Abstract
Nanomedicines are extensively employed in cancer therapy. We here propose four strategic directions to improve nanomedicine performance and translation. (1) Patient stratification has become common practice in oncology drug development. Accordingly, probes and protocols for patient stratification are urgently needed in cancer nanomedicine, to identify individuals suitable for inclusion in clinical trials. (2) Rational drug selection is crucial for clinical and commercial success. Opportunistic choices based on drug availability should be replaced by investments in modular (pro)drug and nanocarrier design. (3) Combination therapies are the mainstay of clinical cancer care. Nanomedicines synergize with pharmacological and physical co-treatments, and should be increasingly integrated in multimodal combination therapy regimens. (4) Immunotherapy is revolutionising the treatment of cancer. Nanomedicines can regulate the behaviour of myeloid and lymphoid cells, thereby empowering anticancer immunity and immunotherapy efficacy. Alone and especially together, these four directions will fuel and foster the development of successful cancer nanomedicine therapies.
Introduction
Nanomedicine holds potential to improve anticancer therapy1. Traditionally, nanomedicines are used to modulate the biodistribution and the target site accumulation of systemically administered chemotherapeutic drugs, thereby improving the balance between their efficacy and toxicity. In preclinical settings, nanomedicines typically increase tumour growth inhibition and prolong survival as compared to non-formulated drugs, but in clinical practice, patients often only benefit from nanomedicines because of reduced or altered side effects2.
Despite the recent approval of several nanomedicinal anticancer drugs, such as Onivyde® (liposomal irinotecan) and Vyxeos™ (liposomal daunorubicin plus cytarabine), the success rate of clinical translation remains relatively low. In this context, the striking imbalance between the ever-increasing number of preclinical studies reporting the development of ever more complex nanomedicines on the one hand, and the relatively small number of nanomedicine products approved for clinical use on the other hand, has become the focus of intense debate3,4.
Multiple biological, pharmaceutical and translational barriers contribute to this imbalance5. Biological barriers include tumor (and metastasis) perfusion, permeability and penetration, as well as delivery to and into target cells, endo/lysosomal escape, and appropriate intracelullar processing and trafficking. Pharmaceutical barriers encompass both nanoformulation- and production-associated aspects. These range from a proper stability in the bloodstream, a beneficial biodistribution, an acceptable toxicity profile, and rational mechanisms for drug release, biodegradation and elimination, to issues related to intellectual property position, cost of goods, cost of manufacturing, upscaling, and batch-to-batch reproducibility. In terms of clinical translation, the key challenge is to select the right drug and the right combination regimen, and to apply them in the right disease indication and the right patient population.
To make sure that we start tackling the right translational challenges, we must define key strategic directions, to guide nanomedicine clinical trial design and ensure clear therapeutic benefits to patients. In this perspective, we conceptualize ‘smart’ cancer nanomedicine as an umbrella term for rational and realistic “Strategies and Materials to Advance and Refine Treatments”. We propose four directions to boost nanomedicine performance and exploitation, i.e. smart patient stratification, smart drug selection, smart combination therapies and smart immunomodulation (Figure 1).
Figure 1. Smart Strategies and Materials to Advance and Refine cancer nanomedicine Treatments.
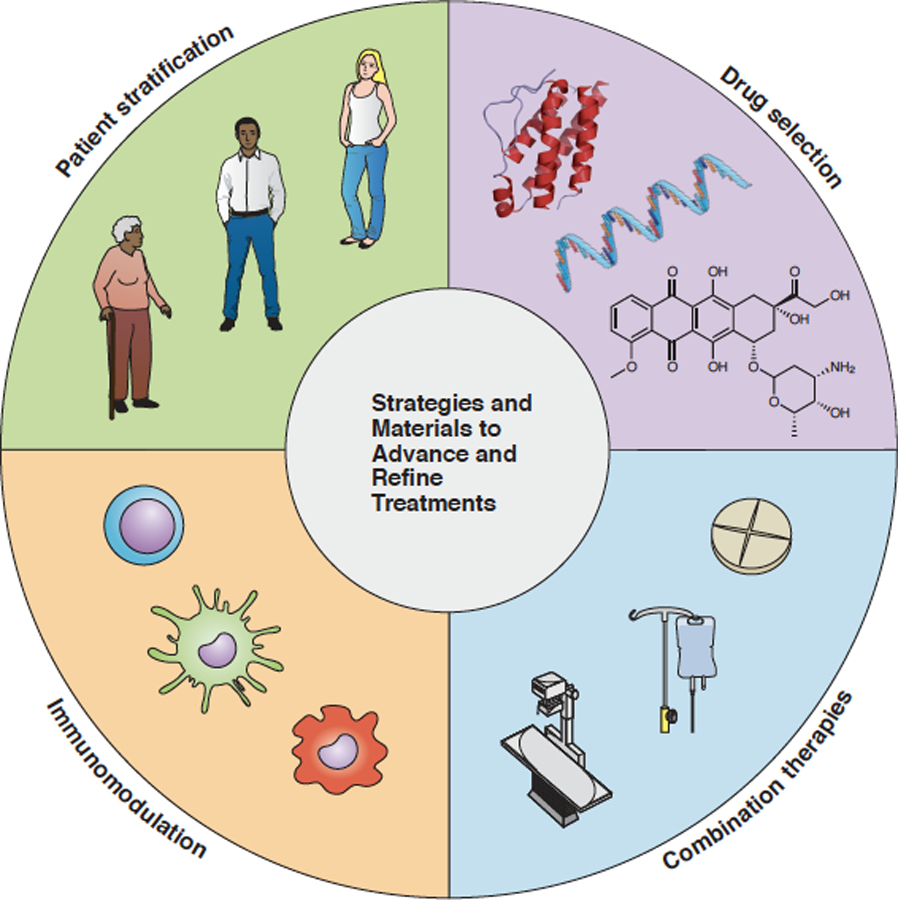
Four directions are proposed that – on their own and especially together – will promote the translation and exploitation of nanomedicinal anticancer drugs.
1. Patient stratification
Patient stratification in oncology drug development
Modern oncology drug development extensively employs biomarkers and companion diagnostics for patient stratification. Companion diagnostics help to address the high heterogeneity that is typical of cancer, and they have been instrumental in the successful clinical translation of molecularly targeted drugs, such as growth factor receptor-blocking antibodies and tyrosine kinase inhibitors. As an example, in the trials that led to the approval of Herceptin® (trastuzumab)6, Perjeta® (pertuzumab)7 and Kadcyla® (ado-trastuzumab emtansine)8, patients with high human epidermal growth factor receptor 2 (HER2) expression levels were pre-selected via pathological stainings and/or fluorescence in situ hybridization, thereby ensuring enrichment of patients likely to respond and excluding expected non-responders. In immuno-oncology, the first ‘general biomarker’, which is not coupled to a particular organ/origin of cancer but instead to a specific genomic signature, has recently been established. This more broadly applicable biomarker is termed microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR), and it is used for patient stratification in case of treatment with immune checkpoint inhibiting antibodies9.
Biomarkers in cancer nanomedicine
Remarkably, neither biomarkers nor companion diagnostics are currently used to tailor nanomedicine treatments in patients (Figure 2). Notable exceptions in this regard are antibody-drug conjugates, which are often excluded from nanomedicine lists because they are more biotechnological than nanotechnological (but should be included according to the generally accepted definition). Four antibody-drug conjugates have recently received regulatory approval: Kadcyla® (ado-trastuzumab emtansine, anti-HER2); Adcetris® (brentuximab vedotin, anti-CD30); Besponsa™ (inotuzumab ozogamicin, anti-CD22) and Mylotarg™ (gemtuzumab ozogamicin, anti-CD33). In all of these cases, the intrinsic availability of biomarkers for patient stratification has played a key role in their successful clinical development.
Figure 2. Smart strategies for patient stratification in cancer nanomedicine.
