Abstract
Extractives are an important class of compounds in plants because they contribute to many of their physicochemical properties such as color, odor, density, strength, permeability, and hygroscopicity. Moreover, they also possess significant biological activity and are thus an important part of the plants’ defense mechanisms against biotic and abiotic stresses. Tree needles are a rich source of extractives, counting for as much as 40% of their dry weight. In this study, chemical fingerprinting of essential oils and solvent extracts, obtained from the needles of four conifer tree species (i.e., pine, spruce, larch, and juniper), was performed by using ultrahigh-resolution Fourier transform ion cyclotron mass spectrometry. A wide variety of compounds were detected in the oil samples, including mono-, sesqui-, and diterpenes, terpenoids, fatty and resin acids, esters, and different phenolic compounds. Although the main compounds were present in all the four essential oil samples, large variations in their relative abundances were observed. In contrast, pine needle hexane and toluene extracts showed a high content of resin acids, including pinifolic acid, a rare labdane-type diterpene diacid, and its mono- and dimethyl esters. Thus, by selecting a suitable solvent, specific types of compounds may be isolated from tree needles for further biotechnological or medicinal applications.
Introduction
Plants serve as important raw materials in food and nonfood industrial products. They are rich sources of nonstructural compounds, which find use in pharmaceutical, cosmetics, agricultural, food, and other related industries.1,2 These bioactive plant compounds (phytochemicals) are known for their pronounced antioxidant, antifungal, antibacterial, insecticidal, and herbicidal character.3,4 Bioactive compounds are also the main constituents of essential oils, which are obtained via steam distillation or solvent extraction of plant materials, and have a characteristic aroma or fragrance. Essential oils are complex chemical mixtures of volatile and nonvolatile constituents (e.g., terpene hydrocarbons, acids, esters, and phenolic compounds). These secondary metabolites are important for plants’ defense mechanisms against biotic and abiotic stresses.5−8 Essential oils are a great reservoir of bioactive compounds, hence an increased interest in them.9
Conifers are a group of cone-bearing gymnosperms, found in most terrestrial habitats. They are seed plants and woody plants that are mostly trees, though a few are shrubs.10 Their ability to habituate in extreme environments and a strong defense system have contributed to their successful colonization of the northern hemisphere.11 They also contribute significantly to the terrestrial photosynthesis and biomass production. Conifers are dominant forest tree species in Finland, with Scots pine (Pinus sylvestris L.) and Norway spruce (Picea abies) being the most abundant, occupying ca. 66 and 24% of the forested areas, respectively.12,13 Conifers have complex secondary metabolite profiles, consisting of volatile and nonvolatile compounds.14 They are widely distributed worldwide, comprising seven families with a total of 600–630 species. Conifer trees are an important resource as solid fuel (wood or charcoal) and a source of other plant-derived products (e.g., resins, essential oils, and ornaments).15,16
Various parts of conifers, such as needles, bark, cones, and pollen, are consumed and they have been shown to promote health and prevent some aging-related chronic diseases.17 Essential oils obtained from conifer needles and bark have been widely used as bathing oils, ointments, or inhaling drugs for treating a wide range of disorders of neuralgic, infectious, and rheumatic origin.18,19 Several studies have reported antioxidant,9 antimicrobial,20 antibacterial,21 larvicidal,22 antifungal,19 herbicidal,23 anti-inflammatory,24 and free radical scavenging25 activity of these oils. Also, resins obtained directly from coniferous trees possess a considerable antibacterial/antifungal activity. For instance, a resin salve from Norway spruce has been used to treat infected skin ulcers.26 These properties are dependent on the chemical composition of the oil, which is influenced by the type of extraction method used.27 Ethanol, methanol, and hexane extracts of conifer needles have been reported and they also possess considerable antioxidant, antimutagenic, antitumor, and anticancer activity.16,17
Several methods, such as hydrodistillation, solvent extraction, simultaneous distillation–extraction, and solid-phase microextraction have been used for essential oil production.28 Hydrodistillation is the most widely used method, although some water-soluble compounds are not obtained and thermolabile compounds can be degraded in the process.29 Hydrodistillation can be divided into water distillation and steam distillation. Steam distillation differs from water distillation so that the steam is passed through the raw material and the oil evaporates, whereas in the case of water distillation, the plant material and water are put to the same vessel and allowed to boil. Direct solvent extraction is an alternative method used. This method is dependent on the solubility of compounds and the extracts are usually mixtures of volatile and nonvolatile constituents. Hexane, toluene, or other nonpolar solvents are often used. In this method, resin acids, fats, waxes, and pigments are often extracted, too.28,29
Chemical compositions of essential oils and solvent extracts obtained from conifer trees have been extensively studied by gas chromatography–mass spectrometry (GC–MS).9,16,20,22,23 The compositions of lipophilic extracts of different pine species have also been characterized.30,31 Even though many compounds can be derivatized prior to the GC–MS measurement (in order to improve thermal stability and enhance volatility) and more polar columns can be used,32 GC–MS is still limited to the most volatile compound fractions. As an alternative strategy, high-performance liquid chromatography (HPLC) coupled to MS has also been used to determine the flavonoid content in buds and young needles of Pinus peuce(33) and P. abies and some other Pinus species.34 Other analytical techniques that have been used in the analysis of essential oils include ultraviolet (UV), Fourier transform infrared, and nuclear magnetic resonance (NMR) spectroscopies,35−37 but each of these techniques have their own intrinsic limitations and they do not provide very detailed information on the complex chemical composition.
In this work, we used ultrahigh-resolution Fourier transform ion cyclotron resonance (FT-ICR) MS for direct chemical fingerprinting of essential oils and solvent extracts obtained from the needles of four conifer tree species, namely Scots pine (P. sylvestris), common juniper (Juniperus communis), Norway spruce (P. abies), and European larch (Larix decidua). Two different ionization techniques, negative-ion electrospray ionization, (−)ESI, and positive-ion atmospheric-pressure photoionization, (+)APPI, were employed to target both polar and nonpolar constituents in the oil samples. Based on the results, conifer needle essential oils possess a rich chemistry, which offer a considerable potential for a variety of biotechnological and medicinal applications.
