Abstract
Obesity and diabetes increase hypertensive disorders of pregnancy (HDP) risk, thus preventive interventions are heavily studied. How pregestational prediabetes and related interventions impact HDP risk is less characterized. Therefore, we searched and reviewed the literature to assess the impact on HDP risk of prediabetes and varied interventions. We identified 297 citations related to pregnancy, prediabetes, and early pregnancy interventions. In addition, we reviewed the references in and citations of included articles. We included five studies assessing HDP outcomes in women with first trimester hemoglobin A1c in the prediabetes range (5.7–6.4%). One prospective observational study demonstrated first trimester hemoglobin A1c (5.9–6.4%) is associated with increased HDP risk, while another prospective observational study and one retrospective observational study had similar trends without achieving statistical significance. A small and underpowered randomized controlled trial demonstrated initiating gestational diabetes mellitus treatment (i.e., diet, monitoring, ± insulin) in response to first trimester hemoglobin A1c (5.7–6.4%) did not statistically reduce HDP compared to standard care. One retrospective observational study suggested metformin, when started early, may reduce HDP risk in patients with prediabetes. Pregestational prediabetes appears to increase HDP risk. Interventions (i.e., metformin, diet/glucose monitoring, and/or exercise) to reduce HDP risk require additional in-trial comparisons.
Keywords: First Trimester Pregnancy, Hemoglobin A1c, Hypertension, Prediabetes, Preeclampsia
INTRODUCTION
Hypertensive disorders of pregnancy (HDP) are a leading cause of perinatal morbidity and mortality, accounting for up to 16% of maternal deaths in developed countries.1–5 HDP encompass chronic hypertension, gestational hypertension, preeclampsia, and eclampsia.6 A large effort has been made to improve the prevention of preeclampsia as it can be accompanied by life-threatening sequelae [e.g. Hemolysis, Elevated Liver enzymes, thrombocytopenia (HELLP) Syndrome; cerebral hemorrhage; pulmonary edema; renal failure; and placental abruption]. In addition to pregnancy related complications, HDP have now been associated with short-term negative effects on cardiac function5 and increased rates of developing cardiovascular disease (CVD) risk factors3 contributing to the overall CVD burden in the United States, which kills one person every 39 seconds.7
Pregestational diabetes is a major risk factor for preeclampsia, increasing its risk 3.6 times.6 Current guidelines emphasize the importance of tight blood glucose control in women before and during pregnancy in order to optimize maternal and infant outcomes.8 While there is a substantial body of literature to guide treatment for pregestational diabetes, less is known regarding the management of prediabetes in pregnancy. Patients with prediabetes have elevated blood glucose below the threshold of diabetes diagnosis, characterized by impaired fasting glucose (100 to 125 mg/dL), impaired glucose tolerance (2-h Plasma Glucose during 75-g OGTT 140 to 199 mg/dL), or elevated hemoglobin A1c (5.7%–6.4%).9 In the United States alone, 84 million adults have prediabetes, at least 10 million of which can be inferred to be women of child bearing age.10 While one study suggested that early pregnancy hemoglobin A1c ≥5.9% increases the risk for preeclampsia,11 pregestational prediabetes was notably absent from the recent Gestational Hypertension and Preeclampsia Practice Bulletin of The American College of Obstetricians and Gynecologists.1
Objectives
Our objective was to complete a review of the literature to evaluate the impact pregestational prediabetes has on HDP risk and to assess the impact of varied lifestyle and medical interventions on this risk.
METHODS
Eligibility criteria, information sources, and search strategy
We performed two searches of the literature using a strategy designed by a research librarian. The strategy combined search headings and keywords to identify studies in Embase assessing pre- and early pregnancy markers of glycemia and outcomes related to HDP, specifically, pregnancy induced hypertension, gestational hypertension, preeclampsia, and eclampsia.1 Our initial search strategy searched for clinical studies including patients with prediabetes prior to pregnancy; our final search strategy added search headings and keywords to identify studies with patients identified with hemoglobin A1c elevations in the prediabetes range in the first trimester of pregnancy. An additional citation and reference review was carried out using Web of Science Core Collection. Search strategies were developed by K.B. and N.C. We did not apply any search limits based on date, language, country of origin, or type of study. The initial search was performed in June of 2018; an update of the initial search was conducted in August of 2018. No date limits were applied. Results were exported to EndNote and de-duplicated first using the automated deduplication function of EndNote (K.B.), then a manual search for duplicates. Full search strategies are provided in the Appendix. After an initial draft of the review was completed, the search was updated on April 23, 2019, and the search strategy used in Embase was translated to search PubMed and Cochrane databases. Following deduplication, we identified an additional 77 articles for review, but no additional studies met our inclusion criteria. Following initial peer review, we completed a final citation review of articles citing the included studies to identify recent studies on July 11, 2019, via Google Scholar using a date filter of 2018 to present and identified one study meeting our inclusion criteria.
