Abstract

Photooxidation utilizing visible light, especially with naturally abundant O2 as the oxygen source, has been well-accepted as a sustainable and efficient procedure in organic synthesis. To ensure the intersystem crossing and triplet quantum yield for efficient photosensitization, we prepared amidated alloxazines (AAs) and investigated their photophysical properties and performance as heavy-atom-free triplet photosensitizers and compared with those of flavin (FL) and riboflavin tetraacetate (RFTA). Because of the difference in the framework structure of AAs and FL and the introduction of carbonyl moiety, the absorption of FL at ∼450 nm is blue-shifted to ∼380 nm and weakened (ε = 8.7 × 103 for FL to ∼6.8 × 103 M–1 cm–1), but the absorption at ∼340 nm is red-shifted to ∼350 nm and enhanced by ∼50% (from ε = 6.4 × 103 for FL to ∼9.9 × 103 M–1 cm–1) in AAs. The intersystem crossing rates from the S1 to T1 are also enhanced in these AAs derivatives, while the fluorescence quantum yield decreases from ∼30 to ∼7% for FL and AAs, respectively, making the triplet excited state lifetime and the singlet oxygen quantum yield of AAs at least comparable to those of FL and RFTA. We examined the performance of these heave-atom-free chromophores in the photooxidation of sulfides to afford sulfoxides. In accordance with the prolonged triplet excited state lifetime and enhanced triplet quantum yield, 2–5-fold performance enhancements were observed for AAs in the photooxidation of sulfides with respect to FL. We proposed that the key reactive oxygen species of AA-sensitized photooxidation are singlet oxygen and superoxide radical anion based on mechanistic investigations. The research highlights the superior performance of AAs in photocatalysis and would be helpful to rationalize the design of efficient heavy-atom-free organic photocatalysts.
Introduction
Photooxidation utilizing visible light, especially with naturally abundant O2 as the oxygen source, has been well-accepted as a sustainable and efficient procedure in synthesis chemistry, for the low energy consumption, high product selectivity, low cost of O2, and limited impact to the environment.1 As the direct oxidation of organic substrates at the singlet ground state with O2 (in triplet state) is spin-forbidden, photosensitizers are commonly required to mediate the formation of singlet oxygen (1O2) or substrate radicals as reactive oxygen species (ROS) via photoinduced energy or electron transfer or their combinations in light irradiation. Kinetically, the concentration of the excited photosensitizer is vital for efficient energy or charge transfer to O2 or the organic substrate independent of the reaction mechanism, and this is determined by the photophysical properties of the photosensitizer. One possible reason for the limited efficiency of conventional organic chromophores in photooxidation is their slow intersystem crossing (ISC) from the short-lived spin-allowed singlet excited states to long-lived triplet excited states for energy transfer, as compared with the competing fast internal conversion and emission. Although this slow ISC can be addressed by the introduction of heavy atoms or halogen bonds,2 it may be of more significance to design heavy-atom-free organic photosensitizers of low toxicity, efficient ISC, and a high triplet quantum yield.
Flavin (FL) derivatives, a kind of nontoxic organic chromophores with the framework structure of isoalloxazines, are important function moieties of redox-active enzymes for a large variety of thermal- or photo-induced biological processes in both animals and plants.3,4 Some FLs, such as riboflavin, may undergo tautomerization in protic solvents when exposed to light irradiation, leading to the formation of alloxazine (AA) derivatives.5 On the other hand, some AA derivatives, such as lumichrome, may also isomerize to be in equilibrium with FLs through excited state proton transfer in protic solvents.6,7 In this sense, AAs are actually directly related to FLs in many photophysical and photochemical processes, including photooxidation.8−11 Compared with FLs, the investigations on photophysical and photochemical properties of AAs were relatively limited. The photophysical properties of AAs are solvent-dependent.11 The amide moiety is sensitive to acid–base properties of the solvent and organic acids were found to catalyze the photoinduced tautomerization of AAs to FLs.12−15 In general, the fluorescence quantum yield of AAs is one order lower than that of FLs because of their fast nonradiative decay and ISC from singlet excited states,16 while triplet quantum yields are ∼0.71 and is obviously superior than those of FLs (∼0.57).10 Considering the vital role of sensitizer in triplet excited states for the sensitized formation of 1O2, it is rather interesting to explore the potential applications of AAs in photooxidation.
The oxidation of sulfides has important applications in many fields, including pharmaceutical production,17 sulfur removal from gasoline and diesel from hydrocracking,18 environment remediation,19 and so on. Although some previously examined organic photosensitizers produce highly oxidative ROS with uncontrolled reaction and poor product selectivity,20−25 riboflavin and riboflavin tetraacetate (RFTA) were reported to be capable of selectively converting sulfides to corresponding sulfoxides, where 1O2 was recognized as the major ROS.26−30 Kinetically, the performance of these photosensitizers can be promoted further if excited state quantum yields of the photosensitizer can be enhanced deliberately. We recently showed that the triplet quantum yield of FL can be enhanced when attached to Ru(II).31,32 Further, we also showed that the Br-introduced heavy atom effect can also promote the singlet-triplet ISC, resulting in an enhanced triplet excited state quantum yield and high catalytic performance in photooxidation of sulfides.33 Considering the reported photophysical properties of AAs, especially the superior triplet excited state quantum yields and singlet oxygen quantum yields as compared with FL, it is interesting to examine the performance of AAs as a sensitizer in photooxidation. To insure efficient ISC of the sensitizer, we attached a carbonyl moiety that is capable of functioning similar to heavy atoms to facilitate ISC34 directly to the amide and imide N atoms and afforded AAs, and expected enhanced catalytic performance of these heavy-atom-free photosensitizers in the photoxidation of sulfides, which is of potential industrial significance in pharmaceutical production, environment remediation, and so on. We also expect that the findings would help to rationalize the design of efficient heavy-atom-free organic photocatalysts.
Results and Discussions
The AAs were synthesized according to Scheme 1. The absorption spectra of AAs, namely, 3a, 3b, and 3c are constituted by 2 bands at ∼350 and ∼380 nm (Figure 1a–c). There are also 2 bands at ∼340 and ∼450 nm, respectively, with vibrational structures, on the absorption spectra of FL and RFTA (Figure 1d,e).35,36 Theoretical calculations were carried out to interpret the absorption spectra of 3a, 3b, and 3c and also the reference compounds FL and RFTA (Tables 1, S1–S3, Figures 2 and S37–S39). The experimental finding for the absorption features of these 3 AAs and the reference compounds, FL and RFTA, in the visible light region were well-reproduced by the density functional theory (DFT)/time-dependent DFT (TD-DFT) results.
