Abstract
Dendritic cell (DC)–based immunotherapies are believed to help eradicate residual tumor cells, including hepatocellular carcinoma (HCC). Here, we assessed the safety and clinical response to OK432-stimulated monocyte-derived DCs (MoDCs) in treating HCC after radiofrequency ablation (RFA). MoDCs were derived from 30 HCC patients in the presence of interleukin-4 and granulocyte-macrophage colony stimulating factor for 5 days and then cultured for 2 more days in the medium (basic protocol) or stimulated with OK432. On day 7, DCs were harvested and percutaneously injected into HCC tumors after RFA. We observed no grade 3 or 4 National Cancer Institute Common Toxicity Criteria adverse events. Kaplan-Meier analysis indicated that patients treated with RFA + OK432-stimulated DCs transfer had longer recurrence-free survival than those treated with RFA + basic-protocol DCs (median: 24.8 vs 13.0 months; P = .003). RFA with DC infusion can enhance various tumor-associated antigen (TAA)–specific T-cell responses. Additionally, the 5-year RFS rate for patients with significantly increased TAA-specific T-cell responses was much higher than for other patients (50.0% vs. 7.7%; P = .030). Our study provides useful information for development of HCC immunotherapies (trial registration: UMIN000001701).
Introduction
Worldwide mortality from hepatocellular carcinoma (HCC) is approximately 818,000 people per year; it is the third most frequent cause of cancer death [1]. Chronic infection with hepatitis C virus (HCV) increases the risk of HCC occurrence; 6.1% to 8.3% of patients with HCV-related liver cirrhosis develop HCC each year [2]. Although HCC has many curative treatments, tumor recurrence rates remain very high. Immunotherapy presents a novel potential therapeutic option for targeting HCC recurrence [3].
Dendritic cells (DCs) are potent antigen-presenting cells that play a central role in regulating innate and acquired immunities [4,5]. Several clinical trials using DCs to present tumor-derived antigens to the immune system have been reported, but clinical responses have been disappointing [6], in part because HCC development can induce immune-suppressive cytokines and cell populations in the tumor microenvironment such as regulatory T cells and myeloid-derived suppressor cells (MDSCs) [[7], [8], [9]].
Radiofrequency ablation (RFA) is a widely used locoregional treatment for HCC [10,11] that destroys local HCC while reportedly creating tumor antigen sources to generate antitumor immunity and enhancing host immune responses [12]. A previous study from our group also showed RFA to enhance tumor-associated antigen-specific T-cell responses and control distant tumor growth in a murine HCC model [13,14].
Recently, we developed a combined therapy, which is transcatheter hepatic arterial embolization (TAE) with intratumoral infusion of monocyte-derived DCs (MoDCs) stimulated with OK432, a Streptococcus-derived anticancer immunotherapeutic agent. As a result, we found that patients treated with TAE and OK432-stimulated DC transfer had longer recurrence-free survival (RFS) than patients who were treated with TAE alone [15]. Therefore, we compared the protocols for preparing MoDCs with and without OK432 in the present study.
This was the first in-human trial to assess the safety and long-term outcomes of MoDC infusion into patients with HCC following RFA.
Materials and Methods
Patients
The Institutional Review Board reviewed and approved the study protocol (Medical Ethics Committee of Kanazawa University, No. 674; trial registration: UMIN000001701). All patients provided written informed consent to participate in the study. This study complied with ethical standards outlined in the Declaration of Helsinki.
This is a randomized phase I/II trial. We enrolled patients with verified radiological diagnoses of primary HCC who were treated at Kanazawa University Hospital between April 2009 and March 2012.
Inclusion criteria were HCV-related HCC, Eastern Cooperative Oncology Group Performance Status 0 or 1, age> 18 years old, informed consent, and the following normal baseline hematological parameters (within 1 week before DC administration): hemoglobin >8.5 g/dl; white cell count >2000/μl; platelet count >50,000/μl; creatinine <1.5 md/dl, and Child-Pugh A or B. The diagnosis of HCC was based on generally accepted imaging criteria, typical hypervascular nodule staining relative to the surrounding liver on the arterial dominant phase, and washout on the equilibrium phase of dynamic computed tomography (CT) and magnetic resonance imaging.
Exclusion criteria included severe cardiac, renal, pulmonary, hematological, or other systemic diseases associated with discontinuation risk; human immunodeficiency virus (HIV) infection; prior history of other malignancies; history of surgery, chemotherapy, or radiation therapy within 4 weeks; immunological disorders including splenectomy and radiation to the spleen; corticosteroid or antihistamine therapy; current lactation; pregnancy; history of organ transplantation; or difficulty in follow-up.
