Abstract
Introduction:
The aims of this study were to assess the renal expression of angiotensin II type 1 receptor (AT1R), angiotensin II type 2 receptor (AT2R), and MAS receptor in human type 2 diabetic nephropathy (DN).
Materials and methods:
In total, 115 patients diagnosed with DN by renal biopsy were enrolled in this study. The protein expression levels of the AT1R, AT2R, and MAS receptors were assessed by immunohistochemistry.
Results:
The protein expression levels of AT1R, AT2R, and MAS receptor in the renal biopsy tissue were correlated with the pathologic classification of DN. Tubulointerstitial AT1R expression in patients of class IIb was significantly stronger than control samples (p < 0.05). Expression of AT2R and MAS receptors were highest with class IIb DN patients. When DN patients were treated with AT1R blocker (ARB), the expression of AT1R was downregulated (p < 0.05), and the MAS receptor was upregulated in tubular interstitial (p < 0.05).
Conclusions:
Our results directly observed that renal expression levels of AT1R increase during the early stages of DN, ARB reducing AT1R while increasing MAS receptor. Therefore, ARB should be used as soon as possible in patients with DN.
Keywords: Diabetic nephropathy, renal biopsy, angiotensin receptor, angiotensin II type 1 receptor blocker, immunohistochemistry
Introduction
The renin–angiotensin system (RAS) plays an important role in the progression of diabetic nephropathy (DN).1–3 The effects of the RAS on DN likely depend on the expression of angiotensin receptors in the kidney, but little information is available regarding angiotensin receptor expression in human subjects. Indeed, existing studies do not show consistent patterns: Wagner et al. reported that angiotensin II type 1 receptor (AT1R) mRNA levels were significantly lower in eight samples from patients with DN,4 and Konoshita et al. found no difference in AT1 mRNA or AT2 mRNA expression levels among diabetic and non-diabetic subjects.5 Complete assessment of angiotensin receptors [angiotensin II type 1 (AT1), angiotensin II type 2 (AT2), and MAS) is necessary for evaluation of the effect of the RAS on the kidney.
Clinical and experimental studies have identified the RAS as a key factor in the progression of DN. A number of large-scale prospective studies have demonstrated that blockade of the system with angiotensin II type 1 receptor blocker (ARB) retards the progression of DN.1,6–8 ARB is a recognized method for treating DN.
Angiotensin (Ang) II acts on two major receptor subtypes: AT1R and AT2R.9 AT1R predominates in most tissues and mediates the classic physiological and pathophysiological actions of Ang II.10 Although a role for AT2R in renal function is currently under investigation, activation of renal AT2R appears to have effects that generally oppose those induced by AT1R.11 Ang (1–7) is generated in the kidney at relatively high levels, often opposing the vasoconstrictive and pro-proliferative actions of Ang II.12 The biological effects of Ang (1–7) in the kidney are mediated primarily by interactions with MAS receptor.13
However, information regarding AT1, AT2, and MAS receptor expression in human DN is very scarce. This prompted us to determine the expression levels of AT1, AT2, and MAS receptors in renal biopsies taken from human type 2 DN samples and to examine the effect of AT1R blockade on the expression of these receptors.
Research design and methods
Patient data
We collected physical and clinical data from 115 patients diagnosed with DN from our database who met the following criteria. Inclusion criteria: 1) between 18 and 70 years of age; 2) renal biopsy (with ⩾10 glomeruli) performed at the Division of Nephrology of China–Japan Friendship Hospital; 3) diagnosed as DN, and 4) written informed consent. The exclusion criteria were as follows: 1) patients treated with angiotensin converting enzyme (ACE) inhibitors or renin inhibitor; 2) patients with endocrine disease with the renin-angiotensin-aldosterone system, for example primary aldosteronism; and 3) patients enrolled on any other type of clinical drug research. The study was approved by the local ethics committee.
Data collected included gender, age, mean arterial pressure (MAP), serum creatinine (SCr), hemoglobin (Hb), estimated glomerular filtration rate (eGFR), and 24-h proteinuria. eGFR was determined using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation. Because normal human kidney tissue is difficult to get, we selected five patients with renal resection due to renal carcinoma and took normal renal tissue next to the cancer as a normal control.
Diagnose and pathological classification of DN
Diagnostic criteria: on renal biopsy, early signs of DN include glomerular hypertrophy and thickening of the glomerular basement membrane (GBM). As the disease progresses, arteriolar hyalinosis, arteriosclerosis, progressive mesangial expansion, and Kimmelstiel-Wilson nodule formation occurs.