Scheme 1. Synthesis of the AAs (3a, 3b, and 3c) and the Molecular Structures of the Reference Sensitizers, FL and RFTA.
Reaction conditions: (i) alloxan, boric acid, acetic acid glacial, 70 °C, 2 h; (ii) caprylyl chloride, Et3N, dimethylformamide, 25 °C, 24 h.
Figure 1.
Absorption spectra of (a) 3a, (b) 3b, (c) 3c, (d) FL, and (e) RFTA in toluene, CH2Cl2, CH3CN, and CH3OH. c = 1 × 10–5 M, 20 °C.
Table 1. Selected Electronic Transitions That Accounts for the Absorption of Amidated Alloxazines, Namely 3a, 3b, and 3c, in the UV–Vis Range.
| transitions | energya | fb | compositionc | CId | character |
|---|---|---|---|---|---|
| 3a: S0 → S1 | 3.39 eV/366 nm | 0.0671 | 77 → 78 | 0.69300 | π → π*, n → π* |
| 3a: S0 → S3 | 3.88 eV/320 nm | 0.2657 | 76 → 78 | 0.66946 | π → π*, n → π* |
| 77 → 79 | 0.14645 | ||||
| 77 → 80 | 0.10979 | ||||
| 3b: S0 → S1 | 3.40 eV/364 nm | 0.0757 | 66 → 67 | 0.69374 | π → π*, n → π* |
| 3b: S0 → S3 | 3.90 eV/318 nm | 0.2714 | 65 → 67 | 0.67110 | π → π*, n → π* |
| 66 → 68 | 0.18749 | ||||
| 3c: S0 → S1 | 3.41 eV/363 nm | 0.0707 | 64 → 67 | 0.12313 | π → π*, n → π* |
| 66 → 67 | 0.68101 | ||||
| 3c: S0 → S3 | 3.90 eV/318 nm | 0.2356 | 65 → 67 | 0.66754 | π → π*, n → π* |
| 66 → 68 | 0.17660 |
Selected electronic transitions with oscillator strength larger than 0.02 are presented.
Oscillator strength. Complete lists of possible transitions can be found in Tables S1–S3.
Composition of the electronic transition.
The CI coefficients are in absolute values.
Figure 2.
Wavefunction of molecular states of 3a, 3b, and 3c involved in the transitions mentioned in Table 1. The contour value is ±0.02 a.u. The C, O, N, and H atoms are in gray, red, blue, and white, respectively.
For 3a, the absorption at ∼380 nm involves electron transitions from MO 77 to MO 78 (S0 → S1) (Figure 2, upper panel). This transition can be assigned to mixed π → π* transition within the AA π and π* symmetry mainly localized on the benzopyrazine moiety and n → π* transition involving nonbonding states on N1 and carbonyl O. The absorption at ∼350 nm (S0 → S3) involves transitions from MO 76 to MO 78, from MO 77 to MO 79, and from MO 77 to MO 80 and is also of mixed π → π* and n → π* character, but also with contribution from carbonyl group introduced by amidation. The removal of the additional carbonyl group from N1 and N3 of 3a does not alter the orbital sequence and the energy levels significantly (Table 1 and Figure 2), so the spectra of 3b and 3c exhibit absorption features similar to that of 3a, with two bands in 300–500 nm (Figure 1b,c and Table 1).
The absorption spectra of AAs are different from those of FL and RFTA (Figure 1d,e). For FL, the absorption bands at ∼450 and ∼330 nm are of mixed character of π → π* transition within FL and also the n → π* involving the imide moiety (Table S4 and Figure S40). The absorption spectrum of RFTA is similar to that of FL because of the similarity in their molecular structures. After a close inspection of the contour plots of wavefunctions involved in the excitations, we noticed that the spacial distribution of highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) of AAs and FL are quite similar, except that C4 and N1 (2, Scheme 1) states have no contribution to the HOMO and LUMO of AAs, respectively (Figures 2 and S40). In this sense, the blue-shift of the absorption band at ∼450 nm of FL to ∼380 nm in AAs, and the red-shift of the band at ∼330 nm of FL to ∼350 of AAs can be attributed to the different conjugations within the FL and AA frameworks and the introduction of the carbonyl moiety.
We previously noticed the solvent-dependent absorption of FL and FL derivatives.31,33 As the AAs are isomers of FL derivatives, we also investigated their absorption in nonpolar solvents including toluene and CH2Cl2 (DCM) and strong polar solvents such as CH3CN (MeCN) and CH3OH (MeOH) (Figure 1). As FL and RFTA contain various functional groups, which will facilitate the formation of interactions with the solvent molecules that affect the absorption.37 The similarity in the structures of the AAs and FL also suggests the absorption of AA derivatives is also solvent-dependent. However, this effect is less pronounced in AAs (Figures 1 and 2)38 probably because of the fact that the conjugation in AAs is mainly within the benzopyrazine moiety and is thus less sensitive to the interactions with the amide moieties.
We also examined the room temperature emission of AAs, FL, and RFTA in toluene, CH2Cl2, CH3CN, and CH3OH (Figure 3). All three AAs show broad emission bands in the range from 400 to 600 nm with a similar shape, with λem,max at 448, 446, and 442 nm for 3a, 3b, and 3c, respectively. This is different from the emission spectra of FL and RFTA featuring 2 emission bands in the range from 450 to 650 nm, with λem,max at 495 and 504 nm, for FL and RFTA, respectively, with observable vibrational fine structures at room temperature. For the AAs investigated, the emission is stronger in CH3OH than in other less polar solvents such as CH2Cl2 and toluene, and there is a blue shift of ∼20 nm in CH3OH with respect to that in toluene. We attributed these two features to the AA–solvent interactions.11,38 Specifically, intermolecular interactions, such as hydrogen bonds and π–π stacking, which are known to modulate the emission properties of a chromophore, can be formed with CH3OH and toluene. The impact of such interactions on the emission properties is also apparent for the emission of reference compounds, namely the FL and RFTA (Figure 3d,e).
Figure 3.
Emission spectra of 3a (a), 3b (b), 3c (c), FL (d), and RFTA (e) in toluene, CH2Cl2, and CH3OH. λex is 341 nm for 3a, 3b, and 3c, 435 and 442 nm for FL and RFTA, respectively. c = 1.0 × 10–5 M, 20 °C.
