Abstract
Background:
Preclinical studies suggest that for complete midsubstance anterior cruciate ligament (ACL) injuries, a suture repair of the ACL augmented with a protein implant placed in the gap between the torn ends (bridge-enhanced ACL repair [BEAR]) may be a viable alternative to ACL reconstruction (ACLR).
Hypothesis:
We hypothesized that patients treated with BEAR would have a noninferior patient-reported outcomes (International Knee Documentation Committee [IKDC] Subjective Score; prespecified noninferiority margin, –11.5 points) and instrumented anteroposterior (AP) knee laxity (prespecified noninferiority margin, +2-mm side-to-side difference) and superior muscle strength at 2 years after surgery when compared with patients who underwent ACLR with autograft.
Study Design:
Randomized controlled trial; Level of evidence, 1.
Methods:
One hundred patients (median age, 17 years; median preoperative Marx activity score, 16) with complete midsubstance ACL injuries were enrolled and underwent surgery within 45 days of injury. Patients were randomly assigned to receive either BEAR (n = 65) or autograft ACLR (n = 35 [33 with quadrupled semitendinosus-gracilis and 2 with bone–patellar tendon–bone]). Outcomes—including the IKDC Subjective Score, the side-to-side difference in instrumented AP knee laxity, and muscle strength—were assessed at 2 years by an independent examiner blinded to the procedure. Patients were unblinded after their 2-year visit.
Results:
In total, 96% of the patients returned for 2-year follow-up. Noninferiority criteria were met for both the IKDC Subjective Score (BEAR, 88.9 points; ACLR, 84.8 points; mean difference, 4.1 points [95% CI, –1.5 to 9.7]) and the side-to-side difference in AP knee laxity (BEAR, 1.61 mm; ACLR, 1.77 mm; mean difference, –0.15 mm [95% CI, –1.48 to 1.17]). The BEAR group had a significantly higher mean hamstring muscle strength index than the ACLR group at 2 years (98.2% vs 63.2%; P < .001). In addition, 14% of the BEAR group and 6% of the ACLR group had a reinjury that required a second ipsilateral ACL surgical procedure (P = .32). Furthermore, the 8 patients who converted from BEAR to ACLR in the study period and returned for the 2-year postoperative visit had similar primary outcomes to patients who had a single ipsilateral ACL procedure.
Conclusion:
BEAR resulted in noninferior patient-reported outcomes and AP knee laxity and superior hamstring muscle strength when compared with autograft ACLR at 2-year follow-up in a young and active cohort. These promising results suggest that longer-term studies of this technique are justified.
Registration:
NCT02664545 (ClinicalTrials.gov identifier)
Keywords: anterior cruciate ligament, human, ACL reconstruction, ACL repair, bridge-enhanced ACL repair, scaffold-enhanced ACL repair, BEAR
Until the mid-1980s, primary repair of the anterior cruciate ligament (ACL) was used to treat ACL injuries. However, failure rates as high as 50% at 2 years have been reported, even recently, particularly in younger active patients.9,13 Thus, replacement of the torn ACL with a tendon graft (ACL reconstruction [ACLR]) serves as the standard of care. Traditional primary repair has been reported to have some success in older patients with proximal tears where the ligament can be reopposed to the femoral bone.27,52 However, these retrospective and uncontrolled studies have been conducted in populations where nonoperative treatment of ACL tears has been demonstrated to be equally effective.44,51 The current study was designed to focus on the young and active patient population, who are at greater risk for ACL injury6 and ACL graft failure.2,23
Over the past 2 decades, preclinical studies have evaluated possible biologic causes of the failure of midsubstance ACL injuries to heal, even with suture repair.31,32,36-39,48 For ligaments that naturally heal with nonoperative treatment (eg, the medial collateral ligament), the gap between the torn ligament ends is bridged by a fibrin clot, which provides a scaffold into which torn ligament ends grow and reunite.11 However, the intra-articular location of the ACL results in the premature dissolution of that fibrin clot, which inhibits gap healing.11,14,30,31,37,38 With that understanding, in vitro and in vivo studies were performed to optimize a scaffold implant that could be positioned in the joint to bridge the gap of a midsubstance ACL tear and facilitate healing.31,39-41 Suturing techniques to facilitate healing were then optimized, including the use of a suture cinch to temporarily reduce the pathologic knee laxity that occurs with acute ACL injury during healing.36
The current technique for bridge-enhanced ACL repair (BEAR) involves placing a resorbable protein-based implant containing autologous blood in the gap between the 2 torn ends of a midsubstance ACL tear in combination with suture repair of the ligament and a suture cinch to reduce the tibiofemoral joint (Figure 1).33,34 The scaffold is used to bridge the gap, so absolute reapproximation of the torn ligament ends is not required for healing.22,28,31 In preclinical animal models, the BEAR technique resulted in a repair with comparable mechanical properties to a healing graft32,53 and in less posttraumatic osteoarthritis than what was observed in ACL-reconstructed animals.32
Figure 1.
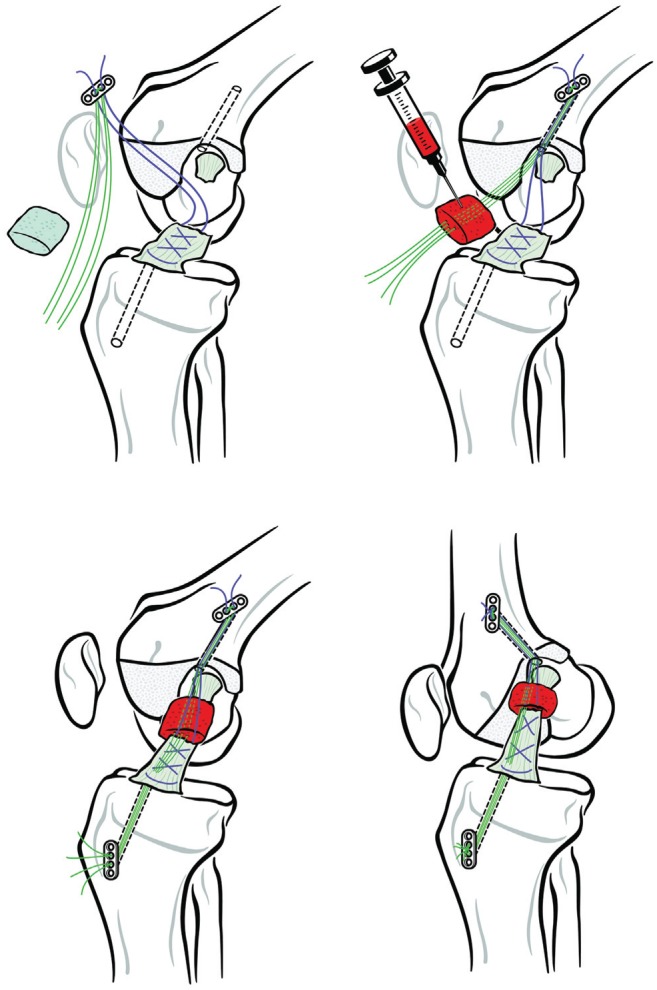
Schematic of the technique used to place the BEAR implant. Upper left panel: A suture (purple) is placed through the tibial stump via a whipstitch and secured with 2 free sutures (green) to an extracortical button. Upper right panel: After a cortical button carrying free sutures (green) is passed up through the femoral tunnel, the BEAR implant is loaded onto them and soaked with up to 10 mL of autologous blood. Lower left panel: The free suture ends (green) at the tibial end of the BEAR implant (which was positioned between the 2 ends of the torn ACL) are passed through the tibial tunnel to be tied over a second extracortical button. Lower right panel: The sutures and extracortical buttons are secured. ACL, anterior cruciate ligament; BEAR, bridge-enhanced ACL repair.