Biopsies diagnosed as DN are classified as follows14: Class I, GBM thickening: GBM >395 nm in females and >430 nm in males. Class II, mesangial expansion, mild (IIa) or severe (IIb): mild or severe mesangial expansion in >25% of the observed mesangium but without nodular sclerosis (Kimmelstiel–Wilson lesions). Class III, nodular sclerosis (Kimmelstiel–Wilson lesions): at least one glomerulus with a nodular increase in the mesangial matrix (Kimmelstiel–Wilson). Class IV, advanced diabetic glomerulosclerosis: more than 50% global glomerulosclerosis with other clinical or pathologic evidence that sclerosis is attributable to DN.
Immunohistochemistry
After fixation, the renal tissues were embedded in paraffin and sectioned at 3-µm thickness. Following dewaxing via a hydration process, antigen repair was performed using the high-pressure hot-repair method, and endogenous antigen was inactivated using peroxidase. The sections were then washed in phosphate-buffered saline (PBS), incubated in goat serum working fluid for 30 min, and then separately with the following primary antibodies. All antibodies were purchased from Abcam (Cambridge, UK; anti-AT1R antibody: ab9391, anti-AT2R antibody: ab19134, anti-MAS1 antibody: ab66030). After an overnight incubation at 4°C, the reactions were visualized by staining with horseradish peroxidase and diaminobenzidine.
The protein expression of AT1, AT2, and MAS receptors was examined quantitatively using the Image-Pro Plus (IPP) 6.0 software program (Media Cybernetics, Silver Spring, MD, USA). First, we select the area of interest (AOI) by setting the color range (yellow or brown), and then measure the average optical density in the AOI. The mean density of the AOI was used to reflect the relative content of the target protein. A mean value was obtained by analysis of 10 different fields. Quantification was done twice in a blinded manner, and interassay variations were not significant.
Data analyses and statistics
Statistical analyses were performed with SPSS 17.0. Data were summarized using means ± standard deviation (SD) or medians as appropriate. Pairwise comparisons were made using Student’s t test. Data with multiple comparisons were analyzed using ANOVA or by the nonparametric Mann–Whitney test. Statistical significance was set at p <0.05.
Results
Clinical and pathological characteristics of patients with DN
In total, 115 patients diagnosed with DN by renal biopsy and 5 normal controls were enrolled in our study. Clinical characteristics are listed in Table 1. There were 87 men and 33women, age range from 26 to 75 years. The pathologic classification of the cases was as follows: 1 case of class I, 12 cases of Class IIa, 23 cases of class IIb, 72 cases of class III, and 7 cases of class IV. A total of 35 patients were receiving ARBs (6 losartan, 11 valsartan, 10 irbesartan, 6 telmisartan, and 2 olmesartan).
Table 1.
Clinical and pathological characteristics of patients with DN.
| Normal control | Class I+IIa | Class IIb | Class III+IV | |
|---|---|---|---|---|
| n | 5 | 13 | 23 | 79 |
| Sex (M/F) | 3/2 | 10/3 | 19/4 | 55/24 |
| Age (years) | 63±8 | 52 ±11a | 48 ± 11a | 51 ± 9a |
| MAP (mmHg) | 98±4 | 101 ± 9 | 101 ± 14 | 102 ± 13 |
| Glucose (mmol/L) | 6.2±1.0 | 7.8±4.8a | 8.6±3.5a | 8.5±4.6a |
| Proteinuria (g/day) | 0.2±0.08 | 2.6 ± 2.4a | 4.3 ± 3.2a | 5.0 ± 2.6ab |
| Serum creatinine (μmol/L) | 83±18 | 93 ± 23 | 128 ± 58a | 177 ± 96abc |
| eGFR (mL/min per 1.73 m²) | 81±9 | 78 ± 16 | 65 ± 21a | 51 ± 19abc |
| Hemoglobin (g/L) | 126±14 | 133 ± 16 | 124 ± 23 | 105 ± 19bc |
| Treated with ARB (n/N) | 0/0 | 3/13 | 12/23 | 20/79 |
p <0.05 vs. normal control; bp <0.05 vs. Class I+IIa; cp <0.05 vs. Class IIb. Data are mean ± SD.
ARB: angiotensin II type 1 receptor blocker; DN, diabetic nephropathy; eGFR, estimated glomerular filtration rate; MAP, mean arterial pressure; SD, standard deviation.
