Abstract
The lateral flow immunoassay (LFIA) is the most successful point-of-care testing (POCT) method to date. In the case of clinical biomarkers that require quantification, it remains a challenge to quantitate those biomarkers using the lateral flow immunoassay remains a challenge due to the cost of the reader and possibly the type of marker used. In the present work, a new concept of a platform LFIA device configuration is proposed in which different, aligned membrane components, some already existing in the classical lateral flow immunoassay, and the others created with special new functions in the present device. As the sample containing the target analyte passes through the aforementioned membranes, the target analyte will initially interact with a target-specific antibody-conjugated to horseradish peroxidase (HRP). Thereafter, the newly formed immunocomplex will diffuse through a proprietary capture membrane (that ensures that the nontarget-bound antibodies do not continue further and thus remain “captured” to that specific area). This is done by having the target molecules (or components thereof) immobilized onto the said capture layer. The target-bound immunocomplexes will then be allowed by the system configuration to continue further to the last layer, where the signal will be generated and quantified. Thus, in the absence of the target analyte in the sample, the free antibodies will be filtered at the capture layer by preimmobilized analyte molecules, thus preventing a false positive signal to occur. We validated the concept in the detection of dengue NS1 protein in view of making a triage test. The sample containing NS1 will first meet HRP-conjugated NS1-specific antibodies and become attached, thus producing an NS1-specific antibody–HRP immunocomplex. The sample then flows through the blocking layer, where the immunocomplex is unchallenged and thus allowed to reach the last “absorbent” pad, incorporating the substrate for the HRP marker. In the case of a positive test, a signal is generated, that is proportional to the amount of immunocomplexes (and therefore the NS1 concentration), and then analyzed and measured at the absorbent pad. Any unbound anti-NS1 antibody will be stopped at the blocking matrix by preimmobilized NS1, so there will be no false positive. As this study is the initial study of a novel configuration, much of the work comprised of optimization steps, such as determining the required NS1 membrane-immobilization concentration and the required target-specific capture antibody concentration. Our immunoassay was tested with spiked buffer and serum samples to mimic the clinical conditions, with a range of NS1 concentrations, and was found, at this time, to be fivefold more sensitive than a gold standard enzyme-linked immunosorbent assay (ELISA) test (5 ng mL–1) performed in our laboratory. This method shows another form of LFIA that has the potential to be quantitative (at least semiquantitative), albeit not solving the reader cost; however, unlike the regular LFIA, we do not use nanobeads but instead enzymes, allowing, in theory, greater sensitivity, while retaining the one-step procedure. The test is accurate and has low production costs.
Introduction
Historical Context
Diagnostics is an essential component of healthcare, enabling the physician to provide proper treatment to the patient. There is a trend toward increasing the use of point-of-care tests (POCT) so as to increase the availability, convenience, speed to test results, and treatment. However, these sometimes have drawbacks, which need to be solved, especially quantitation, cost, and, in some cases, multiplex testing.1 The world of lateral flow immunoassay is filled with patent families, owned by diagnostic companies; however, most seem to be improvements rather than the creation of novel configurations. The present study supports the later attempt and is a daughter study of our previous StackPad work, where the concept of the capture layer was established.2,3
Application
Quantitation of protein-based biomarkers has been proven to be effective in predicting the presence and severity of various clinical disorders.4 However, because of the gap in technology, in some cases, there is difficulty in putting quantitation into practice. Such is the case of dengue fever (DF).
Dengue is an important arthropod-borne (mostly mosquitoes and ticks) viral infection of humans.5,6 Although vaccination and vector control attempts are being made, dengue continues to spread globally and emerge in new areas.7 According to estimations, more than 50 million infections occur annually. Of them, 500 000 are hospitalized for dengue hemorrhagic fever (DHF), mainly among children, and with a case fatality rate exceeding 5% in some areas.6 The group progressing from nonsevere to severe disease is difficult to define. Triage, appropriate treatment, and the decision as to where this treatment should be given (in a healthcare facility or at home) are influenced by the case classification for dengue8,9 and may prevent these patients from developing more severe clinical conditions.10,11 Symptomatic dengue virus infections were grouped into three categories: undifferentiated fever, dengue fever (DF), and dengue hemorrhagic fever (DHF).6
Currently, there is difficulty in the diagnosis of DF, and efficient and accurate diagnosis of dengue is of primary importance for clinical care.6,12−14 The popular methods for dengue detection being used include virus isolation,14,15 nucleic acid amplification test (NAAT) for viral RNA detection,14−16 enzyme-linked immunosorbent assay (ELISA) for antigen,14,16,17 IgM or IgG detection,12,14,18 and traditional lateral flow assay for antigen.19,20 Although these methods have the potential to detect DF, they still have disadvantages whether it is the need for trained personnel to operate, inaccessible in remote locations, lack of specificity,12,18,21−23 inability to provide a quantitative result at an affordable cost.24 There is an urgent need for a robust, cost-effective, point-of-care diagnostics tool that is easy to use for the detection and classification of DF. Our method achieves the mentioned specifications by combining immunoassay, lateral flow technologies, and affinity chromatography.
Dengue NS1 (nonstructural) can be detected in a patient’s serum and allow for differentiation of dengue infections from other infections such as Zika, Yellow fever, West Nile, or others with a specificity of over 94%21,22,25 and indicate the severity of the dengue infection by correlating with the amount of dengue NS1 protein. It was previously suggested that the amount of NS1 correlates to the severity of the infection. In a study, a threshold of 600 ng mL–1 was found as a threshold for the classification of DHF.17 Thus, the detection and quantification of NS1 could provide essential information for medical providers and patients to assess the severity and determine the form of treatment.