Results and Discussion
Mass Spectra of Conifer Needle Essentials Oils and Solvent Extracts
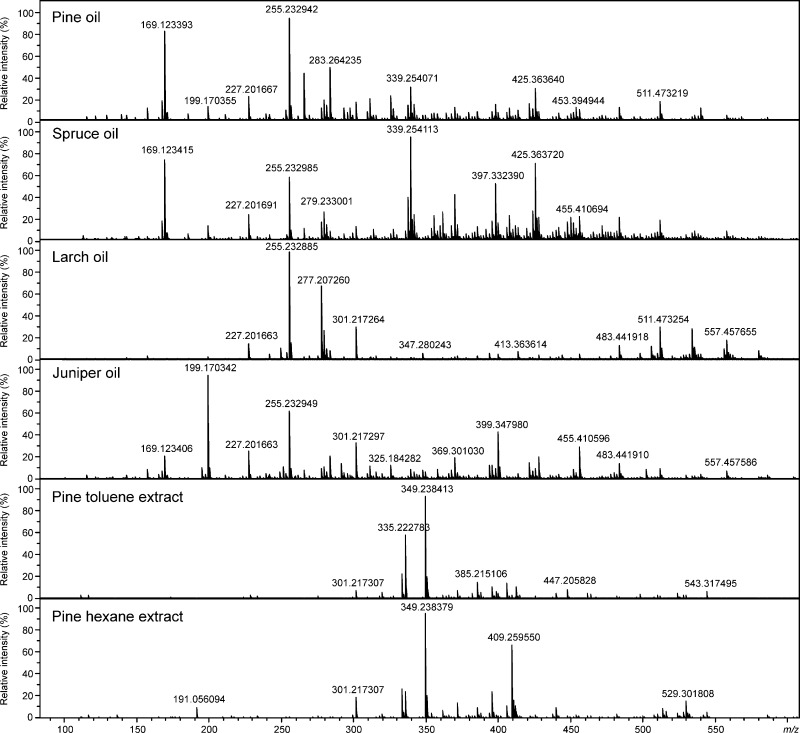
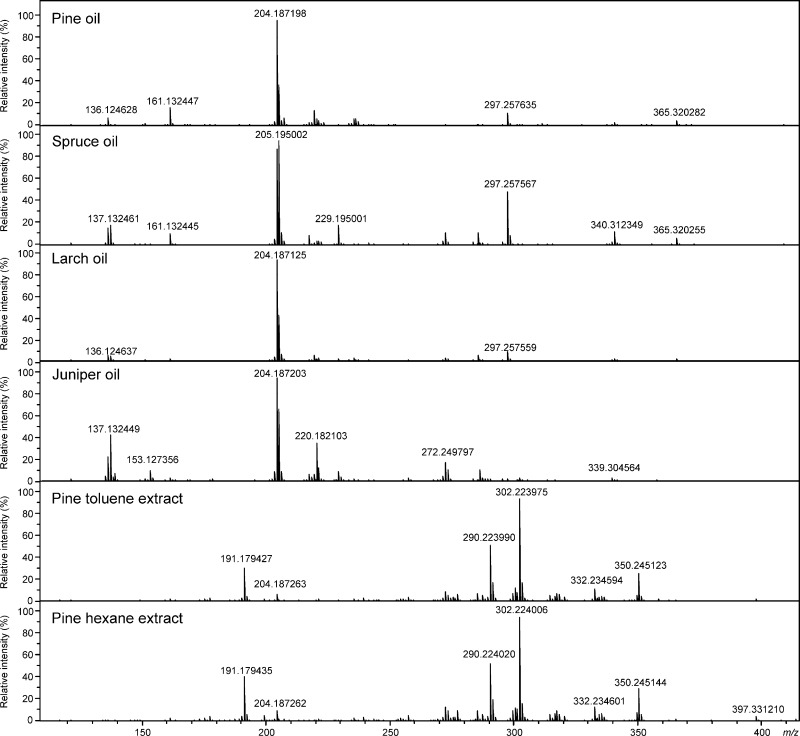
In the (−)ESI FT-ICR mass spectra of conifer needle essential oils (Figure 1), the peaks appeared at m/z 150–600, whereas for the toluene and hexane extracts, the most abundant peaks appeared at m/z 300–450, representing mostly a few resin acids as well as some other diterpenoids. For both solvent extracts, the most abundant ion was observed at m/z 349.238413 and was tentatively identified as pinifolic acid methyl ester (see, discussion below). In the (−)ESI mass spectra, some nonspecific cluster ions (acid dimers) also appeared at a high m/z range. After deisotoping, declustering, and formula assignment, the number of unique monoisotopic peaks detected (at a signal-to-noise ratio of S/N ≥ 5) ranged between 1200 and 1600 in all the samples (Table 1). In contrast, the mass spectra measured with (+)APPI appeared less complex, with the most abundant ions mainly representing monoterpenes, sesquiterpenes, and other hydrocarbons (Figure 2). As compared to (−)ESI, both odd- and even-electron ions (radical cations, M+•, and protonated molecules, [M + H]+) were generated by (+)APPI. The ion observed at m/z 204.187801 was the most abundant in all the oil samples, representing different isomeric sesquiterpenes. This ion also appeared in the mass spectra of the solvent extracts but at much lower abundance. For both solvent extracts, the ion observed at m/z 302.224580, tentatively identified as abietic acid, was the most abundant.
Figure 1.
Negative-ion ESI FT-ICR mass spectra of conifer needle essential oils and solvent extracts.
Table 1. Number of Monoisotopic Peaks (Unique Molecular Formulae)a Assigned for Conifer Needle Essential Oils/Solvent Extracts with (−)ESI and (+)APPI.
| sample | (−)ESI | (+)APPIb |
|---|---|---|
| pine oil | 1513 | 540 (404) |
| spruce oil | 1454 | 828 (571) |
| larch oil | 1637 | 1487 (1017) |
| juniper oil | 1211 | 602 (441) |
| pine toluene extract | 1224 | 1208 (818) |
| pine hexane extract | 1507 | 1011 (690) |
Monoisotopic peaks at S/N ≥ 5.0.
The peak number by counting both [M + H]+ and M•+ ions. The number of unique molecular formulae are given in parentheses.
Figure 2.
Positive-ion APPI FT-ICR mass spectra of conifer needle essential oils and solvent extracts.
Table 1 summarizes the number of unique elemental formulae assigned for all the essential oils and solvent extracts with (−)ESI and (+)APPI.
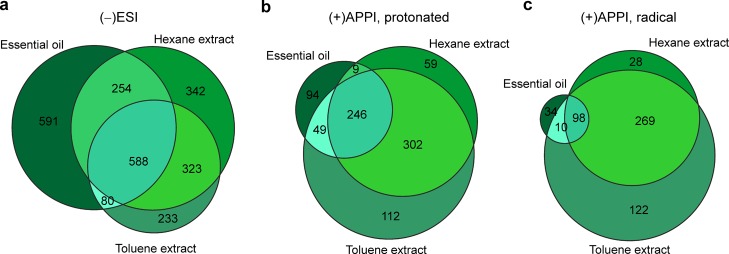
The Venn diagrams in Figure 3 display the number of compounds present in the essential oil and solvent extracts of pine. The Venn diagrams show the number of compounds unique to each extract and the number of compounds found in all extracts, providing a visual means for sample composition differentiation. With (−)ESI, about 24% (588 molecular formulae) of the total assigned formulae were common to all, whereas with (+)APPI, 28 and 17% (249 and 98 formulae), with respect to the protonated molecules and radical cations, respectively, were common to all. More compounds were detected from the solvent extracts as compared to the essential oil. The number of compounds detected by (+)APPI was much less than the number of compounds detected by (−)ESI, except for the pine toluene extract.
Figure 3.
Venn diagrams showing distribution of compounds between pine needle essential oil and two solvent extracts: (a) (−)ESI, (b) (+)APPI (protonated molecules), (c) (+)APPI (radical cations).