We performed RFA in patients with HCC who satisfied the following criteria: ineligible for surgical resection and liver transplantation or had refused surgery, and no extrahepatic metastasis and vascular invasion. Tumor recurrences were followed after RFA administration using dynamic CT and magnetic resonance imaging every 3-4 months. Adverse events (AEs), including fever, vomiting, abdominal pain, encephalopathy, myalgia, ascites, gastrointestinal disorder, bleeding, hepatic abscess, and autoimmune disease were monitored after DC infusion according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (version 3.0).
Preparation and Injection of Autologous DCs
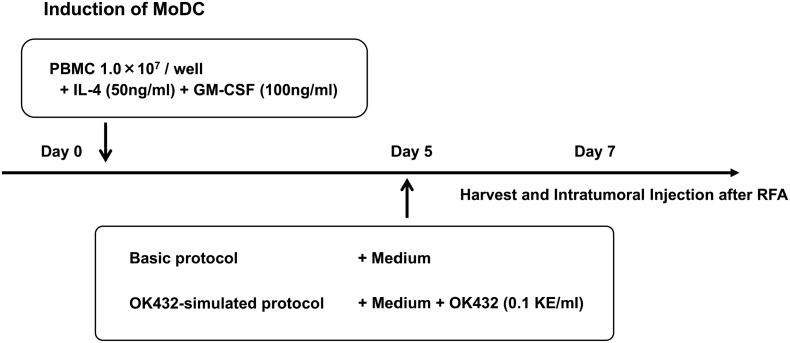
DCs were generated from blood monocyte precursors, as reported previously (Figure 1) [15]. Peripheral blood mononuclear cells (PBMCs) of patients were isolated by centrifugation using Lymphoprep Tubes (Nycomed, Roskilde, Denmark). The cells were resuspended in serum-free medium (GMP CellGro DC Medium; CellGro, Manassas, VA) and allowed to adhere to six-well tissue culture dishes (Costar, Cambridge, MA) at 1.4 × 107 cells in 2 ml per well. After 2 h at 37°C, nonadherent cells were removed, and adherent cells were cultured in the medium with 50 ng/ml recombinant human interleukin (IL)-4 (GMP grade; CellGro) and 100 ng/ml recombinant human granulocyte-macrophage colony stimulating factor (GMP grade; CellGro). After 5 days of culture, the immature DCs induced by the above method were cultured for 2 more days in serum-free medium (basic protocol) or in medium with 0.1 KE/ml of OK432 (Chugai Pharmaceuticals, Tokyo, Japan; OK432-stimulated protocol). On day 7, the cells were harvested for injection (5 × 106 cells suspended in 5 ml normal saline with 1% autologous plasma) and percutaneously injected into patients' HCC tumors with a needle after RFA.
Figure 1.
Preparation of DCs. DCs were derived from patients' PBMCs in the presence of IL-4 and human granulocyte-macrophage colony stimulating factor for 5 days, then cultured for 2 days with serum-free medium only (basic protocol) or with OK432 added to medium (OK432-stimulated protocol), then harvested and evaluated on day 7.
Radiofrequency Ablation
RFA was performed with a cool-tip RFA system consisting of an 18-gauge, cooled-tip electrode with a 2- or 3-cm exposed tip (Radionics, Burlington, MA) and a radiofrequency generator (CC-1 Cosman Coagulator, Radionics). After 2 days post-RFA, complete necrosis was confirmed by dynamic CT. The procedure was performed in the presence of three physicians.
IFN-γ Enzyme-Linked Immunospot (ELISPOT) Assay
ELISPOT assays were performed as described previously with the following modifications [13]. Human leucocyte antigen (HLA)-A24 restricted tumor-associated antigen (TAA)–derived peptides, cyclophilin B (Cyp-B)109 (KFHRVIKDF), squamous cell carcinoma antigen recognized by T cells 2 (SART2)899 (STYRLFLIL), SART3109 (VYDYNCHVDL), p53161 (AIYKQSQHM), multidrug resistance protein 3 (MRP3)765 (VYSDADIFL), alpha-fetoprotein (AFP)357 (EYSRRHPQL), AFP403 (KYIQESQAL), and human telomerase reverse transcriptase (hTERT)461 (VYGFVRACL) were used in this study. Negative controls consisted of an HIV envelope-derived peptide (HIVenv584). Positive controls consisted of 10 ng/ml PMA (Sigma) or a cytomegalovirus (CMV) pp65-derived peptide (CMVpp65328). The colored spots were counted with a KS ELISPOT Reader (Zeiss, Tokyo, Japan). The number of specific spots was determined by subtracting the number of spots in the absence of antigen from the number of spots in its presence. Responses were considered positive if more than 10 specific spots were detected and if the number of spots in the presence of antigen was at least twice more than the number of spots in the absence of antigen.