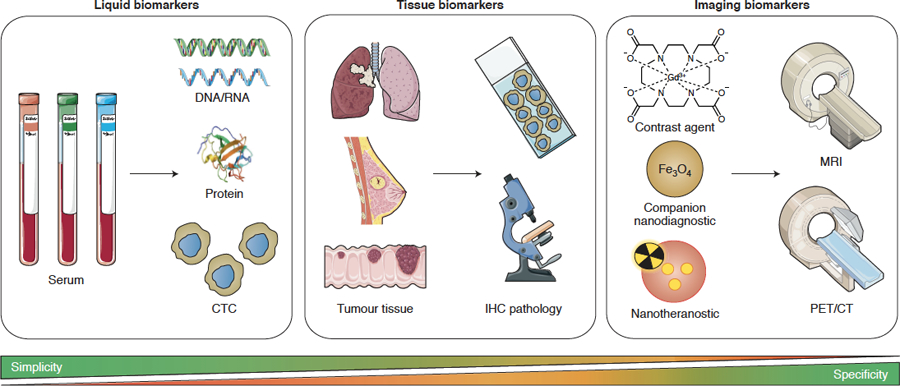
Several probes and protocols can be employed for patient stratification, including circulating tumour cell (CTC) analysis, immunohistochemical assessment of the tumour microenvironment, and direct and indirect imaging of nanomedicine tumour accumulation. These approaches vary in simplicity, specificity and applicability for passively versus actively targeted nanomedicines. Liquid biomarkers are the most straightforward and least invasive, but may not be not predictive enough to serve as standalone biomarkers for tailoring nanomedicine treatment. Tissue biomarkers are easily available, but are likely more useful for actively than for passively targeted nanomedicines, and their predictive power needs to be explored. Imaging biomarkers can rely on approved contrast agents and companion nanodiagnostics, which available off the shelf. This contributes to simplicity, but the information obtained may not be specific enough. Nanotheranostics provide highly specific information on the target site accumulation of the formulation in question, but are more challenging from a translational point of view.
In the case of more traditional cancer nanomedicines, which are based on liposomes, polymeric nanoparticles and micelles, the lack of integrating biomarkers may explain recent failures in the clinic. Notable examples include BIND-014 (docetaxel-loaded prostate-specific membrane antigen (PSMA)-targeted polyethylene glycol-polylactic acid nanoparticles)10, CRLX101 (campthotecin-loaded polyethylene glycol-cyclodextrin nanoparticles)11 and NK105 (paclitaxel-loaded polyethylene glycol-polyaspartate-based micelles)12, which all failed to produce convincing response rates in unstratified patient cohorts. Retrospectively, one can ask whether it was realistic to believe that these nano-drugs could achieve significant response rates in mixed (and heavily pre-treated) patients not stratified in any way. Relatedly, while the FDA-approved nanomedicine formulations Doxil®/Caelyx® and Abraxane®’s toxicity profiles improved thousands of patients’ quality of life, their therapeutic potential might not yet be optimally exploited, as they are applied in non-stratified patient populations13. To improve cancer nanomedicines’ clinical impact, we propose establishing biomarkers for patient stratification, and integrating nanomedicines – ideally already from early clinical development stages onwards – in combination regimens.
Nanomedicine accumulation in solid tumours is generally based on the Enhanced Permeability and Retention (EPR) effect. This concept, however, has come under increasing criticism in recent years. Some nanomedicine investigators even claim that the EPR effect exists only in mice, not in humans. Regardless of whether “EPR” is pathophysiologically the most correct term to describe the phenomena underlying nanocarrier localisation in tumours, passive accumulation definitely occurs in patients but is highly heterogeneous, both inter- and intra-individually. To address this, strategies and materials are needed to monitor and predict nanomedicine accumulation and efficacy14. In the case of ligand-targeted nanomedicines, such as BIND-014, which relies on both EPR and active recognition of receptors overexpressed at pathological sites, patient stratification can in principle be achieved relatively easily, e.g. via immunohistochemically assessing PSMA expression in tumour biopsies seems relatively straightforward15. In addition, PSMA-targeted radiotracers, which are becoming increasingly established in the clinic, can be used to monitor receptor expression on tumour cells and/or angiogenic endothelium via positron emission tomography (PET) or single-photon emission computed tomography (SPECT), allowing for patient stratification also in metastatic patients16. Another approach is circulating tumour cell (CTC) analysis, which, when retrospectively applied in a BIND-014 phase II clinical trial, indicated that efficient treatment coincided with reduced PSMA-positive CTC10. This strategy warrants further investigation, as PSMA-positive (and other) CTC can potentially be employed both as a biomarker for patient stratification and as an indicator of therapeutic responses.
In this context, it is important to keep the basic principles of active tumour targeting in mind. Active targeting relies to a significant extent on passive targeting, which requires prolonged circulation times. Introducing targeting ligands often promotes nanomedicine clearance from the blood stream, via opsonization and accelerated uptake by the mononuclear phagocytic system (MPS)17. Before they can bind to target cells, actively targeted nanomedicines furthermore have to extravasate out of the blood vessels in tumours and metastases, penetrate deep into the interstitium and cross multiple cell layers. The latter can suffer from the so-called binding-site barrier18, which may hinder nanomedicine penetration19. Nanocarrier size is crucial in this context. It has for instance been shown that small-sized (~7 nm) polymeric nanocarriers achieve much higher levels of tumour accumulation when they are functionalized for actively targeting20. Conversely, for larger (~14 nm) nanocarriers, surface functionalization had no effect the overall tumour accumulation levels. Active targeting did, however, change the intratumoural compartmentalisation of the nanocarriers, increasing their uptake by cancer cells. In line with this, it was shown that 70–80 nm-sized HER2-targeted gold nanoparticles21 and 100 nm-sized HER2-targeted liposomes22 accumulate to a higher extent in cancer cells than in macrophages, but do not achieve higher tumour concentrations. These scenarios underline the importance of critically reflecting on the added value of active targeting in cancer nanomedicine applications. In our perspective, actively targeted nanomedicines will be mainly beneficial for the treatment of haematological cancers (in which significant numbers of cancer cells are present in systemic circulation), in setups in which certain immune cells are targeted (in the blood stream and/or in MPS organs), and when the therapeutic payload needs to be delivered intracellularly (e.g. in case of RNA drugs employed to modulate gene expression).
Imaging biomarkers
The use of liquid and tissue biopsy biomarkers is less straightforward in the case of passively targeted nanomedicines, as there are no surface receptors available for immunohistochemical staining or CTC assessment. Accordingly, these nanomedicines likely require imaging probes and protocols for patient stratification (Figure 2). Several recent studies have established companion nanodiagnostics and nanotheranostics to address EPR effect heterogeneity and to predict nanotherapy outcome23,24. For example, ferumoxytol (i.e. FDA-approved 30 nm iron oxide nanoparticles used to treat iron deficiency anaemia) can be used “off-label” to characterize EPR heterogeneity via magnetic resonance imaging (MRI)25. Ferumoxytol-enhanced MRI correlated with therapeutic nanoparticle uptake in tumour-associated macrophages (TAM) and enabled prediction of tumour accumulation and antitumor efficacy25. In the clinic, ferumoxytol-enhanced MRI in patients with mixed solid tumours demonstrated that higher ferumoxytol accumulation levels correlated with greater lesion size reductions following treatment with liposomal irinotecan (Onivyde®)26. Although such MRI-based companion diagnostics are relatively cost effective, they require pre- and post-contrast MR imaging, which complicates the analysis, particularly in regions with variable soft tissue contrast. An alternative companion nanodiagnostic approach is positron emission tomography (PET) imaging with 89Zr-labeled nanoreporter liposomes, which accurately predicted Doxil® accumulation and efficacy using a single PET scan27. Besides predicting tumour accumulation, imaging biomarkers can also be employed to assess responses to nanomedicine therapies. Tumour metabolism – as opposed to that in healthy cells – largely relies on aerobic glycolysis, a phenomenon known as the Warburg effect28. This phenomenon is clinically routinely visualized and quantified using 18F-fluoro-2-deoxyglucose (18F-FDG)-based PET imaging29. 18F-FDG PET imaging can be employed to assess early responses to nanomedicine therapies, e.g. to nano-photothermal therapy30. A related and highly interesting future direction in this regard is imaging and targeting of amino acid metabolism in tumours. Particularly glutamine metabolism31,32, but also the availability and consumption of aspartate33 and asparagine34 have recently been shown to be involved in tumour growth and/or metastasis. Nanomedicine formulations interfering with these pathways and imaging agents capturing these pathways are envisaged to hold significant potential for improving cancer therapy.
Cancer nanomedicines can in principle be co-loaded with drugs and with imaging agents, to directly visualise and quantify target site accumulation. Such theranostic formulations provide the most specific information on biodistribution and tumour accumulation, thereby ruling out issues related to differences in physicochemical and pharmacokinetic properties between nanodiagnostics and nanotherapeutics. However, these formulations are more difficult to translate to the clinic and lack the flexibility and versatility of companion nanodiagnostics, which allow e.g. for decision-making prior to starting treatment. A pioneering clinical study involving nanotheranostics recently showed that PET combined with computed tomography (CT) can assess the tumour accumulation of 64Cu-labeled HER2-targeted liposomal doxorubicin in HER2-positive metastatic breast cancer patients. Higher target site accumulation levels corresponded with more favourable therapeutic outcomes35. Interestingly, molecules such as doxorubicin and alendronate can function as chelators, to enable easy and efficient in vivo monitoring of target site accumulation36. This smart and straightforward approach to obtain radiolabeled liposomal drugs opens up new theranostic opportunities for patient stratification in cancer nanomedicine.