DFT/TD-DFT calculations were also performed to analyze the fluorescent emission properties of AAs and FL (Figure 4, Table 2). The emission of FL rises from ∼450 nm with the λem,max at 482 and 518 nm. As for AAs, the emission rises from ∼400 nm, and the λem,max are at 475, 454, and 444 nm for 3a, 3b, and 3c, respectively (Figure 4a), in a reasonable agreement with experimental results (Figure 3). We projected the energy consumed because of the structure reorganization (Ereorg) accompanying the electronic transition from the first singlet excited state (S1) to the ground state (S0) onto the S0 normal modes of the corresponding compounds (Figure 4b) and analyzed the contribution of specific ground state normal modes to Ereorg (Figure 4c). The calculated Ereorg for 3a, 3b, 3c, and FL are 4339, 4805, 2197, and 1876 cm–1, respectively. The contribution from the C=C and C=N stretching modes, which were known to account for the low fluorescent quantum yield of AA and isoalloxazine derivatives, to Ereorg can be recognized in 3a, 3b, 3c, and FL (Figure 4b,c).31−33 Furthermore, for 3a and 3b, the amide moieties also introduce new normal modes, such as mode 86 of 3a and mode 73 of 3b (Figure 4c), as alternative energy consumption paths competing with the emissive decay, and may partially account for the slight redshift of the emission spectra with respect to 3c.
Figure 4.
Calculated emission spectra (a), projected Ereorg on the S0 vibrational normal modes (b), and the displacement vectors for the S0 normal modes of 3a, 3b, 3c, and FL presenting domination contribution to Ereorg at 298 K in CH2Cl2 (c). In (b), the contribution of S0 normal modes of 3a, 3b, 3c, and FL were marked and number in green, blue, red, and black, respectively, and the corresponding displacement vectors of these modes can be found in (c).
Table 2. Theoretical Emission Properties of 3a, 3b, 3c, and FL at 298 K.
| 298 K | kica | kfb | kISC (S1 → T1)c | kISC (T1 → S0)d | kpe | ΦT (%)f |
|---|---|---|---|---|---|---|
| FL | 9.61 × 108 | 5.50 × 107 | 4.79 × 102 | 4.99 × 102 | 1.42 × 10–1 | 4.71 × 10–5 |
| 3a | 6.61 × 1011 | 8.78 × 106 | 1.77 × 107 | 4.74 × 106 | 1.28 × 10–1 | 2.68 × 10–3 |
| 3b | 6.43 × 1011 | 1.06 × 107 | 8.58 × 106 | 2.17 × 106 | 1.68 × 10–1 | 1.33 × 10–3 |
| 3c | 4.04 × 109 | 2.20 × 107 | 2.43 × 105 | 1.12 × 104 | 3.38 × 10–1 | 5.98 × 10–3 |
Rate constant for nonemissive decay by internal conversion from S1 to S0 in s–1.
Fluorescent emission rate constant in s–1.
ISC rate constant from S1 to T1 in s–1.
ISC rate constant from T1 to S0 in s–1.
Phosphorescent emission rate constant in s–1.
Triplet quantum yield in percentage, calculated as kISC(S1 → T1)/(kic + kf + kISC(S1 → T1)) × 100%.
The calculated kf and kic of FL are 5.50 × 107 and 9.61 × 108 s–1, respectively, and are significantly superior than kISC(S1 → T1), kISC(T1 → S0), and kp, which are 4.79 × 102, 4.99 × 102, and 1.42 × 10–1 s–1, respectively. In this sense, emissive and nonemissive decay to S0 are the major paths for the evolution of excited FL. For the small kISC(S1 → T1), only a small portion of excited FL will evolve into T1. Furthermore, the T1 to S0 nonradiative decay is also dominant over emissive decay, as kISC(T1–S0) is 103 larger than kp. These finely explain the small triplet quantum yield of FL. Compared with those of FL, the kic of AAs are at least 10 times larger, while kf are at nearly the same level. However, the kISC(S1–T1) are drastically increased from 4.79 × 102 for FL to 1.77 × 107, 8.58 × 106, and 2.43 × 105 s–1 for 3a, 3b, and 3c, respectively. In this case, a significant portion of, at least 103 times more, excited AAs would evolve to T1 via ISC and the T1 quantum yield of AAs is thus about 1000 folds with respect to FL (Table 2). Another interesting feature of the AAs is that the kISC(T1–S0) are also increased by at least 100 folds with respect to that of FL. We attributed this to the contribution of the carbonyl group within the amide moiety to the spin–orbital coupling. Furthermore, the calculated kISC(T1–S0) is still ∼10 times lower than that of the corresponding kISC(S1–T1) ISC rates. This is different from the case of FL, where kISC(T1–S0) is at the same level with the kISC(S1–T1). This difference may result in the accumulation of more excited AAs in T1, which would further evolve to S0 via competing nonradiative, radiative decay processes or sensitization with O2. Kinetically, the performance of a chromophore in O2 sensitization is mainly determined by the concentration of excited sensitizers, and is related to ISC and decay rates, etc. Although the kISC(T1–S0) are significantly larger than kp for all of the 4 heavy-atom-free organic chromophores, the relatively smaller kISC(T1–S0) as compared with the kISC(S1–T1) suggests that AAs may potentially exhibit superior performance as O2 sensitizers. This difference between kISC(S1–T1) and kISC(T1–S0) ensures not only the population of a large portion of excited chromophores to T1 but also their accumulation at T1 where they are capable of transferring energy/electrons for the conversion of substrates.
We compared the measured photophysical properties of 3a, 3b, 3c, and FL in Table 3. The emission of the four chromophores is mainly fluorescent emission. Compared with FL, the emission of AAs is significantly weakened according to the measured ΦF. We attributed this to both faster internal conversion and ISC rates (Table 3) in AAs originated from the carbonyl groups within the amide moiety and the different conjugations within the AA framework. Previously, the carbonyl group is known to contribute positively to the spin–orbital coupling and lead to fast ISC for the population and evolution of the organic chromophores in their low-lying triplet excites states,39 which are commonly required for the sensitization of O2 for the formation of singlet O2 as ROS. This is supported by the calculated kISC(S1–T1) and kISC(T1–S0) (Table 2). The observed increase of ΦΔ at the expense of ΦF of AAs with respect to FL is similar to the reported photophysics of FL bromides where Br accounts for the enhanced ISC.33 The measured ΦΔ of AAs are at the same level as compared with those of FL and RFTA, showing that the portion of excited AAs in T1 would be at least comparable to those of FL and RFTA, which accounts for the renowned performance of FL and RFTA as sensitizers for photooxidation.27,40 The measured singlet oxygen quantum yield (ΦΔ) of these 4 organic chromophores are 0.644, 0.715, 0.771, and 0.547 for 3a, 3b, 3c, and FL, respectively. According to the measured ΦΔ in CH2Cl2, the performance of these 4 organic chromophores in photooxidation would be superior or at least similar to that of [Ru(bpy)3]2+ with a ΦΔ of 0.57 and that of RFTA, which was previously proposed to be efficient for photooxidation and are about 20% higher than that of FL.27,33 As inspired by the superior ΦΔ of AAs, we then investigated the performance of these organic chromophores as photosensitizers using the photooxidation of sulfides as a probe reaction (Table 4).