Study selection, data extraction, and assessment of bias risk
Studies were selected if the study population was women with prediabetes or had a measurement of elevated hemoglobin A1c during the first trimester, and which also reported outcomes related to HDP (i.e., pregnancy induced hypertension, gestational hypertension, preeclampsia, and eclampsia) by reported pre- or early pregnancy glycemic status. Specifically, inclusion criteria were: human, pregnancy, clinical outcome during pregnancy or immediate outcome of pregnancy, reported pre-pregnancy prediabetes status or first trimester hemoglobin A1c relevant to prediabetes, reported on HDP; and exclusion criteria were: pregestational diabetes, case studies, case series ≤ n=10, duplicate presentation of data (e.g., choosing full text rather than published abstract), data exclusively in patients with polycystic ovary syndrome or assisted reproductive technology, not available in English. We included reports of randomized controlled trials (RCTs), prospective and retrospective comparative observational reports, as well as noncomparative observational studies. Full published reports, as well as published abstracts, were considered for inclusion. For each study, data abstraction was completed by one author (N.C.) with prior systematic review experience and confirmed by a second author (W.H.). Discrepancies in data abstraction were resolved through additional review and consensus of the abstracting authors. The methodological quality of each study was assessed using the criteria published by the Agency for Healthcare Research and Quality.12 To assess the risk of bias the authors considered study design, study execution, sample size, and precision in reporting glycemic exposure and HDP outcome. Studies were rated as good (A), fair (B), or poor (C) based on risk for bias and confidence the results reflect the true effect. Two authors rated each study and disagreements were resolved by consensus.
Data synthesis
Following data abstraction, summary statements were generated for each included report. Each study was considered in all facets of the overall analysis to which it related. Observational studies and RCT control groups were considered together in assessing the impact of prediabetes on HDP compared to study groups including women without dysglycemia or with late-onset gestational diabetes mellitus (GDM). Prospective and retrospective studies describing interventions to prevent HDP were assessed first individually, then between studies based on the change in HDP risk. Overall, the outcomes assessed were: 1) incidence of HDP in untreated women with prediabetes; 2) risk modification of HDP in lifestyle intervention treated women with prediabetes; 3) risk modification of HDP with medication treatment (± lifestyle intervention) with prediabetes.
CLINICAL EVALUATIONS
Study selection and characteristics
The flow of citations, screening, and inclusion of reports are reported in Figure 1. We screened 134 citations from the initial Embase search and reviewed the full-text of 25 articles. All 25 were excluded based on the following exclusion criteria: 13 did not include first trimester hemoglobin A1c, 8 had no HDP outcome, 2 studied pregestational diabetes, 1 was a long-term follow-up study, and 1 was a trial protocol. We screened 163 citations resulting from the second search strategy and reviewed 16 full-text articles. Three of these were included in the final review.11, 13, 14 Nine studies were excluded for not having an early measure of hemoglobin A1c. Two were excluded for not reporting on HDP outcomes. One was excluded for not reporting HDP outcomes stratified by early glycemic status. Finally, one was excluded because it did not report mean or median gestational age when hemoglobin A1c was measured, and its methods were altered allowing the inclusion of women in their second trimester at multiple study sites following an initial period of slow recruitment.15 An examination of references and citing studies found through searching Web of Science yielded one additional study for inclusion.16 The final update for citing studies via Google Scholar yield one final study.17
Figure 1.
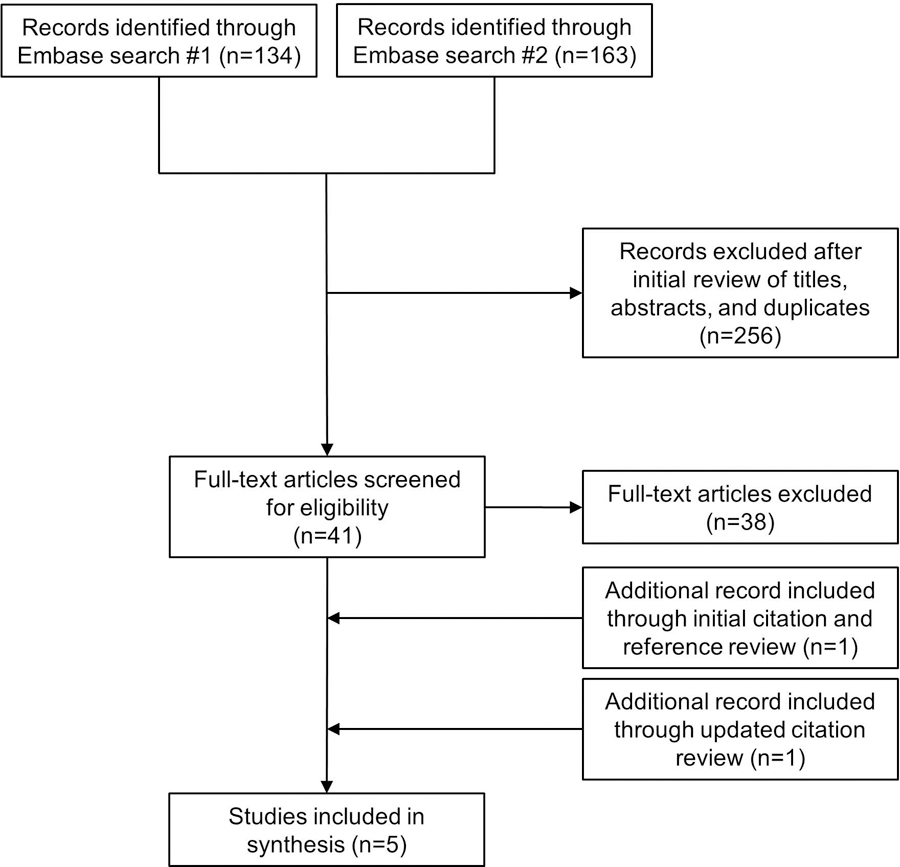
Flow of study screening and inclusion
n, number
Across the five included studies a small variation was found in the hemoglobin A1c range used to define prediabetes (5.7–6.4%, 5.9–6.4%, and 5.9–6.6%).11, 13, 14, 16, 17 The small variation was primarily related to local practice guidelines (United States vs. New Zealand). One study included women with a hemoglobin A1c of 5.9 to 6.6% at baseline.14 In this study mean±SD hemoglobin A1c was 6.1±0.2. Therefore, as two standard deviations above the mean falls within the upper range of the other studies (6.4%), the potential for the varied range to introduce bias is minimal.