The outcomes of the BEAR technique in 10 patients in a first-in-human safety study were recently reported.33,34 Although repair of the torn ACL has some inherent advantages over ACLR—including no need to harvest normal tissue to replace the ACL,27 as well as the decreased risk of postinjury osteoarthritis as suggested in a preclinical study32—these benefits would not outweigh the risks of a procedure that resulted in lower patient-reported outcome scores or significantly greater anteroposterior (AP) knee laxity. The primary hypothesis was that patients treated with BEAR would have noninferior patient-reported outcomes (International Knee Documentation Committee [IKDC] Subjective Score) and AP knee laxity as compared with patients undergoing autograft ACLR at 2-year follow-up. Our secondary hypothesis was that the BEAR group would have superior functional outcomes (hop testing and isometric thigh muscle strength testing) as compared with the ACLR group.
Methods
This randomized controlled trial (BEAR II Trial; ClinicalTrials.gov NCT02664545) consisted of 100 patients undergoing surgery for an acute ACL injury. Patients were blinded to which treatment they received and were unblinded after the 2-year follow-up visit was completed. An independent examiner was blinded to the surgical side and study group assignment when performing the arthrometer testing and physical examination, until the end of each visit, when effusion was assessed after removal of the sleeves. One of the participating surgeons (L.J.M.) performed 10 BEAR procedures in an earlier trial,33,34 while the other 2 (D.E.K., Y.-M.Y.) had not performed a BEAR procedure before this trial.
Patients
Institutional review board and US Food and Drug Administration approval to conduct the trial was obtained before the start of the BEAR II Trial (IDE G150268, IRB P00021470). All patients granted their informed consent. Between May 2016 and June 2017, 100 patients (ages, 13-35 years) who had a complete ACL tear, were <45 days from injury, had closed physes, and had at least 50% of the length of the ACL attached to the tibia were randomized in an approximate 2:1 ratio to undergo either the implant-enhanced ACL repair procedure (ie, BEAR; 65 patients) or autograft ACLR (35 patients) (Figure 2). A permuted block randomization scheme was used with block sizes of 3 and 6. Randomization was stratified by the surgeon’s preference for autograft source (hamstring tendon or bone–patellar tendon–bone) and administered by the research coordinators using sealed envelopes from the statistician. Patients were excluded if they had a history of ipsilateral knee surgery, previous knee infection, or risk factors that could adversely affect ligament healing (nicotine/tobacco use, corticosteroid use in the past 6 months, chemotherapy, diabetes, inflammatory arthritis). Patients were excluded if they had a displaced bucket-handle tear of the medial meniscus requiring repair; patients with any other meniscal injuries were included. Patients were excluded if they had a full-thickness chondral injury, a grade III medial collateral ligament injury, a concurrent complete patellar dislocation, or a posterolateral corner injury requiring operative treatment. All patients were enrolled at Boston Children’s Hospital, and patient recruitment was completed over 12 months.
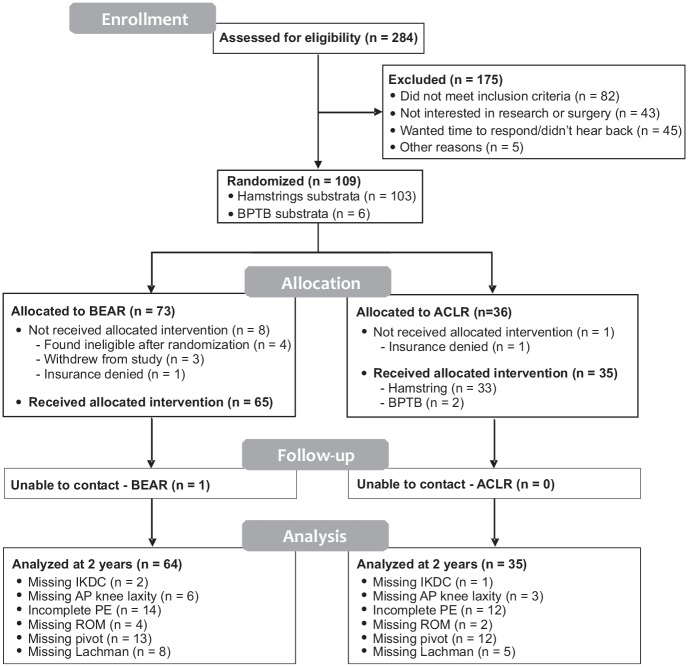
Figure 2.
CONSORT (Consolidated Standards of Reporting Trials) diagram detailing patient flow through the study. ACLR, anterior cruciate ligament reconstruction; AP, anteroposterior; BEAR, bridge-enhanced anterior cruciate ligament repair; BPTB, bone–patellar tendon–bone; IKDC, International Knee Documentation Committee; PE, physical examination; ROM, range of motion.
Implant
The ACL implant (BEAR implant; Boston Children’s Hospital) passed all biocompatibility and sterility testing.48 The implant was composed of extracellular matrix proteins, including collagen, that were obtained from bovine tissue. The efficacy of the implant for stimulating ACL healing was previously demonstrated in preclinical studies.31
BEAR Procedure
A BEAR procedure was performed as previously described.35 In brief, a whipstitch (Vicryl; Ethicon) was placed in the tibial stump and combined with a polyethylene suture cinch (Ethibond; Ethicon) and the BEAR implant (Boston Children’s Hospital) to repair the ACL (Figure 1). The drilling of the tunnels and suture cinch passage were performed arthroscopically, and the BEAR implant was delivered via a mini-arthrotomy.
ACLR Procedure
A standard hamstring autograft procedure was performed using a quadrupled semitendinosus-gracilis graft (n = 33) or central-third bone–patellar tendon–bone autograft (n = 2) with a continuous-loop cortical button (EndoButton; Smith & Nephew) for proximal fixation and a bioabsorbable interference screw (BioRCI HA; Smith & Nephew) for tibial fixation.
Postoperative Rehabilitation
An identical physical therapy protocol adapted from that of MOON (Multicenter Orthopaedics Outcomes Network)55,56 was provided to all patients. The physical therapists were not informed of the treatment assignment. For all patients, a locking hinged brace (TScope; Breg) was applied postoperatively to limit joint range of motion between 0° and 50° of knee flexion for 2 weeks and from 0° to 90° for the next 4 weeks, unless they had a concomitant meniscal repair, in which case the brace range was restricted to 0° to 40° for the first 4 weeks before increasing to 0° to 90° of flexion. Use of a locking hinge brace to restrict range of motion for the first 6 weeks after surgery is the standard of care for ACLR at our institution. All patients were provided with a cold therapy unit (IceMan; DJO Global). Both groups were kept partial weightbearing for 2 weeks and then weightbearing as tolerated with crutches until 4 weeks. Use of a functional ACL brace (CTi brace; OSSUR) was recommended from 6 to 12 weeks and then for cutting and pivoting sports for 2 years after surgery.