Statistical Analysis
Data are expressed as means ± SD. Differences between groups were analyzed for statistical significance using Mann-Whitney U test. Qualitative variables were compared using Fisher's exact test. The estimated probability of tumor RFS and overall survival (OS) were determined using the Kaplan-Meier method. The Mantel-Cox log-rank test was used to compare curves between groups. P < .05 was considered to be significant.
Results
Patient Characteristics and Treatment
We included 30 patients with clinically confirmed HCV-related HCC (solitary or up to 3 nodules, <3 cm in size) who were treated between April 2009 and March 2012, fulfilled the selection criteria, agreed to participate in this study, and were randomly assigned to receive basic-protocol DCs (n = 14) or OK432-stimulated DCs (n = 16) after undergoing RFA. In this report, we evaluated the effects of OK432 on the stimulation of DCs in these two groups. Their clinical characteristics are summarized in Table 1. Of the 30 patients, 50% had primary HCC and the rest had recurrent HCC; 80% had Child-Pugh class A liver function. The two groups did not significantly differ in baseline characteristics.
Table 1.
Patient Characteristics
| RFA + DC | RFA + OK432-DC | P | |
|---|---|---|---|
| No. of patients | 14 | 16 | |
| Age, years | 70.1 ± 7.1 | 69.9 ± 7.4 | .921 |
| Sex, male/female | 9/5 | 9/7 | .722 |
| ECOG PS, 0/1 | 14/0 | 16/0 | >.999 |
| HCV RNA, positive/negative | 1/13 | 4/12 | .336 |
| HLA-A24, positive/negative | 8/6 | 9/7 | >.999 |
| Onset, primary/recurrent | 7/7 | 8/8 | >.999 |
| TMN stage, I/II/III | 7/7/0 | 9/6/1 | .715 |
| BCLC stage, 0/A | 6/8 | 8/8 | .730 |
| Largest tumor diameter, mm | 15.8 ± 3.2 | 15.1 ± 4.6 | .624 |
| Tumor multiplicity, solitary/multiple | 7/7 | 9/7 | >.999 |
| AFP, ng/ml | 25.3 ± 16.6 | 52.8 ± 101.4 | .324 |
| Child-Pugh, A/B | 11/3 | 13/3 | >.999 |
| Prothrombin time, % | 79.7 ± 9.4 | 85.3 ± 17.1 | .281 |
| Platelet count, ×104/μl | 9.3 ± 3.3 | 11.7 ± 5.3 | .165 |
| ALT, IU/l | 46.2 ± 17.5 | 35.5 ± 17.7 | .106 |
| ALP, IU/l | 395.4 ± 222.8 | 292.4 ± 92.8 | .102 |
| T-Bil, mg/dl | 0.97 ± 0.30 | 0.88 ± 0.43 | .550 |
| Albumin, g/dl | 3.69 ± 0.56 | 3.61 ± 0.59 | .733 |
Data are expressed as mean ± SD.
AFP, alpha-fetoprotein; ALP, alkaline phosphatase; ALT, alanine transaminase; BCLC, Barcelona Clinic Liver Cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; HCV RNA, hepatitis C virus ribonucleic acid; DC, dendritic cell; HLA, human leukocyte antigen; RFA, radiofrequency ablation; TNM, tumor-node-metastasis.
Assessment of Safety and Toxicity
We percutaneously injected the DCs into HCC tumors after RFA therapy. The DCs were suspended in 5 ml normal saline that contained 1% autologous plasma. AEs were monitored clinically and biochemically after DC injection (Table 2). This treatment was well tolerated by all patients. No grade 3/4 serious AEs occurred, including hepatic failure and autoimmune response. The most common AE was fever within a few days after treatment (seen in 40% of patients). One patient experienced nausea, and two patients had abdominal pain, which was thought to be related to RFA rather than DC infusion. The two groups did not significantly differ in AEs with or without OK432. Thus, the current treatment of DC infusion was performed safely in patients with cirrhosis and HCC.