The gold standard for the quantitation of proteins in a clinical environment is the ELISA method. However, traditional ELISA requires trained personnel and expensive and central hospital setup.26 Efforts for the development of technology for the detection and quantification of proteins in point-of-care (POC) tests are constantly being made. However, this challenge is still ongoing. The most commonly used POC device is the lateral flow assay (LFA). This method utilizes a paper-based platform to carry the immunoreagents through the required steps to obtain indications to the presence of a protein. The LFA is a well-established technology when applied to POCT and field-use applications.
In this work, a novel design of a point-of-care testing device for the detection of biological analytes is presented. Pads of different materials were layered one next to the other to bring the sample through the setup.
The unique novel capture layer and configuration of reagents allows the construction of a platform for the sample diagnosis in a one-step procedure using a substrate for the enzyme reporter. This arrangement is shared and was inspired by a previous study2,3 and is a logical continuum of this study.
Materials and Methods
Reagents
Phosphate-buffered saline (PBS) tablets (cat. no. P4417), 3-(glycidoxypropyl)trimethoxysilane (GPTMS) (440167, 98% (v/v)), and sodium m-periodate (S1878) were purchased from Sigma-Aldrich. PBS–0.05% (v/v) Tween (PBST) was prepared by adding 0.5 mL of Tween-20 solution (cat. no. P7949) to 1 L of PBS buffer. The 5% (w/v) skim milk (SM) solution was prepared by adding 5 g of SM powder (70166) to 100 mL of PBST solution. Milli-Q ultrafiltered (UF) H2O (with a resistivity of 18.2 MΩ cm at 25 °C) was used in the preparation of all the solutions. The luminol–H2O2 substrate solution (ratio 1:1) (cat. no. 1705040, BioRad) and methyl alcohol (136805) were purchased from Bio-Lab (Israel). Acetic acid (45731, 99.8% (v/v)) was purchased from Fluka. Hydrochloric acid (7647010, 37% (v/v)) and hydrogen peroxide solution (7722841, 35%(v/v)) were purchased from Acros Organic.
Immunoreagents
Dengue NS1 protein (His tag) (Fitzgerald, cat. no. 80-1348) was purchased from Tarom, Israel. Mouse monoclonal antidengue virus, NS1 antibody (IgG), with conjugated horseradish peroxidase (HRP) enzyme (USBiological, cat. no. 143056-HRP) was purchased from Biotest, Israel.
Device Fabrication
Membranes
Conjugate release matrix (cat. no. PT-R5) and absorbent (cat. no. AP-080) pads were purchased from Advanced Microdevices Pvt. Ltd. (India).
Assay Rational
The proposed setup is composed of layers made of materials previously used for diagnostic purposes. At the head of the device, a sample pad will collect the tested sample. Next, a conjugate release matrix has an anti-NS1-HRP. Then, there is a capture layer of a functionalized conjugate matrix with covalently bound dengue NS1 proteins. At the other end, an absorbent pad with a dried substrate generates the signal. The scheme of the design (Figure 1) is such that the putative sample meets the first layer to even the flow rate, and the NS1 in the sample passes to the next functional membrane where the corresponding anti-NS1-HRP conjugates with the NS1 protein to create an NS1-anti-NS1-HRP immunocomplex. This immunocomplex then traverses to a functionalized filter like a capture layer containing the preattached NS1 protein; the immunocomplex formed will pass unreacted and reach the final layer where a dried substrate awaits to react with the reporter and create a readable light signal. In the case of an NS1 free sample, the anti-NS1-HRP will remain available to link to the preattached NS1 in the capture layer. Thus, a false positive signal is prevented, and no signal will be generated at the final layer.
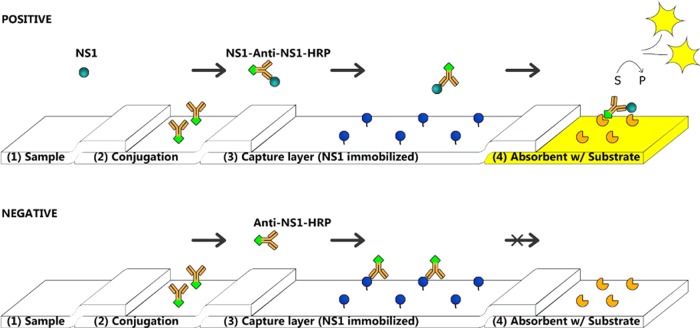
Figure 1.
Schematic presentation of the capture flow assay biosensor. It consists of membranes with specific applications for each. (1) Pad for the collection of a liquid sample. (2) Pad with an antianalyte bioreporter molecule linked to some marker. (3) Blocking pad, with preimmobilized analyte. (4) Measuring pad with a specific substrate. There are two main possibilities that can happen during measurements. In the first test (upper), the sample with a target analyte (NS1), after deposition on the pad (1), will be connected to bioreporter molecules (in this case, antibodies–HRP attached). The complex will then migrate inertly through a pad (3) and reach pad (4) to produce a measured signal since the complex is already formed. The sample without the target analyte (lower) will migrate from the pad (2) and then move unbound antibodies (in this case) to the capture layer (3), where they will be linked to the preimmobilized analyte and stopped from migrating to the next pad. Thus, no visible signal will be observed.