Outcome Measures
IKDC Subjective Score
The IKDC Subjective Score was used per the published instructions.18
Instrumented AP Knee Laxity
Arthrometer testing (KT-1000; MEDMetric) was used to measure the anterior displacement of the tibia with respect to the femur under 130 N of applied anterior force and performed in duplicate on each leg. The results were reported as a side-to-side difference between limbs (mean of the surgical knee minus the mean on the contralateral knee).
Physical Examination
The IKDC Objective Score was calculated for all patients per the published instructions.20 Knee effusion, range of motion, and clinical knee stability measures (Lachman test and pivot-shift test) were evaluated for both knees preoperatively and 2 years after surgery.
Functional Outcomes
Hamstring, quadriceps, and hip abductor muscle isometric strengths were measured with a handheld dynamometer (Microfet 2; Hoggan Scientific, LLC).29 Hamstring strength was measured with the patient prone, the knee in 90° of flexion, and the dynamometer placed proximal to the ankle. Hip abductor strength was measured in a side-lying patient and the dynamometer over the midlateral thigh. Quadriceps strength was measured with the knee in 90° of flexion and the dynamometer at the distal tibia. Patients also performed the following tests: single hop, triple hop, 6-m timed hop, and crossover hop.43 All measures were performed in duplicate on each side, and the duplicate measurements were averaged for further analysis. Results were normalized by expressing the injured knee result as a percentage of the uninjured contralateral knee result (index) for all strength and hop measures.
Additional Knee Surgery
All incidences of ACL failure requiring a second ipsilateral ACL procedure were recorded, as well as any occurrences of a contralateral ACL tear. Patients who had an additional ipsilateral ACL tear in the BEAR group were treated with an ACLR, while those with an additional ACL tear in the ACLR group had revision ACLR. Incidences of arthroscopy to treat arthrofibrosis, meniscal injury not associated with a concomitant ACL injury, or removal of hardware were also recorded.
Methods Used to Minimize Potential, Actual, or Perceived Bias of the Study
Patient recruitment and consent, as well as data collection and statistical analyses, were performed by investigators with no financial stake or compensation from any commercial interest that stood to gain from the results of this study. All physical examination and functional measurements were taken by examiners who were blinded to the procedure and surgical limb via bilateral knee sleeves placed by the research coordinators before the examiner met with the patient. This study was overseen by a Data Safety Monitoring Board, a clinical research manager, and a study monitor, all of whom were independent of the study team.
Statistical Methods
Analyses were performed as a modified intent to treat. t tests and Wilcoxon tests were used for baseline comparisons between BEAR and ACLR treatment groups on continuous variables, and Fisher exact tests were used for categorical measures. Intraoperative findings were compared with Fisher exact tests and Cochran-Armitage tests for ordinal outcomes.
The trial was designed to demonstrate noninferiority of the BEAR group relative to the ACLR group on the IKDC Subjective Score and knee laxity endpoints at 2 years. For the IKDC Subjective Score, noninferiority for BEAR was demonstrated if the entire 95% CI for the difference between groups (BEAR minus ACLR) was to the right of the noninferiority margin of –11.5.19 For knee laxity, a lower side-to-side difference (surgical minus contralateral knee) is desirable, so noninferiority was demonstrated if the 95% CI for the difference (BEAR minus ACLR) was to the left of the noninferiority margin of +2.0 mm.4 The noninferiority margin of –11.5 points for the IKDC Subjective Score was selected according to the work by Irrgang et al,19 who concluded that it represents the optimal threshold for high sensitivity when distinguishing patients who did or did not improve after knee surgery. The noninferiority margin for AP knee laxity was set at +2.0 mm based on the work of Arneja and Leith,4 who suggested a 2- to 3-mm difference as the criterion for a diagnostic test of AP knee laxity. Demonstration of noninferiority required that both endpoints meet the criteria described earlier. Although 95% CIs are 2-sided, only 1 side is relevant for demonstrating noninferiority, which is functionally equivalent to using 1-sided 97.5% CIs and setting α to .025 for each of the 2 primary noninferiority outcomes. One-sided significance tests for noninferiority are also presented for group differences in IKDC Subjective Scores and AP knee laxity, which are based on t tests shifted to the noninferiority margins.
Using a priori power calculations, we estimated that a sample size of 69 (46 BEAR and 23 ACLR) would provide 99% power to test that the IKDC Subjective Score for the BEAR group would be noninferior to that for the ACLR group and additionally provide 80% power to test that AP knee laxity would be noninferior to that for the ACLR group. This assumed true equality of the groups and noninferiority margins equal to those previously specified with an α of .025 for each outcome. The sample size was increased to 100 patients to account for loss to follow-up and to allow for potential subgroup analyses.
Subsequent to demonstrating noninferiority, superiority of the BEAR group relative to ACLR group was performed. t tests were used to compare groups on the 2 primary outcomes and all other secondary functional outcomes. Cochran-Armitage trend tests were used for IKDC objective outcomes, and Fisher exact tests were used for comparing groups on subsequent surgery rates.
Results
Baseline Characteristics and Intraoperative Findings
The 2 groups were similar in age, sex, race, and body mass index (Table 1). Most injuries were noncontact, and all but 1 occurred during sports participation. The median times from injury to surgery were similar between groups. There was no significant difference in baseline IKDC Subjective Scores. One patient in the ACLR group had a medial collateral ligament tear on the preoperative magnetic resonance imaging scan, which was treated nonoperatively. Intraoperatively, all patients had at least 50% of the length of the ACL preserved as a tibial remnant (Table 2). The numbers of patients with concomitant meniscal tears were similar between groups, as was the degree of effusion at the time of surgery. All patients in the BEAR group and 34 of 35 patients in the ACLR group had a positive pivot-shift examination result at their preoperative evaluation.
Table 1.
Baseline Characteristics of the 2 Groups a
| BEAR (n = 65) | ACLR (n = 35) | P Value | |
|---|---|---|---|
| Demographics | |||
| Female | 37 (57) | 19 (54) | .84 |
| White, non-Hispanic b | 55 (86) | 26 (74) | .18 |
| Age, y | 17 (16-20) | 17 (15-23) | .76 |
| Body mass index | 24.7 ± 3.8 | 23.3 ± 4.5 | .11 |
| Noncontact injury | 48 (74) | 29 (83) | .46 |
| Injury to surgery, d | 36 (29-42) | 39 (33-43) | .15 |
| Baseline score | |||
| IKDC b | 50.0 ± 16.7 | 45.5 ± 14.6 | .18 |
| Marx c | 16 (13-16) | 16 (13-16) | .62 |
| MRI findings | |||
| Torn PCL | 00 (0) | 00 (0) | ≥.99 |
| Torn MCL | 00 (0) | 1 (3) | .35 |
| Torn LCL | 00 (0) | 00 (0) | ≥.99 |
Data are presented as No. (%), median (interquartile range), and mean ± SD. ACLR, anterior cruciate ligament reconstruction; BEAR, bridge-enhanced anterior cruciate ligament repair; IKDC, International Knee Documentation Committee; LCL, lateral collateral ligament; MCL, medial collateral ligament; MRI, magnetic resonance imaging; PCL, posterior cruciate ligament.