Compound Class Distributions
A total of 12 different Ox classes (i.e., compounds containing x oxygen atoms and variable amounts of C and H atoms) were detected for the essential oils and solvent extracts (Figures S1 and S2). The Ox classes of O2–O12 were generally observed with (−)ESI; the most abundant Ox classes for all the samples were O2 and O4. For the solvent extracts, O6 (for hexane extract) and O8 (for toluene extract) were also abundant. The classes O11 and O12 were observed only for the solvent extracts except for a small amount of O11 for the pine essential oil. With (+)APPI, both the hydrocarbon class (HC) and the oxygen atom classes (Ox) were observed. A total of seven different Ox class were observed (O1–O7) with (+)APPI. Unlike (−)ESI, (+)APPI also efficiently ionized compounds belonging to the O1 class. For the essential oils, HC was the most abundant class (ca. 55–90%), whereas for the solvent extracts, O2 was the major one. The classes O3 and O4 were also more abundant for the solvent extracts. Again, the highest Ox classes (O5–O7) were only observed for the solvent extracts. For most compounds, (+)APPI produced both protonated molecules and radical cations, not evenly distributed among different compound classes observed. In some instances, these two ion types can be used to distinguish between alicyclic and aromatic compounds.38 The compound class distributions show that ESI generally ionizes more polar compounds, and does not ionize hydrocarbons or lowly oxidized nonpolar species (O1 class); on the other hand, (+)APPI ionizes nonpolar aromatic compounds, including hydrocarbons. Thus, the use of both ESI and APPI provides complementary compositional information on the oil constituents.
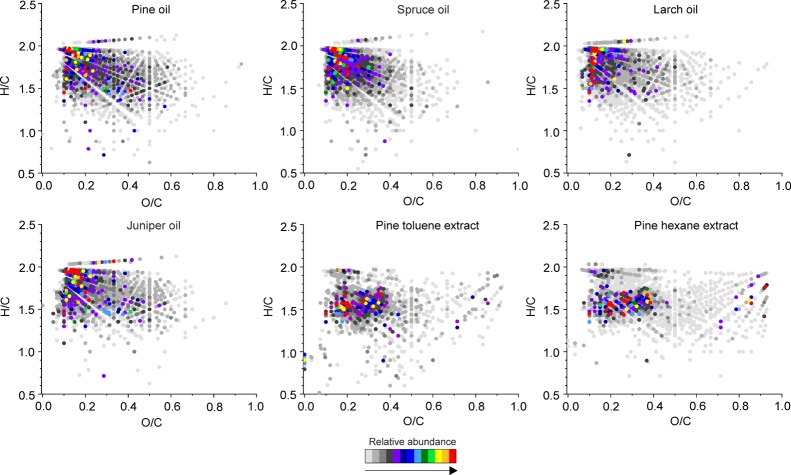
Van Krevelen Diagrams
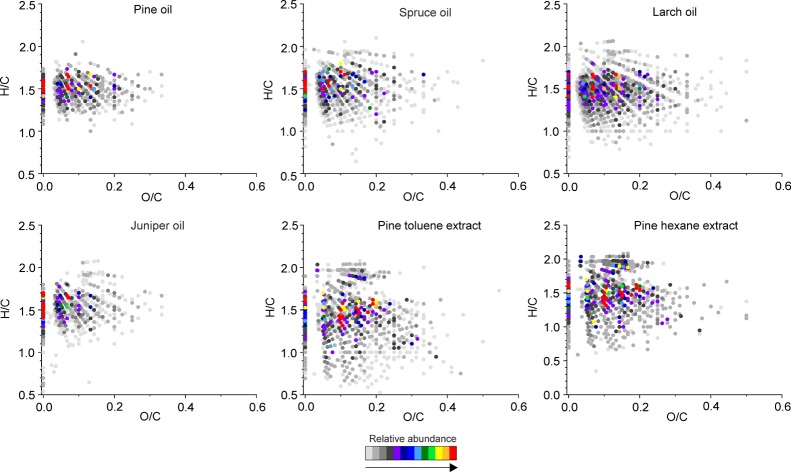
A van Krevelen (VK) diagram is a plot of the atomic H/C versus X/C ratio (where X is a heteroatom, e.g., oxygen) for each detected compound and, thus, a straightforward means for the visualization of complex mass spectrometric data. One can use VK diagrams to have an overall view on the chemical composition of a given sample and to compare different samples. In the VK diagrams of the (−)ESI FT-ICR spectra of the essential oils (Figure 4), the O/C ratio varied between 0 and 0.7, whereas the solvent extracts had a slightly wider range of O/C ≈ 0–1. The most abundant species were centered around O/C ≈ 0.1–0.3 and H/C ≥ 1.5. This is a typical region for lipids (fatty acids, resin acids, diterpenoids). There were no considerable differences between the four essential oil samples. In contrast, the both solvent extracts had generally more condensed (lower H/C) and more oxygenated (higher O/C) species, consistent with compound class distributions (Figure S1). The pine hexane extract also had a few highly abundant species at high O/C ratios (O/C ≈ 0.6–1.0) and H/C ≥ 1.3, representing carbohydrates, whereas the toluene extract had only a few of these compounds at low intensities. Flavonoids were also present around O/C ≈ 0.3–0.5 and H/C ≈ 0.6–1.2 at low intensities in both solvent extracts.
Figure 4.
VK diagrams of the compounds detected in the conifer needle essential oils and solvent extracts by (−)ESI FT-ICR MS.
Unlike by (−)ESI, hydrocarbons were efficiently ionized by (+)APPI (Figure 5). The most abundant species in the essential oils are mono- and sesquiterpene hydrocarbons. They can be seen at O/C = 0 and H/C ≥ 0.5 in the VK diagrams. In addition, many species could be seen around O/C ≈ 0.05–0.1 and H/C ≥ 1, which likely represent different terpenoids. Some resin and fatty acids were also present in the region O/C ≈ 0.1–0.3 and H/C ≈ 1.3–2.3 but there were only a few compounds with high intensity in this region. For all the essential oils, esters were present at low relative intensity, around O/C ≈ 0.1–0.2 and H/C ≈ 1.3–1.5. Diterpenoids (resin acids and their derivatives) were the most dominant compounds in both solvent extracts. The next most abundant were terpene hydrocarbons and then oxygenated terpenes.
Figure 5.
VK diagrams of the compounds detected in the conifer needle essential oils and solvent extracts by (+)APPI FT-ICR MS.
Unique Compounds Detected with (−)ESI
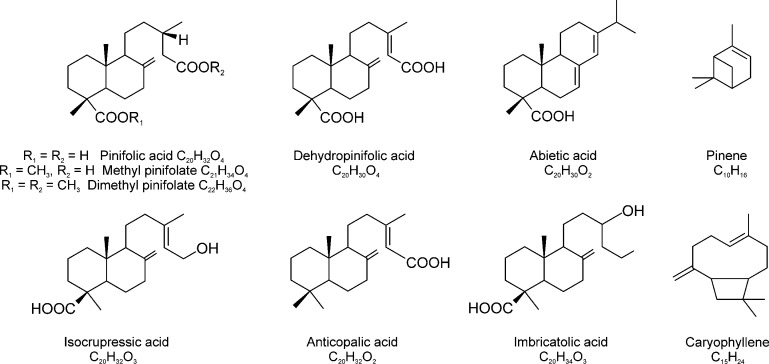
The compounds were tentatively identified by comparing their assigned molecular formulae with the compounds already identified by using other conventional methods. Furthermore, public databases (ChemSpider, ChEBI, PubChem, etc.) were searched to find putative candidates. Only naturally occurring compounds were considered and the original articles were inspected for previously reported structures. As no mass spectrometer can differentiate between isomeric compounds of the same chemical formula, unless hyphenated with chromatographic (GC, LC) or ion mobility separation or by the use of tandem mass spectrometry (MS/MS), the analysis is typically limited to the compound class level, unless a very specific elemental formula arises. In many cases, however, some unique compounds are highly enriched over the other (isomeric) constituents, allowing more confirmatory assignments without chromatographic separation. Structures of some selected compounds detected in the essential oil samples by both (−)ESI and (+)APPI are shown in Figure 6.