Assay Reagent Immobilization Procedures
Substrate pads were made by cutting 40 × 5 mm2 pads from the absorption pads, wetting with 300 μL of luminol–H2O2 substrate solution (ratio 1:1) (cat. no. 1705040, BioRad), and drying for 2 h at 37 °C in the dark. Conjugation pads (10 × 3 mm2) were prepared by wetting pads with 35 μL of antidengue NS1 antibodies–HRP conjugated (diluted with PBS (0.203 g L–1 NaH2PO4, 1.149 g L–1 Na2HPO4, 8.5 g L–1 NaCl) (pH 7.2)) and dried for 20 min at 37 °C. Sample pads were made by cutting 10 × 5 mm2 of empty conjugation pads. The conjugate pads were kept at room temperature until used later the same day. The blocking layer was prepared similarly to the reference protocol described by Liebes et al.27 and Algaar et al.28 Briefly, the conjugate release matrix (cat. no. PT-R5) was exposed to methanol/97% HCl solution for 20 min, cleaned by sonication in DDW for 20 min and treated with 7:3 [v/v] 97% HCl/H202 solution for 10 min at 90 °C to produce surface hydroxyl groups, rinsed with nanopure water, and then dried with nitrogen gas. The membrane surfaces were silanized with (3-glycidoxypropyl)trimethoxysilane for 60 min at 60 °C and then treated with 11.6 mM hydrochloric acid for 60 min at 50 °C (formation of vicinal diols) and 100 mM sodium m-periodate dissolved in 10% (v/v) acetic acid for 60 min at room temperature without exposure to light (oxidation to aldehyde). Blocking layer activation was done up to this point inside a chemical hood for safety. Membranes were then rinsed with deionized water and incubated with 10 mL of 200 ng mL–1 NS1 overnight at 4 °C. The next day, the membranes were washed 3 times using PBST for 5 min each and dried for 40 min at RT.
Assembly of the Membrane-Based Immunoassay Setup
The immunoassay was assembled by placing all prepared pads similar to the traditional lateral flow immunoassay as previously described (Figure 1). The sample, conjugated, blocking, and absorbent (substrate) pads were placed one next to the other with roughly 1 mm overlapping in that order.
Optimization Steps
To assess the potential blocking ability using higher immobilized NS1 protein concentration to prevent a false-positive response in detection, 5 × 15 mm2 conjugate pads were treated according to the protocol previously described (2.4) and incubated overnight with increasingly higher concentrations of NS1 (0, 100, 500, 1000, 1500, 2000 ng mL–1). Next, the pads were washed three times in PBST, incubated with anti-NS1 antibodies-HRP attached 1/15 000 dilution in PBS for 1 h in RT, and washed again three times in PBST. One hundred microliters of luminol–H2O2 was added to each strip (size 0.5 × 1.5 cm2) and then light images were taken using a CCD camera.
The second optimization step included the determination of antibody–HRP concentration. The antibody–HRP concentration is directly related to the credibility of the platform. If the concentration is too high, the blocker layer might overflow and a false positive will occur. When the concentration is too low, the signal will diminish and there is a loss of sensitivity in detection. The setup was prepared, as mentioned in the Assay Reagents Immobilization Procedure section, with different concentrations of anti-NS1-HRP (52, 65, 87, 130 ng mL–1) in PBST 0.05% v/v. Then, the setup was tested once with a negative sample of 360 μL containing PBS clean of NS1 protein and once with a positive sample of 360 μL containing PBS spiked with NS1 in a concentration of 1500 ng mL–1.
Sensitivity Test in Optimized Membrane Setup
After optimization, the sensitivity of membrane-based setup to dengue NS1 was tested and compared to the sensitivity achieved by ELISA. Both methods were tested against the samples of PBS spiked for different concentrations of dengue NS1 (1, 5, 25, 125, 600, 3000 ng mL–1). All immobilization procedures and pad preparation have been previously described in the Assay Reagents Immobilization Procedure section. The setup design was explained in the Assembly of the Membrane-Based Immunoassay Setup section. Three hundred sixty microliters of the samples was applied slowly above the sample pad; after 5 min, measurements were taken using a CCD camera. Each new repetition for all different NS1 concentrations was made on a different day (n = 3). Fit for linear response between the signal strength and the NS1 concentration in the samples was computed between 5 and 600 ng mL–1.
Spiked Serum Sample
Clinical serum samples were tested using the capture flow assay for the presence of dengue NS1. Forty microliters of each sample was diluted with PBS to 360 μL and then used in the setup. The light reaction was recorded using a CCD camera and analyzed by ImageJ.
Measurement Procedure and Data Analysis
The light signal produced was captured using a CCD camera (Retiga-SRV FAST 1394, InterFocus, U.K.) with QCapture pro software. The CCD camera was placed 30 cm above the testing assay, and a picture of 1.5 min exposure time was taken. Measurements were performed 10 min after the sample was applied onto the sample pad. Each measurement was saved as a TIF format file and the total light intensity was measured for each repetition using ImageJ software (US National Institutes of Health).
Results and Discussion
In this work, a novel approach combining the use of a lateral flow membrane-based platform and a unique rearrangement of the reagents in the membranes was tested. It was inspired by a previous study of our group.2,3 This unique rearrangement allows the use of an enzyme reporter and still requires only one step to operate (without an additional extraction, separation, and substrate addition steps). Additionally, a silanization immobilization protocol was tested to functionalize a polyester matrix with an analyte protein. The shown configuration used polyester as a supportive membrane for all of the immunoreactions. This material has properties that greatly benefit the technology since it is a very robust matrix and can be easily shaped to fit different volumes while the linked analyte is safely bound to the matrix. This new method may lead to further developments of other new techniques.
Optimization and Characterization Steps
As described above, the proposed system is constructed from different membranes that are connected together to provide continuous sampled migration from one side to the other. After the sample has been added, it diffuses through the conjugate membrane and releases antibody–HRP molecules to the capture layer. The latter layer plays an important role in preventing further migration of any antibody–HRP molecules that did not meet the target analytes, which would otherwise move onto the absorbent pad and produce a false response. This membrane gives robustness to the platform in a manner similar to that of an affinity column; and much like an affinity column, this layer’s ability to capture antibodies depends on the affinity of the antibodies to the preimmobilized NS1, the quantity of NS1 molecules on the surface, and the strength of NS1 binding to the matrix.29 As described in the section Assay Reagent Immobilization Procedures, the NS1 proteins are covalently bound to the layer’s surface using a silanization binding protocol.27,28,30 In the binding procedure, GPTMS doubles as a cross-linker and a spacer so as to increase the NS1 protein’s availability to interact with the flowing antibodies. Moreover, the strong covalent bond contributes to the prevention of NS1 leakage.