It must be kept in mind, however, that nanomedicine accumulation in tumours must be followed by payload release to achieve therapeutic benefits, since many encapsulated drugs are not pharmacologically active. Drugs can spontaneously release from nanocarriers in tumours, for example free doxorubicin is released from Doxil®37, and this can also be mediated by tumour-associated macrophages38. Monitoring drug release in vivo is possible using contrast-enhanced MRI and optical imaging. Both methods, however, have limited translational relevance, because of the potential toxicity associated with co-loading high amounts of gadolinium in nanomedicines, and because of the limited penetration depth of optical imaging, respectively. Consequently, studies in 2D cell culture, in 3D spheroids and in preclinical mouse models should encompass mechanistic analyses on drug release and target cell uptake upon nanomedicine-mediated delivery.
As an alternative to companion nanodiagnostics and nanotheranostics, clinically established (but intuitively significantly less specific) ‘standard’ imaging protocols could be considered to predict the accumulation and efficacy of passively targeted cancer nanomedicines, such as dynamic contrast-enhanced MRI39, CT40 and ultrasound41. Furthermore, future analyses should set out to evaluate if histopathological assessment of tumour biopsies can help to stratify responders from non-responders. Tumour biopsies are readily available for the vast majority of patients, and scoring e.g. vessel and macrophage density and distribution is hypothesized to be reasonable useful for predicting nanomedicine accumulation and efficacy.
2. Drug selection
Rational drug selection is crucial for making cancer nanomedicines clinically and commercially successful. Preclinically, most nanomedicine publications deal with re-formulating established chemotherapeutic drugs, and all clinically approved cancer nanomedicines (antibody-drug conjugates excluded) are based on standard cytostatics, such as doxorubicin, daunorubicin, paclitaxel, vincristine and irinotecan42. For all of these agents, nanomedicine re-formulation improves the therapeutic index, but typically mostly by attenuating side effects, not by inducing significantly improved therapeutic responses. In this context, specific nanomedicine-related side effects, such as complement-mediated infusion reactions43, have to be kept in mind, and a better mechanistic understanding of these effects, and the identification of efficient ways of dealing with them, is crucial for maximizing nanomedicines’ impact on the therapeutic index5. In addition, future efforts in cancer nanomedicine drug development should focus more on the use of nanocarrier materials for the delivery non-standard drugs such as biologics, and they should increasingly encompass smart strategies such as drug derivatisation, modular nanocarrier design and library screening (Figure 3).
Figure 3. Smart drug selection in cancer nanomedicine.
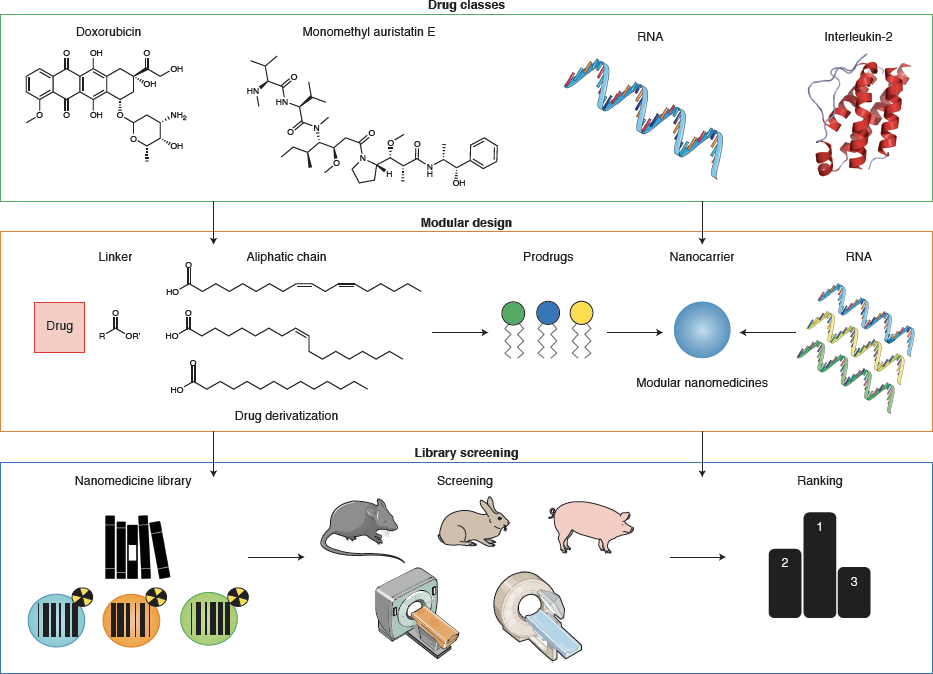
Rational drug selection is crucial to ensure clinical and commercial success. Multiple strategies can be envisaged to connect the right drug to the right nanocarrier for the right indication. Drug classes: Nanomedicines can be loaded with different types of anticancer drugs, including standard chemotherapeutics, highly potent toxins, biologics and nucleic acids. Prodrugs can be engineered to ensure optimal compatibility with nanocarrier formulations, including e.g. drug coupling via ester linkers to an aliphatic chain for efficient incorporation in lipid-based nanomedicines. Modularity: Nanomedicines can be designed to encapsulate payloads with comparable / compatible properties, such as RNA or small molecule prodrugs. Screening: Nanomedicine libraries can be produced via high-throughput technologies. Various labelling and analytical techniques can be employed to identify nanomedicine candidates with optimal properties for in vivo performance.
Drug classes
Standard chemotherapeutic drugs, such as doxorubicin and paclitaxel, do come with side effects, but are overall reasonably well-tolerated by patients; otherwise they would not have become drug products. Newer, more potent agents, such as auristatins, are too toxic to be administered to patients in free form. To enable the in vivo use of auristatins, antibody-drug conjugates have been developed, such as brentuximab vedotin (Adcetris®), which is approved to treat CD30+ haematological cancers. Antibody-drug conjugates, however, typically have very low payloads. To explore formulations with higher auristatin payloads, polymeric nanoparticles containing thousands of drug molecules were developed. These nanoparticles achieved efficient tumour growth inhibition and prolonged survival as compared to standard-of-care (cisplatin) in a patient-derived xenograft model of ovarian cancer, without significant systemic toxicity44. Similarly, a potent Aurora B kinase inhibitor – which in free form caused unacceptable side effects in a phase II clinical trial – increased efficacy and reduced toxicity in multiple preclinical models upon reformulation in PEG-PLA-based nanoparticles 45.
Nanocarrier technologies also play a prominent role in the development of DNA- and RNA-based drugs. Nucleic acid agents critically rely on protection against degradation in systemic circulation and they generally require intracellular delivery. The nanotechnology used in Onpattro™ (patisiran; liver-targeted siRNA-containing lipid nanoparticles for the treatment of transthyretin-related hereditary amyloidosis46) is facilitating the development of other genetic nanomedicines, such as RNA-based vaccines for treating infections and cancer47,48. These vaccines contain RNA encoding for therapeutic proteins and upon delivery to hepatocytes, the liver is utilized as a bio-factory for protein production49. Similarly, nanoparticles carrying RNA encoding for tumour antigens can be delivered to antigen-presenting cells in liver and spleen to elicit T cell-mediated anti-tumour effects50–52. Several such nucleic acid-based nano-vaccines have recently entered clinical trials for cancer immunotherapy47,50,53.
Proteins are another class of therapeutics that benefit from nanocarrier delivery, for instance by abrogating the systemic toxicity of immunostimulatory agents such as interleukin-2 (IL2) for immuno-oncology applications54. NKTR-214 (bempegaldesleukin), i.e. a biologic prodrug comprising IL2 with 6 conjugated releasable PEG chains, significantly inhibited tumour growth in a melanoma mouse model compared to unmodified IL2 (aldesleukin) and it synergised with checkpoint inhibitor therapy55. NKTR-214 treatment was furthermore found to be well tolerated in non-human primates55 and in patients with advanced or metastatic solid tumors56.