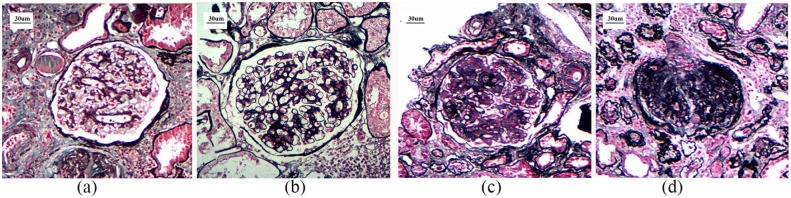
Typical changes in glomeruli in patients with each classification of DN are shown in Figure 1.
Figure 1.
Typical changes in glomeruli in patients with each classification of DN (PAS, 200×).
(a) class I, (b) class II, (c) class III, (d) class IV.
DN, diabetic nephropathy; PAS, periodic acid-Schiff.
AT1, AT2, and MAS receptor protein expression levels of tubulointerstitial in biopsy samples from DN patients
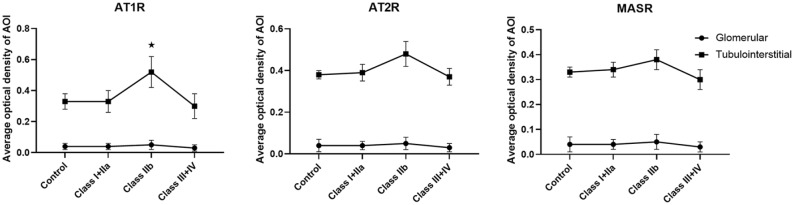
We assessed the renal expression levels of AT1, AT2, and MAS receptors in 80 human kidneys with DN. No patients were receiving angiotensin-converting enzyme inhibitors (AECI) or ARB. AT1, AT2 and MAS receptors were not significantly different between each classification of DN. Tubulointerstitial AT1R expression in patients of class IIb was significantly stronger than in control samples, Class I+IIa subjects, and Class III+IV subjects (p < 0.05) (Figure 2a). Tubulointerstitial AT2, and MAS receptors expression levels of Class IIb subjects, tended to be higher than those of control samples, Class I+IIa subjects, and Class III+IV subjects, but these differences were not statistically significant (Figure 2b,c).
Figure 2.
Angiotensin II receptor protein expression levels of glomerular and tubulointerstitial in biopsy samples from DN patients.
*p <0.05 compared with control samples.
AOI, area of interest; AT1R, angiotensin II type 1 receptor; AT2R, angiotensin II type 2 receptor; DN, diabetic nephropathy; MASR, MAS receptor.
ARB regulates renal expression of angiotensin receptors in biopsy samples from DN patients
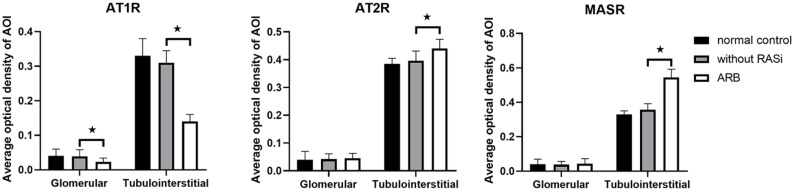
We assessed the effect of ARB on the renal expression of AT1, AT2, and MAS receptors in human DN (35 patients with ARB, 80 patients without RAS inhibitors). In the 35 patients receiving ARBs (6 losartan, 11 valsartan, 10 irbesartan, 6 telmisartan, and 2 olmesartan), protein expression levels of AT1Rs were downregulated in glomeruli, and tubulointerstitial (p < 0.05), and the expression level of MAS receptors was upregulated in tubulointerstitial (p < 0.05), but there was no difference in AT2R expression levels (Figures 3 and 4). Pre-treatment proteinuria was 4.7±2.9 g/day and reduced to 3.9±2.4 g/day after a 2-month ARB treatment in 35 patients.
Figure 3.
ARB regulates renal expression of angiotensin receptors in biopsy samples from DN patients.
*p <0.05 compared with samples without RAS inhibitors
AOI, area of interest; ARB, AT1R blocker; AT1R, angiotensin II type 1 receptor; AT2R, angiotensin II type 2 receptor; DN, diabetic nephropathy; MASR, MAS receptor; RAS, renin–angiotensin system.