Table 2.
Immune-Related Adverse Events
| RFA + DC(n = 14) | RFA + OK432-DC(n = 16) | aP | ||||
|---|---|---|---|---|---|---|
| n (%) | n (%) | |||||
| Adverse event grade | Any | 3 or 4 | Any | 3 or 4 | Any | 3 or 4 |
| Fever | 4 (28.5) | 0 | 8 (50.0) | 0 | .284 | – |
| Nausea | 1 (7.1) | 0 | 0 (0) | 0 | .467 | – |
| Abdominal pain | 1 (7.1) | 0 | 1 (6.2) | 0 | >.999 | – |
DC, dendritic cell; RFA, radiofrequency ablation.
Per chi-squared test.
Clinical Outcomes
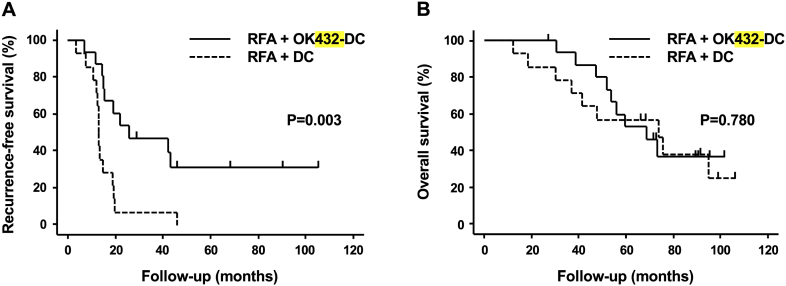
A further objective of this study was to determine clinical outcomes after DC infusion. No other anticancer treatment was given to these patients after RFA until recurrence. Median follow-up period was 62 months (range: 30-105 months). All patients were followed for 2 or more years. Median RFS was 11.8 months longer in the OK432-stimulated DC group than in patients treated with basic protocol DCs (24.8 vs. 13.0 months; P = .003; Figure 2A). However, the two groups did not significantly differ in OS (67.8 vs. 73.4 months; P = .780; Figure 2B).
Figure 2.
Outcomes of DC-based immunotherapy: (A) PFS and (B) OS. Time zero: date of RFA.
Immune Responses to Cytotoxic T Lymphocyte Epitopes Derived from Tumor Antigens
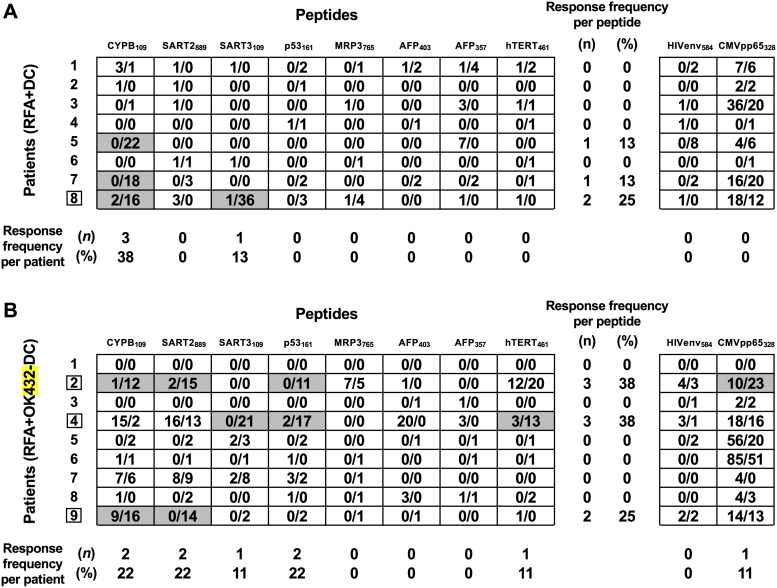
To assess the effects on T-cell responses to tumor antigens, PBMCs were obtained before and 1 month after DC administration. IFN-γ producing T cells responding to HLA-A24–restricted cytotoxic T lymphocyte epitopes derived from AFP, MRP3, SART2, SART3, and hTERT (which we previously identified as HCC-specific TAAs) were assessed by ELISPOT assay in 17 HLA-A24–positive patients [16]. The magnitude of TAA-specific T-cell responses determined by the frequency of T cells and the proportion of the patients who showed a positive increase of TAA-specific T cells are shown in Figure 3. Six of 17 (35.3%) patients showed positive responses to at least one TAA-derived peptide, and most of them showed responses to one to three TAA-derived peptides. Five of eight (62.5%) TAA-derived peptides were recognized by T cells in at least one patient. Significant T-cell responses (i.e., against ≥2 peptides) were observed in patient 8, who received basic-protocol DCs (Figure 3A), and in patients 2, 4 and 8, who received OK432-stimulated DCs (Figure 3B).