BEAR, n = 64; ACLR, n = 35.
BEAR, n = 64; ACLR, n = 34.
Table 2.
Intraoperative Findings and Additional Procedures a
| BEAR (n = 65) | ACLR (n = 35) | P Value | |
|---|---|---|---|
| Length of ACL tibial remnant, % | .38 | ||
| <50 | 00 (0) | 00 (0) | |
| 50-74 | 57 (88) | 28 (80) | |
| 75-100 | 8 (12) | 7 (20) | |
| ≥1 meniscal tears | |||
| Medial | 5 (8) | 6 (17) | .19 |
| Lateral | 26 (40) | 20 (57) | .14 |
| Treatment of meniscal tears b | .48 | ||
| Repair | 15 (56) | 15 (68) | |
| Abrasion/trephination | 2 (7) | 1 (5) | |
| Excision | 6 (22) | 3 (14) | |
| No surgical treatment | 4 (15) | 3 (14) | |
| Effusion grade c | .12 | ||
| None | 17 (26) | 15 (44) | |
| Mild | 38 (58) | 15 (44) | |
| Moderate | 10 (15) | 4 (12) | |
| Severe | 00 (0) | 00 (0) | |
| Firm Lachman endpoint c | 1 (2) | 1 (3) | ≥.99 |
| Pivot shift | .67 | ||
| Negative | 00 (0) | 1 (3) | |
| Glide | 13 (20) | 5 (14) | |
| Clunk | 41 (63) | 25 (71) | |
| Gross | 11 (17) | 4 (11) |
Data are presented as No. (%). ACL, anterior cruciate ligament; ACLR, ACL reconstruction; BEAR, bridge-enhanced ACL repair.
If patients had >1 treatment, they were categorized as the first type listed. For example, if patients had both repair and excision, they were categorized as repair. Analysis of meniscal treatment is restricted to patients with ≥1 meniscal tears (BEAR, n = 27; ACLR, n = 22).
BEAR, n = 65; ACLR, n = 34.
Patients at 2 Years Postoperatively
Three patients in the BEAR group and 1 in the ACLR group were unable to come on-site for their 2-year follow-up examinations. However, 3 of these 4 patients were contacted by phone to verify if they had additional knee surgery. Sample sizes also varied for some outcomes owing to missing data, as not all patients were able to complete all outcome measures (Figure 2).
Primary Outcomes
At 2 years, there were no significant differences between the BEAR and ACLR groups in terms of IKDC Subjective Score or AP knee laxity (Table 3). For the primary analysis of noninferiority, the 95% CI for the difference in mean IKDC Subjective Scores between groups did not exceed the noninferiority threshold of –11.5. Additionally, the 95% CI for the group difference in AP knee laxity (expressed as side-to-side difference) did not exceed the noninferiority criterion of +2.0. Thus, inferiority was rejected in favor of noninferiority (P < .001) for IKDC Subjective Score and AP knee laxity.
Table 3.
Primary Outcomes at 2 Years: IKDC Subjective Score and AP Knee Laxity a
| BEAR | ACLR | P Value | |||||
|---|---|---|---|---|---|---|---|
| No. | Mean (SD) | No. | Mean (SD) | Mean Difference (95% CI) b | Noninferiority c | Superiority/Inferiority d | |
| IKDC Subjective Score | 62 | 88.9 (13.2) | 34 | 84.8 (13.2) | 4.1 (–1.5 to 9.7) | <.001 | .15 |
| AP knee laxity, mm | 58 | 1.61 (3.16) | 32 | 1.77 (2.79) | −0.15 (–1.48 to 1.17) | <.001 | .82 |
ACLR, anterior cruciate ligament reconstruction; AP, anteroposterior; BEAR, bridge-enhanced anterior cruciate ligament repair; IKDC, International Knee Documentation Committee.
Positive difference for IKDC Subjective Score and negative difference for AP laxity favor BEAR.
P values (1-sided) correspond to testing primary research hypothesis of noninferiority vs null hypothesis of inferiority based on predetermined inferiority thresholds (–11.5 and +2.0 for IKDC and laxity, respectively).
P values (2-sided) correspond to secondary hypothesis of superiority/inferiority vs null hypothesis of equality.
IKDC Objective Score
Fifty of 65 (77%) patients in the BEAR group and 25 of 35 (71%) in the ACLR group returned for a complete IKDC physical examination at 2 years (Table 4). For the patients tested, 93% of the BEAR group and 90% of the ACLR group had a grade A Lachman. In addition, 80% of the BEAR group and 92% of the ACLR group had a grade A pivot examination. There were no significant differences in IKDC Objective Scores.
Table 4.
IKDC Objective Score Outcomes at 2 Years After Surgery a
| BEAR | ACLR | P Value | |
|---|---|---|---|
| Effusion | 57 | 30 | .48 |
| A | 53 (93) | 29 (97) | |
| B | 4 (7) | 1 (3) | |
| C | 00 (0) | 00 (0) | |
| D | 00 (0) | 00 (0) | |
| Range of motion | 60 | 33 | .42 |
| A | 32 (53) | 18 (55) | |
| B | 20 (33) | 13 (39) | |
| C | 5 (8) | 2 (6) | |
| D | 3 (5) | 00 (0) | |
| Lachman | 56 | 30 | .41 |
| A | 52 (93) | 27 (90) | |
| B | 3 (5) | 1 (3) | |
| C | 1 (2) | 2 (7) | |
| D | 00 (0) | 00 (0) | |
| Pivot | 51 b | 25 | .19 |
| A | 41 (80) | 23 (92) | |
| B | 10 (20) | 2 (8) | |
| C | 00 (0) | 00 (0) | |
| D | 00 (0) | 00 (0) | |
| Overall c | 50 | 25 | .64 |
| A | 19 (38) | 11 (44) | |
| B | 25 (50) | 11 (44) | |
| C | 5 (10) | 3 (12) | |
| D | 1 (2) | 00 (0) |
Values are presented as No. (%). ACLR, anterior cruciate ligament reconstruction; BEAR, bridge-enhanced anterior cruciate ligament repair; IKDC, International Knee Documentation Committee.
Three patients in the BEAR group had undergone a second anterior cruciate ligament surgical procedure <6 mo before the 2-y follow-up visit, and a pivot-shift examination was not performed per the study protocol.
Overall score was computed for patients with complete data for all IKDC components; the worse of the Lachman or Pivot scores were used for the Ligament component of the calculation.
Functional Outcomes
The hamstring index was significantly different in the BEAR and ACLR groups at 2 years (Table 5). The ACLR group had a mean of 63% hamstring strength on the operated side versus the contralateral side. There were no differences in quadriceps and hip abductor indices. The hamstring:quadriceps ratio was larger in the BEAR group. The only significant difference between the groups on hop testing was for the 6-m timed hop test, where the BEAR group took 4% longer to hop 6 m on the operative leg as compared with the nonoperative leg. There were no other significant differences in hop testing between the BEAR and ACLR groups at 2 years.
Table 5.