Figure 6.
Structures of some selected compounds detected in the conifer needle essential oils and solvent extracts.
The major compounds detected in the essential oil samples with (−)ESI were different acids, esters, and alcohols. Many acidic compounds present in all the four samples have not been reported earlier. The complete list of the putative compounds and their elemental formulae are given in Table S1. As (−)ESI preferentially ionizes polar (acidic) compounds, terpenes or terpenoids present in the oil samples were not efficiently ionized or were detected at very low abundance.
Citronellic acid, palmitic acid, oleic acid, and stearic acid were the most abundant fatty acids present in the essential oil samples. In addition, resin acids were detected as well. Abietane-type resin acids were more abundant than the labdane-type resin acids except in the juniper oil. Some of the resin acids tentatively identified were abietic acid, pinifolic acid and its monomethyl ester, isocupressic acid, and anticopalic acid. To the best of our knowledge, these compounds have not been reported in the literature to be present in the essential oils of these plant species, albeit in their organic solvent extracts. Some acidic compounds were unique to certain samples; coumaric acid was present only in pine and spruce. An earlier study reported the presence of this compound in the callus resins of pine and spruce.39 In addition, 3-methylbutanoic acid was only detected in the pine oil. The ion with the molecular formula of C10H16O2 was most likely geranic acid (3,7-dimethyl-2,6-octadienoic acid), a polyunsaturated fatty acid that has been reported to be a tyrosinase inhibitor.40 This monoterpenoid was detected at high abundance for all the samples except for the larch oil. Another class of compounds, which was present in all the samples, were phenylpropanoids, which are known for their antioxidant and anti-inflammatory activities.41 They were present at low abundance in all the samples. Eugenol, methyl eugenol, and safrole are the examples of phenylpropanoids that are present in conifer species.42
Table S2 shows the list of putative compounds present in the hexane and toluene extracts of pine. Given the nonpolarity of the solvents used, the extracts were dominated by lipophilic compounds, for example, resin acids, free fatty acids, diterpenyl alcohols, diterpenyl aldehydes, sitosterols, and phenolic compounds. The major constituents in both toluene and hexane extracts were resin acids and the most abundant compounds were the labdane-type secondary metabolites. The compounds found in these two extracts were quite similar. The most abundant compound in both was methyl pinifolate (C21H34O4; m/z 349.238396), a monomethyl ester of pinifolic acid (13S-labd-8,17-ene-15,18-dioic acid; C20H32O4), a rare labdane-type diterpene diacid (Figure 6).43 Pinifolic acid was reported as early as 1962 by Enzell and Theander100 and was shown to be highly enriched in pine needles (∼65% of the acid fraction of the needle acetone extract). A major portion of pinifolic acid has been reported to exist as its monomethyl ester.44 A free pinifolic acid was also detected at m/z 335.222739 at high abundance. In addition, dehydropinifolic acid, first reported by Norin et al.45 in 1971, was also present at m/z 333.207094. Isocupressic acid, a diterpene acid that has been found to induce abortion in cattle,46 was also detected at m/z 319.227841. Another labdane-type acid, imbricatolic acid (also known as dihydroisocupressic acid), was present at moderate intensity. This compound was first reported as a natural compound in Pinus elliottii(47) and it has since then been reported in several conifer species. Basas-Jaumandreu et al. reported it as the most abundant compound found in the extract of J. communis needles.48 Structures of these acids are presented in Figure 6. Bornyl p-coumarate, which was reported as the major compound in the acid fraction of Pinus koraiensis needle lipophilic extract,31 was present at low abundance. Other labdanoids, which have been reported in other studies and were also detected in this work, include anticopalic acid, first isolated from the bark and sap wood of western white pine,49 and acetylisocupressic acid.48
The other classes of resin acids present in the solvent extracts were abietane and the pimarane-type resin acids. As these two types of acids are structural isomers, their differentiation is impossible without chromatographic separation. However, some studies have shown that abietic-type diterpenoids are more dominant in Pinaceae species50,51 The tentative identifications include abietic acid, dehydroabietic acid, and hydroxyabietic acid.
A homologous series of long-chain fatty acids, ranging from C12 to C32, were also tentatively identified in the extracts. Both saturated and unsaturated fatty acids were observed. In the hexane extract, the most abundant homolog of these fatty acids was α-linolenic acid, which agrees with the results reported by Makarenko et al.52 and Berg et al.50 In contrast, the most abundant fatty acid in the toluene extract was palmitic acid; this is in line with the fatty acid composition of 137 gymnosperm species reported by Mongrand et al.53 Other fatty acids identified in the extracts were oleic acid, linoleic acid, tridecanoic acid, and myristic acid. A few hydroxy fatty acids were also detected at very low abundance with 16-hydroxypalmitic acid (juniperic acid) as the most abundant compound in this group. A homologous series of monoglycerides were also identified in the extracts. These monoglycerides have been reported in common juniper.48
Phenolic compounds such as phenolic acids, flavonoids, and lignins were also tentatively identified. Phenolics are a class of secondary metabolites with a different chemical nature. Phenolic acids identified in the extracts were quinic acid, benzoic acid, salicylic acid, and ferulic acid (present only in the toluene extract). An earlier study identified quinic acid in Scots pine needles.33
A few flavonols (quercetin, isorhamnetin, kaempferol, taxifolin) were present at very low abundance in the toluene extract but were absent in the hexane extract. A study by Oleszek et al. identified quercetin and taxifolin in the needles of Scots pine from Brazil.54 Lignins, which are phenolic compounds that contain dimers of phenylpropane, were present only in the toluene extract. Hydroxymatairesinol, matairesinol, and nortrachelogenin were tentatively identified; these compounds have been previously reported in the knot and stem wood of Scots pine.55 Some sugaric compounds (6-O-heptopyranosyl-d-glucopyranose, and cinnacassiol D1 glucoside) were also present (detected at m/z 371.119456 and m/z 513.269973, respectively) but these compounds were detected at low abundance in the toluene extract only. Low-molecular-weight carbohydrates, such as arabinose, glucose, rhamnose, raffinose, and pinitol, were also present in the extracts. These compounds were first isolated from Scots pine needles by Assarsson et al.56
Unique Compounds Detected with (+)APPI
APPI ionizes both polar and nonpolar compounds and is less sensitive to ion suppression effects than ESI; thus, it is less selective toward certain compound types, for example, acids. Therefore, the combination of both ionization methods provides complementary data on essential oils and plant extracts. In this work, (+)APPI analysis targeted nonpolar species and it preferentially ionized terpene hydrocarbons and their derivatives. For all the essential oils (Table S3), the most abundant peak appeared at m/z 205.195077, representing different sesquiterpene hydrocarbons (C15H24). Some studies have reported β-caryophyllene and germacrene D as the most abundant sesquiterpenes in the essential oils of some conifer species, like Pinus canariensis and Pinus pinaster, and the second most abundant to monoterpenes.57,58 An abundant compound in the pine and larch oils was a sesquiterpenoid C15H22O1, tentatively identified as nootkatone. Adams et al.59 and Stewart et al.42 reported only a trace amount of nootkatone in the essential oils of juniper species, consistent with our results. Most studies report α-pinene, a monoterpene hydrocarbon (C10H16), as the main component in the conifer essential oils.16,21−23,57 A peak representing monoterpenes (m/z 137.132464) was also observed in the (+)APPI spectra of all the essential oils. A variability of terpene hydrocarbons may be attributed to many factors, including climate, geographical location, harvesting season, genotype, and also the extraction technique used.58 In addition to mono- and sesquiterpenes, a few other presently unidentified hydrocarbons were detected at m/z 297.257567 (C22H32; DBE = 7), m/z 340.312349 (C25H40; DBE = 6) and m/z 365.320255 (C27H40; DBE = 8). No reasonable hits by the database search were found for these compounds, except for C25H40, which could represent relatively rare sesterterpenes, possessing a C25 skeleton.
