, and degradation rate were characterized by our previous methods.19,34 To investigate the anti-inflammatory function and pro-skin cell growth function of the benlysta-loaded SA hydrogels, macrophages, fibroblasts (L929 cell line), and epidermal cells (HaCat cell line) were seeded on the benlysta-loaded SA hydrogel, the single SA hydrogel, and the control surface and cultured for 24 and 72 h.35 Then, the cells were stained with acridine orange and ethidium bromide (AO/EB) double-staining kit (Solabio) and observed by laser confocal microscopy (Nikon C2 Plus, Tokyo, Japan).36 The CCK-8 kit was used to investigate cell proliferation, and the detected OD value at 450 nm had a positive correlation with the cell number.37 To preliminarily evaluate the angiogenic ability of the benlysta-loaded SA hydrogel, endothelial cells, as an important part of blood vessels, were inoculated on the surface of the benlysta-loaded SA hydrogel, the single SA hydrogel, and the control group with a density of 4 × 104 cells/mL and then cultured under standard conditions for 24 and 72 h. After the endothelial cells had been washed with normal saline and fixed with 4% paraformaldehyde, they were stained with phalloidin and 4,6-diamino-2-phenyl indole (DAPI), and 15 pictures were taken for counting and statistics.38
4.2. Statistical Analysis
All of the values are presented as mean ± standard deviation using Origin8 software. Statistical analysis was performed by t test.
This research was funded by the National Natural Science Foundation of China (NSFC51671175), the Key Scientific and Technological Research Projects in Henan Province (grant number 182102310076), and the Top Doctor Program of Zhengzhou University (grant number 32210475).
The authors declare no competing financial interest.
References
- Qu J.; Zhao X.; Liang Y. P.; Zhang T. L.; Ma P. X.; Guo B. L. Antibacterial adhesive injectable hydrogels with rapid self-healing, extensibility and compressibility as wound dressing for joints skin wound healing. Biomaterials 2018, 183, 185–199. 10.1016/j.biomaterials.2018.08.044. [DOI] [PubMed] [Google Scholar]
- Liu J.; Chen Z. Q.; Wang J.; Li R. H.; Li T. T.; Chang M. Y.; Yan F.; Wang Y. F. Encapsulation of Curcumin Nanoparticles with MMP9-Responsive and Thermos-Sensitive Hydrogel Improves Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 16315–16326. 10.1021/acsami.8b03868. [DOI] [PubMed] [Google Scholar]
- Zheng Y. Y.; Liang Y. Q.; Zhang D. Q.; Sun X. Y.; Liang L.; Li J.; Liu Y. N. Gelatin-Based Hydrogels Blended with Gellan as an Injectable Wound Dressing. ACS Omega 2018, 3, 4766–4775. 10.1021/acsomega.8b00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H.; Zheng M.; Liu X.; Zhang S.; Wang X.; Chen Y.; Hou M.; Zhu J. Feasibility Study of Tissue Transglutaminase for Self-Catalytic Cross-Linking of Self-Assembled Collagen Fibril Hydrogel and Its Promising Application in Wound Healing Promotion. ACS Omega 2019, 4, 12606–12615. 10.1021/acsomega.9b01274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.; Chen Y.; Rehman H. U.; Chen Z.; Yang Z.; Wang M.; Li H.; Liu H. Ultratough, Self-Healing, and Tissue-Adhesive Hydrogel for Wound Dressing. ACS Appl. Mater. Interfaces 2018, 10, 33523–33531. 10.1021/acsami.8b10064. [DOI] [PubMed] [Google Scholar]
- Son Y. J.; Tse J. W.; Zhou Y.; Mao W.; Yim E. K. F.; Yoo H. S. Biomaterials and controlled release strategy for epithelial wound healing. Biomater. Sci. 2019, 7, 4444–4471. 10.1039/C9BM00456D. [DOI] [PubMed] [Google Scholar]
- Zhu Y.; Zhang J.; Song J.; Yang J.; Xu T.; Pan C.; Zhang L. One-step synthesis of an antibacterial and pro-healing wound dressing that can treat wound infections. J. Mater. Chem. B 2017, 5, 8451–8458. 10.1039/C7TB02477K. [DOI] [PubMed] [Google Scholar]
- Li H.; Yang J.; Hu X.; Liang J.; Fan Y.; Zhang X. Superabsorbent polysaccharide hydrogels based on pullulan derivate as antibacterial release wound dressing. J. Biomed. Mater. Res., Part A 2011, 98A, 31–39. 10.1002/jbm.a.33045. [DOI] [PubMed] [Google Scholar]