At the first optimization stage, the effect of increasing the concentration of the immobilized capture agent on the membrane’s potential to capture antibodies was tested.
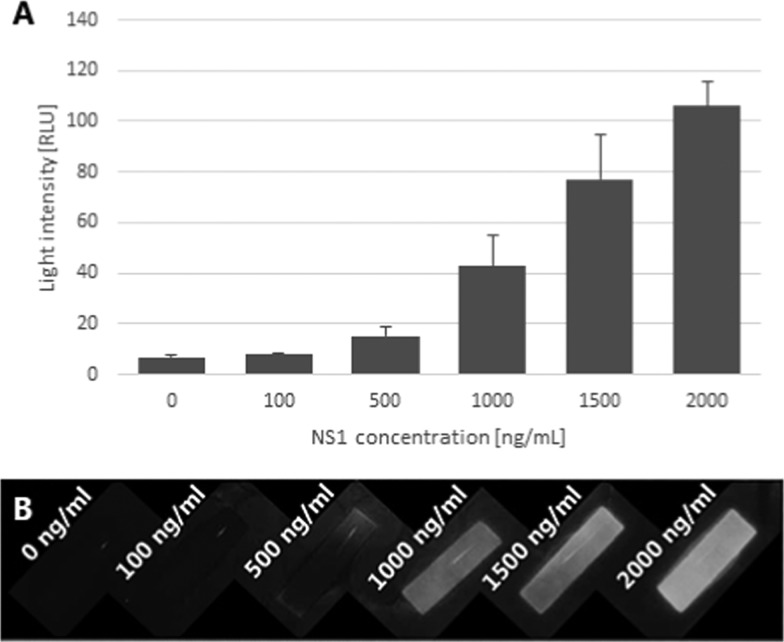
The amount of capturing agent (dengue NS1 protein), immobilized onto the blocking layer, affects the blocking layer potential to filter unbound antibodies in a dose-dependent manner, as shown in Figure 2. With higher concentrations of NS1 protein immobilized, there is an increase in the signal formed by the reporter on the anti-NS1-HRP complex, which, in turn, shows a higher attachment of antibodies onto the NS1 protein of the membrane. It appears that the increase in the light intensity occurs within the whole tested range (up to 2000 ng mL–1).
Figure 2.
Effect of NS1 concentration on immobilization efficiency. From left to right, blocker with increased NS1 concentration incubated in 0, 100, 500, 1000, 1500, 2000 ng mL–1: (A) numerical presentation of a signal generated with ImageJ. (B) Photograph was taken using the CCD camera, n = 4.
The platform’s function depends on the ability of the capture membrane to prevent free antibody reporter from reaching the absorbent pad. If the number of free antibodies is too great for the capture layer to hold, then those free antibodies will reach the absorbent pad and generate a false-positive signal. As seen in Figure 2, the potential of the capture layer to filter out the free antibodies is dependent on the quantity of the capturing agent (in this case, dengue NS1 protein). As more NS1 proteins are immobilized onto the membrane, more antibodies could be filtered. However, as our goal is to detect a small amount of analyte, it follows that the amount of antibodies will also be minimal, so there is no need for an extreme concentration of capture agents on the surface. It is important to note that the potential of the capture layer was tested by incubating the antibodies with the membrane so as to reach an equilibrium between the antibodies and proteins. However, in a flow-based interaction, the time of reagents is limited. Higher interaction will occur with higher concentrations of both immunoreagents.31
Ideally, it is reasonable to assume that higher amounts of capturing agent would ensure minimal false positives and maximum capturing ability of the antibodies; however, to reduce the production cost, it is preferable to immobilize the efficient minimum required. In later experiments, a concentration of 200 ng mL–1 was used.
The next step in the development of the assay was the optimization of the number of antibodies on the conjugate pad. The antibodies awaiting NS1 in the sample determine the potential sensitivity of the setup. Optimal antibody’s concentration allows their higher conjugation efficiency to the NS1 in the sample. Such an efficiency not only generates strong response signals but also prevents uncontrolled migration of the unbound antibodies through the capture layer, which would proceed to generate false-positive responses.
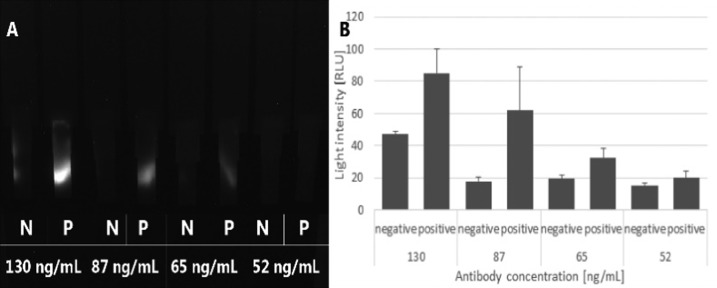
In the concentration range of the tested antibodies (52–130 ng mL–1), dose-dependent responses were observed (Figure 3). Increasing the antibody’s concentration induced light responses. However, the negative signal remains constant up to a concentration of 87 ng mL–1; this might be referred to as a background noise caused by limitations of the measurement instruments. At a concentration of 130 ng mL–1, there is an increase in the signal even in the negative test, which is, in fact, a false-positive signal caused by the saturation of the capture layer. This means that in the current configuration, the capture layer was able to hold around 3.0 ng of free-anti-NS1-HRP complexes from reaching the absorbent pad. The number of free antibodies passing the negative test can be calculated by subtracting 87 from 130 ng mL–1. This optimization step suggests that the highest number of antibodies that may have generated a false signal was 43 ng mL–1. However, the light intensity is much higher than the positive signal generated even at 52 ng mL–1. This may be explained by the fact that not all of the antibodies are being released from the conjugate pad, so the positive signal generated at 52 ng mL–1 is caused by fewer antibodies. It should be mentioned that the antibodies used here are native IgG molecules and may have more than one valency, which may cause the capture of attached antibodies and produce a false-negative reaction. In the current setup, it does not seem to induce such an undesirable result in the range of concentrations that were tested. However, in adapting the concept to different analytes, the use of monovalent fragments of IgG should be considered.