Modular design
Nanomedicine design has traditionally been formulation-driven; for every new nanodrug, the carrier material and formulation process need to be adjusted to the payload’s physicochemical properties. While this approach is inherently inflexible, it can be applied to therapeutics with similar characteristics. For example, pH gradient-based remote loading has been widely employed to entrap amphiphilic and ionizable drugs, such as doxorubicin, in liposomes57. As an alternative, drug molecules can be (re-)engineered to improve their compatibility with nanocarriers. For example, rational chemical derivatization of doxorubicin modified the drug’s hydrophobicity and miscibility with PLGA nanoparticles to improve drug-nanocarrier compatibility and therapeutic efficacy. Analogously, a hydrolysable ester linker conjugated to docetaxel enabled stable incorporation in core-crosslinked polymeric micelles while still allowing for controlled drug release. A single intravenous injection of polymeric micelles loaded with the docetaxel derivative induced complete tumour regression in a xenograft model of breast cancer58. This platform is currently being evaluated in a phase II trial for treatment of ovarian cancer59. Using a similar ester linker technology, fatty acids conjugated to cabazitaxel inducted prodrugs and PEG-lipids to self-assemble into nanoparticles. The resulting PEGylated cabazitaxel-prodrug had reduced systemic toxicity and superior therapeutic efficacy when compared to free cabazitaxel in mice bearing breast cancer xenografts60.
Drug derivatization techniques are increasingly used to develop modular nanomedicine platforms. Synthesizing prodrugs that stably incorporate in nanocarriers reduces differences among drug molecules’ physicochemical properties, thereby improving the encapsulation predictability of drugs with diverse characteristics. In addition, modular prodrug nanomedicines can be employed to control localized drug release, which reduces systemic exposure and side effects. For example, a smart prodrug-based modular nanomedicine platform stably co-encapsulated doxorubicin and MMAE (which are physicochemically very different), and allowed for prodrug activation via the administration of a separate nanoparticle, together achieving improved selectivity and treatment efficacy in fibrosarcoma-bearing mice61.
Screening nanomedicine libraries
Drug derivatization and modular concepts combined with rapid and scalable production methods have been expediting the formation of nanomedicine libraries for candidate selection during preclinical nanodrug development. Accordingly, efficient in vitro and in vivo screening methods need to be established to evaluate such libraries. Traditional approaches only vary a few parameters, such as size and surface charge, when exploring a new nanomedicine’s pharmaceutical properties and pharmacological performance. Novel production methods, such as microfluidics, facilitate the generation of large nanomedicinal libraries and thereby promote systematic screening of multiple parameters. Such microfluidic production protocols were employed in the development of BIND-014 (i.e. PSMA-targeted polymeric nanoparticles containing docetaxel)62. To optimize BIND-014, a combinatorial polymeric nanoparticle library was formed and subsequently screened for nanoparticle size, ligand density, drug encapsulation and release kinetics. Formulations with the most favourable characteristics were taken forward for pharmacokinetic, biodistribution, tolerability and therapeutic efficacy studies in animal models, and the most optimal formulation was eventually tested in patients62.
In pursuit of innovative nano-immunotherapeutics, PET imaging and flow cytometry were used to evaluate the biodistribution and immune cell specificity of a library of high-density lipoprotein-mimicking nanoparticles. To advance cancer vaccine development, libraries of lipid nanoparticles containing mRNA encoding for the model protein ovalbumin52 were screened for the percentages of ovalbumin-specific CD8+ T cells 7 days after subcutaneous injection. After a two-step library selection to optimize lipid components and molar composition, the formulation that induced the highest number of specific CD8+ T cells was loaded with mRNA encoding for tumour-associated antigens, resulting in improved survival of mice bearing B16.F10 melanoma tumours52. Barcoding methods are useful in library screening. Deep sequencing of nucleic acid-based barcodes in tissues upon the intravenous administration of 30 barcoded lipid nanoparticles helped to identify a nanoparticle formulation capable of delivering functional siRNA and sgRNA to bone marrow endothelial cells 63,64. Another smart barcoding approach involved the co-loading of different chemotherapeutics together with DNA barcodes in PEGylated liposomes that were administered to mice, followed by tumour biopsy 48 h later. PCR barcode detection and cell viability assessment in isolated tumour cells could predict the most effective chemotherapeutic treatment in a mouse model of breast cancer65.
3. Combination therapies
Depending on their location, grade and stage, cancers are treated with a combination of surgery, radiotherapy, chemotherapy and/or immunotherapy. Strikingly, however, clinical trials involving nanomedicines are generally designed for evaluation in monotherapy settings. This makes it difficult to compare nanodrugs to standard-of-care in terms of efficacy and, more importantly, it does not optimally exploit some of the intrinsic capabilities of nanomedicine formulations. Indeed, nanomedicines combine well with standard chemotherapy treatments, they can co-encapsulate multiple drugs in one formulation and they interact with external stimuli more effectively than drugs in free form. Regarding the latter, (1) nanomedicines depend more on vascular perfusion and permeability than small molecule drugs, and these features profit from using pharmacological and physical combination therapies; (2) nanomedicines deliver higher amounts of drugs to pathological sites, and keep them there for prolonged periods of time, thereby beneficially affecting locally applied physical combination therapies; and (3) nanomedicines can be designed to specifically respond to locally applied physical triggers, e.g. via inducing drug release. These aspects should be exploited more extensively to create more effective anticancer combination therapies (Figure 4).
Figure 4. Smart cancer nanomedicine-based combination therapies.
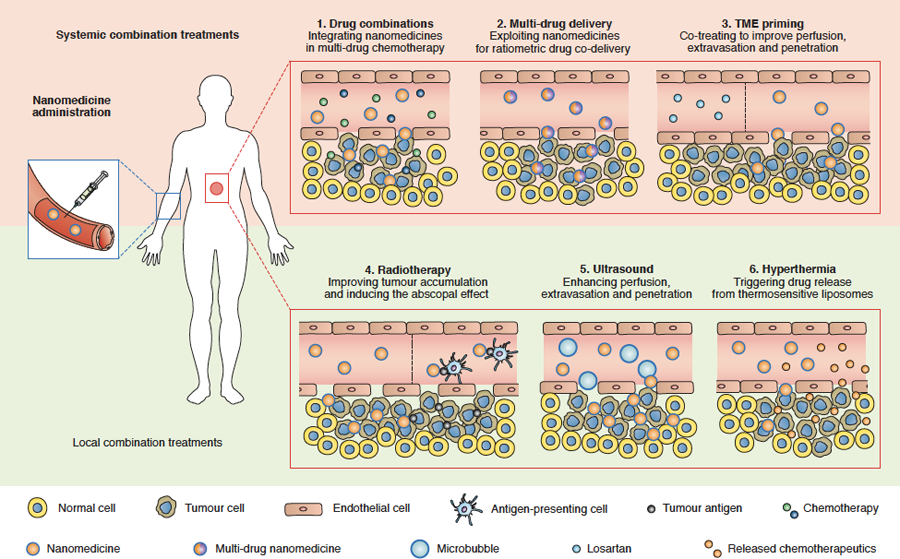
Systemic combinations: (1) Integrating nanomedicines in multimodal chemotherapy regimens results in reduced side effects, better patient compliance and improved quality of life; (2) Co-treatment with approved drugs, such as losartan, helps prime blood vessels and the tumour microenvironment (TME) for improved delivery, thereby enhancing the accumulation, penetration and efficacy of cancer nanomedicines; (3) Nanomedicines enable ratiometric multi-drug delivery, which contributes to synergistic drug effects by improving control over pharmacokinetic and pharmacodynamic interactions. Local combination: (4) Radiotherapy treatment alters the TME to improve the accumulation, penetration, retention and efficacy of cancer nanomedicines. In addition, nanomedicines can potentiate the abscopal effect upon radiotherapy; (5) Ultrasound and microbubbles can induce sonopermeation, thereby increasing vascular perfusion and permeability, and nanomedicine accumulation and efficacy; (6) Hyperthermia can be used to locally trigger payload release from temperature-sensitive liposomes, resulting in increased drug concentrations at the pathological site and less systemic drug exposure, thereby improving the balance between drug efficacy and toxicity.
Systemic combination therapies
The value of incorporating nanomedicines in systemic combination therapies is exemplified by liposomal irinotecan (Onivyde®), which has recently been approved in combination with 5-fluorouracil (5-FU) and leucovorin (LV) to treat pancreatic cancer. Although irinotecan is a very potent anticancer drug, its application in free form is hampered by severe side effects that lead to poor patient compliance. While liposomal irinotecan alone did not improve overall survival as compared to 5-FU/LV in pancreatic cancer patients, the combination of liposomal irinotecan and 5-FU/LV significantly improved overall survival with very acceptable toxicity profiles66,67. A similarly promising systemic combination therapy is nanoparticle albumin-bound paclitaxel (Abraxane®) combined with the immune checkpoint inhibitor atezolizumab, which together induced unprecedented therapeutic responses in triple-negative breast cancer patients68.