Other oxygenated monoterpenes were present in disparate intensities; a monoterpenoid C10H18O1 (either borneol, citronellal, eucalyptol, linalool, caraneol, or a mixture of those) was distinct to the juniper and spruce oils. Oxygenated compounds, such as pinocarvone, thymol and its methyl ether, carvacrol, verbenone, thujone, and piperitone, have been reported to occur in conifer species.57,59,60 Oxygenated sesquiterpenes were more abundant than the oxygenated monoterpenes. The reports have shown that caryophyllene oxide, germacrene-4-ol, nootkatone, nerolidol, farnesol, nerolidol, and levomenol are among the most abundant sesquiterpernoids in conifer needle essential oils.57,59,60 Sesquiterpenoids showed higher abundance in spruce oil as compared to the other oils, and were less abundant in the solvent extracts. The amount of diterpenoids was exiguous as compared to monoterpene and sesquiterpene hydrocarbons in all the four samples. These results agree well with the previous reports.57,61,62 Cembrene, abietal, abietadiene, abietatriene, ferruginol, isopimarol, and dehydroabietal are common diterpenoids in essential oils.57,59−62 Phenylpropanoids were present at low abundance among all the compounds. Esters, except sesquiterpenoid esters, were present also at low abundance. The complete list of the compounds detected in the essential oils with (+)APPI is given in Table S3.
A resin acid with the molecular formula of C20H30O2, which is likely abietic acid (see structure in Figure 6), was the most abundant acid present in both solvent extracts. Pinifolic acid and its methyl ester were also present in significant amounts. Other resin acids that were efficiently ionized by (+)APPI include dehydroabietic acid, dehydropinifolic acid, isocupressic acid, and anticopalic acid. Diterpene hydrocarbons and diterpenoids were also found in the solvent extracts. Isoabienol, a labdane alcohol (a main diterpene alcohol present in Scots pine needles63) was present at a moderate abundance. Other identified diterpenoids include isopimarol, dehydroabietal, and ferruginol. Karapandzova et al.60 also found these compounds from the essential oil of Macedonian pine needles (P. peuce).
Diterpene hydrocarbons, such as abietatriene, norabietatriene, and norabietatetraene, were also detected. Resin acids are used in various pharmaceutical applications to produce microcapsules and nanoparticles because of their extremely good film-forming and coating properties, whereas their parent hydrocarbons are frequently used to modify the concept of skin adhesion.64 Sesquiterpenes were present in the extracts in moderate amounts but their oxygenated derivatives were present only in minor quantities. Monoterpene hydrocarbons and their oxygenated compounds were present at low abundance as compared to the other types of terpenoids present in both samples, possibly because of their high volatility.
All the compounds belonging to the phenylpropanoid class were present at low abundance. Diterpenoid esters were the only esters present at high abundance. β-Sitosterol was the only phytosterol identified in the extracts. β-Sitosterol is the most common sterol and it has a broad bioactivity spectrum, possessing antioxidant, anti-inflammatory, antiapoptotic, hypocholesterolemic, and antihyperglycemic effects.66 Tocopherol, a natural source of vitamin E, which is present in almost all plant leaves, was also detected in both hexane and toluene extracts. Resin acids and diterpernoids were abundant in the solvent extracts but were less abundant in the essential oils. The complete list of compounds identified from the solvent extracts with (+)APPI is presented in Table S4.
Conclusions
In this work, high-resolution FT-ICR mass spectrometry, combined with (−)ESI and (+)APPI was successfully used for direct chemical fingerprinting of essential oils and solvent extracts obtained from four conifer tree species. Based on the results, conifer needles possess a rich chemistry which differs considerably from that of the sap/heart wood. Some compounds are only found in the needles and may be present in high quantities (e.g., pinifolic acid and its derivatives). Whereas (−)ESI targeted mainly both the polar constituents of the oils, including different acids, esters, alcohols, and phenolic compounds, (+)APPI targeted more preferentially the nonpolar compounds, especially terpene hydrocarbons. The hydrodistillation process resulted in the extraction of most volatile constituents, whereas organic solvents extracted the more resinous part of the plant. All the main compounds identified were predominant in all the four essential oil samples although their relative abundances varied considerably. Resin acids were dominated by labdane- and abietane-types of compounds. Other detected compounds included different carbohydrates, flavanols, and lignans. Some of the detected compounds possess considerable bioactivity and may serve as potential ingredients in different nutritional or health products. In conclusion, a direct-infusion ultrahigh-resolution FT-ICR MS serves as a powerful technique for nontargeted analysis of complex organic mixtures, like plant extracts and essential oils, capable of detecting even thousands of compounds in a single run without chromatographic separation.
Experimental Section
Plant Materials
Branches of pine (P. sylvestris) and spruce (P. abies) were collected in April, and the branches of juniper (J. communis) and larch (L. decidua) were collected in June from the Ylä-Valtimo region, Eastern Finland, and stored in a cold room (at 4 °C) to avoid the loss of volatile components. The needles were picked out of the branches and cut into pieces for hydrodistillation or solvent extraction. All the solvents used were obtained from VWR Chemicals (Darmstadt, Germany) and they were of HPLC grade.
Hydrodistillation
The hydrodistillation device was assembled using a Clevenger apparatus and a condenser. Briefly, 100 g of needles was placed into a 500 mL round-bottom flask with 150 mL of distilled water and subjected to hydrodistillation for 3 h. The separated essential oil was then stored in the refrigerator for further analysis. For simplicity, the obtained essential oils are referred to as “pine oil”, “spruce oil”, “larch oil”, and “juniper oil”.
Solvent Extraction
Solvent extraction of pine needles was carried out with a Buchi extraction system B-811 (BÜCHI Labortechnik AG, Flawil, Switzerland). Hexane and toluene were used for the extraction. For each extraction experiment, 20 g of pine needles were cut and placed into the small sample tube, screwed onto the condenser tube. Of the solvent, 100 mL was poured into the solvent cup. During the heating of the solvent, a lower temperature was used for hexane as compared to toluene because of the higher boiling point of toluene. The solvent was then evaporated to dryness. The extraction time was 2 h 30 min for both solvents.