Figure 3.
Effect of concentrations of antibodies on signal resolution: (A) photograph taken using a CCD camera and (B) numerical presentation of the signal generated with ImageJ, n = 3.
Under the current configuration, the concentration of 87 ng mL–1 was chosen as the optimized concentration for the highest sensitivity and lowest noise. Further experiments were conducted using this concentration.
Sensitivity Test in Optimized Membrane Setup
The assessment of the current optimized setup was made by comparing it with the ELISA test, which is a gold standard for the quantification of protein biomarkers26,32 and is used extensively for the quantification of dengue NS1 protein in a clinical environment.33,34
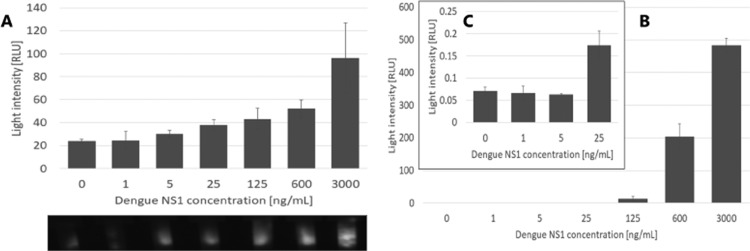
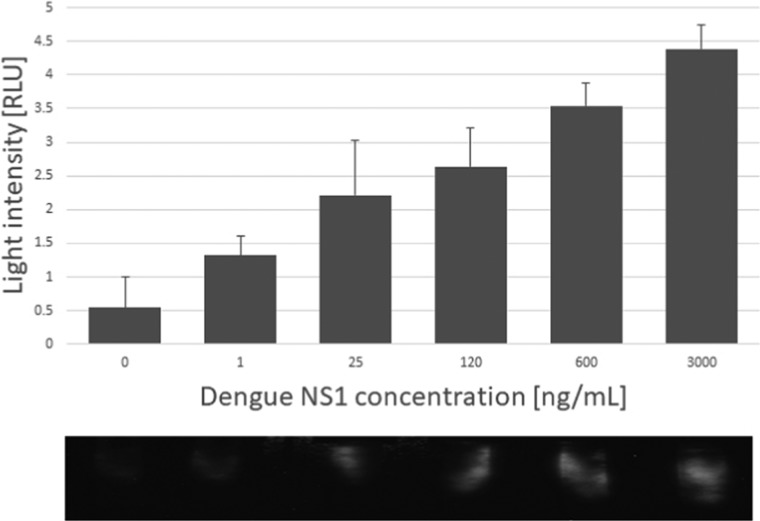
Reaction to the range of NS1 protein is clearly dose-dependent; the lowest concentration detected using our setup was 5 ng mL–1 (Figure 4A), which is 5 times lower than the sensitivity limit reached with ELISA (5 ng mL–1) (Figure 4B,C). The increase in sensitivity may be caused by the design of the ELISA test where the active area is only the solid interface, where the capture antibodies lie, and the rest of the test volume, containing the analyte, is lost.35−37 Here, in the capture-layer lateral flow assay, all of the sample volumes are in contact with the antibodies, so potentially all of the analyte proteins should connect, and all should generate a signal. As mentioned, the threshold found in the literature for differentiating between a mild case of dengue and dengue hemorrhagic fever is 600 ng mL–1 of NS1 in the patient’s serum.17,33,34 A linear fit for response in the capture-layer lateral flow was built in the range between 5 and 600 ng mL–1 with R2 = 0.9921 (data not shown), compared to R2 = 0.6539 in the ELISA test. However, a linear fit for the ELISA test in the range between 125 and 3000 ng mL–1 produced R2 = 0.9883, showing that the capture-layer lateral flow produces more linear results in lower concentration than that in the ELISA method.
Figure 4.
(A) Response of the setup to samples spiked with NS1. (B) Chemiluminescence test with ELISA to different NS1 concentrations. (C) Enlargement of (B) showing the concentration range 0–25 ng mL–1. Dengue NS1, n = 3.
The sensitivity test used serum sample. In the clinical setting, the dengue NS1 biomarker is detected in the patient’s serum, so it is crucial for the platform to perform with the serum sample.6 The platform was tested for the serum spiked with NS1 protein in the range of concentrations.
Detection in a serum sample might show difficulties because of proteins, antibodies, sugars, cholesterol, and other contents.38−41 These materials may interfere with the correct interaction between the antibodies and antigens in the platform. To reduce the interfering influence, it is sometimes customary to dilute the serum sample.42,43 As shown in Figure 5, the performance of the setup was not affected using a serum sample compared to the PBS buffer. It shows a clear dose-dependent response to the presence of dengue NS1 in the putative sample; also, the sensitivity of the platform was maintained even for testing serum samples. A linear fit for response in the range between 1 and 300 ng mL–1 was built with R2 = 0.9798 (data not shown).
Figure 5.
Response of the setup to serum samples spiked with NS1, n = 3.