Of the five included studies, 1 was a RCT and 4 were observational (Tables 1 and 2).11, 13, 14, 16, 17 The RCT was small (n=83) and compared early-treatment of GDM (i.e., diet, glucose monitoring, ± insulin) versus standard care in women with hemoglobin A1c of 5.7–6.4% at < 14 weeks of gestation.13 Neither of the two prospective observational studies included early treatment for dysglycemia.11, 16 One of the two prospective observational studies included women with early pregnancy hemoglobin A1c of 5.9–6.4%, but excluded women who at any point were treated for GDM.11 The other prospective observational study included women with early pregnancy hemoglobin A1c 5.9–6.4% as well, but also included women who were subsequently diagnosed with GDM at 24–28 weeks of gestation, therefore assessing an inherently higher risk group.16 One of the retrospective observational studies compared women with early pregnancy hemoglobin A1c of < 5.7% to 5.7–6.4%.17
Table 1:
Abstracted data from included studies
| Study | Study design | Study quality | Women, n | Interventions or groups | Mean age of subjects, y, ±SD or median (IQR) | Mean BMI of subjects, kg/m2, ±SD or median (IQR) | Mean gestational age of glucose assessment (weeks), ±SD or median (IQR) | Hypertensive Disorder of Pregnancy Outcomes number (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Chen et al, 2018 | Retr nRCS |
C | With early pregnancy HbA1c ≤ 6.4% 7020 |
HbA1c<5.7 n=6781 HbA1c 5.7–6.4 n=239 |
31.0±5.1 32.5±5.2 |
25%OW 21%OB 31%OW 46%OB |
8 (7–9) 8 (7–10) |
Preeclampsia 249 (3.7) 14 (5.9) Multivariate adjusted RR (95% CI) 1.11 (0.66–1.87) |
|
| Mañé et al, 2017 | Pros nRCS |
C | Without DM and with pregnancy outcome available 1228 |
HbA1c<5.9 n=1180 HbA1c 5.9–6.4 n=48 |
32.6±5.7 33.7±5.2 |
25.3±5.0 28.1±5.4 |
Entire cohort, first antenatal visit (first trimester, wks not reported) | Preeclampsia 43 (3.9) 4 (9.3) P=0.092 |
|
| Osmundson et al, 2016 | RCT | B | HbA1c 5.7–6.4and<14wks gestation 95 (83 with data for analysis) |
Early GDM treatment (diet±insulin), n=42 Usual care, n=41 |
32.4±5.1 34.3±5.2 |
27.2(24.8- 33.2) 27.4 (22.6- 32.7) |
9.1±2.0 9.3±2.3 |
All HDPs 3/38 (7.9) 3/36 (8.3) P=0.95 |
|
| Rowan et al, 2016 | Retr nRCS |
C | GDM and HbA1c at GDM dx≤6.6 Lifestyle advice± metformin± insulin 952 |
Early GDM: n=134 HbA1c 5.9–6.6 Late GDM: n=151 HbA1c 5.9–6.6 Late GDM: n=661 HbA1c<5.9 |
33.5±4.8 32.4±5.1 32.6±4.9 |
32.1±7.9 31.8±8.5 26.3±6.4 |
10 (6–13) 29 (26–32) 28 (25–30) |
Preeclampsia a. 2 (1.5) b. 12 (8.0) a vs. b. P=0.01 c. 16 (2.4) a vs. c P=0.5 b vs. c P=0.001 |
gHTN 5 (3.7) 10 (6.6) a vs. b. P=0.3 25 (3.8) a vs. c P=1.0 b vs. c P=0.12 |
| Hughes et al, 2014 | Pros nRCS |
B | Early preg HbA1c≤6.4, not referred for GDM treatment 8187 |
HbA1c 5.9–6.4 n=200 HbA1c<5.9 n=7987 |
31.5±6.3 30.3±5.9 |
28.9±7.4 25.9±5.7 |
Entire cohort, 6.7 (5.4–8.9) | Preeclampsia 11 (5.5) 181 (2.3) RR (95% CI) 2.42 (1.34–4.38) |
|
BMI, body mass index; DM, diabetes mellitus; dx, diagnosis; GDM, gestational diabetes mellitus; gHTN, gestational hypertension; HbA1c, Hemoglobin A1c; HDP, hypertensive disorders of pregnancy; IQR, interquartile range; nRCS, not randomized controlled study; OW, overweight; OB, obese; PIH, pregnancy induced hypertension; Pros, prospective; RCT, randomized controlled trial; Retr, retrospective; wk, week; RR, relative risk; SD, standard deviation; y, year
Table 2:
Summary conclusions of included studies with relation to hypertensive disorders of pregnancy
| Study | Summary Statements |
|---|---|
| Chen et al, 2018 | In women with hemoglobin A1c measured in early pregnancy, this study did not find a significantly increased risk for preeclampsia among women with hemoglobin A1c 5.7–6.4% versus hemoglobin A1c < 5.7%. The lack of significance may be explained by more women with baseline hemoglobin A1c 5.7–6.4% receiving diabetes medications, fewer experiencing excessive weight gain, and 67% having minimally elevated hemoglobin A1c (5.7% or 5.8%). |
| Mañé et al, 2017 | In women with hemoglobin A1c measured in early pregnancy, this study suggests an increased risk of preeclampsia in women with hemoglobin A1c 5.9–6.4% versus hemoglobin A1c < 5.9% |