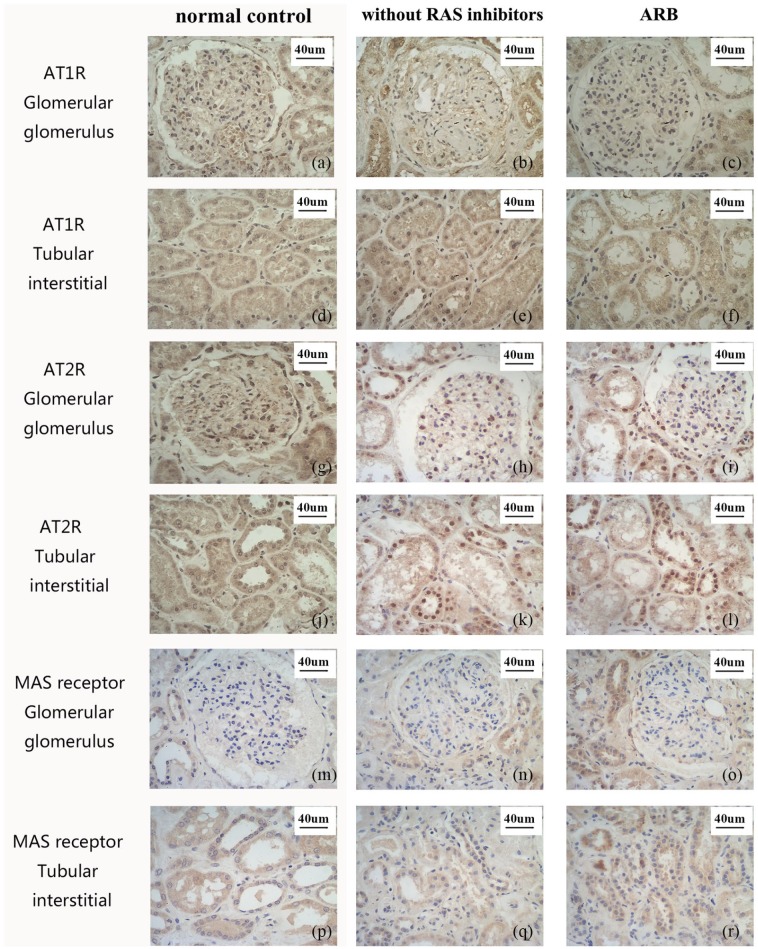
Figure 4.
Representative immunohistochemistry staining images of kidney from DN patients (200x).
(a)–(f) AT1R, (g)–(l) AT2R, (m)–(r) MAS receptor. a,d, g, j, m, p normal controls; b, e, h, k, n, q specimens without RAS inhibitors; c, f, i, l, o, r ARB group.
ARB, AT1R blocker; AT1R, angiotensin II type 1 receptor; AT2R, angiotensin II type 2 receptor; DN, diabetic nephropathy; MASR, MAS receptor; RAS, renin–angiotensin system.
Discussion
The importance of the RAS in the regulation of blood pressure and fluid and electrolyte balance has been recognized for decades.15 Its role in the pathogenesis of cardiorenal diseases is also widely accepted, based largely on results from clinical studies using drugs that interfere with the RAS.7,8 Tissue RAS is thought to be controlled independently of traditional circulating RAS.
Compared with human tissues, considerably more is known with regard to animal models, especially the streptozotocin (STZ) diabetes model. The kidney of diabetic mice displayed upregulated protein expression levels of AT1 and AT2 and downregulated protein expression levels of MAS in most studies,16–19 but there were also some exceptions showing decreased protein of AT2R.20 Both AT1 and AT2R mRNA levels in the kidney were reduced in diabetic spontaneously hypertensive rats (SHRs) compared with non-diabetic SHRs.21 But, in diabetic WKY rats, no such reduction in AT1 expression was observed, although there was a trend toward reduced AT2R expression.21 Because the STZ-induced diabetic animal is a model of type 1 diabetes, it is possible that expression of its genes differs from that in type 2 diabetes.