Table 3. Measured Photophysical Properties of 3a, 3b, 3c, FL, and RFTA.
| λabs/nma | εb | λem/nmc | ΦFd | τF/nse | ΦΔf | τT/μsg | |
|---|---|---|---|---|---|---|---|
| 3a | 350; 379 | 0.062; 0.040 | 448 | 0.063 | 0.0168 | 0.644 | 256 |
| 3b | 348; 384 | 0.099; 0.068 | 446 | 0.066 | 0.0391 | 0.715 | 383 |
| 3c | 347; 381 | 0.093; 0.067 | 442 | 0.068 | 0.0629 | 0.771 | 186 |
| FL | 334; 440 | 0.064; 0.087 | 495 | 0.285 | 0.2689 | 0.547 | 218 |
| RFTA | 348; 447 | 0.067; 0.092 | 504 | 0.300 | 6.2806 | 0.701 | 722 |
In CH2Cl2 (c = 1.0 × 10–5 M).
Molar absorption coefficient. ε: 105 M–1 cm–1.
Maximal emission wavelength in CH2Cl2 (c = 1.0 × 10–5 M).
The fluorescence quantum yields with anthracene (ΦF = 0.27, in ethanol) as the standard.
At 293 K, measured in air in CH2Cl2.
Singlet oxygen quantum yields in CH2Cl2; Ru(bpy)32+ was used as standard (ΦΔ = 0.57, in CH2Cl2).
Measured by transient absorption in CH2Cl2 (4.0 × 10 –5 M).
Table 4. Photooxidation of Thioanisole with AAs, FL, and RFTA as Catalystsa.
| entry | reaction condition | yield (%) |
|---|---|---|
| 1 | 0.5 mol % 3a, 300 min | 52.2 |
| 2 | 0.5 mol % 3b, 300 min | 100.0 |
| 3 | 0.5 mol % 3c, 300 min | 72.2 |
| 4 | 0.5 mol % FL, 300 min | 22.6 |
| 5 | 0.5 mol % RFTA, 300 min | 75.0 |
| 6 | 0.5 mol % 3b, 300 min, CH3CN/H2O (v/v = 9:1) | 100.0 |
| 7 | 2 mol % 3b, 200 min | 100.0 |
| 8 | 1 mol % 3b, 200 min | 100.0 |
| 9 | no 3b, 300 min | 0 |
| 10 | 0.5 mol % 3b, 300 min, no light | 0 |
| 11 | no 3b, 300 min, no light | 0 |
Reaction conditions: The 3.0 mL reaction mixture was formed by mixing 0.02 mmol thioanisole and catalyst in CH2Cl2/CH3OH (v/v = 9 : 1), otherwise specified. The liquid mixture was exposed to 35 W xenon lamp (250 W/m2) at 20 °C under continuous stirring. The progress of the reaction was monitored by thin-layer chromatography (TLC) analysis. The product yields were determined by 1H NMR spectra of the mixture at the end of the reaction.
We first inspected the photooxidation of 0.20 mmol thioanisol in 3 mL of CH2Cl2/CH3OH (v/v = 9:1) solution with 0.5 mol % of AAs, FL, and RFTA as sensitizers (entries 1–5, Table 4). Full conversion was achieved only when 3b was used as the photosensitizer (entry 2, Table 4), while the conversions with other chromophores are at least 25% lower than that of 3b. It should be noted that the conversion with RFTA for this reaction is 75.0% and is higher than 3a, 3c, and FL with conversion of 52.2, 72.2, and 22.6%, respectively. In this sense, the performance of 3b is at least comparable to that of RFTA in sensitization and the formation of ROS for the oxidation of thioanisol.26,27,30,41 Although the photophysical properties of these AAs, FL, and RFTA are strongly solvent-dependent, the conversion of an organic substrate in photooxidation depends strongly on the solvability of both the sensitizer and the substrate. As protonic solvents, such as C2H5OH, CH3OH, H2O, and so forth, are known to stabilize the intermediate for the formation of 1O2 and keep the reaction proceed efficiently,26,27,30 we also examined the performance of 3b in CH3CN/H2O (v/v = 9:1) (entry 6, Table 4) and the conversion of thioanisole was also 100%. In this sense, 3b would exhibit comparable performance in CH3CN/H2O and CH2Cl2/CH3OH (v/v = 9:1) mixtures.26,27,30,41 The optimized concentration of the catalyst and reaction time were set as 0.5 mol % and 300 min (entries 2, 7–8, Table 4) as they are already enough to reach full conversion. The vital roles of 3b and light radiation were supported by parallel experiments without catalyst or light irradiation or both, where the nonobservable conversion of the substrate was found (entries 9–11, Table 4). It should be noted that, although 3c has a higher ΦT, its performance in photooxidation is not excellent among 3 AAs. This can be attributed to the complexity of the photooxidation of sulfides sensitized by AAs and FL. The excited sensitizers can sensitize the formation of not only singlet oxygen but also the substrate radicals by electron transfer. In this sense, the overall measured performance is determined by the contribution from all competing mechanisms. Although the performance of 3b in CH3CN/H2O and CH2Cl2/CH3OH mixtures are similar (entries 2 and 6, Table 4), we focused our efforts on the photooxidation in CH2Cl2/CH3OH solutions where the absorption of 3b is reasonable, considering the solvability of aromatic and aliphatic sulfides in these systems. Parallel experiments were also performed using FL as the catalyst under the same reaction conditions for comparison. According to the product yield, the performance of 3b is significantly higher than that of FL (Table 5).