| Osmundson et al, 2016 | In women with first trimester hemoglobin A1c of 5.7–6.4%, this underpowered study suggests no decrease in hypertensive disorders of pregnancy risk from a diet-based lifestyle intervention, with blood glucose monitoring, ± insulin versus usual care. |
| Rowan et al, 2016 | In women with hemoglobin A1c of 5.9–6.6% at gestational diabetes mellitus diagnosis, this study suggests early identification and treatment of gestational diabetes mellitus (63.4% included metformin) is associated with a lower risk for preeclampsia compared to later identification and treatment of gestational diabetes mellitus (56.3% included metformin). |
| Hughes et al, 2014 | In women with hemoglobin A1c measured in early pregnancy who were not treated for gestational diabetes mellitus during the index pregnancy, this study suggests an increased risk of preeclampsia in women with hemoglobin A1c 5.9–6.4% versus hemoglobin A1c < 5.9% |
The final retrospective observational study assessed only women who were diagnosed with GDM and analyzed them according to hemoglobin A1c at GDM diagnosis and time of GDM diagnosis (i.e., early versus late).14 The 3 distinct groups analyzed were: 1) early-treatment GDM with hemoglobin A1c 5.9–6.6% at the time of GDM diagnosis; 2) later-treatment GDM with hemoglobin A1c 5.9–6.6% at the time of GDM diagnosis; and 3) later-treatment GDM with hemoglobin A1c < 5.9% at GDM diagnosis. Inclusion was based on referrals to a multidisciplinary diabetes clinic. Therefore, the potential exists that some patients in the “late-treatment” GDM group might have had early-onset GDM that was not diagnosed. However, this risk is lessened as hemoglobin A1c measurement at first antenatal visit was the standard of care at the study site. All women received written and/or in person lifestyle advice within 1–3 weeks of referral and medications were initiated when clinically indicated. Women requiring medication were given the choice between metformin or insulin, with supplemental insulin following metformin if required. Notably, more than half of the study population were treated with metformin±insulin.
HDP Risk
Relative to the expected epidemiology of HDP,4 early pregnancy hemoglobin A1c in the prediabetes range was associated with a relatively high risk of HDP (Table 1). Standard care in one RCT of patients with hemoglobin A1c of 5.7–6.4% at < 14 weeks of gestation resulted in 3/36 (8.3%) experiencing HDP.13 Preeclampsia was more common in women with early pregnancy hemoglobin A1c of 5.9–6.4% in both prospective observational studies,11, 16 although the difference in one study only became statistically significant after multivariable adjustment,16 likely related to sample size. However as expected, the study excluding patients treated for GDM demonstrated a lower rate of preeclampsia compared to the study including these patients (5.5% vs. 9.32%, between studies). One retrospective observational study did not address HDP risk in untreated patients, as all patients received some form of treatment when identified clinically.14
The other retrospective observational study by Chen et al did not demonstrate a significant increase in preeclampsia in women with hemoglobin A1c of 5.7–6.4% compared to women with hemoglobin A1c <5.7%, yet multiple factors suggest the lack of significant difference may be the result of confounding. Women with hemoglobin A1c of 5.7–6.4% were slightly older (mean +1.5 years), more from racial or ethnic minority backgrounds (37.2% non-Hispanic white vs. 70.3% non-Hispanic white), and fewer to have a prepregnancy body mass index <25 kg/m2 (23.1% vs. 53.8%). However, these women also received diabetes treatment more frequently (62% vs. 40%), had less excessive gestational weight gain (37% vs. 48%), and were tested for GDM 2 weeks earlier on average. Moreover, 67% of women with hemoglobin A1c of 5.7–6.4% had hemoglobin A1cs of 5.7 or 5.8%, likely representing a lower risk population compared to hemoglobin A1cs of 5.9–6.4%.11 Despite the mitigating factors suggesting more intensive care, women with hemoglobin A1c of 5.7–6.4% still had a numerically larger percent with preeclampsia (5.9% vs. 3.7%) and the wide confidence interval suggests the study was underpowered to assess the observed difference in this population [Multivariate adjusted relative risk and 95% CI, 1.11 (0.66, 1.87)].