Compared with numerous studies on animals, only a small number of studies have examined the expression of renal tissue angiotensin receptors in human DN. AT1R mRNA levels were significantly lower in eight samples from patients with DN.4 In another study, AT1 mRNA expression of non-diabetic subjects tended to be higher than that of diabetic subjects, but this difference did not reach statistical significance, and the AT2 mRNA expression level of diabetic subjects was comparable to that of non-diabetic subjects.5 Only one study used a semiquantitative histochemical method to assay AT1 protein expression, and this study found AT1R expression was lower than AT2R expression in the 10 samples from patients with DN.22
Our study is the first to assess angiotensin receptors (AT1, AT2, and MAS) protein expression in a large sample of human patients with DN. Taking into account the ethical standards of the responsible committee on human experimentation and the Declaration of Helsinki, we selected five patients with renal resection due to renal carcinoma, and took normal renal tissue next to the cancer as a normal control. As whole biopsies are a composite of various renal structural components, we analyzed AT1, AT2, and MAS receptor expression levels in glomerular, and tubulointerstitial. We assessed the expression of AT1, AT2, and MAS receptors in two groups of DN patients: one group was not receiving any RAS inhibitor, another group was treated with ARB only. AT1R mediates the classic physiological and pathophysiological actions of Ang II. AT1 protein expression levels increased during the early stages of DN, but decreased during the late stage. Elevated angiotensin II immunohistostaining was observed in tubular and infiltrating cells in diabetic human kidneys23. It is therefore tempting to speculate that a decrease in AT1R and AT2R levels mirrors high intrarenal Ang II concentrations, provoking a negative feedback response. Previous studies have shown a decrease in AT1 mRNA expression, which may be related to the enrollment of diabetic patients.
After treatment with ARB, AT1R protein expression levels were downregulated in glomerular and tubulointerstitial, and MAS receptor expression levels were upregulated in tubulointerstitial, but there was no difference in AT2R expression, which is consistent with a previous animal study.24 It should be noted that, as seen in Figure 2, AT1R expression increased significantly only in class IIb DN patients; AT2 and MAS have an increasing trend, and there were no significant change in angiotensin II receptor expression in class I+IIa and class III+IV. In this study, only 20% (23/115) DN patients were class IIb, and 80% (95/115) patients were class I, IIa, III, or IV. We believe that the severe lack of members of class IIb may be the main cause of similarity between the group of DN patients without RAS inhibitors and normal controls.
The MAS receptor is considered part of the protective arm of the RAS and has been implicated in improving disease phenotypes, such as obesity hypertension and chronic kidney diseases, as supported by pharmacological and knockout animal studies.25 Our previous research also found that MAR receptor expression levels were significantly higher in the group of mesangial hypercellularity.26 Currently, we believe that the local MAS receptor in the kidney is a protective receptor, and its expression is increased in the case of kidney damage.
Due to the small volume of the renal tissue obtained from the patient’s renal biopsy, there is not enough protein to carry out a western blot on the premise of completing the clinical pathological diagnosis, so we chose to replace this step with immunohistochemistry. If there is any opportunity to obtain more kidney tissue samples in the future, we will perform quantitative detection of proteins. Also, we plan to collect blood samples and test for Ang II, Ang 1–7 and other indicators in the future.
In summary, our study directly observed the expression of AT1R, AT2R, MAS and confirmed the local role of ARB in kidney: expression levels of AT1R increase during the early stages of DN. Blockade of the AT1R by ARB had disparate effects on the renal expression of angiotensin receptors, reducing AT1R while increasing MAS receptor expression.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Beijing Municipal Science and Technology Project (D171100002817003, D171100002817004), National Natural Science Foundation of China (81600547) and Beijing Natural Science Foundation (7202179).
ORCID iDs: Lin Liu  https://orcid.org/0000-0001-9690-1302
https://orcid.org/0000-0001-9690-1302
Wen-Ge Li  https://orcid.org/0000-0002-4891-2469
https://orcid.org/0000-0002-4891-2469
References
- 1. Barnett AH, Bain SC, Bouter P, et al. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med 2004; 351: 1952–1961. [DOI] [PubMed] [Google Scholar]