There are other antibody-based platforms that can detect the dengue NS1 protein, some studies even demonstrate the use of enzymatic enhancement to increase the sensitivity by up to 10-fold compared to those of gold nanoparticles. However, not all of them reach the sensitivity shown in the paper; they require more than one step to operate, usually substrate addition;42,44−49 they need sophisticated machinery;34 or they are able to give qualitative but not quantitative50,51 results.
Conclusions
In the present work, we present a new approach for the lateral flow immunoassay platform. All membranes in the platform are common and affordable. A third membrane, which is traditionally a test membrane and contains test and control lines, was used here as a functionalized capture layer designed to filter the molecules (free-anti-dengue NS1-HRP in this case) out of stream, and a fourth membrane doubled as the test pad, with a dried substrate, where the signal is produced and also provides a pulling force for the liquid sample. This concept was tested and found to be useful for the detection of dengue NS1 protein, but it can easily be adapted for the detection of other analytes. The unique configuration allows for the use of substrate in a one-step LFA and so facilitates quantitation in LFA.
Acknowledgments
The authors wish to thank Kamin funding source [8771221] for their generous support.
Author Contributions
T.A. and R.S.M. conceived the presented original idea. T.A. performed the experimental research, including working out the technical details. T.A., E.E., and R.S.M. verified the analytical methods. R.S.M. supervised, helped plan and encourage T.A. to investigate the various aspects of the study, and supervised the findings of this work. All authors provided critical feedback, discussed the results, helped shape the research, and contributed to the final manuscript.
The Kamin funding source had no role in the design, conduct, and execution of the empirical research, collection of the data, data analyses, interpretation, or manuscript writing. It is a governmental institution with no direct commercial conflicting interests. It retained the role of periodic report supervision and final decision in funding continuation.
The authors declare the following competing financial interest(s): The authors all declare that they do not have any conflicts of interest to declare, including financial, non-financial interests, relationships, direct employment nor stock ownership with a private sector entity connected to the present study, intellectual property or honoraria. The present study does form part of a patent filed in the name of two of the authors (TA, RM) in the ownership of their institution, including a third-party patent (PCT/SG2016/050578) (EE, RM).
Notes
The field of lateral flow immunoassay tests is cluttered with numerous patents listing many claims that are subject to corporate conflicts but also support the investment and creation of a continuum of new products in different application areas. Our study strives to provide, as indicated in the “Innovation” and “Significance of the study” sections, alternative properties resulting in new claims. Furthermore, this study finds that it depends on our previous “CAPTURE FLOW ASSAY DEVICE AND METHODS” patent BGU-P-085-USP, which acts as a third-party patent.
References
- Urusov A. E.; Zherdev A. V.; Dzantiev B. B. Towards Lateral Flow Quantitative Assays: Detection Approaches. Biosensors 2019, 9, 89 10.3390/bios9030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzov E.; Marks R. S. Miniaturized Flow Stacked Immunoassay for Detecting Escherichia coli in a Single Step. Anal. Chem. 2016, 88, 6441–6449. 10.1021/acs.analchem.6b01034. [DOI] [PubMed] [Google Scholar]
- Eltzov E.; Marks R. S. Colorimetric stack pad immunoassay for bacterial identification. Biosens. Bioelectron. 2017, 87, 572–578. 10.1016/j.bios.2016.08.044. [DOI] [PubMed] [Google Scholar]
- Boschetti E.; D’Amato A.; Candiano G.; Righetti P. G. Protein biomarkers for early detection of diseases: The decisive contribution of combinatorial peptide ligand libraries. J. Proteomics 2018, 188, 1–14. 10.1016/j.jprot.2017.08.009. [DOI] [PubMed] [Google Scholar]
- Mairuhu A. T.; Wagenaar J.; Brandjes D. P.; van Gorp E. C. Dengue: an arthropod-borne disease of global importance. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 425–33. 10.1007/s10096-004-1145-1. [DOI] [PubMed] [Google Scholar]
- WHO Guidelines Approved by the Guidelines Review Committee. In Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition; World Health Organization: Geneva, 2009. [PubMed] [Google Scholar]
- Bhatt S.; Gething P. W.; Brady O. J.; Messina J. P.; Farlow A. W.; Moyes C. L.; Drake J. M.; Brownstein J. S.; Hoen A. G.; Sankoh O.; Myers M. F.; George D. B.; Jaenisch T.; Wint G. R. W.; Simmons C. P.; Scott T. W.; Farrar J. J.; Hay S. I. The global distribution and burden of dengue. Nature 2013, 496, 504–507. 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalayanarooj S.; Rothman A. L.; Srikiatkhachorn A. Case Management of Dengue: Lessons Learned. J. Infect. Dis. 2017, 215, S79–s88. 10.1093/infdis/jiw609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco L. R.; Leo Y. S.; Cook A. R.; Lee V. J.; Thein T. L.; Go C. J.; Lye D. C. Predictive Tools for Severe Dengue Conforming to World Health Organization 2009 Criteria. PLoS Neglected Trop. Dis. 2014, 8, e2972 10.1371/journal.pntd.0002972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morens D. M. Dengue fever and dengue hemorrhagic fever. Pediatr. Infect. Dis. J. 2009, 28, 635–6. 10.1097/INF.0b013e3181afcd5b. [DOI] [PubMed] [Google Scholar]