Lifestyle Intervention with or without Insulin
In the RCT, women with a hemoglobin A1c in the prediabetes range at baseline were randomly assigned to early GDM treatment or standard care.13 All women received written education regarding healthy weight gain and physical activity. Women in the intervention group met with a certified diabetes educator and were counseled on diet, keeping a food log, and self-monitoring blood glucose. Patients were to self-monitor blood glucose four times daily with goals values of < 92 mg/dL fasting and < 135 mg/dL one-hour postprandial. Insulin was initiated if > 20% of self-monitored blood glucose values were above goal. Notation of additional medications was not identified. Overall the study reflects an early, diet-based, lifestyle intervention with insulin rescue when indicated. The lifestyle intervention was not associated with a lower rate of positive third trimester oral glucose tolerance test or requiring insulin prior to the test (intervention, 45.2% vs. control, 56.1%; P=0.32), although the difference trended toward a benefit, and was significant in patients who were nonobese at baseline (early GDM treatment 29.6% vs. standard care 60.9%; RR 0.49; 95% CI 0.25–0.95). Early treatment was not associated with a reduction in the risk of HDP (intervention, 7.9% vs. control, 8.3%; P=0.95).
Metformin with or without Insulin
One retrospective observational study included women with hemoglobin A1c in the prediabetes range (5.9–6.6%) in the first trimester who were treated with metformin, among other women in the study.14 In this study, women were included at the time of GDM diagnosis and analyzed in 3 groups. First and representative of prediabetes, women with hemoglobin A1c of 5.9–6.6% at the time of GDM diagnosis who received early-treatment for GDM were diagnosed with GDM at a median of 10 weeks of gestation (IQR, 6–13) and 63.4% received metformin alone or with insulin. Second, women with hemoglobin A1c of 5.9–6.6% at the time of GDM diagnosis who received later-treatment for GDM were diagnosed with GDM at a median of 29 weeks of gestation (IQR, 26–32) and 56.3% received metformin alone or with insulin. Third, women with hemoglobin A1c of <5.9% at the time of GDM diagnosis (28 weeks of gestation, IQR, 25–35) also initiated treatment later (30 weeks of gestation, IQR 25–32) and 46.4% received metformin alone or with insulin. In this study women with GDM indicated for medication treatment choose between insulin or metformin with subsequent insulin as needed; indicating that women taking both would have initiated metformin first, and in the early-treatment group, typically before 20 weeks of gestation. While a higher rate of HDP would have been expected in the “early-treatment” group, this was not observed. Of patients with hemoglobin A1c of 5.9–6.6% at GDM diagnosis, significantly fewer early-treatment patients developed preeclampsia compared to “late-treatment” (1.5% vs. 8.0%; P=0.01). While this is counter to the prospective observational studies of untreated patients, one possible explanation is the presence and timing of metformin use.
While both groups had high rates of metformin use (early-treatment, 63.4%; late-treatment, 56.3%), it is reasonable to expect the earlier initiation would be more likely to prevent HDP.18 Although the specific timing of medication initiation was not reported, the authors stated that most women requiring medication treatment were started on an antidiabetic medication at the first or second diabetes visit. The first visit occurring at a median of 14 weeks of gestation (IQR, 12–17) in the early-treatment group and a median of 31 weeks of gestation (IQR, 28–33) in the late-treatment group. Alternatively, differences between groups could be related to the potential for the “later-treatment” group to be heterogenous, including patients who had early-onset GDM that were not identified early. However, as stated, hemoglobin A1c measurement at first antenatal visit was standard of care, partially limiting the risk for misclassification. Additionally, the potential that this difference is related to bias is less likely as the rate of preeclampsia in the early-treatment group (1.5%) is also low compared to the expected rate in GDM.4
DISCUSSION
Overall, first trimester hemoglobin A1c in the prediabetes range appears to be associated with an increased risk for HDP relative to later-onset GDM or healthy pregnancy. In one prospective observational study, the risk of HDP was more than doubled compared to women with hemoglobin A1c <5.9%.11 Another prospective observational study trended toward a similar finding but was limited by small sample size.16 The retrospective observational study also trended toward a difference despite confounding which suggested patients with hemoglobin A1c received more intensive care.17
Studies examining interventions to improve outcomes in women with first trimester hemoglobin A1c in the prediabetes range are limited. A single RCT suggests diet-based lifestyle interventions may reduce GDM risk in nonobese patients without an impact on HDP.13 However, the study was small and underpowered. Therefore, firm conclusions cannot be made regarding the intervention’s impact overall and particularly with regard to preeclampsia. An observational study suggests that metformin may reduce the risk for HDP.14 Additional research is needed regarding the role of lifestyle intervention (particularly with varied intensities of exercise), metformin, and their combination in women with pregestational prediabetes. Moreover, additional clarity is needed regarding their impact on varied outcomes (e.g., GDM, HDP, excessive gestational weight gain, large for gestational age).