Functional Measures at 2 Years After Surgery a
| BEAR | ACLR | |||||
|---|---|---|---|---|---|---|
| No. | Mean (SD) | No. | Mean (SD) | Mean Difference (95% CI) b | P Value | |
| Index | ||||||
| Hamstring | 59 | 98.2 (26.5) | 31 | 63.2 (15.5) | 35.0 (26.1 to 43.8) | <.001 |
| Quadriceps | 59 | 100.1 (12.2) | 31 | 101.5 (12.4) | −1.4 (–6.6 to 4.0) | .61 |
| Hamstring:quadriceps ratio (surgical side) | 59 | 0.43 (0.12) | 32 | 0.27 (0.08) | 0.16 (0.11 to 0.21) | <.001 |
| Hip abductor index | 56 | 105.3 (15.3) | 31 | 107.9 (22.5) | −2.6 (–11.7 to 6.6) | .58 |
| Hop | ||||||
| Single-leg | 42 | 94.4 (13.0) | 23 | 96.9 (13.4) | −2.4 (–9.2 to 4.4) | .48 |
| Triple | 41 | 94.9 (9.7) | 22 | 98.0 (6.9) | −3.0 (–7.7 to 1.6) | .20 |
| 6-m timed | 40 | 103.9 (10.6) | 22 | 98.0 (6.7) | 5.9 (1.5 to 10.3) | .009 |
| Crossover | 39 | 96.6 (9.8) | 22 | 96.0 (7.3) | 0.6 (–4.2 to 5.4) | .81 |
Values are presented as percentages, unless otherwise stated. ACLR, anterior cruciate ligament reconstruction; BEAR, bridge-enhanced anterior cruciate ligament repair.
Positive difference favors BEAR, and negative difference favors ACLR for all outcomes except 6-m timed hop.
Additional Knee Surgery Rates
Conversion to ACLR was performed in 9 of 64 (14%) patients in the BEAR group, and a revision ACLR was required in 2 of 35 (6%) patients in the ACLR group, a difference that was not statistically significant (P = .32) (Table 6). Half of the retears occurred in both groups during the first year. There were no significant differences between groups related to other additional surgeries for the ipsilateral or contralateral knee.
Table 6.
Additional Ipsilateral and Contralateral Knee Surgical Procedures Within the First 2 Postoperative Years for the BEAR and ACLR Groupsa
| BEAR (n = 64) | ACLR (n = 35) | P Value | |
|---|---|---|---|
| Ipsilateral ACL surgery—all | 9 (14.1) | 2 (5.7) | .32 |
| Isolated | 1 (1.6) | 1 (2.9) | ≥.99 |
| With meniscus | 8 (12.5) | 1 (2.9) | .15 |
| Non-ACL ipsilateral knee surgery | |||
| Arthrofibrosis | 00 (0.0) | 2 (5.7) | .12 |
| Meniscus | 7 (10.9) | 2 (5.7) | .49 |
| Removal of hardware | 1 (1.6) | 00 (0.0) | ≥.99 |
| Total patients with ipsilateral knee surgeryb | 16 (25.0) | 5 (14.3) | .30 |
| Contralateral ACL surgery | 2 (3.1) | 1 (2.9) | ≥.99 |
Values are presented as No. (%). ACL, anterior cruciate ligament; ACLR, ACL reconstruction; BEAR, bridge-enhanced ACL repair.
Two patients (BEAR, n = 1; ALCR, n = 1) had both ACL and non-ACL ipsilateral knee surgery.
Outcomes in Patients Converting to ACLR in the First 2 Postoperative Years
Eight of 9 patients in the BEAR group who converted to an ACLR returned for their 2-year postoperative visit from the index procedure. These patients had a mean IKDC Subjective Score of 85.5 points (95% CI, 77.1-93.9) at 2 years. For the 5 patients in the BEAR group who converted to an ACLR and had their 2-year follow-up visit >6 months after their second surgical procedure (and thus could have an instrumented AP knee laxity test), the mean laxity value (ie, difference between surgical and contralateral) was 1.40 mm (95% CI, –4.2 to 7.0).
Discussion
This randomized controlled trial demonstrated that the IKDC Subjective Scores and AP knee laxity values in the BEAR group were noninferior to those of the ACLR group at 2 years after surgery based on the a priori established margins. In addition, there was no statistically significant difference in failure rate with the BEAR technique when compared with autograft ACLR, which is in contrast to the significantly higher risk (with a concomitant 10× hazard ratio) previously reported with nonaugmented suture repair.13 Hamstring strength was significantly better in the BEAR group. These findings—in a young athletic patient population (median age, 17 years; median Marx activity score, 16) and with the very early experience of surgeons and physical therapists with this new technique—suggest that ACL repair with the BEAR implant is worthy of additional study. It is important to note that we selected this population because it is at greater risk for ACL graft failure2,23 and because we wanted to minimize the potential bias of studying patients who would not challenge the ACL graft or repair and who could do well with nonoperative treatment.12
There was no significant difference in IKDC Subjective Scores between groups at 2 years. The mean 2-year IKDC Subjective Score was 85 points in the ACLR group in this trial, which is consistent with the 2-year ACLR scores reported by the MOON group (82 points)8 and others (86 points).49 The BEAR group had mean scores at 2 years similar to those in the ACLR group and similar to that reported for an age-matched control cohort (88 points).3 Given that the lower bound of the confidence interval for the difference was −1.5, the pre-specified noninferiority lower bound (−11.5) for the IKDC Subjective Score could have been set much tighter and still been satisfied.
Instrumented AP knee laxity values were also similar between the groups, with a mean side-to-side difference <2 mm, similar those values reported for other ACLR studies at 2 years (1.1-2.5 mm).10,15 Given the bounds of the confidence intervals for the group difference of this primary outcome measure (ie, 1.17-mm side-to-side difference), the noninferiority bound could have been set much tighter and still been satisfied. The knees in both groups were also largely stable on clinical examination, with no side-to-side difference in the Lachman grade for >90% of the patients in both groups. The pivot-shift examination at 2 years had no side-to-side difference for 80% of the patients tested in the BEAR group, similar to the percentage in the ACLR group and to those previously reported 2 years after autograft ACLR (49%-84%).24,50
The morbidity of autograft tendon harvest includes muscle weakness from graft harvest.25 In this study, the weakness of the hamstring muscles, when tested by dynamometer at 90° of flexion, persisted to 2 years for the patients treated with ACLR, of which most were 4-stranded hamstring tendon autografts. Isometric deficits, as measured here, are associated with slower walking speeds and altered knee mechanics during walking and running gait.1 Taking these measurements at higher knee flexion angles better isolates the contributions of the gracilis and semitendinosis muscles,42 and the results here suggest that the harvest of these tendons may result in a loss of strength for these 2 muscles for at least 2 years after ACLR. However, the lack of hamstring strength in the ACLR group did not appear to detrimentally affect the hop testing results, as the values were similar to those in the BEAR group, and the ACLR group was able to hop a 6m distance faster than the BEAR group. This difference between groups, although statistically significant, was relatively small in magnitude (6%) and of unclear clinical importance.