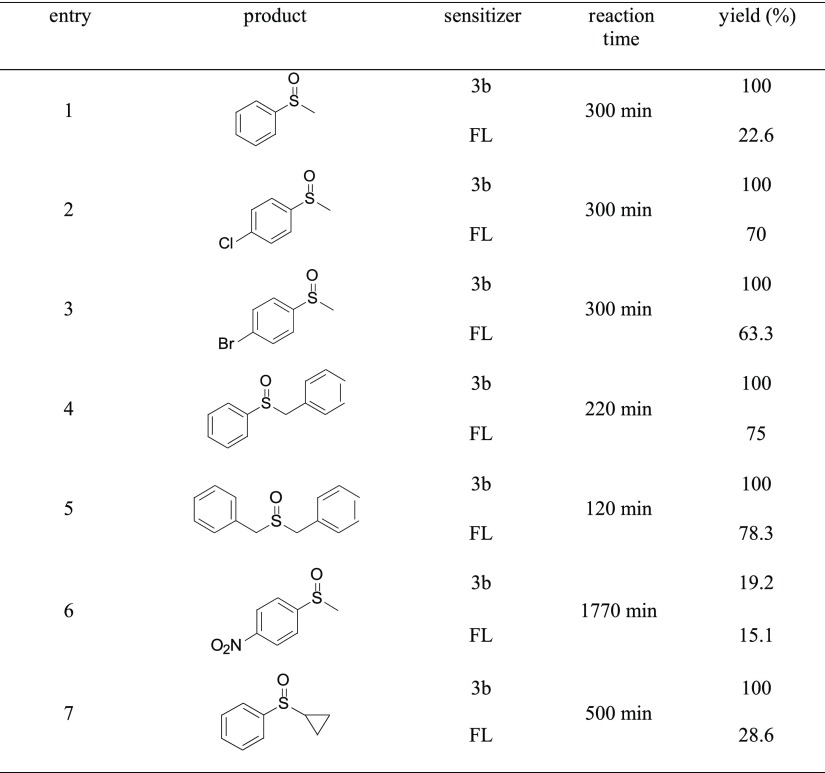
Table 5. Photooxidation of Various Sulfides with 3b and FL as Catalystsa.
Reaction conditions: the 3.0 mL reaction mixture was formed by mixing 0.02 mmol sulfides and catalyst in CH2Cl2/CH3OH (v/v = 9:1). The liquid mixture was exposed to 35 W xenon lamp (250 W/m2) at 20 °C under continuous stirring. The progress of the reaction was monitored by TLC analysis, and the product yields were determined by 1H NMR spectra of the mixture at the end of the reaction.
According to the product yields, the full conversion of most of the substrates, including 4-chlorothioanisole, 4-bromothioanisole, benzyl phenyl sulfide, dibenzyl sulfide, and so forth, can be reached in less than 300 min (entries 1–5, Table 5). In this sense, the performance of 3b is already comparable or even superior than other reported catalysts, such as Rose Bengal,20 riboflavin and RFTA,27 bromo-flavin derivatives,33 and so forth, which require longer reaction time or the introduction of promoters. We noticed that the conversion of 4-nitrothioanisole is still low (19.2%) even when the reaction time was elongated to 1770 min (entry 6, Table 5) and the conversion is only 15.1% when FL was used as the sensitizer. Nitro-aromatic compounds are conventionally used as the radical/spin trap to investigate reaction mechanisms.20,42 This ultralow conversion suggests the photooxidation of 4-nitrothioanisole with 3b may involve a radical-based intermediate, which is inevitably quenched by the NO2-containing substrate. We also noticed that the conversion of cyclopropyl phenyl sulfide can be fully converted in 500 min when 3b was used, while the conversion with FL as the sensitizer is only 28.6% (Table 5, entry 7). According to the conversion, 3b is already superior over previously reported bromo-FL that reached a conversion of 80.6% within 1025 min33 and the Pt(II) complex that fully converts the substrate in 2880 min.43 Furthermore, only peaks of the substrate and desired product were observed in the 1H NMR spectrum of the final reaction mixture (Figures S29–S30), providing direct evidence for the high selectivity of photooxidation sensitized by 3b.
We also performed experiments to highlight the ROSs and the mechanism for the photooxidation of sulfides sensitized by 3b (Table 6). The conversion of thioanisole is only 33.3% in pure CH2Cl2, and this value increases to 100% in CH2Cl2/CH3OH (v/v = 9:1) mixture (entries 1 and 2, Table 6). This is due to the fact that protons within the protonic solvents may help to stabilize the reaction intermediates and promote the formation of 1O2 as ROS and in turn accelerates the photooxidation.30,42 The difference in conversion when CH2Cl2 and CH2Cl2/CH3OH (v/v = 9:1) were used as solvents also suggests that 1O2 can be assigned as one of the ROS in the photooxidation of thioanisole.27 Another well-recognized ROS is O2•– radical and its existence and a potential role in the reaction system can be identified by the decrease of the product yield with the addition of benzoquinone.44 In the parallel experiment with 5 mol % benzoquinone, the substrate conversion was significantly decreased to 15.6% while that without benzoquinone is 100%, suggesting that O2•– radical is also one of the ROS (entry 3, Table 6). We also noticed that even in the parallel experiments without CH3OH and with 5 mol % benzoquinone, the substrate conversion was not zero, implying that the mechanism would be very complicated (entries 1–3, Table 6).45 Therefore, the photooxidation with 3b as the sensitizer was also investigated in CH3OH, deuterated CH3OH, and with 0.5 mol % DABCO that was used as a selective scavenger for 1O2 (entries 4–6, Table 6). Without any scavenger, the substrate conversion was 70% in CH3OH (entry 4, Table 6), and this value was further decreased to 48.4% when 0.5 mol % DABCO was added to identify for 1O2 (entry 5, Table 6), proving the ROS role of 1O2 in this photooxidation.43 We also investigated the photooxidation with deuterated CH3OH as the solvent,27 as deuterated solvents may promote the reactions with 1O2 as ROS. The substrate yield reached 100% in deuterated CH3OH as compared with the yield of 70% when CH3OH was used, confirming again the ROS role of 1O2 (Table 6, entry 6). According to these evidences, we proposed that both 1O2 and O2•– are formed as ROSs in the photooxidation of sulfides sensitized by 3b.
Table 6. Photooxidation of Thioanisole with 3b as Catalysta.
| entry | catalysts | solvents | scavengers | reaction time (min) | yield (%) |
|---|---|---|---|---|---|
| 1 | 3b | CH2Cl2 | none | 300 | 33.3 |
| 2 | 3b | CH2Cl2/CH3OH | none | 300 | 100.0 |
| 3 | 3b | CH2Cl2/CH3OH | 5 mol % benzoquinone | 300 | 15.6 |
| 4 | 3b | CH3OH | none | 300 | 70.0 |
| 5 | 3b | CH3OH | 0.5 mol % DABCO | 300 | 48.4 |
| 6 | 3b | deuterated CH3OH | none | 300 | 100.0 |
Reaction conditions: the 3.0 mL reaction mixture was formed by mixing 0.02 mmol thioanisole and catalyst in CH2Cl2/CH3OH (v/v = 9:1) unless specified. The liquid mixture was exposed to 35 W xenon lamp (250 W/m2) at 20 °C under continuous stirring. The progress of the reaction was monitored by TLC analysis, and the product yields were determined by 1H NMR spectra of the mixture at the end of the reaction.