- Heilman J. M.; De Wolff J.; Beards G. M.; Basden B. J. Dengue fever: a Wikipedia clinical review. Open Med. 2014, 8, e105–e115. [PMC free article] [PubMed] [Google Scholar]
- Senanayake S. Dengue fever and dengue haemorrhagic fever--a diagnostic challenge. Aust. Fam. Physician 2006, 35, 609–612. [PubMed] [Google Scholar]
- Gómez-Dantés H.; Willoquet J. R. Dengue in the Americas: challenges for prevention and control. Cadernos de Saúde Pública 2009, 25, S19–S31. 10.1590/S0102-311X2009001300003. [DOI] [PubMed] [Google Scholar]
- Kumar R. Evaluation of diagnostic tests. Clin. Epidemiol. Global Health 2016, 4, 76–79. 10.1016/j.cegh.2015.12.001. [DOI] [Google Scholar]
- Guzmán M. G.; Kourí G. Dengue diagnosis, advances and challenges. Int. J. Infect. Dis. 2004, 8, 69–80. 10.1016/j.ijid.2003.03.003. [DOI] [PubMed] [Google Scholar]
- Hoang L. T.; Lynn D. J.; Henn M.; Birren B. W.; Lennon N. J.; Le P. T.; Duong K. T.; Nguyen T. T.; Mai L. N.; Farrar J. J.; Hibberd M. L.; Simmons C. P. The early whole-blood transcriptional signature of dengue virus and features associated with progression to dengue shock syndrome in Vietnamese children and young adults. J. Virol. 2010, 84, 12982–12994. 10.1128/JVI.01224-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libraty D. H.; Young P. R.; Pickering D.; Endy T. P.; Kalayanarooj S.; Green S.; Vaughn D. W.; Nisalak A.; Ennis F. A.; Rothman A. L. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J. Infect. Dis. 2002, 186, 1165–1168. 10.1086/343813. [DOI] [PubMed] [Google Scholar]
- Houghton-Triviño N.; Montana D.; Castellanos J. Dengue-yellow fever sera cross-reactivity; challenges for diagnosis. Revista Salud Publica 2008, 10, 299–307. 10.1590/S0124-00642008000200010. [DOI] [PubMed] [Google Scholar]
- Linares E. M.; Kubota L. T.; Michaelis J.; Thalhammer S. Enhancement of the detection limit for lateral flow immunoassays: Evaluation and comparison of bioconjugates. J. Immunol. Methods 2012, 375, 264–270. 10.1016/j.jim.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Yen C.-W.; de Puig H.; Tam J. O.; Gómez-Márquez J.; Bosch I.; Hamad-Schifferli K.; Gehrke L. Multicolored silver nanoparticles for multiplexed disease diagnostics: distinguishing dengue, yellow fever, and Ebola viruses. Lab Chip 2015, 15, 1638–1641. 10.1039/C5LC00055F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang J.; Chia P. Y.; Lye D. C.; Leo Y. S. Progress and Challenges towards Point-of-Care Diagnostic Development for Dengue. J. Clin. Microbiol. 2017, 55, 3339–3349. 10.1128/JCM.00707-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulin E.; Selby K.; Cherpillod P.; Kaiser L.; Boillat-Blanco N. Simultaneous outbreaks of dengue, chikungunya and Zika virus infections: diagnosis challenge in a returning traveller with nonspecific febrile illness. New Microbes New Infect. 2016, 11, 6–7. 10.1016/j.nmni.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low J. G. H.; Ong A.; Tan L. K.; Chaterji S.; Chow A.; Lim W. Y.; Lee K. W.; Chua R.; Chua C. R.; Tan S. W. S.; Cheung Y. B.; Hibberd M. L.; Vasudevan S. G.; Ng L.-C.; Leo Y. S.; Ooi E. E. The early clinical features of dengue in adults: challenges for early clinical diagnosis. PLoS Neglected Trop. Dis. 2011, 5, e1191. 10.1371/journal.pntd.0001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoudi T.; de la Guardia M.; Baradaran B. Lateral flow assays towards point-of-care cancer detection: A review of current progress and future trends. TrAC, Trends Anal. Chem. 2020, 125, 115842 10.1016/j.trac.2020.115842. [DOI] [Google Scholar]
- Matheus S.; Boukhari R.; Labeau B.; Ernault V.; Bremand L.; Kazanji M.; Rousset D. Specificity of Dengue NS1 Antigen in Differential Diagnosis of Dengue and Zika Virus Infection. Emerging Infect. Dis. 2016, 22, 1691–1693. 10.3201/eid2209.160725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed N. H.; Broor S. Comparison of NS1 antigen detection ELISA, real time RT-PCR and virus isolation for rapid diagnosis of dengue infection in acute phase. J. Vector Borne Dis. 2014, 51, 194–199. [PubMed] [Google Scholar]
- Liebes Y.; Amir L.; Marks R. S.; Banai M. Immobilization strategies of Brucella particles on optical fibers for use in chemiluminescence immunosensors. Talanta 2009, 80, 338–345. 10.1016/j.talanta.2009.06.070. [DOI] [PubMed] [Google Scholar]
- Algaar F.; Eltzov E.; Vdovenko M. M.; Sakharov I. Y.; Fajs L.; Weidmann M.; Mirazimi A.; Marks R. S. Fiber-Optic Immunosensor for Detection of Crimean-Congo Hemorrhagic Fever IgG Antibodies in Patients. Anal. Chem. 2015, 87, 8394–8398. 10.1021/acs.analchem.5b01728. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P.; Wilchek M.; Anfinsen C. B. Selective enzyme purification by affinity chromatography. Proc. Natl. Acad. Sci. U.S.A. 1968, 61, 636–643. 10.1073/pnas.61.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldini F.; Bracci S. Optical-fibre sensors by silylation techniques. Sens. Actuators, B 1993, 11, 353–360. 10.1016/0925-4005(93)85275-F. [DOI] [Google Scholar]