Cancer nanomedicines are increasingly combined with systemically administered drugs that help overcome barriers associated with tumour-targeted drug delivery. Multiple biological, immunological and translational barriers have been identified that need to be tackled when aiming to increase cancer nanomedicine’s clinical impact and performance5. Biological barriers include the dense extracellular matrix (ECM), the high interstitial fluid pressure (IFP) and the high metabolism in tumours. The angiotensin II receptor inhibitor losartan can be used to inhibit TGFβ signalling and attenuate collagen production by cancer-associated fibroblasts, thereby reducing the ECM content and the IFP in tumours, and improving the delivery and efficacy of both conventional nanomedicines and 100 nm-sized oncolytic viruses69,70. Recently, losartan has been shown to substantially increase the fraction of R0 resections upon FOLFIRINOX-based neoadjuvant chemotherapy in pancreatic cancer, likely via alleviating the impact of some of the above biological barriers71,72. The enhanced cellular metabolism within tumours results in a relatively low extracellular pH (pH 6.5–7) and re-adjusting this metabolic phenotype can help to promote the performance of drug and drug delivery systems. As an example, in a recent study, 100 nm liposomes loaded with sodium bicarbonate were shown to be able to increase the intratumoural pH to ~7.4, thereby enhancing the cellular uptake of doxorubicin via preventing its protonation and hydrophilization73.
Nanomedicines are also intrinsically very useful for systemic combination therapy. For example, Vyxeos™ is a liposomal nanomedicine formulation co-loaded with cytarabine and daunorubicin at a synergistic drug ratio of 5:1, ensuring controllable pharmacokinetics and co-delivery for both entrapped agents, that can be used to ‘ratiometrically’ kill cancer cells. In a phase III trial in patients with acute myeloid leukaemia, Vyxeos™ improved survival as compared to standard-of-care from 6 to 10 months74, resulting in fast-track FDA approval.
Local combination therapies
Cancer nanomedicines combine well with locally confined treatment modalities, such as radiotherapy, ultrasound and hyperthermia. Pre-treating tumours with radiotherapy increases nanomedicine accumulation and penetration, and vice versa, nanocarrier materials improve radiochemotherapy outcomes75,76. Radiotherapy combined with cyclophosphamide can prime tumours for more efficient delivery and efficacy of liposomal irinotecan77. In addition, nanomedicines can capture tumour antigens released by radiotherapy and subsequently deliver them to antigen-presenting cells, resulting in significantly improved immunotherapy outcomes via promotion of the abscopal effect78.
Ultrasound can be combined with microbubbles to enhance tumour-targeted drug delivery via sonopermeation79, the effects of which include opening inter-endothelial junctions, promoting endocytosis and transcytosis, and – particularly in highly stromal tumours – improving vascular perfusion5. Initial clinical proof-of-concept for sonopermeation showed that combining gemcitabine with ultrasound and microbubbles in pancreatic cancer patients resulted in a median survival time of 18 months, as compared to 9 months for a historical cohort treated with gemcitabine alone80. When combined with intravenously administered microbubbles, a 1 cm-sized skull-implantable ultrasound device termed SonoCloud can open up the blood-brain barrier (BBB), promoting carboplatin delivery in patients with glioblastoma81. For externally applied ultrasound, magnetic resonance-guided focused ultrasound can feasibly and safely improve (nano-) drug delivery in patients with primary brain tumours 82.
The clinically most advanced approach combining nanomedicines with locally applied physical treatments involves the use of temperature-sensitive doxorubicin-loaded liposomes (Thermodox®). In combination with radiofrequency ablation-based hyperthermia, Thermodox® did not meet its primary endpoint in a phase III trial for treatment of hepatocellular carcinoma (HCC), for a number of reasons. It did however demonstrate improved progression-free and overall survival in a subset of patients83. A follow-up phase III trial in HCC, with rigorously refined RFA settings, has recently been completed, and the outcomes of this study are eagerly awaited84. Furthermore, in the last couple of years, several additional trials have recently been initiated in which Thermodox® is combined with focused ultrasound-based hyperthermia, e.g. for breast cancer and certain paediatric tumours79,85.
4. Immunomodulation
Immune checkpoint inhibitors86 and chimeric antigen receptor (CAR) T cell therapies87 are radically changing the cancer therapy landscape, as evidenced by remarkable recent clinical results. Empowering the body’s immune system has produced long-lasting anti-tumour responses in patients with cancer types and stages that were deemed very difficult or impossible to treat. Most impressively, long-term follow-up studies show that a subset of patients have experienced complete remission, thus far for up to a decade. Unfortunately, however, the majority of cancer patients do not respond to immunotherapy. To improve immunotherapy outcomes, we have to expand our understanding of both systemic anti-tumour immunity and the local tumour immune microenvironment (TIME)88. This knowledge will guide the development of drugs and drug delivery systems that boost the efficacy of immuno-oncological interventions5.
Since its inception in the late 1990s, cancer nanomedicine development has been based on designing drug delivery systems that evade the immune system and transport therapeutic payloads directly to tumour cells. We now understand that nanomedicines’ true potential may actually lie in their ability to engage the immune system. Deploying nanotherapeutics for immuno-oncological purposes89–91 necessitates nanoformulations that can smartly modulate the adaptive and/or the innate immune system (Figure 5). We anticipate it will soon become feasible to engineer nanomedicines that boost the immune system’s ability to fight cancer and/or improve the efficacy of existing immunotherapies while mitigating some of their side effects, which can be quite severe92,93.
Figure 5. Smart immunomodulation involving cancer nanomedicine.
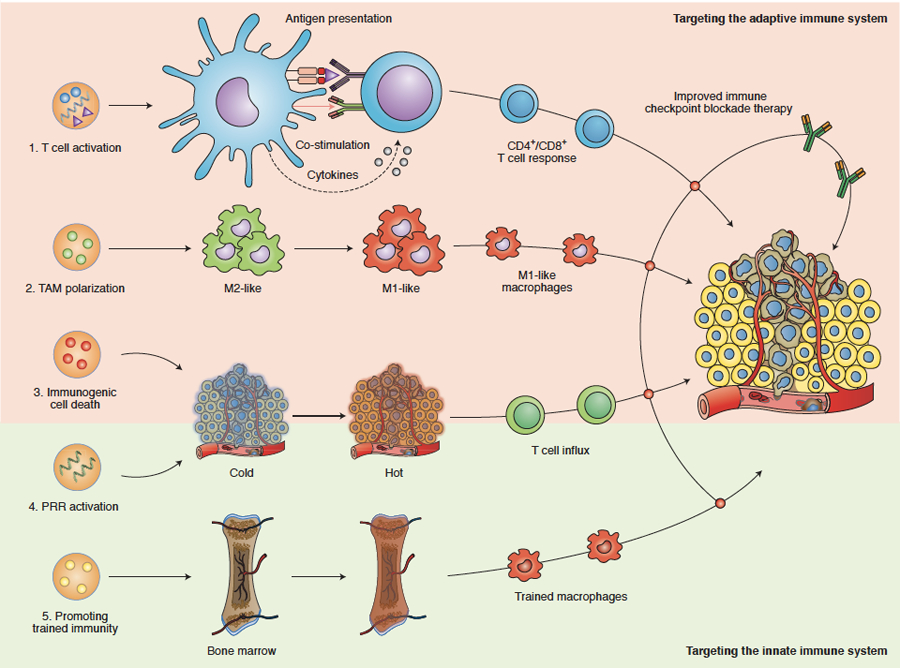
Nanomedicines can be designed to target and modulate components of the adaptive and innate immune systems, thereby improving the outcome of immune checkpoint inhibition therapy. Adaptive immune system-targeted approaches include: (1) Directly targeting antigen-presenting cells by using nanomedicines to deliver (RNA encoding for) tumour antigens and/or drug molecules that modulate co-stimulation and cytokine production. These approaches result in anti-tumour effects mediated via generating and/or activating CD4+ and CD8+ T cells. (2) Nanomedicines can change the polarization of tumour-associated macrophages (TAM) from a pro-tumour and anti-immunotherapy (M2-like) phenotype into a more anti-tumour and pro-immunotherapy (M1-like) phenotype. (3) Nanomedicines containing chemotherapeutic drugs, such as doxorubicin and oxaliplatin, can boost the induction of immunogenic cell death (ICD), which helps reprogram immunogenically ‘cold’ tumours into ‘hot’ tumours. Innate immune system-targeted approaches include: (4) Reprogramming tumours into an immunogenically ‘hot’ phenotype via the activation of pattern recognition receptors (PRRs), eliciting a type I interferon response and inducing anti-tumour T cell immunity. (5) Nanomedicines can be developed to deliver drugs to myeloid cells and their progenitors in the bone marrow, resulting in specific metabolic and epigenetic changes. The ensuing myeloid cells’ hyperresponsiveness towards secondary stimuli has become known as “trained immunity” and may help improve the efficacy of (checkpoint inhibition-based) cancer immunotherapy.