While the primary outcome measures for the BEAR procedure came reasonably close to restoring a normal knee, 14% of the patients in this group required additional ipsilateral ACL surgery, in comparison with 6% in the ACLR group, a difference that was not statistically significant. Evaluating these results in the context of other studies of patients with a similar patient demographic (median age, 17 years; median Marx activity score, 16) is important, as younger age and higher activity level have been found to be correlated with a higher ACL reinjury rate.2,45 In evaluating reinjury rates in similarly young and athletic patient populations, the risk for reinjury for a primary repair without a scaffold, even in carefully selected patients, is 49% at 2 years.13 Revision rates for ACLR in similar patient populations have ranged from 10% to 28%.5,7,17,21,23,46 Thus, the revision rate of 14% would compare favorably with the rate noted for suture repair without a scaffold in this patient population13 and may be similar to that reported for autograft ACLR for this group.5,7,17,21,23,46 Also interesting was that patients in the BEAR group who had a revision ACLR had a mean IKDC Subjective Score at 2 years similar to that of patients who had only a primary ACLR (85.5 vs 84.8 points) and that the AP knee laxity values were also similar (1.4 vs 1.8 mm). This is in contrast to previous reports of revision ACL surgery of a primary ACLR, which indicated poorer IKDC Subjective Scores (8-point difference at 2 years)54 than those of patients who did not have that second procedure.
The rate for subsequent surgical procedures, other than ipsilateral ACL surgery, in the adolescent and young adult population has been reported to be 9.4%16; for arthrofibrosis/lysis of adhesions, 4.8%; and for removal of tibial hardware, 1.3%.16 In studies with a mean patient age <18 years, a reoperation rate for meniscal repair, even when performed with concurrent ACLR, has been reported to be 10% at 2 years23,26 and to range from 26% to 35% at 5 years.26,47 Thus, while an 11% postrepair meniscal surgery rate is not desirable, it is consistent with that previously reported for a young and active cohort undergoing ACLR.
There were several study limitations. While the 2:1 ratio enabled enrollment of a larger number of patients in the BEAR arm, facilitated recruitment, and improved power to detect the occurrence of adverse events that might occur with a low frequency, it also led to the enrollment of only 35 patients in the ACLR group, which lessened the power of the study when comparing differences in the rarer outcomes, including reoperation rates. In addition, the majority of patients in the autograft ACLR group had hamstring autografts; however, multiple randomized controlled trials have shown no significant difference in patient-reported outcomes, AP knee laxity, and failure rates between hamstring and bone–patellar tendon–bone autografts.57 In addition, while all surgeons in this study had significant experience performing ACLR, only 1 (L.J.M.) had previously performed 10 BEAR procedures.33,34 Thus, if a learning curve for BEAR was present, this study would still have been early within that curve.
In conclusion, ACL repair with the BEAR implant produced outcomes similar to those of ACLR for patient-reported outcomes and AP knee laxity at 2 years after surgery in a young and active cohort. The inherent benefits of this procedure—including no need for autograft harvest, and a decreased risk of posttraumatic osteoarthritis according to preclinical studies of the repair procedure— should be weighed carefully by individual patients and surgeons against the risk of requiring conversion to an ACLR in the first 2 years after surgery. The results here suggest that ACL repair with the BEAR implant is a safe and promising technique that is deserving of further study.
Acknowledgments
The authors acknowledge the significant contributions of the clinical trial team, including Bethany Trainor, Andrea Hale, Elizabeth Carew, Brett Flutie, Laura Thurber, and Shanika Coney. The authors also acknowledge the contributions of their medical safety monitoring team of Joseph DeAngelis, Peter Nigrovic, and Carolyn Hettrich; data monitors Maggie Malsch, Megan Fitzgerald, and Erica Denhoff; and the clinical care team for the trial patients, including Kathryn Ackerman, Alyssa Aguiar, Judd Allen, Michael Beasley, Jennifer Beck, Dennis Borg, Nicole Bottino, Jeff Brodeur, Stephanie Burgess, Melissa Christino, Andrea Cianci, Sara Cline, Sarah Collins, Gianmichel Corrado, Corey Dawkins, Pierre D’Hemecourt, Peter Fabricant, Jon Ferguson, Michele Flannery, Joseph Founds, Casey Gavin, Ellen Geminiani, George Georgoudis, Stacey Gigante, Christine Gonzalez, Annie Griffin, Emily Hanson, Elspeth Hart, Jackie Hastings, Pamela Horne-Goffigan, Leslie Kalish, Meghan Keating, Elizabeth Killkelley, Elizabeth Kramer, Pamela Lang, Hayley Lough, Kathleen Maguire, Chaimae Martin, Steven Mathew, Michael McClincy, William Meehan, Ariana Moccia, Jen Morse, Mariah Mullen, Stacey Murphy, Emily Niu, Michael O’Brien, Nikolas Paschos, Katrina Plavetsky, Bridget Quinn, Brianna Quintiliani, Lauren Redler, Shannon Savage, Edward Schleyer, Benjamin Shore, Cynthia Stein, Andrea Stracciolini, Kathleen Strawn, Dai Sugimoto, Dylan Taylor, Ashleigh Thorogood, Jessica Travers, Natasha Trentacosta, Patrick Vavken, Lisa Vopat, Kevin Wenner, Cecily Whitehead, and Lenise Young. The authors thank the perioperative and operating room staff and the members of the Department of Anesthesia, who were extremely helpful in developing the peri- and intraoperative protocols. The authors acknowledge the efforts of the scaffold manufacturing team, including Gabe Perrone, Gordon Roberts, Doris Peterkin, and Jakob Sieker. The authors are grateful for the study design guidance provided by the Division of Orthopedic Devices, Center for Devices and Radiological Health, US Food and Drug Administration, under the guidance of Laurence Coyne and Mark Melkerson, particularly the efforts of Casey Hanley, Peter Hudson, Jemin Dedania, Pooja Panigrahi, and Neil Barkin. The authors are especially grateful to the patients and their families who participated in this study. Their willingness to participate in research that may help others in the future inspires all of us.
Footnotes
Submitted October 12, 2019; accepted February 6, 2020.
Presented at the annual meeting of the AOSSM, Seattle, Washington, July 2020.
The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Medical School, Harvard University or its affiliated academic health care centers, the National Football League Players Association, Boston Children’s Hospital, Rhode Island Hospital, or the National Institutes of Health.