- 2. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345: 851–860. [DOI] [PubMed] [Google Scholar]
- 3. Parving HH, Brenner BM, McMurray JJ, et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 2012; 367: 2204–2213. [DOI] [PubMed] [Google Scholar]
- 4. Wagner J, Gehlen F, Ciechanowicz A, et al. Angiotensin II receptor type 1 gene expression in human glomerulonephritis and diabetes mellitus. J Am Soc Nephrol 1999; 10: 545–551. [DOI] [PubMed] [Google Scholar]
- 5. Konoshita T, Wakahara S, Mizuno S, et al. Tissue gene expression of renin-angiotensin system in human type 2 diabetic nephropathy. Diabetes Care 2006; 29: 848–852. [DOI] [PubMed] [Google Scholar]
- 6. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet 2000; 355: 253–259. [PubMed] [Google Scholar]
- 7. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869. [DOI] [PubMed] [Google Scholar]
- 8. Parving HH, Lehnert H, Brochner-Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 2001; 345: 870–878. [DOI] [PubMed] [Google Scholar]
- 9. Siragy HM, Carey RM. Role of the intrarenal renin-angiotensin-aldosterone system in chronic kidney disease. Am J Nephrol 2010; 31: 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kobori H, Nangaku M, Navar LG, et al. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev 2007; 59: 251–287. [DOI] [PubMed] [Google Scholar]
- 11. Carey RM, Wang ZQ, Siragy HM. Role of the angiotensin type 2 receptor in the regulation of blood pressure and renal function. Hypertension 2000; 35: 155–163. [DOI] [PubMed] [Google Scholar]
- 12. Zimmerman D, Burns KD. Angiotensin-(1–7) in kidney disease: a review of the controversies. Clin Sci (Lond) 2012; 123: 333–346. [DOI] [PubMed] [Google Scholar]
- 13. Santos RA, Simoes e Silva AC, Maric C, et al. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA 2003; 100: 8258–8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tervaert TW, Mooyaart AL, Amann K, et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol 2010; 21: 556–563. [DOI] [PubMed] [Google Scholar]
- 15. Ruster C, Wolf G. Renin-angiotensin-aldosterone system and progression of renal disease. J Am Soc Nephrol 2006; 17: 2985–2991. [DOI] [PubMed] [Google Scholar]
- 16. Lakshmanan AP, Watanabe K, Thandavarayan RA, et al. Telmisartan attenuates oxidative stress and renal fibrosis in streptozotocin induced diabetic mice with the alteration of angiotensin-(1–7) mas receptor expression associated with its PPAR-gamma agonist action. Free Radic Res 2011; 45: 575–584. [DOI] [PubMed] [Google Scholar]
- 17. Lakshmanan AP, Thandavarayan RA, Watanabe K, et al. Modulation of AT-1R/MAPK cascade by an olmesartan treatment attenuates diabetic nephropathy in streptozotocin-induced diabetic mice. Mol Cell Endocrinol 2012; 348: 104–111. [DOI] [PubMed] [Google Scholar]
- 18. He M, Zhang L, Shao Y, et al. Angiotensin II type 2 receptor mediated angiotensin II and high glucose induced decrease in renal prorenin/renin receptor expression. Mol Cell Endocrinol 2010; 315: 188–194. [DOI] [PubMed] [Google Scholar]
- 19. Xu ZG, Miao LN, Cui YC, et al. Angiotensin II type 1 receptor expression is increased via 12-lipoxygenase in high glucose-stimulated glomerular cells and type 2 diabetic glomeruli. Nephrol Dial Transplant 2009; 24: 1744–1752. [DOI] [PubMed] [Google Scholar]
- 20. Wehbi GJ, Zimpelmann J, Carey RM, et al. Early streptozotocin-diabetes mellitus downregulates rat kidney AT2 receptors. Am J Physiol Renal Physiol 2001; 280: F254–F265. [DOI] [PubMed] [Google Scholar]
- 21. Bonnet F, Candido R, Carey RM, et al. Renal expression of angiotensin receptors in long-term diabetes and the effects of angiotensin type 1 receptor blockade. J Hypertens 2002; 20: 1615–1624. [DOI] [PubMed] [Google Scholar]
- 22. Mezzano S, Droguett A, Burgos ME, et al. Renin-angiotensin system activation and interstitial inflammation in human diabetic nephropathy. Kidney Int Suppl 2003: S64–S70. [DOI] [PubMed] [Google Scholar]
- 23. Ruggenenti P, Gambara V, Perna A, et al. The nephropathy of non-insulin-dependent diabetes: predictors of outcome relative to diverse patterns of renal injury. J Am Soc Nephrol 1998; 9: 2336–2343. [DOI] [PubMed] [Google Scholar]
- 24. Yu QX, Zhang H, Xu WH, et al. Effect of irbesartan on chemerin in the renal tissues of diabetic rats. Kidney Blood Press Res 2015; 40: 467–477. [DOI] [PubMed] [Google Scholar]
- 25. Patel SN, Ali Q, Samuel P, et al. Angiotensin II type 2 receptor and receptor Mas are colocalized and functionally interdependent in obese zucker rat kidney. Hypertension 2017; 70: 831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang Z, Jiang SM, Ma YP, et al. Expression of the intrarenal angiotensin receptor and the role of renin-angiotensin system inhibitors in IgA nephropathy. Mol Cell Biochem 2019; 453: 103–110. [DOI] [PubMed] [Google Scholar]