- Mohamad N.; Buang F.; Lazim A. M.; Ahmad N.; Martin C.; Amin M. C. I. M. Characterization and biocompatibility evaluation of bacterial cellulose-based wound dressing hydrogel: effect of electron beam irradiation doses and concentration of acrylic acid. J. Biomed. Mater. Res., Part B 2017, 105, 2553–2564. 10.1002/jbm.b.33776. [DOI] [PubMed] [Google Scholar]
- Carvalho I. C.; Mansur H. S. Engineered 3D-scaffolds of photocrosslinked chitosan-gelatin hydrogel hybrids for chronic wound dressings and regeneration. Mater. Sci. Eng., C 2017, 78, 690–705. 10.1016/j.msec.2017.04.126. [DOI] [PubMed] [Google Scholar]
- Zhang F.; Hu C.; Kong Q.; Luo R.; Wang Y. Peptide-/Drug-Directed Self-Assembly of Hybrid Polyurethane Hydrogels for Wound Healing. ACS Appl. Mater. Interfaces 2019, 11, 37147–37155. 10.1021/acsami.9b13708. [DOI] [PubMed] [Google Scholar]
- Schanuel F. S.; Santos K. S. R.; Monte-Alto-Costa A.; de Oliveira M. G. Combined nitric oxide-releasing poly(vinyl alcohol) film/F127 hydrogel for accelerating wound healing. Colloids Surf., B 2015, 130, 182–191. 10.1016/j.colsurfb.2015.04.007. [DOI] [PubMed] [Google Scholar]
- Mohandas A.; Deepthi S.; Biswas R.; Jayakumar R. Chitosan based metallic nanocomposite scaffolds as antimicrobial wound dressings. Bioact. Mater. 2018, 3, 267–277. 10.1016/j.bioactmat.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currao M.; Malara A.; Buduo C. A. D.; Abbonante V.; Tozzi L.; Balduini A. Alessandra Balduini. Hyaluronan based hydrogels provide an improved model to study megakaryocyte–matrix interactions. Exp. Cell Res. 2016, 346, 1–8. 10.1016/j.yexcr.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y.; Zhang H.; Zhang X.; Chen Z.; Zhao D.; Ma J. A comparative study of two porous sponge scaffolds prepared by collagen derived from porcine skin and fish scales as burn wound dressings in a rabbit model. Regener. Biomater. 2020, 7, 63–70. 10.1093/rb/rbz036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.; Cao X.; Jiang H.; Che X.; Xu X.; Ma B.; Zhang J.; Huang T. SIKVAV-Modified Chitosan Hydrogel as a Skin Substitutes for Wound Closure in Mice. Molecules 2018, 23, 2611. 10.3390/molecules23102611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He D.; Zhao A.; Su H.; Zhang Y.; Wang Y.; Luo D.; Gao Y.; Li J.; Yang P. An injectable scaffold based on temperature responsive hydrogel and factors loaded nano-particles for potential application of vascularization in tissue engineering. J. Biomed. Mater. Res., Part A 2019, 107, 2123–2134. 10.1002/jbm.a.36723. [DOI] [PubMed] [Google Scholar]
- Chen Y.; Sun P. pH-Sensitive Polyampholyte Microgels of Poly(Acrylic Acid-co-Vinylamine) as Injectable Hydrogel for Controlled Drug Release. Polymers 2019, 11, 285 10.3390/polym11020285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K.; Shi Z. Q.; Zhou J. K.; Xing Q.; Ma S. S.; Li Q. H.; Zhang Y. T.; Yao M. H.; Wang X. F.; Li Q.; Li J. A.; Guan F. X. Potential application of an injectable hydrogel scaffold loaded with mesenchymal stem cells for treating traumatic brain injury. J. Mater. Chem. B 2018, 6, 2982–2992. 10.1039/C7TB03213G. [DOI] [PubMed] [Google Scholar]
- Cui L.; Liang J.; Liu H.; Zhang K.; Li J. Nanomaterials for angiogenesis in skin tissue engineering. Tissue Eng., Part B 2020, 10.1089/ten.teb.2019.0337. [DOI] [PubMed] [Google Scholar]
- Xu R.; Su C.; Cui L. L.; Zhang K.; Li J. A. Preparing sodium alginate/polyethyleneimine spheres for potential application of killing tumor cells by reducing the concentration of copper ions in the lesions of colon cancer. Materials 2019, 12, 1570 10.3390/ma12091570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y.; Niu L.; Yu Z.; Wang M.; Shang Z.; Yang Y. Sodium alginate-grafted β-cyclodextrins as a matrix for immobilized Arthrobacter simplex for cortisone acetate biotransfromation. Appl. Surf. Sci. 2018, 444, 42–47. 10.1016/j.apsusc.2018.03.028. [DOI] [Google Scholar]
- Bhutani U.; Laha A.; Mitra K.; Majumdar S. Sodium alginate and gelatin hydrogels: Viscosity effect on hydrophobic drug release. Mater. Lett. 2016, 164, 76–79. 10.1016/j.matlet.2015.10.114. [DOI] [Google Scholar]
- Horák D.; Plichta Z.; Starykovych M.; Myronovskij S.; Kit Y.; Chopyak V.; Stoika R. Calf thymus histone-conjugated magnetic poly(2-oxoethyl methacrylate) microspheres for affinity isolation of anti-histone IgGs from the blood serum of patients with systemic lupus erythematosus. RSC Adv. 2015, 5, 63050–63055. 10.1039/C5RA09280A. [DOI] [Google Scholar]