Mass Spectrometry
All essential oil samples were analyzed on a 12-T Bruker solariX XR FT-ICR mass spectrometer (Bruker Daltonik GmbH, Bremen, Germany), equipped with a dynamically harmonized ICR cell (Paracell) and an Apollo-II atmospheric pressure ion source (serving both ESI and APPI). For the (−)ESI measurements, 10 μL of each sample was diluted with 190 μL of methanol. The samples were directly infused into the ion source at a flow rate of 2 μL/min using a syringe pump. For the (+)APPI measurements, 10 μL of each sample was diluted with 90 μL methanol and 10 μL toluene (serving as a dopant). For the APPI measurements, the flow rate was increased to 4 μL/min. Dry nitrogen was used as the drying (4.0 L/min, 220 °C) and nebulizing gas (0.8 bar). The mass spectra were first calibrated externally by using either sodium trifluoroacetate clusters67 (for ESI) or APCI-L Tuning Mix (part no. G1969-85010; Agilent Technologies, Santa Clara, CA) (for APPI). Compass ftmsControl software was used for the data acquisition and the mass spectra were further processed and analyzed with DataAnalysis 5.0 software (Bruker Daltonik GmbH, Bremen, Germany). A total of 300 time-domain transients (8 Mword each) were co-added and zero-filled once prior to full-sine apodization, fast Fourier transform, and magnitude calculation. For the peak picking, the signal-to-noise (S/N) ratio was set at 5.0 and the relative intensity threshold was 0.01%. For the molecular formula assignment, the parameters were as follows: DBE, 0–80; mass error, ≤1 ppm; maximum number of formulae, 50; elemental formula, 12C1–1001H1–20014N0–232S0–116O0–25. The data sorting and visualization was done with Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA) and OriginPro 2019 (Originlab Corporation, Northampton, MA) software. Structure assignments (tentative identifications) were made with the help of the Bruker CompoundCrawler database search engine and the original articles.
Acknowledgments
This work was supported by the Strategic Research Council at the Academy of Finland (FORBIO project; grant 293380) and the European Network of Fourier Transform Ion Cyclotron Resonance Mass Spectrometry Centers (EU FT-ICR MS; grant agreement 731077). The FT-ICR MS facility is supported by the Biocenter Finland/Biocenter Kuopio and the Regional Council or North Karelia (grant 70135). O.M. acknowledges the funding from the Faculty of Natural Sciences and Forestry, University of Eastern Finland.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c00901.
List of compounds tentatively identified by (−)ESI and (+)APPI; and compound class plots (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Machalova Z.; Sajfrtova M.; Pavela R.; Topiar M. Extraction of botanical pesticides from Pelargonium graveolens using supercritical carbon dioxide. Ind. Crops Prod. 2015, 67, 310–317. 10.1016/j.indcrop.2015.01.070. [DOI] [Google Scholar]
- Lubbe A.; Verpoorte R. Cultivation of medicinal and aromatic plants for specialty industrial materials. Ind. Crops Prod. 2011, 34, 785–801. 10.1016/j.indcrop.2011.01.019. [DOI] [Google Scholar]
- Asl M. N.; Hosseinzadeh H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother Res. 2008, 22, 709–724. 10.1002/ptr.2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egigu M. C.; Ibrahim M. A.; Yahya A.; Holopainen J. K. Cordeauxia edulis and Rhododendron tomentosum extracts disturb orientation and feeding behavior of Hylobius abietis and Phyllodecta laticollis. Entomol. Exp. Appl. 2011, 138, 162–174. 10.1111/j.1570-7458.2010.01082.x. [DOI] [Google Scholar]
- Figueiredo A. C.; Barroso J. G.; Pedro L. G.; Scheffer J. J. C. Factors affecting secondary metabolite production in plants: volatile components and essential oils. Flavour Fragrance J. 2008, 23, 213–226. 10.1002/ffj.1875. [DOI] [Google Scholar]
- Tang C.-S.; Cai W.; Kohl K.; Nishimoto R. K. Plant stress and allelopathy, In Allelopathy. ACS Symp. Ser. 1995, 582, 142–157. 10.1021/bk-1995-0582.ch011. [DOI] [Google Scholar]
- Bakkali F.; Averbeck S.; Averbeck D.; Idaomar M. Biological effects of essential oils–a review. Food Chem. Toxicol. 2008, 46, 446–475. 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- Brenes A.; Roura E. Essential oils in poultry nutrition: Main effects and modes of action. Anim. Feed Sci. Technol. 2010, 158, 1–14. 10.1016/j.anifeedsci.2010.03.007. [DOI] [Google Scholar]
- Pérez-Rosés R.; Risco E.; Vila R.; Peñalver P.; Cañigueral S. Biological and nonbiological antioxidant activity of some essential oils. J. Agric. Food Chem. 2016, 64, 4716–4724. 10.1021/acs.jafc.6b00986. [DOI] [PubMed] [Google Scholar]
- Campbell N. A.; Reece J. B.. Biology, 7th ed.; Pearson/Benjamin Cummings: San Francisco, 2005, pp 8−10. [Google Scholar]
- Fäldt J.Volatile constituents in conifers and conifer related wood-decaying fungi. Biotic influences on monoterpene compositions in pines. Ph.D. Thesis, Royal Institute of Technology, Department of Chemistry, Organic Chemistry, Stockholm, Sweden, 2000. [Google Scholar]
- Vaario L.-M.; Pennanen T.; Sarjala T.; Savonen E.-M.; Heinonsalo J. Ectomycorrhization of Tricholoma matsutake and two major conifers in Finland—an assessment of in vitro mycorrhiza formation. Mycorrhiza 2010, 20, 511–518. 10.1007/s00572-010-0304-8. [DOI] [PubMed] [Google Scholar]
- Rinne J.; Hakola H.; Laurila T.; Rannik Ü. Canopy scale monoterpene emissions of Pinus sylvestris dominated forests. Atmos. Environ. 2000, 34, 1099–1107. 10.1016/s1352-2310(99)00335-0. [DOI] [Google Scholar]
- Stolter C.; Niemelä P.; Ball J. P.; Julkunen-Tiitto R.; Vanhatalo A.; Danell K.; Varvikko T.; Ganzhorn J. U. Comparison of plant secondary metabolites and digestibility of three different boreal coniferous trees. Basic Appl. Ecol. 2009, 10, 19–26. 10.1016/j.baae.2007.12.001. [DOI] [Google Scholar]
- Setzer W.; Satyal P.; Paudel P.; Raut J.; Deo A.; Dosoky N. S. Volatile constituents of Pinus roxburghii from Nepal. Pharmacogn. Res. 2013, 5, 43–48. 10.4103/0974-8490.105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raal A.; Hoai N. T.; Duc H. V.; Thao d. T.; Orav A. Selectivity of Pinus sylvestris extract and essential oil to estrogen-insensitive breast cancer cells Pinus sylvestris against cancer cells. Pharmacogn. Mag. 2015, 11, S290–S295. 10.4103/0973-1296.166052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak C. S.; Moon S. C.; Lee M. S. Antioxidant, antimutagenic, and antitumor effects of pine needles (Pinus densiflora). Nutr. Canc. 2006, 56, 162–171. 10.1207/s15327914nc5602_7. [DOI] [PubMed] [Google Scholar]
