Abstract
Background:
The purpose of this study was to systematically evaluate the effect of renin–angiotensin–aldosterone system blockers on the incidence of contrast-induced nephropathy in patients undergoing coronary angiography or percutaneous coronary intervention.
Methods:
A systematic literature search of several databases was conducted to identify studies that met the inclusion criteria. A total of 12 studies with 14 trials that performed studies on a total of 4864 patients (2484 treated with renin–angiotensin–aldosterone system blockers and 2380 in the control group) were included. The primary endpoint was the overall incidence of contrast-induced nephropathy. Analyses were performed with STATA version 12.0.
Results:
The overall contrast-induced nephropathy incidence in renin–angiotensin–aldosterone system blocker and control groups was 10.43% and 6.81%, respectively. The pooled relative risk of contrast-induced nephropathy incidence was 1.22 (95% confidence interval: 0.81–1.84) in the renin–angiotensin–aldosterone system blocker group. An increased risk of developing contrast-induced nephropathy in the renin–angiotensin–aldosterone system blocker group was observed among older people, non-Asians, chronic users, and studies with larger sample size, and the pooled RRs and 95% confidence intervals were 2.02 (1.21–3.36), 2.30 (1.41–3.76), 1.69 (1.10–2.59) and 1.83 (1.28–2.63), respectively.
Conclusions:
Intervention with renin–angiotensin–aldosterone system blockers was associated with an increased risk of contrast-induced nephropathy among non-Asians, chronic users, older people, and studies with larger sample size. Large clinical trials with strict inclusion criteria are needed to confirm our results and to evaluate the effect further.
Keywords: Contrast-induced nephropathy, renin–angiotensin–aldosterone system blockers, meta-analysis
Introduction
With the wide use of contrast media (CM), contrast-induced nephropathy (CIN) has become an important cause of hospital-acquired kidney injury, which accounts for increase in morbidity, in-hospital stays, and mortality.1–5 CIN is defined as an absolute rise in the serum creatinine (Scr) level by at least 44 μmol/l (0.5 mg/dl) or an increase in Scr level of >25% over baseline within 3 days following intravascular CM exposure.6 The incidence of CIN has been reported to be <2% in general population, but it can rise up to 20% or more in high-risk groups such as the elderly patients and patients with diabetes mellitus (DM) or chronic kidney disease (CKD).7 With the continuous increase in the treatment of coronary angiography (CAG) or percutaneous coronary intervention (PCI), and the number of elderly patients and the patients with DM or CKD, the incidence of CIN will be much higher in the future.8 Therefore, it is important and urgent to find a way to prevent CIN.
Due to the increased usage of renin-angiotensin-aldosterone system (RAAS) blockers, including angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs), in patients with hypertension, heart failure, renal glomerular disease, and diabetic nephropathy, the effect of them on CIN is of increasing concern.9–11 However, results from studies on the effect of ACEIs or ARBs on the incidence of CIN are conflicting. Some reported that RAAS blockers increased the risk of developing CIN, while others suggested they reduced the risk.12,13 Since the available data is conflicting, it is still not clear whether people should withhold ACEI or ARB use in order to prevent CIN. Therefore, we performed this meta-analysis to investigate the influence of ACEIs or ARBs on CIN incidence. Compared with other reviews, we excluded the trials that used high osmolar CM (HOCM) which is an identified risk factor for CIN. Furthermore, we performed more extensive subgroup analyses based on mean age, race, type of intervention, type of RAAS blockers, and sample size.
Methods
Data sources and search strategies
The databases of PubMed, EMBASE, Cochrane Central Register of Controlled Trials, the China National Knowledge Infrastructure (CNKI) Database, Wanfang Digital Periodicals Database (WFDP), Chinese Biological and Medical Database (CBM), Chinese Journal Full-text Database (CJFD), China Doctoral and Masters Dissertations Full-text Database were searched. The following keywords and MeSH terms were applied: “angiotensin-converting enzyme inhibitor,” “ACE inhibitor,” “angiotensin receptor blocker,” “renin angiotensin aldosterone system,” “contrast-induced nephropathy,” “kidney injury,” “renal failure.” The search included all relevant studies published before 9 December 2016 with no language limitation. We also screened the reference lists of relevant review articles and included studies for additional information.
Selection standards
We included studies investigating the effect of RAAS blockers on CIN incidence. The studies had to meet the following criteria: (a) adult patients received CM during the procedure of CAG or PCI; (b) the comparison included chronic administration of an ACEI/ARB versus control or withdrawal of the ACEI/ARB prior to the procedure, and new administration of ACEI/ARB versus control; (c) the study design was a randomized controlled trial (RCT), non-randomized prospective registry analysis or retrospective analysis; (d) the primary outcome of interest is CIN incidence that defined as an absolute increase in Scr values (>0.5 mg/dl) or by a relative increase as compared with the baseline value (>25%) within 2–3 days after exposure to CM. Additionally, the values of Scr after exposure to CM 72 h were also pooled; (e) all patients received hydration therapy. Studies that used HOCM or did not report what kind of CM was used were excluded. Furthermore, if multiple publications were available for a study, we included the most recent or the most detailed one.