Figure 3.
Enhancement of TAA-derived peptide-specific T-cell responses after RFA with DC (A) and OK432-DC (B) injections. Magnitude of TAA-specific T-cell responses was examined by IFN-γ ELISPOT assay. The frequency of T cells responsive to each peptide before RFA (the number of left side) and after RFA with DC injection (the number of right side) is shown. Responses to peptides were considered positive (gray boxes) if equal and more than 10 specific spots per 300,000 PBMCs were detected and if the numbers of spots after RFA with DC injection were at least two-fold that before. Boxes: patients with significantly increased TAA-specific T-cell responses.
Effect on Outcomes by Increased TAA-Specific T Cells After RFA with DC Infusion
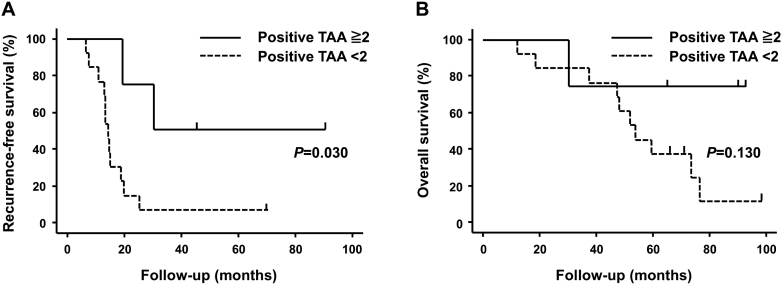
We analyzed the relationships between number of positive TAAs, and RFS and OS. First, we divided patients by their ELISPOT assay results into those with ≥2 (high) or 1 or 0 (low) positive TAAs after treatment. We found that number of positive TAAs after treatment correlated significantly with the length of RFS (P = .030; Figure 4A). Five-year RFS rates were as follows: high group, 50.0%; low groups, 7.7%. However, number of positive TAAs after treatment did not correlate with OS (P = .130; Figure 4B).
Figure 4.
Outcomes of DC-based immunotherapy. (A) PFS and (B) OS in patients who could be evaluated by IFN-γ ELISPOT assay. Time zero: date of RFA.
Discussion
Despite advances in the treatment of HCC, recurrence is extremely common in the background of the high-carcinogenesis condition of the liver cirrhosis, even after curative treatment [17]. However, unlike other carcinomas, treatment selection for recurrent HCC is equal to that in the onset. Therefore, it is important to consider the therapeutic strategy for recurrence as well as for newly diagnosed HCC.
After radical treatment for hepatitis B and C–related HCC, maintenance of liver function by antiviral therapies (including interferon) may indirectly improve prognosis, but evidence to show recurrence can be suppressed directly is lacking [18]. Furthermore, the utilization of direct antitumor therapy (including adjuvant chemotherapy) after radical treatment deteriorates hepatic function and worsens prognosis rather than preventing recurrence in HCC patients [19]. Therefore, strict follow-up with testing for tumor markers and imaging studies is the standard approach after curative treatment.
Use of immunotherapy to treat cancer is increasing, and a certain effect of immune checkpoint inhibitors has been reported [20]. Although HCC recurrence has been reportedly suppressed by postoperative adoptive immunotherapy, it has not significantly improved OS [21]. Previously, we combined RFA with intratumoral injection of OK432-stimulated DCs in a murine cancer model, which showed improved outcomes compared with RFA alone [14]. In humans, we had also reported safety and efficacy in preventing HCC recurrence after TAE with intratumoral administration of OK432-stimulated DCs [15]. Therefore, in this study, we used RFA, which is a more radical treatment for HCC.
In general, tumor cells secrete several immunosuppressive cytokines, which could induce immune tolerance in the tumor microenvironment [22]. These cytokines (including transforming growth factor-β, vascular endothelial growth factor, and IL-10) inhibit maturation of DCs [23]. The function of DCs has been shown to be impaired in several types of cancer, including HCC, with mature and activated DCs almost absent in cancer nodules [24,25]. In this study, we used RFA, a curative treatment for HCC, because it destroys the local microenvironment and creates a tumor antigen source for generation of antitumor immunity. RFA has been shown to induce TAA-specific T-cell responses, which is known as the abscopal effect [26,27]. For tumor-specific immunity, RFA can reportedly enhance TAA-specific T-cell responses; the number of T cells induced is associated with RFS in HCC [13]. This means that thermally induced necrosis can act as a permanent source of tumor antigens, which can induce systemic antitumor immunity.