Conclusions
We investigated photophysical properties and the photooxidation performance as single-atom-free sensitizers of AAs and compared with those of FL and RFTA. Because of the difference in the framework structure of AAS and FL and the introduction of the amide moiety, the absorption of FL at ∼450 nm is blue-shifted to ∼380 nm and weakened with ε decrease from 8.7 × 103 for FL to ∼6.8 ×103 M–1 cm–1 for AAs, but the absorption at ∼340 nm is red-shifted to ∼350 nm and enhanced by more than 50% with ε increases from 6.4 × 103 for FL to ∼9.9 × 103 M–1 cm–1 in AAs. The ISC rate from the S1 to T1 is also enhanced significantly in these AA derivatives while the fluorescence quantum yield decreases from ∼30 to ∼7% for FL and AAs, respectively, making the triplet excited state lifetime and the singlet oxygen quantum yield of AAs to be at least comparable to those of FL and RFTA. We examined the performance of these organic chromophores in the photooxidation of sulfides to afford sulfoxides. In accordance with the elongated triplet excited state lifetime and enhanced triplet quantum yield, 2–5 fold performance enhancements were observed for AAs in the photooxidation of sulfides with respect to FL. In the mechanistic investigations, both 1O2 and O2•– were observed as ROS. The findings highlight the potential superior performance of AAs in photocatalysis, and we expect these would help to rationalize the design of efficient organic photocatalysts.
Experimental Section
Scheme 1 briefs the synthesis of AAs. The theoretical calculations were performed with Gaussian 1646 and MOMAP.47−51 Detailed information on synthesis, characterization, and theoretical calculations can be found in the Supporting Information.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC, nos: 21573034, 21771029, 21373036, and 21103015). The supercomputer time was provided by the National Supercomputing Center in Guangzhou, China, and the High Performance Computing Center at the Dalian University of Technology.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c01087.
Detailed information on synthesis, structural characterization of 3a, 3b, 3c, FL, and RFTA, NMR spectra for the photooxidation products, and theoretical calculations(PDF)
Author Contributions
H.G. conceived this research and contributed materials and analysis tools. H.X. performed the experimental investigation with the guidance of H.G. and K.C. X.M. and C.D. performed the theoretical research with the guidance of H.G. H.X., X.M. and C.D. are responsible for the results presented. The manuscript was written through contributions of all authors. B.D. and J.Z. commented the manuscript and polished the language. H.G. finalized the manuscript. All authors have given approval to the final version of the manuscript. The authors declare no competing financial interest.
The authors declare no competing financial interest.
Supplementary Material
References
- Sheldon R. A. Catalysis: The key to waste minimization. J. Chem. Technol. Biotechnol. 1997, 68, 381–388. . [DOI] [Google Scholar]
- Giachino G. G.; Kearns D. R. Nature of External Heavy-atom Effect on Radiative and Nonradiative Singlet-Triplet Transitions. J. Chem. Phys. 1970, 52, 2964–2974. 10.1063/1.1673425. [DOI] [Google Scholar]
- Murakami M.; Ohkubo K.; Fukuzumi S. Inter- and Intramolecular Photoinduced Electron Transfer of Flavin Derivatives with Extremely Small Reorganization Energies. Chem.—Eur. J. 2010, 16, 7820–7832. 10.1002/chem.200903236. [DOI] [PubMed] [Google Scholar]
- Kaim W.; Schwederski B. Non-innocent ligands in bioinorganic chemistry-An overview. Coord. Chem. Rev. 2010, 254, 1580–1588. 10.1016/j.ccr.2010.01.009. [DOI] [Google Scholar]
- Koziol J. Absorption spectra of riboflavin lumiflavin and lumichrome in organic solvents. Experientia 1965, 21, 189–190. 10.1007/bf02141876. [DOI] [PubMed] [Google Scholar]
- Song P.-S.; Sun M.; Koziolowa A.; Koziol J. Phototautomerism of lumichromes and alloxazines. J. Am. Chem. Soc. 1974, 96, 4319–4323. 10.1021/ja00820a045. [DOI] [Google Scholar]
- Choi J. D.; Fugate R. D.; Song P.-S. Nanosecond time-resolved fluorscence of phototautomeric lumichrome. J. Am. Chem. Soc. 1980, 102, 5293–5297. 10.1021/ja00536a029. [DOI] [Google Scholar]
- Sikorska E.; Khmelinskii I.; Hoffmann M.; Machado I. F.; Ferreira L. F. V.; Dobek K.; Karolczak J.; Krawczyk A.; Insińska-Rak M.; Sikorski M. Ground- and excited-state double proton transfer in lumichrome/acetic acid system: Theoretical and experimental approach. J. Phys. Chem. A 2005, 109, 11707–11714. 10.1021/jp053951d. [DOI] [PubMed] [Google Scholar]
- Sikorski M.; Prukała D.; Insińska-Rak M.; Khmelinskii I.; Worrall D. R.; Williams S. L.; Hernando J.; Bourdelande J. L.; Koput J.; Sikorska E. Spectroscopy and photophysics of dimethyl-substituted alloxazines. J. Photochem. Photobiol., A 2008, 200, 148–160. 10.1016/j.jphotochem.2008.07.006. [DOI] [Google Scholar]
- Salzmann S.; Marian C. M. The photophysics of alloxazine: a quantum chemical investigation in vacuum and solution. Photochem. Photobiol. Sci. 2009, 8, 1655–1666. 10.1039/b9pp00022d. [DOI] [PubMed] [Google Scholar]
- Moyon N. S.; Mitra S. Fluorescence Solvatochromism in Lumichrome and Excited-State Tautomerization: A Combined Experimental and DFT Study. J. Phys. Chem. A 2011, 115, 2456–2464. 10.1021/jp1102687. [DOI] [PubMed] [Google Scholar]