Targeting the adaptive immune system
In line with currently approved anticancer immunotherapeutics, most immunomodulating nanomedicines target the adaptive immune system. These formulations are generally designed to elicit anti-tumour effects via generating CD4+ and CD8+ T cell responses, inducing or improving antigen presentation (signal 1), modulating co-stimulatory signals (signal 2) and/or triggering cytokine production (signal 3). In addition, immunomodulating nanomedicines can be devised to alter the TIME and thereby increase susceptibility to immunotherapy.
Nanomedicines that deliver chemotherapeutics such as doxorubicin and oxaliplatin to tumours can promote anti-tumour immunity by inducing immunogenic cell death (ICD), thereby potentiating the effects of checkpoint blockade immunotherapeutics94. A key reason for why ICD-inducing nanomedicines generate better immunotherapy outcomes than ICD-inducing free drugs is that systemic drug exposure is reduced in case of the former, resulting in less systemic immunodepression. Nanomedicines can also curtail the activity of soluble immuno-inhibitors, like indoleamine 2,3-dioxygenase (IDO). IDO plays a key role in cancer immune metabolism, promoting the conversion of tryptophan to kynurenine, thereby inducing immunosuppression95. Nanomedicines containing IDO inhibitors inactivate this immunosuppressive metabolic pathway. As an example, nanoparticles containing oxaliplatin and a small molecule IDO inhibitor induced regression in pancreatic ductal adenocarcinoma mouse models following both local and systemic administration96. Similarly, in a mouse model of metastatic breast cancer, the therapeutic efficacy of anti-PD-1 therapy was improved by a nano-co-formulation of doxorubicin and an IDO inhibitor97.
As described in the Drug selection section, developing personalized cancer vaccines is the most clinically advanced genetic drug application in oncology. These vaccines consist of an ionizable lipid-based carrier system loaded with mRNA encoding for patient-specific tumour antigens50–52. In such setups, the nanocarrier formulation protects the payload following subcutaneous52 or intravenous50,51 administration, thereby ensuring efficient antigen delivery to antigen-presenting cells and subsequently inducing effective anti-tumour T cell responses. The interchangeable RNA payloads can be designed to encode either for tumour-associated self-antigens shared by patients with a specific tumour type or for patient-specific neoepitopes, thus creating truly personalized cancer vaccines47.
Analogous to some of the results described in the Combination therapies section, studies show that under certain circumstances, radiotherapy effects can synergize with immune checkpoint blockade-based interventions98. In this regard there is growing evidence that the abscopal effect, whereby local radiotherapy treatment at the primary tumour site can induce regression in distant metastatic lesions, can augment immunotherapy, particularly when combined with smart nanomedicine formulations99.
Targeting the innate immune system
Most nano-immunotherapies target the adaptive immune system; modulating components of the innate immune system has thus far remained largely unexplored91,100. The innate immune system functions as a rapid and relatively non-specific first line of defence against infections, mediated by the complement system and by cells such as phagocytes and natural killer cells. These cells contain pattern recognition receptors (PRRs), which detect pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs). PAMPs and DAMPs can induce an innate immune response and subsequently activate the adaptive immune system91. It is becoming increasingly clear that metabolic and epigenetic modifications underlying these responses coordinate a primitive form of innate immune memory referred to as ‘trained immunity’101,102, which is systemically regulated and preserved by hematopoietic stem cells and progenitor cells in the bone marrow. Accordingly, nanomedicines with bone marrow avidity may help to induce trained immunity to suppress local and metastatic tumour growth91.
Alternatively, nanomedicine-based approaches can be designed to induce innate anti-tumour immune responses via activation of PRRs on dendritic cells (DCs) in the TIME, including toll-like receptors (TLRs), retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs) and stimulator of interferon genes (STING). Activating PRRs results in multiple signalling cascades such as transcription of type I interferons (IFN-1). In turn, IFN-1 activates DCs and induces anti-tumour T cell immunity, providing opportunities to reprogram immunologically ‘cold’ tumours into ‘hot’ tumours that are susceptible to checkpoint inhibitor therapy. As an example, cyclodextrin nanoparticles encapsulating the TLR7/8 agonist R848 have been shown to enhance cancer immunotherapy, by promoting the polarization of TAM towards an M1-like phenotype95. As a monotherapy, the nanoparticles modulated the immunosuppressive TIME to inhibit tumour growth. The R848-containing formulation furthermore boosted checkpoint inhibitor therapy, in a tumour model unresponsive to anti-PD-1 monotherapy. RLRs are sensor proteins that recognize cytosolic double-stranded RNA. RIG-1 preferentially binds 5’-triphosphorylated RNA and short double-stranded RNA. As such, bispecific siRNAs with 5’-triphosphate ends can be designed to specifically silence an oncogene and to also activate RIG-1. Exploiting this dual strategy, treatment with lipid-coated calcium phosphate nanoparticles containing double-stranded 5’-triphosphorylated anti-Bcl2 siRNA inhibited tumour growth and prolonged survival in mice with orthotopic pancreatic cancer96. STING is a key regulatory protein that triggers an IFN-1 response upon detection of cytosolic double-stranded DNA (dsDNA). DsDNA is recognized by cyclic GMP-AMP synthase (cGAS), which induces the production of cyclic guanosine monophosphate-adenosine monophosphate (cGAMP; i.e. the high affinity ligand for STING), resulting in a type I interferon response103. Nanomedicines are very useful for activating this type of immune response, as cGAMP and other STING agonists are cyclic dinucleotides, and need to be present intracellularly to be recognized by cGAS. As an example, polymersomes designed to enhance cytosolic cGAMP delivery potently inhibited tumour growth and prolonged survival in a mouse melanoma model (alone and in combination with checkpoint inhibitor therapy)104.
Outlook
Improving the clinical impact of cancer nanomedicines requires smart thinking and rational and realistic reasoning. In this perspective, we present four strategic directions to boost cancer nanomedicine performance, translation and exploitation. Like in other areas of oncology drug development, probes and protocols for patient stratification are urgently needed to refine cancer nanomedicine clinical trials. Smart strategies for modular (pro)drug and nanocarrier design as well as library screening will help to maximize the chances that those formulations developed and tested preclinically will eventually perform well in patients. Rationally designed pharmacological and physical combination regimens will amplify the pharmacokinetic and/or pharmacodynamic benefits conferred by entrapping drugs in nanomedicine formulations. Finally, uncovering which pathophysiological features constrain the efficacy of current cancer immunotherapies, and developing immunomodulatory nanomedicines accordingly, will help to improve the outcomes of immuno-oncological interventions and increase the number of long-term survivors.
Beyond the strategies discussed above, several additional directions can be considered to assist in improving the impact of cancer nanomedicine. For instance, the field may profit from implementing guidelines for minimal information reporting to standardize preclinical nanomedicine research and thus promote reproducibility, quantitative comparisons, meta-analyses and modelling105. Furthermore, while the majority of nanomedicine formulations are developed for intravenous application, it may be advantageous to also explore other administration routes. For example, certain antibody-based therapeutics, such as Humira® (i.e. adalimumab; anti-TNF), work well upon subcutaneous self-administration, which provides the advantage of at-home self-administration versus clinical intravenous administration, thereby mitigating the need for hospitalization. Such alternative route of administration, which have thus far received relatively little consideration in case of nanomedicines, may provide significant advantages in terms of applicability, cost, efficacy and toxicity.
The cancer nanomedicine field has expanded exponentially in recent years. In stark contrast to the numerous new materials and papers that are being produced, only about a dozen nanomedicinal anticancer drugs (antibody-drug conjugates included) have thus far made it to the market. To change this situation, we must move away from continuously making increasingly complex nanomedicine materials and critically reconsider how we are doing translational cancer nanomedicine research. We must establish smart strategies to make nanomedicines work, in as many patients as possible. This shift requires rational and realistic thinking, and integrated and concerted efforts from consortia comprising academics, clinicians, pharmaceutical companies and regulatory authorities. The strategic directions outlined in this manuscript aim to streamline translational cancer nanomedicine research and they will help to promote the clinical impact and patient performance of nanomedicinal anticancer drugs.