- Grassmann J.; Hippeli S.; Vollmann R.; Elstner E. F. Antioxidative properties of the essential oil from Pinus mugo. J. Agric. Food Chem. 2003, 51, 7576–7582. 10.1021/jf030496e. [DOI] [PubMed] [Google Scholar]
- Motieju̅naitė O.; Dalia Pečiulytė D. Fungicidal properties of Pinus sylvestris L. for improvement of air quality. Medicina 2004, 8, 787–794. [PubMed] [Google Scholar]
- Zeng W.-C.; Zhang Z.; Gao H.; Jia L.-R.; He Q. Chemical composition, antioxidant, and antimicrobial activities of essential oil from pine needle (Cedrus deodara). J. Food Sci. 2012, 77, C824–C829. 10.1111/j.1750-3841.2012.02767.x. [DOI] [PubMed] [Google Scholar]
- Kačániová M.; Vukovič N.; Horská E.; šalamon I.; Bobková A.; Hleba L.; Mellen M.; Vatl’ák A.; Petrová J.; Bobko M. Antibacterial activity against Clostridium genus and antiradical activity of the essential oils from different origin. J. Environ. Sci. Health, Part B 2014, 49, 505–512. 10.1080/03601234.2014.896673. [DOI] [PubMed] [Google Scholar]
- Koutsaviti K.; Giatropoulos A.; Pitarokili D.; Papachristos D.; Michaelakis A.; Tzakou O. Greek Pinus essential oils: larvicidal activity and repellency against Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 2015, 114, 583–592. 10.1007/s00436-014-4220-2. [DOI] [PubMed] [Google Scholar]
- Amri I.; Lamia H.; Gargouri S.; Hanana M.; Mahfoudhia M.; Fezzani T.; Ezzeddine F.; Jamoussi B. Chemical composition and biological activities of essential oils of Pinus patula. Nat. Prod. Commun. 2011, 6, 1531–1536. 10.1177/1934578x1100601031. [DOI] [PubMed] [Google Scholar]
- Süntar I.; Tumen I.; Ustün O.; Keleş H.; Küpeli Akkol E. Appraisal on the wound healing and anti-inflammatory activities of the essential oils obtained from the cones and needles of Pinus species by in vivo and in vitro experimental models. J. Ethnopharmacol. 2012, 139, 533–540. 10.1016/j.jep.2011.11.045. [DOI] [PubMed] [Google Scholar]
- Maric S.; Jukic M.; Katalinic V.; Milos M. Comparison of Chemical Composition and Free Radical Scavenging Ability of Glycosidically Bound and Free Volatiles from Bosnian Pine (Pinus heldreichii Christ. var. leucodermis). Molecules 2007, 12, 283–289. 10.3390/12030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipponen A.; Kuokkanen O.; Tiihonen R.; Kauppinen H.; Jokinen J. J. Natural coniferous resin salve used to treat complicated surgical wounds: pilot clinical trial on healing and costs. Int. J. Dermatol. 2012, 51, 726–732. 10.1111/j.1365-4632.2011.05397.x. [DOI] [PubMed] [Google Scholar]
- Orav A.; Kailas T.; Koel M. Simultaneous distillation, extraction and supercritical fluid extraction for isolating volatiles and other materials from conifer needles. J. Essent. Oil Res. 1998, 10, 387–393. 10.1080/10412905.1998.9700928. [DOI] [Google Scholar]
- Richter J.; Schellenberg I. Comparison of different extraction methods for the determination of essential oils and related compounds from aromatic plants and optimization of solid-phase microextraction/gas chromatography. Anal. Bioanal. Chem. 2007, 387, 2207–2217. 10.1007/s00216-006-1045-6. [DOI] [PubMed] [Google Scholar]
- Jun Y.; Lee S.; Ju H.; Lee H.; Choi H.-K.; Jo G.; Kim Y.-S. Comparison of the profile and composition of volatiles in coniferous needles according to extraction methods. Molecules 2016, 21, 363. 10.3390/molecules21030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpatov A. V.; Popov S. A.; Salnikova O. I.; Shmidt E. N.; Kang S. W.; Kim S. M.; Um B. H. Lipophilic Extracts from Needles and Defoliated Twigs of Pinus pumila from Two Different Populations. Chem. Biodivers. 2013, 10, 198–208. 10.1002/cbdv.201200009. [DOI] [PubMed] [Google Scholar]
- Shpatov A. V.; Popov S. A.; Salnikova O. I.; Kukina T. P.; Shmidt E. N.; Um B. H. Composition and Bioactivity of Lipophilic Metabolites from Needles and Twigs of Korean and Siberian Pines (Pinus koraiensis Siebold & Zucc. and Pinus sibirica Du Tour). Chem. Biodivers. 2017, 14, e1600203 10.1002/cbdv.201600203. [DOI] [PubMed] [Google Scholar]
- Schummer C.; Delhomme O.; Appenzeller B.; Wennig R.; Millet M. Comparison of MTBSTFA and BSTFA in derivatization reactions of polar compounds prior to GC/MS analysis. Talanta 2009, 77, 1473–1482. 10.1016/j.talanta.2008.09.043. [DOI] [PubMed] [Google Scholar]
- Karapandzova M.; Stefkov G.; Cvetkovikj I.; Stanoeva J. P.; Stefova M.; Kulevanova S. Flavonoids and Other Phenolic Compounds in Needles of Pinus peuce and Other Pine Species from the Macedonian Flora. Nat. Prod. Commun. 2015, 10, 987–990. 10.1177/1934578x1501000647. [DOI] [PubMed] [Google Scholar]
- Slimestad R. Flavonoids in buds and young needles of Picea, Pinus and Abies. Biochem. Syst. Ecol. 2003, 31, 1247–1255. 10.1016/s0305-1978(03)00018-8. [DOI] [Google Scholar]
- Li Y.-q.; Kong D.-x.; Wu H. Analysis and evaluation of essential oil components of cinnamon barks using GC–MS and FTIR spectroscopy. Ind. Crops Prod. 2013, 41, 269–278. 10.1016/j.indcrop.2012.04.056. [DOI] [Google Scholar]
- Do T. K. T.; Hadji-Minaglou F.; Antoniotti S.; Fernandez X. Authenticity of essential oils. Trends Anal. Chem. 2015, 66, 146–157. 10.1016/j.trac.2014.10.007. [DOI] [Google Scholar]
- Oumzil H.; Ghoulami S.; Rhajaoui M.; Ilidrissi A.; Fkih-Tetouani S.; Faid M.; Benjouad A. Antibacterial and antifungal activity of essential oils of Mentha suaveolens. Phytother Res. 2002, 16, 727–731. 10.1002/ptr.1045. [DOI] [PubMed] [Google Scholar]
- Miettinen I.; Kuittinen S.; Paasikallio V.; Mäkinen M.; Pappinen A.; Jänis J. Characterization of fast pyrolysis oil from short-rotation willow by high-resolution Fourier transform ion cyclotron resonance mass spectrometry. Fuel 2017, 207, 189–197. 10.1016/j.fuel.2017.06.053. [DOI] [Google Scholar]
- Holmbom T.; Reunanen M.; Fardim P. Composition of callus resin of Norway spruce, Scots pine, European larch and Douglas fir. Holzforschung 2008, 62, 417–422. 10.1515/hf.2008.070. [DOI] [Google Scholar]
- Masuda T.; Odaka Y.; Ogawa N.; Nakamoto K.; Kuninaga H. Identification of geranic acid, a tyrosinase inhibitor in lemongrass (Cymbopogon citratus). J. Agric. Food Chem. 2008, 56, 597–601. 10.1021/jf072893l. [DOI] [PubMed] [Google Scholar]