- Hulme E. C.; Trevethick M. A. Ligand binding assays at equilibrium: validation and interpretation. Br. J. Pharmacol. 2010, 161, 1219–1237. 10.1111/j.1476-5381.2009.00604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl P. F.; Turner P. C.; Sutcliffe A. E.; Sylla A.; Diallo M. S.; Friesen M. D.; Groopman J. D.; Wild C. P. Quantitative comparison of aflatoxin B1 serum albumin adducts in humans by isotope dilution mass spectrometry and ELISA. Cancer Epidemiol., Biomarkers Prev. 2006, 15, 823–826. 10.1158/1055-9965.EPI-05-0890. [DOI] [PubMed] [Google Scholar]
- Paranavitane S. A.; Gomes L.; Kamaladasa A.; Adikari T. N.; Wickramasinghe N.; Jeewandara C.; Shyamali N. L.; Ogg G. S.; Malavige G. N. Dengue NS1 antigen as a marker of severe clinical disease. BMC Infect. Dis. 2014, 14, 570 10.1186/s12879-014-0570-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes P.; Watterson D.; Parmvi M.; Burger R.; Boisen A.; Young P.; Cooper M. A.; Hansen M. F.; Ranzoni A.; Donolato M. Quantification of NS1 dengue biomarker in serum via optomagnetic nanocluster detection. Sci. Rep. 2015, 5, 16145 10.1038/srep16145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baid A. ELISA - a mini review. J. Pharm. Anal. 2016, 118–125.29404026 [Google Scholar]
- Engvall E.; Perlmann P. The enzyme-linked immunosorbent assay (ELISA). Bull. World Health Organ. 1976, 54, 129–139. [PMC free article] [PubMed] [Google Scholar]
- Aydin S. A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides 2015, 72, 4–15. 10.1016/j.peptides.2015.04.012. [DOI] [PubMed] [Google Scholar]
- Nissum M.; Foucher A. L. Analysis of human plasma proteins: a focus on sample collection and separation using free-flow electrophoresis. Expert Rev. Proteomics 2008, 5, 571–587. 10.1586/14789450.5.4.571. [DOI] [PubMed] [Google Scholar]
- Stewart G. N. The relations of the electrolytes to the non-electrolytes in the blood-corpuscles and blood-serum. J. Boston Soc. Med. Sci. 1897, 1, 18–21. [PMC free article] [PubMed] [Google Scholar]
- Shope R. E. Sugar and cholesterol in the blood serum as related to fasting. J. Biol. Chem. 1927, 75, 101–113. [Google Scholar]
- Sesardic D.; Corbel M. J. Testing for neutralising potential of serum antibodies to tetanus and diphtheria toxin. Lancet 1992, 340, 737–738. 10.1016/0140-6736(92)92284-M. [DOI] [PubMed] [Google Scholar]
- Young P. R.; Hilditch P. A.; Bletchly C.; Halloran W. An antigen capture enzyme-linked immunosorbent assay reveals high levels of the dengue virus protein NS1 in the sera of infected patients. J. Clin. Microbiol. 2000, 38, 1053–1057. 10.1128/JCM.38.3.1053-1057.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey C. Y.; Pace-Templeton J. G.; Millard C. B.; Wannemacher R. W.; Hewetson J. F. Validation of ELISA for the determination of anti-ricin immunoglobulin G concentration in mouse sera. Biologicals 2006, 34, 33–41. 10.1016/j.biologicals.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Parolo C.; de la Escosura-Muñiz A.; Merkoçi A. Enhanced lateral flow immunoassay using gold nanoparticles loaded with enzymes. Biosens. Bioelectron. 2013, 40, 412–416. 10.1016/j.bios.2012.06.049. [DOI] [PubMed] [Google Scholar]
- Deng J.; Yang M.; Wu J.; Zhang W.; Jiang X. A Self-Contained Chemiluminescent Lateral Flow Assay for Point-of-Care Testing. Anal. Chem. 2018, 90, 9132–9137. 10.1021/acs.analchem.8b01543. [DOI] [PubMed] [Google Scholar]
- Tominaga T. Enhanced sensitivity of lateral-flow test strip immunoassays using colloidal palladium nanoparticles and horseradish peroxidase. LWT 2017, 86, 566–570. 10.1016/j.lwt.2017.08.027. [DOI] [Google Scholar]
- Zhang J. A.; Gui X.; Zheng Q.; Chen Y.; Ge S.; Zhang J.; Xia N. An HRP-labeled lateral flow immunoassay for rapid simultaneous detection and differentiation of influenza A and B viruses. J. Med. Virol. 2019, 91, 503–507. 10.1002/jmv.25322. [DOI] [PubMed] [Google Scholar]
- Panferov V. G.; Safenkova I. V.; Varitsev Y. A.; Zherdev A. V.; Dzantiev B. B. Enhancement of lateral flow immunoassay by alkaline phosphatase: a simple and highly sensitive test for potato virus X. Mikrochim. Acta 2017, 185, 25. 10.1007/s00604-017-2595-3. [DOI] [PubMed] [Google Scholar]
- Akter S.; Kustila T.; Leivo J.; Muralitharan G.; Vehniäinen M.; Lamminmäki U. Noncompetitive Chromogenic Lateral-Flow Immunoassay for Simultaneous Detection of Microcystins and Nodularin. Biosensors 2019, 9, 79 10.3390/bios9020079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang V. T.; Nguyet N. M.; Trung D. T.; Tricou V.; Yoksan S.; Dung N. M.; Van Ngoc T.; Hien T. T.; Farrar J.; Wills B.; Simmons C. P. Diagnostic accuracy of NS1 ELISA and lateral flow rapid tests for dengue sensitivity, specificity and relationship to viraemia and antibody responses. PLoS Neglected Trop. Dis. 2009, 3, e360. 10.1371/journal.pntd.0000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. M.; Sekaran S. D. Early diagnosis of Dengue infection using a commercial Dengue Duo rapid test kit for the detection of NS1, IGM, and IGG. Am. J. Trop. Med. Hyg. 2010, 83, 690–695. 10.4269/ajtmh.2010.10-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]