Acknowledgements
R.v.d.M. is supported by The Netherlands Organization for Scientific Research (NWO; Veni STW grant #14385). E.S. is supported by the Central Norway Regional Health Authority (#46084000). Y.S. and T.L. acknowledge support by the European Union (EFRE: I3-STM (#0800387)). F.K. and T.L. are supported by the German Research Foundation (DFG: GRK2375: #331065168). W.J.M.M. is supported by the National Institutes of Health (NIH: R01 HL118440, R01 HL125703, P01 HL131478) and by the Netherlands Organization for Scientific Research (NWO; ZonMW Vici grant #016.176.622). T.L. is supported by the European Research Council (ERC: #309495, #680882, #813086), the European Commission (ERA-NET: NSC4DIPG), the German Research Foundation (DFG: SFB1066, SFB/TRR57) and the Aachen Interdisciplinary Center for Clinical Research (IZKF: #O3–2).
Footnotes
Financial and Non-Financial Competing Interest statement
The authors have no financial and non-financial competing interests to declare.
References
- 1.Peer D et al. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2, 751–760 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Shi J, Kantoff PW, Wooster R & Farokhzad OC Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer 17, 20–37 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Björnmalm M, Thurecht KJ, Michael M, Scott AM & Caruso F Bridging bio-nano science and cancer nanomedicine. ACS Nano 11, 9594–9613 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Van der Meel R, Lammers T & Hennink WE Cancer nanomedicines: oversold or underappreciated? Expert Opin. Drug Deliv 14, 1–5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anchordoquy TJ et al. Mechanisms and barriers in cancer nanomedicine: addressing challenges, looking for solutions. ACS Nano 11, 12–18 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cobleigh MA et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J. Clin. Oncol 17, 2639–48 (1999). [DOI] [PubMed] [Google Scholar]
- 7.Baselga J et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N. Engl. J. Med 366, 109–119 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verma S et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med 367, 1783–1791 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le DT et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science (80-.). 357, 409–413 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Autio KA et al. Safety and efficacy of BIND-014, a docetaxel nanoparticle targeting prostate-specific membrane antigen for patients with metastatic castration-resistant prostate cancer: a phase 2 clinical trial. JAMA Oncol 4, 1344–1351 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voss MH et al. A randomized phase II trial of CRLX101 in combination with bevacizumab versus standard of care in patients with advanced renal cell carcinoma. Ann. Oncol 28, 2754–2760 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Fujiwara Y et al. A multi-national, randomised, open-label, parallel, phase III non-inferiority study comparing NK105 and paclitaxel in metastatic or recurrent breast cancer patients. Br. J. Cancer 120, 475–480 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petersen GH, Alzghari SK, Chee W, Sankari SS & La-Beck NM Meta-analysis of clinical and preclinical studies comparing the anticancer efficacy of liposomal versus conventional non-liposomal doxorubicin. J. Control. Release 232, 255–264 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Lammers T, Rizzo LY, Storm G & Kiessling F Personalized nanomedicine. Clin. Cancer Res 18, 4889–4894 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Bravaccini S et al. PSMA expression: A potential ally for the pathologist in prostate cancer diagnosis. Sci. Rep 8, 1–8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han S, Woo S, Kim YJ & Suh CH Impact of 68Ga-PSMA PET on the management of patients with prostate cancer: a systematic review and meta-analysis. Eur. Urol 74, 179–190 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Kunjachan S et al. Passive versus active tumor targeting using RGD- and NGR-modified polymeric nanomedicines. Nano Lett 14, 972–981 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juweid M et al. Micropharmacology of monoclonal antibodies in solid tumors: direct experimental evidence for a binding site barrier. Cancer Res 52, 5144–53 (1992). [PubMed] [Google Scholar]
- 19.Allen TM & Cullis PR Liposomal drug delivery systems: from concept to clinical applications. Adv. Drug Deliv. Rev 65, 36–48 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Tsvetkova Y et al. Balancing passive and active targeting to different tumor compartments using riboflavin-functionalized polymeric nanocarriers. Nano Lett 17, 4665–4674 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Choi CHJ, Alabi CA, Webster P & Davis ME Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles. Proc. Natl. Acad. Sci. U. S. A 107, 1235–40 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirpotin DB et al. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res 66, 6732–6740 (2006). [DOI] [PubMed] [Google Scholar]
- 23.Miller MA, Arlauckas S & Weissleder R Prediction of anti-cancer nanotherapy efficacy by imaging. Nanotheranostics 1, 296–312 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen H, Zhang W, Zhu G, Xie J & Chen X Rethinking cancer nanotheranostics. Nat. Rev. Mater 2, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller MA et al. Predicting therapeutic nanomedicine efficacy using a companion magnetic resonance imaging nanoparticle. Sci. Transl. Med 7, 314ra183–314ra183 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramanathan RK et al. Correlation between ferumoxytol uptake in tumor lesions by MRI and response to nanoliposomal irinotecan in patients with advanced solid tumors: a pilot study. Clin. Cancer Res 23, 3638–3648 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Perez-Medina C et al. Nanoreporter PET predicts the efficacy of anti-cancer nanotherapy. Nat. Commun 7, 11838 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vander Heiden MG, Cantley LC & Thompson CB Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–33 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsu PP & Sabatini DM Cancer cell metabolism: Warburg and beyond. Cell 134, 703–707 (2008). [DOI] [PubMed] [Google Scholar]
- 30.Jørgensen JT, Norregaard K, Martín MS, Oddershede LB & Kjaer A Non-invasive early response monitoring of nanoparticle-assisted photothermal cancer therapy using 18F-FDG, 18F-FLT, and 18F-FET PET/CT imaging. Nanotheranostics 2, 201–210 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phillips E et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci. Transl. Med 6, 260ra149 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altman BJ, Stine ZE & Dang CV From Krebs to clinic: glutamine metabolism to cancer therapy. Nat. Rev. Cancer 16, 619–634 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Bermudez J et al. Aspartate is a limiting metabolite for cancer cell proliferation under hypoxia and in tumours. Nat. Cell Biol 20, 775–781 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knott SRV et al. Asparagine bioavailability governs metastasis in a model of breast cancer. Nature 554, 378–381 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee H et al. 64Cu-MM-302 positron emission tomography quantifies variability of enhanced permeability and retention of nanoparticles in relation to treatment response in patients with metastatic breast cancer. Clin. Cancer Res 23, 4190–4202 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edmonds S et al. Exploiting the metal-chelating properties of the drug cargo for in vivo positron emission tomography imaging of liposomal nanomedicines. ACS Nano 10, 10294–10307 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barenholz Y Doxil® - the first FDA-approved nano-drug: lessons learned. J. Control. Release 160, 117–134 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Miller MA et al. Tumour-associated macrophages act as a slow-release reservoir of nano-therapeutic Pt(IV) pro-drug. Nat. Commun 6, 1–13 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karageorgis A et al. An MRI-based classification scheme to predict passive access of 5 to 50-nm large nanoparticles to tumors. Sci. Rep 6, 21417 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sulheim E et al. Multi-modal characterization of vasculature and nanoparticle accumulation in five tumor xenograft models. J. Control. Release 279, 292–305 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Theek B et al. Histidine-rich glycoprotein-induced vascular normalization improves EPR-mediated drug targeting to and into tumors. J. Control. Release 282, 25–34 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hare JI et al. Challenges and strategies in anti-cancer nanomedicine development: an industry perspective. Adv Drug Deliv Rev 108, 25–38 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Szebeni J, Simberg D, González-Fernández Á, Barenholz Y & Dobrovolskaia MA Roadmap and strategy for overcoming infusion reactions to nanomedicines. Nat. Nanotechnol 13, 1100–1108 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qi R et al. Nanoparticle conjugates of a highly potent toxin enhance safety and circumvent platinum resistance in ovarian cancer. Nat. Commun 8, 2166 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ashton S et al. Aurora kinase inhibitor nanoparticles target tumors with favorable therapeutic index in vivo. Sci. Transl. Med 8, 325ra17 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Adams D et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med 379, 11–21 (2018). [DOI] [PubMed] [Google Scholar]