- Wu M.; Zhang Y.; Liu Q.; Huang H.; Wang X.; Shi Z.; Li Y.; Liu S.; Xue L.; Lei Y. A smart hydrogel system for visual detection of glucose. Biosens. Bioelectron. 2019, 142, 111547 10.1016/j.bios.2019.111547. [DOI] [PubMed] [Google Scholar]
- Tsutsumi M.; Denda S.; Inoue K.; Ikeyama K.; Denda M. Calcium ion gradients and dynamics in cultured skin slices of rat hindpaw in response to stimulation with ATP. J. Invest. Dermatol. 2009, 129, 584–589. 10.1038/jid.2008.299. [DOI] [PubMed] [Google Scholar]
- Shin S. W.; Jang Y. D.; Ko K. W.; Kang E. Y.; Han J. H.; Bedair T. M.; Kim I. H.; Son T. I.; Park W.; Han D. K. PCL microspheres containing magnesium hydroxide for dermal filler with enhanced physicochemical and biological performances. J. Ind. Eng. Chem. 2019, 80, 854–861. 10.1016/j.jiec.2019.07.043. [DOI] [Google Scholar]
- Kimoto A.; Aoki D. A layered sensor for simultaneous measurement of skin water content and softness. Sens. Actuators, A 2014, 217, 111–115. 10.1016/j.sna.2014.06.016. [DOI] [Google Scholar]
- Téllez S. C. A.; Pereira L.; dos Santos L.; Fávero P.; Martin A. A. RM1 semi empirical and DFT: B3LYP/3-21G theoretical insights on the confocal Raman experimental observations in qualitative water content of the skin dermis of healthy young, healthy elderly and diabetic elderly women’s. Spectrochim. Acta, Part A 2015, 149, 1009–1019. 10.1016/j.saa.2015.06.110. [DOI] [PubMed] [Google Scholar]
- Xu M.; Khan A.; Wang T.; Song Q.; Han C.; Wang Q.; Gao L.; Huang X.; Li P.; Huang W. Mussel-Inspired Hydrogel with Potent in Vivo Contact-Active Antimicrobial and Wound Healing Promoting Activities. ACS Appl. Bio Mater. 2019, 2, 3329–3340. 10.1021/acsabm.9b00353. [DOI] [PubMed] [Google Scholar]
- Li H. Q.; Jiang H. B.; Yin X. Z.; Bard J. E.; Zhang B. R.; Feng J. Attenuation of PRRX2 and HEY2 enables efficient conversion of adult human skin fibroblasts to neurons. Biochem. Biophys. Res. Commun. 2019, 516, 765–769. 10.1016/j.bbrc.2019.06.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akutsu T.; Ikegaya H.; Watanabe K.; Miyasaka S. Immunohistochemical staining of skin-expressed proteins to identify exfoliated epidermal cells for forensic purposes. Forensic Sci. Int. 2019, 303, 109940 10.1016/j.forsciint.2019.109940. [DOI] [PubMed] [Google Scholar]
- Li J. A. Constructing a spatially ordered composite system on cardiovascular biomaterials surface to create preferable endothelial microenvironment. Basic Clin. Pharmacol. Toxicol. 2018, 123, 18–19. [Google Scholar]
- Zhou J. K.; Zhang K.; Ma S. S.; Liu T. F.; Yao M. H.; Li J. A.; Wang X. F.; Guan F. X. Preparing an injectable hydrogel with sodium alginate and Type I collagen to create better MSCs growth microenvironment. e-Polym. 2019, 19, 87–91. 10.1515/epoly-2019-0011. [DOI] [Google Scholar]
- Zhang K.; Bai Y. X.; Wang X. F.; Li Q.; Guan F. X.; Li J. A. Surface modification of esophageal stent materials by a polyethylenimine layer aiming at anti-cancer function. J. Mater. Sci.: Mater. Med. 2017, 28, 125 10.1007/s10856-017-5939-y. [DOI] [PubMed] [Google Scholar]
- Wang S.; Zhu S. J.; Zhang X. Q.; Li J. A.; Guan S. K. Effects of degradation products of biomedical magnesium alloys on nitric oxide release from vascular endothelial cells. Med. Gas Res. 2019, 9, 153–159. 10.4103/2045-9912.266991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. A.; Wu F.; Zhang K.; He Z. K.; Zou D.; Luo X.; Fan Y. H.; Yang P.; Zhao A. S.; Huang N. Controlling Molecular Weight of Hyaluronic Acid Conjugated on Amine-rich Surface: Towards Better Multifunctional Biomaterials for Cardiovascular Implants. ACS Appl. Mater. Interfaces 2017, 9, 30343–30358. 10.1021/acsami.7b07444. [DOI] [PubMed] [Google Scholar]
- Wu F.; Li J. A.; Zhang K.; He Z. K.; Yang P.; Zou D.; Huang N. Multi-Functional Coating Based on Hyaluronic Acid and Dopamine Conjugate for Potential Application on Surface Modification of Cardiovascular Implanted Devices. ACS Appl. Mater. Interfaces 2016, 8, 109–121. 10.1021/acsami.5b07427. [DOI] [PubMed] [Google Scholar]