One or more of the authors has declared the following potential conflict of interest or source of funding: This study received funding support from the Translational Research Program at Boston Children’s Hospital, the Children’s Hospital Orthopaedic Surgery Foundation, the Children’s Hospital Sports Medicine Foundation, the Football Players Health Study at Harvard University, and the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases through grants R01-AR065462 and R01-AR056834. M.M.M. is a founder, paid consultant, and equity holder in Miach Orthopaedics, Inc, which was formed to work on upscaling production of the BEAR scaffold. M.M.M. maintained a conflict-of-interest management plan that was approved by Boston Children’s Hospital and Harvard Medical School during the conduct of the trial, with oversight by both conflict-of-interest committees and the institutional review board of Boston Children’s Hospital, as well as the US Food and Drug Administration. B.C.F. is an assistant editor for The American Journal of Sports Medicine, the spouse of M.M.M. with the inherently same conflicts. D.E.K., L.J.M., and Y.-M.Y. all have disclosures as listed in the American Academy of Orthopaedic Surgeons database, none of which are related to this current project or technology. These include educational payments from Kairos Surgical (D.E.K., Y.-M.Y.) and food, beverage, and travell reimbursements from 5 companies (each <$500). L.J.M. also has received multiple payments for food and beverage from various companies. For the BEAR Trial Team, B.P. has manufactured the scaffolds used in the trials at Boston Children’s Hospital and is a paid consultant and equity holder in Miach Orthopaedics at this time, as he assists with transfer of the manufacturing process to the contract manufacturing organization that Miach has engaged to do the manufacturing. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Abourezk MN, Ithurburn MP, McNally MP, et al. Hamstring strength asymmetry at 3 years after anterior cruciate ligament reconstruction alters knee mechanics during gait and jogging. Am J Sports Med. 2017;45:97-105. [DOI] [PubMed] [Google Scholar]
- 2. Andernord D, Desai N, Bjornsson H, Ylander M, Karlsson J, Samuelsson K. Patient predictors of early revision surgery after anterior cruciate ligament reconstruction: a cohort study of 16,930 patients with 2-year follow-up. Am J Sports Med. 2015;43:121-127. [DOI] [PubMed] [Google Scholar]
- 3. Anderson AF, Irrgang JJ, Kocher MS, Mann BJ, Harrast JJ; International Knee Documentation Committee. The International Knee Documentation Committee Subjective Knee Evaluation Form: normative data. Am J Sports Med. 2006;34:128-135. [DOI] [PubMed] [Google Scholar]
- 4. Arneja S, Leith J. Review article: validity of the KT-1000 knee ligament arthrometer. J Orthop Surg (Hong Kong). 2009;17:77-79. [DOI] [PubMed] [Google Scholar]
- 5. Astur DC, Cachoeira CM, da Silva Vieira T, Debieux P, Kaleka CC, Cohen M. Increased incidence of anterior cruciate ligament revision surgery in paediatric verses adult population. Knee Surg Sports Traumatol Arthrosc. 2018;26:1362-1366. [DOI] [PubMed] [Google Scholar]
- 6. Beck NA, Lawrence JT, Nordin JD, DeFor TA, Tompkins M. ACL tears in school-aged children and adolescents over 20 years. Pediatrics. 2017;139(3):e20161877. [DOI] [PubMed] [Google Scholar]
- 7. Cordasco FA, Black SR, Price M, et al. Return to sport and reoperation rates in patients under the age of 20 after primary anterior cruciate ligament reconstruction: risk profile comparing 3 patient groups predicated upon skeletal age. Am J Sports Med. 2019;47(3):628-639. [DOI] [PubMed] [Google Scholar]
- 8. Cox CL, Huston LJ, Dunn WR, et al. Are articular cartilage lesions and meniscus tears predictive of IKDC, KOOS, and Marx Activity Level outcomes after anterior cruciate ligament reconstruction? A 6-year multicenter cohort study. Am J Sports Med. 2014;42:1058-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Feagin JA, Curl WW. Isolated tear of the anterior cruciate ligament: 5-year follow-up study. Am J Sports Med. 1976;4:95-100. [DOI] [PubMed] [Google Scholar]
- 10. Feller JA, Webster KE. A randomized comparison of patellar tendon and hamstring tendon anterior cruciate ligament reconstruction. Am J Sports Med. 2003;31:564-573. [DOI] [PubMed] [Google Scholar]
- 11. Frank C, Schachar N, Dittrich D. Natural history of healing in the repaired medial collateral ligament. J Orthop Res. 1983;1:179-188. [DOI] [PubMed] [Google Scholar]
- 12. Frobell RB, Roos EM, Roos HP, Ranstam J, Lohmander LS. A randomized trial of treatment for acute anterior cruciate ligament tears. N Engl J Med. 2010;363:331-342. [DOI] [PubMed] [Google Scholar]
- 13. Gagliardi AG, Carry PM, Parikh HB, Traver JL, Howell DR, Albright JC. ACL repair with suture ligament augmentation is associated with a high failure rate among adolescent patients. Am J Sports Med. 2019;47(3):560-566. [DOI] [PubMed] [Google Scholar]
- 14. Harrold AJ. The defect of blood coagulation in joints. J Clin Pathol. 1961;14:305-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heijne A, Werner S. A 2-year follow-up of rehabilitation after ACL reconstruction using patellar tendon or hamstring tendon grafts: a prospective randomised outcome study. Knee Surg Sports Traumatol Arthrosc. 2010;18:805-813. [DOI] [PubMed] [Google Scholar]
- 16. Hettrich CM, Dunn WR, Reinke EK, Group M, Spindler KP. The rate of subsequent surgery and predictors after anterior cruciate ligament reconstruction: two- and 6-year follow-up results from a multicenter cohort. Am J Sports Med. 2013;41:1534-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ho B, Edmonds EW, Chambers HG, Bastrom TP, Pennock AT. Risk factors for early ACL reconstruction failure in pediatric and adolescent patients: a review of 561 cases. J Pediatr Orthop. 2018;38:388-392. [DOI] [PubMed] [Google Scholar]
- 18. Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med. 2001;29:600-613. [DOI] [PubMed] [Google Scholar]
- 19. Irrgang JJ, Anderson AF, Boland AL, et al. Responsiveness of the International Knee Documentation Committee Subjective Knee Form. Am J Sports Med. 2006;34:1567-1573. [DOI] [PubMed] [Google Scholar]
- 20. Irrgang JJ, Ho H, Harner CD, Fu FH. Use of the International Knee Documentation Committee Guidelines to assess outcome following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 1998;6:107-114. [DOI] [PubMed] [Google Scholar]
- 21. Jacobs CA, Burnham JM, Makhni E, Malempati CS, Swart E, Johnson DL. Allograft augmentation of hamstring autograft for younger patients undergoing anterior cruciate ligament reconstruction. Am J Sports Med. 2017;45:892-899. [DOI] [PubMed] [Google Scholar]
- 22. Joshi S, Mastrangelo A, Magarian E, Fleming BC, Murray MM. Collagen-platelet composite enhances biomechanical and histologic healing of the porcine ACL. Am J Sports Med. 2009;37:2401-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaeding CC, Aros B, Pedroza A, et al. Allograft versus autograft anterior cruciate ligament reconstruction: predictors of failure from a MOON prospective longitudinal cohort. Sports Health. 2011;3:73-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kondo E, Yasuda K, Kitamura N, et al. Effects of initial graft tension on clinical outcome after anatomic double-bundle anterior cruciate ligament reconstruction: comparison of two graft tension protocols. BMC Musculoskelet Disord. 2016;17:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Konrath JM, Vertullo CJ, Kennedy BA, Bush HS, Barrett RS, Lloyd DG. Morphologic characteristics and strength of the hamstring muscles remain altered at 2 years after use of a hamstring tendon graft in anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44:2589-2598. [DOI] [PubMed] [Google Scholar]