- Tyagi A.; Penzkofer A. Absorption and Emission Spectroscopic Characterization of Lumichrome in Aqueous Solutions. Photochem. Photobiol. 2011, 87, 524–533. 10.1111/j.1751-1097.2010.00836.x. [DOI] [PubMed] [Google Scholar]
- Penzkofer A. Absorption and emission spectroscopic investigation of alloxazine in aqueous solutions and comparison with lumichrome. J. Photochem. Photobiol., A 2016, 314, 114–124. 10.1016/j.jphotochem.2015.08.011. [DOI] [Google Scholar]
- Prukala D.; Sikorska E.; Koput J.; Khmelinskii I.; Karolczak J.; Gierszewski M.; Sikorski M. Acid-Base Equilibriums of Lumichrome and its 1-Methyl, 3-Methyl, and 1,3-Dimethyl Derivatives. J. Phys. Chem. A 2012, 116, 7474–7490. 10.1021/jp300522h. [DOI] [PubMed] [Google Scholar]
- Marchena M.; Gil M.; Martín C.; Organero J. A.; Sanchez F.; Douhal A. Stability and Photodynamics of Lumichrome Structures in Water at Different pHs and in Chemical and Biological Caging Media. J. Phys. Chem. B 2011, 115, 2424–2435. 10.1021/jp110134f. [DOI] [PubMed] [Google Scholar]
- Sikorska E.; Khmelinskii I. V.; Prukała W.; Williams S. L.; Patel M.; Worrall D. R.; Bourdelande J. L.; Koput J.; Sikorski M. Spectroscopy and photophysics of lumiflavins and lumichromes. J. Phys. Chem. A 2004, 108, 1501–1508. 10.1021/jp037048u. [DOI] [Google Scholar]
- Bian C.; Singh A. K.; Niu L.; Yi H.; Lei A. Visible-Light-Mediated Oxygenation Reactions using Molecular Oxygen. Asian J. Org. Chem. 2017, 6, 386–396. 10.1002/ajoc.201600563. [DOI] [Google Scholar]
- Otsuki S.; Nonaka T.; Takashima N.; Qian W.; Ishihara A.; Imai T.; Kabe T. Oxidative desulfurization of light gas oil and vacuum gas oil by oxidation and solvent extraction. Energy Fuels 2000, 14, 1232–1239. 10.1021/ef000096i. [DOI] [Google Scholar]
- Marin M. L.; Santos-Juanes L.; Arques A.; Amat A. M.; Miranda M. A. Organic Photocatalysts for the Oxidation of Pollutants and Model Compounds. Chem. Rev. 2012, 112, 1710–1750. 10.1021/cr2000543. [DOI] [PubMed] [Google Scholar]
- Gu X.; Li X.; Chai Y.; Yang Q.; Li P.; Yao Y. A simple metal-free catalytic sulfoxidation under visible light and air. Green Chem. 2013, 15, 357–361. 10.1039/c2gc36683e. [DOI] [Google Scholar]
- Li W.; Xie Z.; Jing X. BODIPY photocatalyzed oxidation of thioanisole under visible light. Catal. Commun. 2011, 16, 94–97. 10.1016/j.catcom.2011.09.007. [DOI] [Google Scholar]
- Guerrero-Corella A.; María Martinez-Gualda A.; Ahmadi F.; Ming E.; Fraile A.; Alemán J. Thiol-ene/oxidation tandem reaction under visible light photocatalysis: synthesis of alkyl sulfoxides. Chem. Commun. 2017, 53, 10463–10466. 10.1039/c7cc05672a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Wang M.; Jiang X. Controllable Sulfoxidation and Sulfenylation with Organic Thiosulfate Salts via Dual Electron- and Energy-Transfer Photocatalysis. ACS Catal. 2017, 7, 7587–7592. 10.1021/acscatal.7b02735. [DOI] [Google Scholar]
- Wojaczyńska E.; Wojaczynski J. Enantioselective Synthesis of Sulfoxides: 2000-2009. Chem. Rev. 2010, 110, 4303–4356. 10.1021/cr900147h. [DOI] [PubMed] [Google Scholar]
- Silvi M.; Melchiorre P. Enhancing the potential of enantioselective organocatalysis with light. Nature 2018, 554, 41–49. 10.1038/nature25175. [DOI] [PubMed] [Google Scholar]
- Jensen R. L.; Arnbjerg J.; Ogilby P. R. Temperature Effects on the Solvent-Dependent Deactivation of Singlet Oxygen. J. Am. Chem. Soc. 2010, 132, 8098–8105. 10.1021/ja101753n. [DOI] [PubMed] [Google Scholar]
- Dad’ová J.; Svobodová E.; Sikorski M.; König B.; Cibulka R. Photooxidation of Sulfides to Sulfoxides Mediated by Tetra-O-Acetylriboflavin and Visible Light. ChemCatChem 2012, 4, 620–623. 10.1002/cctc.201100372. [DOI] [Google Scholar]
- Megerle U.; Wenninger M.; Kutta R.-J.; Lechner R.; König B.; Dick B.; Riedle E. Unraveling the flavin-catalyzed photooxidation of benzylic alcohol with transient absorption spectroscopy from sub-pico- to microseconds. Phys. Chem. Chem. Phys. 2011, 13, 8869–8880. 10.1039/c1cp20190e. [DOI] [PubMed] [Google Scholar]
- Dad’ová J.; Kummel S.; Feldmeier C.; Cibulkova J.; Pazout R.; Maixner J.; Gschwind R. M.; Konig B.; Cibulka R. Aggregation Effects in Visible-Light Flavin Photocatalysts: Synthesis, Structure, and Catalytic Activity of 10-Arylflavins. Chem.—Eur. J. 2013, 19, 1066–1075. 10.1002/chem.201202488. [DOI] [PubMed] [Google Scholar]
- Neveselý T.; Svobodová E.; Chudoba J.; Sikorski M.; Cibulka R. Efficient Metal-Free Aerobic Photooxidation of Sulfides to Sulfoxides Mediated by a Vitamin B-2 Derivative and Visible Light. Adv. Synth. Catal. 2016, 358, 1654–1663. 10.1002/adsc.201501123. [DOI] [Google Scholar]
- Guo H.; Zhu L.; Dang C.; Zhao J.; Dick B. Synthesis and photophysical properties of ruthenium(II) polyimine complexes decorated with flavin. Phys. Chem. Chem. Phys. 2018, 20, 17504–17516. 10.1039/c8cp02358a. [DOI] [PubMed] [Google Scholar]
- Guo H.; Dang C.; Zhao J.; Dick B. Lighting the Flavin Decorated Ruthenium(II) Polyimine Complexes: A Theoretical Investigation. Inorg. Chem. 2019, 58, 8486–8493. 10.1021/acs.inorgchem.9b00713. [DOI] [PubMed] [Google Scholar]