- da Silveira e Sá R. d. C.; Andrade L.; Dos Reis Barreto de Oliveira R.; De Sousa D. A review on anti-inflammatory activity of phenylpropanoids found in essential oils. Molecules 2014, 19, 1459–1480. 10.3390/molecules19021459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. D.; Jones C. D.; Setzer W. N. Essential oil compositions of Juniperus virginiana and Pinus virginiana, two important trees in Cherokee traditional medicine. Am. J. Essent. Oil Nat. Prod. 2014, 2, 17–24. [Google Scholar]
- Gref R.; Lindgren D. The inheritance of pinifolic acid in Scots pine (Pinus sylvestris L.) needles. Silvae Genet. 1984, 33, 235–237. [Google Scholar]
- Enzell C.; Theander O.; Lindberg B.; McKay J.; Theander O.; Flood H. The Constituents of Conifer Needles. II. Pinofolic Acid, a new Diterpene Acid Isolated from Pinus silvestris L.. Acta Chem. Scand. 1962, 16, 607–614. 10.3891/acta.chem.scand.16-0607. [DOI] [Google Scholar]
- Bardyshev I. I.; Degtyarenko A. S.; Pekhk T. I.; Makhnach S. A. A study of the properties and 13 C NMR spectra of pinifolic acid and its derivatives. Chem. Nat. Compd. 1981, 17, 408–410. 10.1007/bf00565151. [DOI] [Google Scholar]
- Norin T.; Sundin S.; Theander O.; Lindberg A. A.; Lagerlund I.; Ehrenberg L. The constituents of conifer needles. Acta Chem. Scand. 1971, 25, 607–610. 10.3891/acta.chem.scand.25-0607. [DOI] [Google Scholar]
- Wang S.; Panter K. E.; Gardner D. R.; Evans R. C.; Bunch T. D. Effects of the pine needle abortifacient, isocupressic acid, on bovine oocyte maturation and preimplantation embryo development. Anim. Reprod. Sci. 2004, 81, 237–244. 10.1016/j.anireprosci.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Spalding B. P.; Zinkel D. F.; Roberts D. R. New labdane resin acids from Pinus elliottii. Phytochemistry 1971, 10, 3289–3292. 10.1016/s0031-9422(00)97394-1. [DOI] [Google Scholar]
- Basas-Jaumandreu J.; López J. F.; de las Heras F. X. C. Xavier C Labdane-type diterpenoids from Juniperus communis needles. Ind. Crops Prod. 2015, 76, 333–345. 10.1016/j.indcrop.2015.07.005. [DOI] [Google Scholar]
- Zinkel D. F.; Toda J. K.; Rowe J. W. Occurrence of anticopalic acid in Pinus monticola. Phytochemistry 1971, 10, 1161–1163. 10.1016/s0031-9422(00)89956-2. [DOI] [Google Scholar]
- Berg B.; Hannus K.; Popoff T.; Theander O. Chemical components of Scots pine needles and needle litter and inhibition of fungal species by extractives. Ecol. Bull. 1980, 32, 391–400. [Google Scholar]
- Roshchin V. I.; Skachkova N. M.; Lyandres G. V.; Maksimchuk P. L. Carbon dioxide extract from woody verdure of the Scotch pine. Group composition and acids. Chem. Nat. Compd. 1988, 24, 447–452. 10.1007/bf00598529. [DOI] [Google Scholar]
- Makarenko S. P.; Konstantinov Y. M.; Shmakov V. N.; Konenkina T. A. Fatty acid composition of lipids in the calluses of two pine species Pinus sibirica and Pinus sylvestris. Russ. J. Plant Physiol. 2010, 57, 739–743. 10.1134/s1021443710050183. [DOI] [Google Scholar]
- Mongrand S.; Badoc A.; Patouille B.; Lacomblez C.; Chavent M.; Cassagne C.; Bessoule J.-J. Taxonomy of gymnospermae: multivariate analyses of leaf fatty acid composition. Phytochemistry 2001, 58, 101–115. 10.1016/s0031-9422(01)00139-x. [DOI] [PubMed] [Google Scholar]
- Oleszek W.; Stochmal A.; Karolewski P.; Simonet A. M.; Macias F. A.; Tava A. Flavonoids from Pinus sylvestris needles and their variation in trees of different origin grown for nearly a century at the same area. Biochem. Syst. Ecol. 2002, 30, 1011–1022. 10.1016/s0305-1978(02)00060-1. [DOI] [Google Scholar]
- Willför S.; Hemming J.; Reunanen M.; Holmbom B. Phenolic and lipophilic extractives in Scots pine knots and stemwood. Holzforschung 2003, 57, 359–372. 10.1515/hf.2003.054. [DOI] [Google Scholar]
- Assarsson A.; Theander O.; Bergson G.; Grönvall A.; Zaar B.; Diczfalusy E. The constituents of conifer needles. Acta Chem. Scand. 1958, 12, 1319–1322. 10.3891/acta.chem.scand.12-1319. [DOI] [Google Scholar]
- Ioannou E.; Koutsaviti A.; Tzakou O.; Roussis V. The genus Pinus: a comparative study on the needle essential oil composition of 46 pine species. Phytochem. Rev. 2014, 13, 741–768. 10.1007/s11101-014-9338-4. [DOI] [Google Scholar]
- Mimoune N. A.; Mimoune D. A.; Yataghene A. Chemical composition and antimicrobial activity of the essential oils of Pinus pinaster. J. Coastal Life Med. 2013, 1, 54–58. [Google Scholar]
- Adams R. P.; Beauchamp P. S.; Dev V.; Bathala R. M. The leaf essential oils of Juniperus communis L. varieties in North America and the NMR and MS data for isoabienol. J. Essent. Oil Res. 2010, 22, 23–28. 10.1080/10412905.2010.9700258. [DOI] [Google Scholar]
- Karapandzova M.; Stefkov G.; Kulevanova S. Essential oils composition of Pinus peuce Griseb.(Pinaceae) growing on Pelister Mtn., Republic of Macedonia. Maced. Pharm. Bull. 2010, 56, 13–22. 10.33320/maced.pharm.bull.2010.56.002. [DOI] [Google Scholar]
- Cavaleiro C.; Pinto E.; Goncalves M. J.; Salgueiro L. Antifungal activity of Juniperus essential oils against dermatophyte, Aspergillus and Candida strains. J. Appl. Microbiol. 2006, 100, 1333–1338. 10.1111/j.1365-2672.2006.02862.x. [DOI] [PubMed] [Google Scholar]
- Kupcinskiene E.; Stikliene A.; Judzentiene A. The essential oil qualitative and quantitative composition in the needles of Pinus sylvestris L. growing along industrial transects. Environ. Pollut. 2008, 155, 481–491. 10.1016/j.envpol.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Gref R. Variation in isoabienol content in Pinus sylvestris needles. Can. J. Bot. 1981, 59, 831–835. 10.1139/b81-116. [DOI] [Google Scholar]
- Mathiowitz E.; Chickering D. E. III; Lehr C.. Bioadhesive Drug Delivery Systems: Fundamentals, Novel Approaches, and Development; CRC Press, 1999. [Google Scholar]
- Miettinen I.; Mäkinen M.; Vilppo T.; Jänis J. Compositional characterization of phase-separated pine wood slow pyrolysis oil by negative-ion electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Energy Fuels 2015, 29, 1758–1765. 10.1021/ef5025966. [DOI] [Google Scholar]
- Moini M.; Jones B. L.; Rogers R. M.; Jiang L. Sodium trifluoroacetate as a tune/calibration compound for positive-and negative-ion electrospray ionization mass spectrometry in the mass range of 100–4000 Da. J. Am. Soc. Mass Spectrom. 1998, 9, 977–980. 10.1016/s1044-0305(98)00079-8. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.