While the study we critically reviewed regarding lifestyle intervention (diet, monitoring, ± insulin as indicated) demonstrated no statistical impact on HDP in pregnant women with a first trimester hemoglobin A1c in the prediabetes range, it was small and underpowered.13 However, this result is consistent with prior findings of lifestyle intervention in pregnancy which demonstrate a reduced risk for GDM, but not preeclampsia.19, 20 These results are also consistent with a study by Sweeting et al., which found a high rate of HDP (26.3%) in women with GDM onset before 12 weeks of gestation and borderline hemoglobin A1c (mean±SD, 5.7%±1.3) despite intervention (diet, exercise, monitoring, ± insulin as indicated).21 However, these results somewhat contrast a recent subgroup analysis of the Lifestyle in Pregnancy (LiP) study by Vinter et al22 and a recent feasibility study by Hughes et al.15
Specifically, Vinter et al. assessed a subgroup of women from the Lifestyle in Pregnancy (LiP) study with early-onset GDM.22 In this study a diet and exercise intervention trended toward reducing pregnancy induced hypertension versus control (11.1% vs. 16.7%; P=0.46) but not preeclampsia (5.6% vs. 5.6%; P=0.92). However, the study by Vinter et al. was limited by small sample size (n=90), similar to the included RCT by Osmundson et al. (n=83) which assessed early intervention for hemoglobin A1c levels of 5.7–6.4%. A key difference between these two studies was the exercise component in the LiP study (56% attended at least half of aerobic classes), which may be important in addressing the pathogenesis of HDP.22 However, the analysis by Vinter et al did not report hemoglobin A1c, and therefore the difference between study findings could also be related to differing clinical study populations.
The results of a recent feasibility study by Hughes et al.15 also contrast the included RCT by Osmundson et al.13 Both studies assessed similar populations, although Hughes et al. included some women in the early second trimester.15 While similarly limited by small sample size, Hughes et al. demonstrated that the early intervention for women with hemoglobin A1c 5.9–6.4 numerically decreased preeclampsia (0 of 23 vs. 3 of 21). However, this study was also insufficiently powered. A key difference between studies was Hughes et al. permitting metformin use, which was more common in the early intervention group (61% vs. 14%).
The findings for lifestyle intervention in our analysis contrast those of metformin, which demonstrated that prevention of HDP may be possible with early metformin treatment.14 The retrospective study which suggested metformin’s benefit was also limited by the varied use of metformin between groups.14 However, the results are similar to those of The Metformin in Obese Nondiabetic Pregnant Women (MOP) trial, in which metformin was associated with a reduced rate of preeclampsia.23 However, both results contrast the Effect of metformin on maternal and fetal outcomes in obese pregnant women (EMPOWaR) trial24, in which metformin did not reduce the rate of preeclampsia. While the differences between the MOP23 and EMPOWar trials24 have been explored previously,18 notably absent from this discussion was the EMPOWaR trial’s exclusion of women with early-onset GDM. In light of our findings, we proposed this exclusion of early-onset GDM patients as the key difference between the MOP and EMPOWaR trials. Given the known impact of lifestyle intervention on preventing GDM,20 our analysis suggests that a single intervention approach to preventing HDP and GDM (at 24–28 weeks of gestation) may be less practicable for women with first trimester hemoglobin A1c in the prediabetes range.