More interestingly, ELISPOT assay of this study showed that infusing DCs into HCC tumors after RFA induced immune responses to unprimed tumor antigens, which implies that antigen-nonspecific DC injection into the treated tumor enhanced tumor immunity. Cancer tissue necrosis and DC infusion directly into HCC after RFA might provide maturational signals and maintain TAA-specific T-cell responses.
In this study, we used OK432 for DC maturation. DC maturation with a proinflammatory cytokine cocktail composed of tumor necrosis factor-α, IL-1β, IL-6, and prostaglandin E2 is by far the most commonly used in clinical trials [28]. However, in our previous basic research, the production of cytokines, such as IL-12, and allostimulatory capacity were greater in MoDCs derived from patients with HCC that were stimulated with OK432 than with proinflammatory cytokine cocktails [29]. The ability of DCs to produce IL-12p70, which favors induction of a protective T-helper type 1 immune response, was reported to be an important predictor of favorable clinical outcome in cancer immunotherapies [30]. Additionally, in our previous clinical study, TAE with intratumoral infusion of OK432-stimulated DC had longer RFS than patients who were treated with TAE alone. Therefore, we consider OK432 to be a key drug for immunotherapy of HCC and decide to use OK432 for DC maturation in HCC immunotherapy.
All of our basic and clinical studies mentioned in the previous section only included HCV patients since HCV is the most common cause of HCC in Japan. In addition, we previously showed that host immune responses are different between HCC caused by hepatitis B virus (HBV) and HCV infection [31]. Therefore, we targeted HCV-related HCC patients in this study. But in the future, we will broaden the target patients to cover HBV-related and non–HBV/non–HCV-related HCCs because HCV infection is now well controllable and HCV-related HCC cases will decrease due to the development of direct-acting antiviral treatment.
Overall survival was not significantly better in the RFA + OK-432–stimulated DC group or in the immune reactive group. This might be due to the replete second- and third-line therapies that the participants received after their relapses; these responses might dilute the impact on OS. On the other hand, patients with RFA and OK432-stimulated DCs had a significantly longer RFS. Based on the results of ELISPOT assay, RFS was favorable in the group with tumor antigen-specific T-cell responses, regardless of the additional stimulation of OK432. In the last period, several studies have focused their application on discovering predictive biomarkers for HCC [32]. These results of ELISPOT assay may be used to predict the prognosis of patients who have a good response to this treatment.
Percutaneously infused DCs, using a needle, after RFA did not cause additional AEs in the current study. The safety of DC-based immunotherapy has been well documented in many phase 1 clinical studies [33,34]. This study demonstrated, for the first time, that the combination of RFA and intratumoral injection of MoDC is safe in patients with HCC.
In conclusion, these results suggest that MoDC administration to necrotic tumor with curative treatments is safe against HCV-related HCC. We are planning a clinical trial to investigate the efficacy of this treatment for a wide range of HCC patients.
Author Contributions
Masaaki Kitahara: analysis of data and drafting of the article; Eishiro Mizukoshi: conception and design of the article; Takeshi Terashima, Hidetoshi Nakagawa, Rika Horii, Noriho Iida, Kuniaki Arai, Taro Yamashita, Yoshio Sakai, Tatuya Yamashita, Masao Honda, and Yasunari Nakamoto: critical revision of the article for important intellectual content; Shuichi Kaneko: final approval of the article.
Acknowledgments
Acknowledgements
The authors thank Kazumi Fushimi and Maki Kawamura for technical assistance. We also thank the patients for participating in this study.
Conflict of Interest
The authors have declared no conflict of interest exists.