- 47.Sahin U & Türeci Ö Personalized vaccines for cancer immunotherapy. Science (80-.). 359, 1355–1360 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Pardi N, Hogan MJ, Porter FW & Weissman D mRNA vaccines - a new era in vaccinology. Nat. Rev. Drug Discov 17, 261–279 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pardi N et al. Administration of nucleoside-modified mRNA encoding broadly neutralizing antibody protects humanized mice from HIV-1 challenge. Nat. Commun 8, 14630 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kranz LM et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534, 396–401 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Kreiter S et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature 520, 692–696 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oberli MA et al. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett 17, 1326–1335 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sahin U et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547, 222–226 (2017). [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y, Li N, Suh H & Irvine DJ Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nat. Commun 9, 6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Charych DH et al. NKTR-214, an engineered cytokine with biased IL2 receptor binding, increased tumor exposure, and marked efficacy in mouse tumor models. Clin. Cancer Res 22, 680–690 (2016). [DOI] [PubMed] [Google Scholar]
- 56.Bentebibel S-E et al. A first-in-human study and biomarker analysis of NKTR-214, a novel IL2Rβγ-biased cytokine, in patients with advanced or metastatic solid tumors. Cancer Discov 9, 711–721 (2019). [DOI] [PubMed] [Google Scholar]
- 57.Cern A, Marcus D, Tropsha A, Barenholz Y & Goldblum A New drug candidates for liposomal delivery identified by computer modeling of liposomes’ remote loading and leakage. J. Control. Release 252, 18–27 (2017). [DOI] [PubMed] [Google Scholar]
- 58.Hu Q et al. Complete regression of breast tumour with a single dose of docetaxel-entrapped core-cross-linked polymeric micelles. Biomaterials 53, 370–378 (2015). [DOI] [PubMed] [Google Scholar]
- 59.Therapeutics Cristal. Efficacy study of CPC634 (CriPec® docetaxel) in platinum resistant ovarian cancer (CINOVA). (2018). Available at: https://clinicaltrials.gov/ct2/show/NCT03742713. (Accessed: 15th March 2019)
- 60.Wang H et al. New generation nanomedicines constructed from self-assembling small-molecule prodrugs alleviate cancer drug toxicity. Cancer Res 77, 6963–6974 (2017). [DOI] [PubMed] [Google Scholar]
- 61.Miller MA et al. Modular nanoparticulate prodrug design enables efficient treatment of solid tumors using bioorthogonal activation. ACS Nano 12, 12814–12826 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hrkach J et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci. Transl. Med 4, 128ra39 (2012). [DOI] [PubMed] [Google Scholar]
- 63.Wang ET et al. Barcoded nanoparticles for high throughput in vivo discovery of targeted therapeutics. Proc. Natl. Acad. Sci 114, 2060–2065 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sago CD et al. Nanoparticles that deliver RNA to bone marrow identified by in vivo directed evolution. J. Am. Chem. Soc 140, 17095–17105 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yaari Z et al. Theranostic barcoded nanoparticles for personalized cancer medicine. Nat. Commun 7, 13325 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang-Gillam A et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 387, 545–557 (2016). [DOI] [PubMed] [Google Scholar]
- 67.Chen LT et al. Final results of NAPOLI-1: a phase 3 study of nal-IRI (MM-398) ± 5-fluorouracil and leucovorin (5-FU/LV) vs 5-FU/LV in metastatic pancreatic cancer (mPAC) previously treated with gemcitabine-based therapy. Ann. Oncol 27, 622PD–622PD (2016). [Google Scholar]
- 68.Schmid P et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med 379, 2108–2121 (2018). [DOI] [PubMed] [Google Scholar]
- 69.Chauhan VP et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Commun 4, 2516 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y & Jain RK Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc. Natl. Acad. Sci. U. S. A 108, 2909–2914 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murphy JE et al. Potentially curative combination of TGF-b1 inhibitor losartan and FOLFIRINOX (FFX) for locally advanced pancreatic cancer (LAPC): R0 resection rates and preliminary survival data from a prospective phase II study. J. Clin. Oncol 36, 4116 (2018). [Google Scholar]
- 72.Murphy JE et al. Total neoadjuvant therapy with FOLFIRINOX in combination with losartan followed by chemoradiotherapy for locally advanced pancreatic cancer. JAMA Oncol 5, 1020 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abumanhal-Masarweh H et al. Sodium bicarbonate nanoparticles modulate the tumor pH and enhance the cellular uptake of doxorubicin. J. Control. Release 296, 1–13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lancet JE et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J. Clin. Oncol 36, 2684–2692 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davies CDL et al. Radiation improves the distribution and uptake of liposomal doxorubicin (Caelyx) in human osteosarcoma xenografts. Cancer Res 64, 547–553 (2004). [DOI] [PubMed] [Google Scholar]
- 76.Lammers T et al. Image-guided and passively tumour-targeted polymeric nanomedicines for radiochemotherapy. Br. J. Cancer 99, 900–910 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miller MA et al. Radiation therapy primes tumors for nanotherapeutic delivery via macrophage-mediated vascular bursts. Sci. Transl. Med 9, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Min Y et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat Nanotechnol 12, 877–882 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Snipstad S et al. Sonopermeation to improve drug delivery to tumors: from fundamental understanding to clinical translation. Expert Opinion on Drug Delivery 15, 1249–1261 (2018). [DOI] [PubMed] [Google Scholar]
- 80.Dimcevski G et al. A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer. J. Control. Release 243, 172–181 (2016). [DOI] [PubMed] [Google Scholar]
- 81.Carpentier A et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci. Transl. Med 8, 343re2–343re2 (2016). [DOI] [PubMed] [Google Scholar]
- 82.Mainprize T et al. Blood-brain barrier opening in primary brain tumors with non-invasive MR-guided focused ultrasound: a clinical safety and feasibility study. Sci. Rep 9, 321 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tak WY et al. Phase III HEAT study adding lyso-thermosensitive liposomal doxorubicin to radiofrequency ablation in patients with unresectable hepatocellular carcinoma lesions. Clin. Cancer Res 24, 73–83 (2018). [DOI] [PubMed] [Google Scholar]
- 84.Celsion. Study of ThermoDox with standardized radiofrequency ablation (RFA) for treatment of hepatocellular carcinoma (HCC). (2014). Available at: https://clinicaltrials.gov/ct2/show/NCT02112656. (Accessed: 15th March 2019)
- 85.Kim AeRang. A phase I study of lyso-thermosensitive liposomal doxorubicin and MR-HIFU for pediatric refractory solid tumors. (2015). Available at: https://clinicaltrials.gov/ct2/show/NCT02536183?term=thermodox&rank=10. (Accessed: 15th March 2019)
- 86.Ribas A & Wolchok JD Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.June CH et al. CAR T cell immunotherapy for human cancer. Science (80-.). 359, 1361–1365 (2018). [DOI] [PubMed] [Google Scholar]
- 88.Jain RK et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med 24, 541–550 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Riley RS, June CH, Langer R & Mitchell MJ Delivery technologies for cancer immunotherapy. Nature Reviews Drug Discovery 18, 175–196 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun Q et al. Nanomedicine and macroscale materials in immuno-oncology. Chemical Society Reviews 48, 351–381 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mulder WJM, Ochando J, Joosten LAB, Fayad ZA & Netea MG Therapeutic targeting of trained immunity. Nat. Rev. Drug Discov 18, 553–566 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jiang W et al. Designing nanomedicine for immuno-oncology. Nat. Biomed. Eng 1, 29 (2017). [Google Scholar]
- 93.Friedman CF, Proverbs-Singh TA & Postow MA Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA oncology 2, 1346–1353 (2016). [DOI] [PubMed] [Google Scholar]
- 94.Pfirschke C et al. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity 44, 343–354 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Prendergast GC, Malachowski WP, DuHadaway JB & Muller AJ Discovery of IDO1 inhibitors: from bench to bedside. Cancer Res 77, 6795–6811 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lu J et al. Nano-enabled pancreas cancer immunotherapy using immunogenic cell death and reversing immunosuppression. Nat. Commun 8, 1811 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lu J et al. Breast cancer chemo-immunotherapy through liposomal delivery of an immunogenic cell death stimulus plus interference in the IDO-1 pathway. ACS Nano 12, 11041–11061 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 98.Hwang WL, Pike LRG, Royce TJ, Mahal BA & Loeffler JS Safety of combining radiotherapy with immune-checkpoint inhibition. Nature Reviews Clinical Oncology 15, 477–494 (2018). [DOI] [PubMed] [Google Scholar]
- 99.Ngwa W et al. Using immunotherapy to boost the abscopal effect. Nat. Rev. Cancer 18, 313 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Borden EC Interferons α and β in cancer: therapeutic opportunities from new insights. Nature Reviews Drug Discovery 18, 219–234 (2019). [DOI] [PubMed] [Google Scholar]
- 101.Netea MG, Latz E, Mills KHG & O’Neill LAJ Innate immune memory: a paradigm shift in understanding host defense. Nat. Immunol 16, 675–679 (2015). [DOI] [PubMed] [Google Scholar]
- 102.Netea MG et al. Trained immunity: A program of innate immune memory in health and disease. Science (80-.). 352, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen Q, Sun L & Chen ZJ Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nature Immunology 17, 1142–1149 (2016). [DOI] [PubMed] [Google Scholar]
- 104.Shae D et al. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat. Nanotechnol 14, 269–278 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Faria M et al. Minimum information reporting in bio–nano experimental literature. Nat. Nanotechnol 13, 777–785 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]