Our current analysis has notable limitations. First, we did not identify any studies which assessed pregnancy outcomes in women with biochemically identified prediabetes prior to conception or with documented prediabetes prior to conception. However, Hinkle et al. recently demonstrated that regardless of subsequent gestational diabetes mellitus diagnosis, hemoglobin A1c decreases until approximately weeks of gestation 16–22 of pregnancy.25 Therefore, despite the difficulty in hemoglobin A1c interpretation due to the physiologic responses to pregnancy (e.g., hemodilution with increases in plasma volume, increased erythrocyte production and turnover, natural insulin resistance of pregnancy), women without pregestational diabetes, but with a first trimester hemoglobin A1c of 5.7%–6.4% are highly likely to represent prediabetes. Second, there were a limited number of studies for review, some of the included studies were limited by small sample size, and the number of quality studies was inadequate to perform a meta-analysis, which is currently the gold standard. However, the finding that women with first trimester hemoglobin A1c in the prediabetes range are at a higher risk for HDP seems unlikely to change. Conversely, these data which suggest that a diet-based lifestyle intervention is ineffective derive from a single underpowered RCT, and therefore, no firm conclusion can be made. Importantly, these conclusions cannot be applied to other populations, where these interventions have shown different effects. Indeed, lifestyle intervention for HDP prevention is still an area of interest. Of specific interest would be pregestational or early-gestational lifestyle interventions with an emphasis on relatively more vigorous physical activity, with the goal of restoring early-gestation vasodilator (e.g. Prostacyclin/Thromboxane, Nitric Oxide) or angiogenic (e.g. VEGF, PlGF, sFlt-1 and sEndoglin) balance. The weakest evidence in the current review pertains to metformin’s role in preventing HDP, which is based on a single observational study. However, this weakness is diminished by the body of related literature.18, 23, 26
CONCLUSION
Overall, our analysis suggests that first trimester hemoglobin A1c in the prediabetes range is associated with an increased risk for HDP. Additionally, based on our analysis we suspect metformin to be a good if not more viable option than lifestyle intervention in reducing the risk for HDP in these women. However, due to limited data, both metformin and lifestyle intervention require further study, particularly in conjunction, to prevent HDP. Regardless, it is reasonable to inform women who are attempting to become pregnant with pregestational prediabetes or early pregnancy hemoglobin A1c (5.7–6.4%) that they have an increased risk of HDP and to encourage them to adopt a healthy lifestyle.
ACKNOWLEDGMENTS
NIH HL117341(RRM) funding supported time for the completion of this manuscript. The funding agency played no role in design, data collection, data analysis, or data interpretation; or in the writing of the report or the decision to submit for publication.
APPENDIX: FINAL SEARCH STRATEGIES
EMBASE
((‘impaired glucose tolerance’/exp OR ‘chemical diabetes’ OR ‘chemical diabetes mellitus’ OR ‘diabetes mellitus, potential’ OR ‘diabetes, chemical’ OR ‘diabetes, latent’ OR ‘genetic prediabetes’ OR ‘glucose tolerance impairment’ OR ‘glucose tolerance, potentially impaired’ OR ‘impaired glucose tolerance’ OR ‘impaired glucose tolerance, potential’ OR ‘latent diabetes’ OR ‘latent diabetes mellitus’ OR ‘potential diabetes’ OR ‘potential diabetes mellitus’ OR ‘potential glucose tolerance impairment’ OR ‘pre diabetes mellitus’ OR ‘prediabetes’ OR ‘prediabetes mellitus’ OR ‘prediabetic stage’ OR ‘prediabetic state’) AND (‘prepregnancy’/exp OR prepregnan* OR pregestat*)) OR ((‘pregnancy’/exp OR ‘pregnancy’) AND (‘early treatment’/exp OR ‘early treatment’ OR ‘early intervention’) AND (‘gestational diabetes’/exp OR ‘gestational diabetes’ OR ‘oral glucose tolerance test’/exp OR ‘oral glucose tolerance test’))
PubMed
(pregestational OR prepregnancy OR prepregnan* OR pre-pregnan* OR pregestat*) AND (prediabetes OR “impaired glucose tolerance” OR “latent diabetes” OR “potential diabetes”) OR ((“pregnancy”[Mesh] OR pregnancy) AND (“diabetes, gestational”[MeSH] OR “gestational diabetes” OR “glucose tolerance test”) AND (“Early Medical Intervention”[Mesh] OR “early medical intervention”))
Cochrane
-
#1
pregestational OR prepregnancy OR prepregnan* OR pre-pregnan* OR pregestat*
-
#2
prediabetes OR “impaired glucose tolerance” OR “latent diabetes” OR “potential diabetes”
-
#3
#1 AND #2
-
#4
MeSH descriptor: [Pregnancy] explode all trees
-
#5
pregnancy
-
#6
#4 OR #5
-
#7
MeSH descriptor: [Diabetes, Gestational] explode all trees
-
#8
MeSH descriptor: [Glucose Tolerance Test] explode all trees
-
#9
“gestational diabetes” OR “glucose tolerance test”
-
#10
#7 OR #8 OR #9
-
#11
MeSH descriptor: [Early Medical Intervention] explode all trees
-
#12
“early treatment” OR “early medical intervention”
-
#13
#11 OR #12
-
#14
#6 AND #10 AND #13
-
#15
#3 OR #14
Footnotes
Disclosure
The authors report no conflict of interest
REFERENCES
- 1.Committee on Practice Bulletins–Obstetrics. ACOG Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obstet Gynecol 2019;133(1):e1–e25 [DOI] [PubMed] [Google Scholar]