- Dang C.; Zhu L.; Guo H.; Xia H.; Zhao J.; Dick B. Flavin Dibromide as an Efficient Sensitizer for Photooxidation of Sulfides. ACS Sustain. Chem. Eng. 2018, 6, 15254–15263. 10.1021/acssuschemeng.8b03729. [DOI] [Google Scholar]
- Soep B.; Mestdagh J.-M.; Briant M.; Gaveau M.-A.; Poisson L. Direct observation of slow intersystem crossing in an aromatic ketone, fluorenone. Phys. Chem. Chem. Phys. 2016, 18, 22914–22920. 10.1039/c6cp04308a. [DOI] [PubMed] [Google Scholar]
- Korvinson K. A.; Hargenrader G. N.; Stevanovic J.; Xie Y.; Joseph J.; Maslak V.; Hadad C. M.; Glusac K. D. Improved Flavin-Based Catalytic Photooxidation of Alcohols through Intersystem Crossing Rate Enhancement. J. Phys. Chem. A 2016, 120, 7294–7300. 10.1021/acs.jpca.6b08405. [DOI] [PubMed] [Google Scholar]
- Sichula V.; Kucheryavy P.; Khatmullin R.; Hu Y.; Mirzakulova E.; Vyas S.; Manzer S. F.; Hadad C. M.; Glusac K. D. Electronic Properties of N(5)-Ethyl Flavinium Ion. J. Phys. Chem. A 2010, 114, 12138–12147. 10.1021/jp106288s. [DOI] [PubMed] [Google Scholar]
- Heelis P. F. The photophysical and photochemical properties of flavines (Isoalloxazines). Chem. Soc. Rev. 1982, 11, 15–39. 10.1039/cs9821100015. [DOI] [Google Scholar]
- Sikorska E.; Khemlinskii I. V.; Worrall D. R.; Williams S. L.; Gonzalez-Moreno R.; Bourdelande J. L.; Koput J.; Sikorski M. Photophysics of 1-methyllumichrome. J. Photochem. Photobiol., A 2004, 162, 193–201. 10.1016/s1010-6030(03)00353-8. [DOI] [Google Scholar]
- Zhao J.; Ji S.; Wu W.; Wu W.; Guo H.; Sun J.; Sun H.; Liu Y.; Li Q.; Huang L. Transition metal complexes with strong absorption of visible light and long-lived triplet excited states: from molecular design to applications. RSC Adv. 2012, 2, 1712–1728. 10.1039/c1ra00665g. [DOI] [Google Scholar]
- König B.; Kümmel S.; Svobodová E.; Cibulka R.. Flavin photocatalysis. Phys. Sci. Rev. 2018, 3, 20170168. 10.1515/psr-2017-0168 [DOI] [Google Scholar]
- Iida H.; Imada Y.; Murahashi S.-I. Biomimetic flavin-catalysed reactions for organic synthesis. Org. Biomol. Chem. 2015, 13, 7599–7613. 10.1039/c5ob00854a. [DOI] [PubMed] [Google Scholar]
- Bonesi S. M.; Manet I.; Freccero M.; Fagnoni M.; Albini A. Photosensitized oxidation of sulfides: Discriminating between the singlet-oxygen mechanism and electron transfer involving superoxide anion or molecular oxygen. Chem.—Eur. J. 2006, 12, 4844–4857. 10.1002/chem.200501144. [DOI] [PubMed] [Google Scholar]
- Casado-Sánchez A.; Gómez-Ballesteros R.; Tato F.; Soriano F. J.; Pascual-Coca G.; Cabrera S.; Alemán J. Pt(II) coordination complexes as visible light photocatalysts for the oxidation of sulfides using batch and flow processes. Chem. Commun. 2016, 52, 9137–9140. 10.1039/c6cc02452a. [DOI] [PubMed] [Google Scholar]
- Bonesi S. M.; Protti S.; Albini A. Reactive Oxygen Species (ROS)-vs Peroxyl-Mediated Photosensitized Oxidation of Triphenylphosphine: A Comparative Study. J. Org. Chem. 2016, 81, 11678–11685. 10.1021/acs.joc.6b02088. [DOI] [PubMed] [Google Scholar]
- Feldmeier C.; Bartling H.; Magerl K.; Gschwind R. M. LED-Illuminated NMR Studies of Flavin-Catalyzed Photooxidations Reveal Solvent Control of the Electron-Transfer Mechanism. Angew. Chem. Int. Ed. 2015, 54, 1347–1351. 10.1002/anie.201409146. [DOI] [PubMed] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Petersson G. A.; Nakatsuji H.; Li X.; Caricato M.; Marenich A. V.; Bloino J.; Janesko B. G.; Gomperts R.; Mennucci B.; Hratchian H. P.; Ortiz J. V.; Izmaylov A. F.; Sonnenberg J. L.; Williams; Ding F.; Lipparini F.; Egidi F.; Goings J.; Peng B.; Petrone A.; Henderson T.; Ranasinghe D.; Zakrzewski V. G.; Gao J.; Rega N.; Zheng G.; Liang W.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Throssell K.; Montgomery J. A. Jr.; Peralta J. E.; Ogliaro F.; Bearpark M. J.; Heyd J. J.; Brothers E. N.; Kudin K. N.; Staroverov V. N.; Keith T. A.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A. P.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Millam J. M.; Klene M.; Adamo C.; Cammi R.; Ochterski J. W.; Martin R. L.; Morokuma K.; Farkas O.; Foresman J. B.; Fox D. J.. Gaussian 16, Rev. A.03, Wallingford, CT, 2016.
- Peng Q.; Yi Y.; Shuai Z.; Shao J. Excited state radiationless decay process with Duschinsky rotation effect: Formalism and implementation. J. Chem. Phys. 2007, 126, 114302. 10.1063/1.2710274. [DOI] [PubMed] [Google Scholar]
- Peng Q.; Yi Y.; Shuai Z.; Shao J. Toward quantitative prediction of molecular fluorescence quantum efficiency: Role of Duschinsky rotation. J. Am. Chem. Soc. 2007, 129, 9333–9339. 10.1021/ja067946e. [DOI] [PubMed] [Google Scholar]
- Niu Y.; Peng Q.; Deng C.; Gao X.; Shuai Z. Theory of Excited State Decays and Optical Spectra: Application to Polyatomic Molecules. J. Phys. Chem. A 2010, 114, 7817–7831. 10.1021/jp101568f. [DOI] [PubMed] [Google Scholar]
- Niu Y.; Peng Q.; Shuai Z. Promoting-mode free formalism for excited state radiationless decay process with Duschinsky rotation effect. Sci. China, Ser. B: Chem. 2008, 51, 1153–1158. 10.1007/s11426-008-0130-4. [DOI] [Google Scholar]
- Peng Q.; Niu Y.; Shi Q.; Gao X.; Shuai Z. Correlation Function Formalism for Triplet Excited State Decay: Combined Spin-Orbit and Nonadiabatic Couplings. J. Chem. Theory Comput. 2013, 9, 1132–1143. 10.1021/ct300798t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.