- 26. Krych AJ, Pitts RT, Dajani KA, Stuart MJ, Levy BA, Dahm DL. Surgical repair of meniscal tears with concomitant anterior cruciate ligament reconstruction in patients 18 years and younger. Am J Sports Med. 2010;38:976-982. [DOI] [PubMed] [Google Scholar]
- 27. Marshall JL, Warren RF, Wickiewicz TL. Primary surgical treatment of anterior cruciate ligament lesions. Am J Sports Med. 1982;10:103-107. [DOI] [PubMed] [Google Scholar]
- 28. Mastrangelo AN, Vavken P, Fleming BC, Harrison SL, Murray MM. Reduced platelet concentration does not harm PRP effectiveness for ACL repair in a porcine in vivo model. J Orthop Res. 2011;29:1002-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mentiplay BF, Perraton LG, Bower KJ, et al. Assessment of lower limb muscle strength and power using hand-held and fixed dynamometry: a reliability and validity study. PLoS One. 2015;10:e0140822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murray MM, Bennett R, Zhang X, Spector M. Cell outgrowth from the human ACL in vitro: regional variation and response to TGF-beta1. J Orthop Res. 2002;20:875-880. [DOI] [PubMed] [Google Scholar]
- 31. Murray MM, Fleming BC. Biology of anterior cruciate ligament injury and repair: Kappa Delta Ann Doner Vaughn Award Paper 2013. J Orthop Res. 2013;31:1501-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Murray MM, Fleming BC. Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes posttraumatic osteoarthritis after surgery. Am J Sports Med. 2013;41:1762-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Murray MM, Flutie BM, Kalish LA, et al. The bridge-enhanced anterior cruciate ligament repair (BEAR) procedure: an early feasibility cohort study. Orthop J Sports Med. 2016;4:2325967116672176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Murray MM, Kalish LA, Fleming BC, et al. Bridge-enhanced anterior cruciate ligament repair: two-year results of a first-in-human study. Orthop J Sports Med. 2019;7:2325967118824356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Murray MM, Kiapour AM, Kalish LA, Ecklund K, Team BT, Fleming BC. Predictors of healing ligament size and magnetic resonance signal intensity at 6 months after bridge-enhanced anterior cruciate ligament repair. Am J Sports Med. 2019;47:1361-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Murray MM, Magarian E, Zurakowski D, Fleming BC. Bone-to-bone fixation enhances functional healing of the porcine anterior cruciate ligament using a collagen-platelet composite. Arthroscopy. 2010;29:S49-S57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Murray MM, Martin SD, Martin TL, Spector M. Histological changes in the human anterior cruciate ligament after rupture. J Bone Joint Surg Am. 2000;82:1387-1397. [DOI] [PubMed] [Google Scholar]
- 38. Murray MM, Martin SD, Spector M. Migration of cells from human anterior cruciate ligament explants into collagen-glycosaminoglycan scaffolds. J Orthop Res. 2000;18:557-564. [DOI] [PubMed] [Google Scholar]
- 39. Murray MM, Spindler KP, Abreu E, et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25:81-91. [DOI] [PubMed] [Google Scholar]
- 40. Murray MM, Spindler KP, Ballard P, Welch TP, Zurakowski D, Nanney LB. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25:1007-1017. [DOI] [PubMed] [Google Scholar]
- 41. Murray MM, Spindler KP, Devin C, et al. Use of a collagen-platelet rich plasma scaffold to stimulate healing of a central defect in the canine ACL. J Orthop Res. 2006;24:820-830. [DOI] [PubMed] [Google Scholar]
- 42. Nomura Y, Kuramochi R, Fukubayashi T. Evaluation of hamstring muscle strength and morphology after anterior cruciate ligament reconstruction. Scand J Med Sci Sports. 2015;25:301-307. [DOI] [PubMed] [Google Scholar]
- 43. Noyes FR, Barber SD, Mangine RE. Abnormal lower limb symmetry determined by function hop tests after anterior cruciate ligament rupture. Am J Sports Med. 1991;19:513-518. [DOI] [PubMed] [Google Scholar]
- 44. Odensten M, Hamberg P, Nordin M, Lysholm J, Gillquist J. Surgical or conservative treatment of the acutely torn anterior cruciate ligament: a randomized study with short-term follow-up observations. Clin Orthop Relat Res. 1985;198:87-93. [PubMed] [Google Scholar]
- 45. Parkinson B, Robb C, Thomas M, Thompson P, Spalding T. Factors that predict failure in anatomic single-bundle anterior cruciate ligament reconstruction. Am J Sports Med. 2017;45:1529-1536. [DOI] [PubMed] [Google Scholar]
- 46. Perkins CA, Busch MT, Christino M, Herzog MM, Willimon SC. Allograft augmentation of hamstring anterior cruciate ligament autografts is associated with increased graft failure in children and adolescents. Am J Sports Med. 2019;47:1576-1582. [DOI] [PubMed] [Google Scholar]
- 47. Proffen BL, Nielson JH, Zurakowski D, Micheli LJ, Curtis C, Murray MM. The effect of perioperative ketorolac on the clinical failure rate of meniscal repair. Orthop J Sports Med. 2014;2(5):2325967114529537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Proffen BL, Perrone G, Roberts G, Murray MM. Bridge-enhanced ACL repair: a review of the science and the pathway through FDA investigational device approval. Ann Biomed Engin. 2015;43:805-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Razi M, Moradi A, Safarcherati A, et al. Allograft or autograft in skeletally immature anterior cruciate ligament reconstruction: a prospective evaluation using both partial and complete transphyseal techniques. J Orthop Surg Res. 2019;14:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sadoghi P, Muller PE, Jansson V, van Griensven M, Kropfl A, Fischmeister MF. Reconstruction of the anterior cruciate ligament: a clinical comparison of bone–patellar tendon–bone single bundle versus semitendinosus and gracilis double bundle technique. Int Orthop. 2011;35:127-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sandberg R, Balkfors B, Nilsson B, Westlin N. Operative versus non-operative treatment of recent injuries to the ligaments of the knee: a prospective randomized study. J Bone Joint Surg Am. 1987;69:1120-1126. [PubMed] [Google Scholar]
- 52. Sherman MF, Lieber L, Bonamo JR, Podesta L, Reiter I. The long-term followup of primary anterior cruciate ligament repair: defining a rationale for augmentation. Am J Sports Med. 1991;9:243-255. [DOI] [PubMed] [Google Scholar]
- 53. Vavken P, Fleming BC, Mastrangelo AN, Machan JT, Murray MM. Biomechanical outcomes after bioenhanced anterior cruciate ligament repair and anterior cruciate ligament reconstruction are equal in a porcine model. Arthroscopy. 2012;28:672-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wright R, Spindler K, Huston L, et al. Revision ACL reconstruction outcomes: MOON cohort. J Knee Surg. 2011;24:289-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wright RW, Preston E, Fleming BC, et al. A systematic review of anterior cruciate ligament reconstruction rehabilitation. Part I: continuous passive motion, early weight bearing, postoperative bracing, and home-based rehabilitation. J Knee Surg. 2008;21:217-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wright RW, Preston E, Fleming BC, et al. A systematic review of anterior cruciate ligament reconstruction rehabilitation. Part II: open versus closed kinetic chain exercises, neuromuscular electrical stimulation, accelerated rehabilitation, and miscellaneous topics. J Knee Surg. 2008;21:225-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xie X, Liu X, Chen Z, Yu Y, Peng S, Li Q. A meta-analysis of bone–patellar tendon–bone autograft versus four-strand hamstring tendon autograft for anterior cruciate ligament reconstruction. Knee. 2015;22:100-110. [DOI] [PubMed] [Google Scholar]