References
- 1.Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease StudyLancet. 2013;385(2015):117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruix J., Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Longo V., Gnoni A., Casadei Gardini A., Pisconti S., Licchetta A., Scartozzi M., Memeo R., Palmieri V.O., Aprile G., Santini D., Nardulli P., Silvestris N., Brunetti O. Immunotherapeutic approaches for hepatocellular carcinoma. Oncotarget. 2017;8:33897–33910. doi: 10.18632/oncotarget.15406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 5.Palucka K., Banchereau J. Dendritic cells: a link between innate and adaptive immunity. J. Clin. Immunol. 1999;19:12–25. doi: 10.1023/a:1020558317162. [DOI] [PubMed] [Google Scholar]
- 6.Butterfield L.H. Dendritic cells in cancer immunotherapy clinical trials: are we making progress? Front. Immunol. 2013;4:454. doi: 10.3389/fimmu.2013.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Becker J.C., Andersen M.H., Schrama D., Thor Straten P. Immune-suppressive properties of the tumor microenvironment. Cancer Immunol. Immunother. 2013;62:1137–1148. doi: 10.1007/s00262-013-1434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arihara F., Mizukoshi E., Kitahara M., Takata Y., Arai K., Yamashita T., Nakamoto Y., Kaneko S. Increase in CD14+HLA-DR−/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol. Immunother. 2013;62:1421–1430. doi: 10.1007/s00262-013-1447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takata Y., Nakamoto Y., Nakada A., Terashima T., Arihara F., Kitahara M., Kakinoki K., Arai K., Yamashita T., Sakai Y., Mizukoshi E., Kaneko S. Frequency of CD45RO+ subset in CD4+CD25(high) regulatory T cells associated with progression of hepatocellular carcinoma. Cancer Lett. 2011;307:165–173. doi: 10.1016/j.canlet.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 10.Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010;52:762–773. doi: 10.1002/hep.23725. [DOI] [PubMed] [Google Scholar]
- 11.Tiong L., Maddern G.J. Systematic review and meta-analysis of survival and disease recurrence after radiofrequency ablation for hepatocellular carcinoma. Br. J. Surg. 2011;98:1210–1224. doi: 10.1002/bjs.7669. [DOI] [PubMed] [Google Scholar]
- 12.Zerbini A., Pilli M., Fagnoni F., Pelosi G., Pizzi M.G., Schivazappa S., Laccabue D., Cavallo C., Schianchi C., Ferrari C., Missale G. Increased immunostimulatory activity conferred to antigen-presenting cells by exposure to antigen extract from hepatocellular carcinoma after radiofrequency thermal ablation. J. Immunother. 2008;31:271–282. doi: 10.1097/CJI.0b013e318160ff1c. [DOI] [PubMed] [Google Scholar]
- 13.Mizukoshi E., Yamashita T., Arai K., Sunagozaka H., Ueda T., Arihara F., Kagaya T., Yamashita T., Fushimi K., Kaneko S. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology. 2013;57:1448–1457. doi: 10.1002/hep.26153. [DOI] [PubMed] [Google Scholar]
- 14.Nakagawa H., Mizukoshi E., Iida N., Terashima T., Kitahara M., Marukawa Y., Kitamura K., Nakamoto Y., Hiroishi K., Imawari M., Kaneko S. In vivo immunological antitumor effect of OK-432-stimulated dendritic cell transfer after radiofrequency ablation. Cancer Immunol. Immunother. 2014;63:347–356. doi: 10.1007/s00262-013-1514-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakamoto Y., Mizukoshi E., Kitahara M., Arihara F., Sakai Y., Kakinoki K., Fujita Y., Marukawa Y., Arai K., Yamashita T., Mukaida N., Matsushima K., Matsui O., Kaneko S. Prolonged recurrence-free survival following OK432-stimulated dendritic cell transfer into hepatocellular carcinoma during transarterial embolization. Clin. Exp. Immunol. 2011;163:165–177. doi: 10.1111/j.1365-2249.2010.04246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizukoshi E., Nakamoto Y., Arai K., Yamashita T., Sakai A., Sakai Y., Kagaya T., Yamashita T., Honda M., Kaneko S. Comparative analysis of various tumor-associated antigen-specific t-cell responses in patients with hepatocellular carcinoma. Hepatology. 2011;53:1206–1216. doi: 10.1002/hep.24149. [DOI] [PubMed] [Google Scholar]
- 17.Tateishi R., Shiina S., Yoshida H., Teratani T., Obi S., Yamashiki N., Yoshida H., Akamatsu M., Kawabe T., Omata M. Prediction of recurrence of hepatocellular carcinoma after curative ablation using three tumor markers. Hepatology. 2006;44:1518–1527. doi: 10.1002/hep.21408. [DOI] [PubMed] [Google Scholar]