- 2.Khan KS, Wojdyla D, Say L, Gulmezoglu AM, Van Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet 2006;367(9516):1066–74 [DOI] [PubMed] [Google Scholar]
- 3.Stuart JJ, Tanz LJ, Missmer SA, et al. Hypertensive Disorders of Pregnancy and Maternal Cardiovascular Disease Risk Factor Development: An Observational Cohort Study. Annals Intern Med 2018;169(4):224–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umesawa M, Kobashi G. Epidemiology of hypertensive disorders in pregnancy: prevalence, risk factors, predictors and prognosis. Hypertens Res 2017;40(3):213–20 [DOI] [PubMed] [Google Scholar]
- 5.Vaught AJ, Kovell LC, Szymanski LM, et al. Acute Cardiac Effects of Severe Pre-Eclampsia. J Am Coll Cardiol 2018;72(1):1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohkuchi A, Hirashima C, Takahashi K, Suzuki H, Matsubara S. Prediction and prevention of hypertensive disorders of pregnancy. Hypertens Res 2017;40(1):5–14 [DOI] [PubMed] [Google Scholar]
- 7.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017;135(10):e146–e603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Diabetes Association. 13. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018;41(Suppl 1):S137–S143 [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018;41(Suppl 1):S13–S27 [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017 [Google Scholar]
- 11.Hughes RC, Moore MP, Gullam JE, Mohamed K, Rowan J. An early pregnancy HbA1c >/=5.9% (41 mmol/mol) is optimal for detecting diabetes and identifies women at increased risk of adverse pregnancy outcomes. Diabetes Care 2014;37(11):2953–9 [DOI] [PubMed] [Google Scholar]
- 12.Owens DK, Lohr KN, Atkins D, et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions--agency for healthcare research and quality and the effective health-care program. J Clin Epidemiol 2010;63(5):513–23 [DOI] [PubMed] [Google Scholar]
- 13.Osmundson SS, Norton ME, El-Sayed YY, Carter S, Faig JC, Kitzmiller JL. Early Screening and Treatment of Women with Prediabetes: A Randomized Controlled Trial. Am J Perinatol 2016;33(2):172–9 [DOI] [PubMed] [Google Scholar]
- 14.Rowan JA, Budden A, Ivanova V, Hughes RC, Sadler LC. Women with an HbA1c of 41–49 mmol/mol (5.9–6.6%): a higher risk subgroup that may benefit from early pregnancy intervention. Diabet Med 2016;33(1):25–31 [DOI] [PubMed] [Google Scholar]
- 15.Hughes RCE, Rowan J, Williman J. Prediabetes in pregnancy, can early intervention improve outcomes? A feasibility study for a parallel randomised clinical trial. BMJ Open 2018;8(3):e018493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mane L, Flores-Le Roux JA, Benaiges D, et al. Role of First-Trimester HbA1c as a Predictor of Adverse Obstetric Outcomes in a Multiethnic Cohort. J Clin Endocrinol Metab 2017;102(2):390–7 [DOI] [PubMed] [Google Scholar]
- 17.Chen L, Pocobelli G, Yu O, et al. Early Pregnancy Hemoglobin A1C and Pregnancy Outcomes: A Population-Based Study. Am J Perinatol 2018. November 30. doi: 10.1055/s-0038-1675619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero R, Erez O, Huttemann M, et al. Metformin, the aspirin of the 21st century: its role in gestational diabetes mellitus, prevention of preeclampsia and cancer, and the promotion of longevity. Am J Obstet Gynecol 2017;217(3):282–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Syngelaki A, Sequeira Campos M, Roberge S, Andrade W, Nicolaides KH. Diet and exercise for preeclampsia prevention in overweight and obese pregnant women: systematic review and meta-analysis. J Matern Fetal Neonatal Med 2019;32(20):3495–501 [DOI] [PubMed] [Google Scholar]
- 20.Shepherd E, Gomersall JC, Tieu J, Han S, Crowther CA, Middleton P. Combined diet and exercise interventions for preventing gestational diabetes mellitus. Cochrane Database syst Rev 2017;11:Cd010443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sweeting AN, Ross GP, Hyett J, et al. Gestational Diabetes Mellitus in Early Pregnancy: Evidence for Poor Pregnancy Outcomes Despite Treatment. Diabetes Care 2016;39(1):75–81 [DOI] [PubMed] [Google Scholar]
- 22.Vinter CA, Tanvig MH, Christensen MH, et al. Lifestyle Intervention in Danish Obese Pregnant Women With Early Gestational Diabetes Mellitus According to WHO 2013 Criteria Does Not Change Pregnancy Outcomes: Results From the LiP (Lifestyle in Pregnancy) Study. Diabetes Care 2018;41(10):2079–85 [DOI] [PubMed] [Google Scholar]
- 23.Syngelaki A, Nicolaides KH, Balani J, et al. Metformin versus Placebo in Obese Pregnant Women without Diabetes Mellitus. N Engl J Med 2016;374(5):434–43 [DOI] [PubMed] [Google Scholar]
- 24.Chiswick C, Reynolds RM, Denison F, et al. Effect of metformin on maternal and fetal outcomes in obese pregnant women (EMPOWaR): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2015;3(10):778–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hinkle SN, Tsai MY, Rawal S, Albert PS, Zhang C. HbA1c Measured in the First Trimester of Pregnancy and the Association with Gestational Diabetes. Sci Rep 2018;8(1):12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Committee on Practice Bulletins–Obstetrics. ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet Gynecol 2018;131(2):e49–e64 [DOI] [PubMed] [Google Scholar]