- 18.Shen Y.C., Hsu C., Chen L.T., Cheng C.C., Hu F.C., Cheng A.L. Adjuvant interferon therapy after curative therapy for hepatocellular carcinoma (HCC): a meta-regression approach. J. Hepatol. 2010;52:889–894. doi: 10.1016/j.jhep.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 19.Hasegawa K., Takayama T., Ijichi M., Matsuyama Y., Imamura H., Sano K., Sugawara Y., Kokudo N., Makuuchi M. Uracil-tegafur as an adjuvant for hepatocellular carcinoma: a randomized trial. Hepatology. 2006;44:891–895. doi: 10.1002/hep.21341. [DOI] [PubMed] [Google Scholar]
- 20.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takayama T., Sekine T., Makuuchi M., Yamasaki S., Kosuge T., Yamamoto J., Shimada K., Sakamoto M., Hirohashi S., Ohashi Y., Kakizoe T. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356:802–807. doi: 10.1016/S0140-6736(00)02654-4. [DOI] [PubMed] [Google Scholar]
- 22.Gajewski T.F., Schreiber H., Fu Y.X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rutkowski M.R., Stephen T.L., Conejo-Garcia J.R. Anti-tumor immunity: myeloid leukocytes control the immune landscape. Cell. Immunol. 2012;278:21–26. doi: 10.1016/j.cellimm.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kakumu S., Ito S., Ishikawa T., Mita Y., Tagaya T., Fukuzawa Y., Yoshioka K. Decreased function of peripheral blood dendritic cells in patients with hepatocellular carcinoma with hepatitis B and C virus infection. J. Gastroenterol. Hepatol. 2000;15:431–436. doi: 10.1046/j.1440-1746.2000.02161.x. [DOI] [PubMed] [Google Scholar]
- 25.Wada Y., Nakashima O., Kutami R., Yamamoto O., Kojiro M. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology. 1998;27:407–414. doi: 10.1002/hep.510270214. [DOI] [PubMed] [Google Scholar]
- 26.Siva S., Macmanus M.P., Martin R.F., Martin O.A. Abscopal effects of radiation therapy: A clinical review for the radiobiologist. Cancer Lett. 2015;356:82–90. doi: 10.1016/j.canlet.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Frey B., Rubner Y., Kulzer L., Werthmoller N., Weiss E.M., Fietkau R., Gaipl U.S. Antitumor immune responses induced by ionizing irradiation and further immune stimulation. Cancer Immunol. Immunother. 2014;63:29–36. doi: 10.1007/s00262-013-1474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tacken P.J., de Vries I.J., Torensma R., Figdor C.G. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007;7:790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- 29.Kitahara M., Mizukoshi E., Nakamoto Y., Mukaida N., Matsushima K., Kaneko S. Efficient generation of highly immunocompetent dendritic cells from peripheral blood of patients with hepatitis C virus-related hepatocellular carcinoma. Int. Immunopharmacol. 2014;21:346–353. doi: 10.1016/j.intimp.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 30.Kirkwood J.M., Butterfield L.H., Tarhini A.A., Zarour H., Kalinski P., Ferrone S. Immunotherapy of cancer in 2012. CA Cancer J. Clin. 2012;62:309–335. doi: 10.3322/caac.20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inada Y., Mizukoshi E., Seike T., Tamai T., Iida N., Kitahara M., Yamashita T., Arai K., Terashima T., Fushimi K., Yamashita T., Honda M., Kaneko S. Characteristics of immune response to tumor-associated antigens and immune cell profile in hepatocellular carcinoma patients. Hepatology. 2019;69:653–665. doi: 10.1002/hep.30212. [DOI] [PubMed] [Google Scholar]
- 32.Casadei Gardini A., Scarpi E., Faloppi L., Scartozzi M., Silvestris N., Santini D., de Stefano G., Marisi G., Negri F.V., Foschi F.G., Valgiusti M., Ercolani G., Frassineti G.L. Immune inflammation indicators and implication for immune modulation strategies in advanced hepatocellular carcinoma patients receiving sorafenib. Oncotarget. 2016;7:67142–67149. doi: 10.18632/oncotarget.11565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palucka K., Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity. 2013;39:38–48. doi: 10.1016/j.immuni.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anguille S., Smits E.L., Lion E., van Tendeloo V.F., Berneman Z.N. Clinical use of dendritic cells for cancer therapy. Lancet Oncol. 2014;15:e257–e267. doi: 10.1016/S1470-2045(13)70585-0. [DOI] [PubMed] [Google Scholar]