Data extraction and quality assessment
Two of our authors extracted the following data independently, using a pre-defined standardized data extraction form: first author name, publication year, country of origin of the population studied, study design, inclusion criteria, sample size, participant characteristics, mean and standard deviation of the value of Scr, glomerular filtration rate (GFR) and blood urea nitrogen (BUN) before and after exposure to CM, detailed preprocedural hydration protocols, name and dose of CM, name and dose of ACEI/ARB, the usage methods of ACEI/ARB (chronic or new), definition of the CIN, CIN events in case and control group. The differences between these were resolved through discussion and consensus with another of our authors.
We assessed the quality of RCTs using the Jadad Scale,14 which features three principal assessment domains: randomization, blinding, and participant dropout. Total scores range from 0–7, studies scoring fewer than three points were considered to be of low quality. As for observational studies, the Newcastle-Ottawa Quality Assessment Scale (NOS) was used, which is a validated scale for non-randomized studies in meta-analyses containing nine items.15 Each item is assigned with a star if a study meets the criteria of the item. Studies can be awarded a maximum score of nine stars, and be considered of high quality with a score of five stars or more.
Statistical analysis
The relative risk (RR) and 95% confidence interval (CI) was considered as the effect size for CIN incidence, while the standard mean difference (SMD) was used to summarize results for the values of Scr after exposure to CM 72 h. A fixed-effects model was used if there was low heterogeneity existing between studies, otherwise, a random-effects model was used. Heterogeneity was evaluated by the Chi-square-based Q statistic test and quantified by I2, classified as low (I2<25%), moderate (25%⩽I2<50%), or high (I2⩾50%).16 Potential publication bias was evaluated by funnel plots by Begg’s test and Egger’s regression test.17,18 Sensitivity analyses were conducted to test whether the pooled RRs were influenced by certain individual studies by omitting a study each time and recalculating the pooled RR of the rest of the studies. Subgroup analyses based on mean age, race, type of intervention (chronic or new use), type of RAAS blockers (ACEI or ARB), and sample size (⩾200 or <200) were conducted to assess possible sources of statistical heterogeneity. Analyses were performed with STATA version 12.0 (StataCorp, College Station, Texas, USA) and all tests were two-sided with a significance level of 0.05.
Results
Search results and study characteristics
We identified 713 citations, of which 12 studies including 14 trials met the inclusion criteria (Figure 1). The population for our meta-analysis was composed of 4864 patients (2484 treated with ACEI or ARB and 2380 in the control group).19–30 The characteristics of the individual studies are summarized in Table 1. There were eight studies (10 trials) performed in Asians.21,23,25–30 Nine RCTs were identified in this study,21,22,24,25,27,29,30 while the other five studies were a non-randomized prospective registry analysis or retrospective analysis.19,20,23,26,28 Patients in six trials received ACEIs,19,21,25,27,29 in four trials received ARBs,22,26,30 and in four studies received either ACEIs or ARBs.20,23,24,28 In six trials patients received chronic intervention of ACEIs or ARBs.19,20,23–25 There were five studies with a sample size greater than 200.19,20,23,25,26 Assessment of quality of the studies found that nine RCTs scored 3–5, and five observational studies scored six stars, suggesting a moderate quality.
Figure 1.
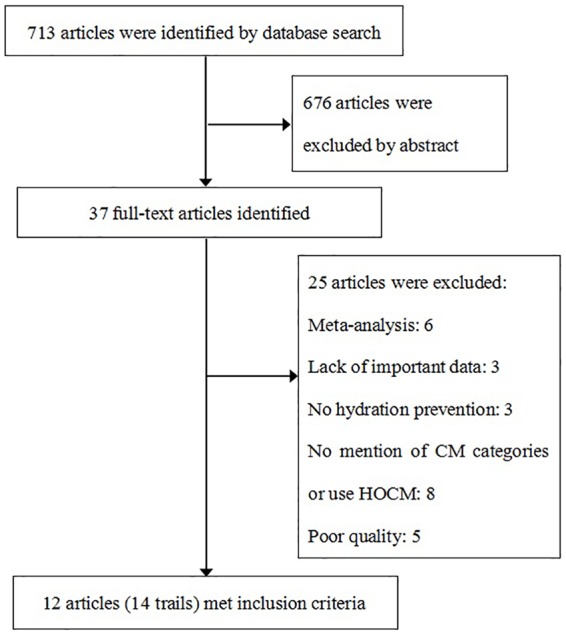
Flow chart of study selection.
CM: contrast media; HOCM: high osmolar contrast media.
Table 1.
Study characteristics.
| First author, year | Sample size (n) | Country | RCT or not | Inclusion criteria | Age, years (T/C) |
DM (n) (T/C) |
CM name | ACEI/ARB dosing | ACEI/ARB chronic use or not | Quality evaluation |
|---|---|---|---|---|---|---|---|---|---|---|
| Cirit, 200619 |
230 | Turkey | N | ⩾65 years age; with mild to moderate renal insufficiency |
71.38/71.29 | 11/10 | Iohexol | Enalapril 10–20 mg qd; fosinopril 10–20 mg qd; perindopril 4–8 mg qd; ramipril 2.5–5 mg qd; quinapril 5–20 mg qd; other type of ACEI | Y | 6 starsa |
| Kiski, 201020 |
412 | Germany | N | Scr 1.3–3.5 mg/dl | 67.8/65.9 | 83/37 | Iopromide | – | Y | 6 starsa |
| Li, 201121 |
114 | China | Y | Scr<176 μmol/l | 60.77/61.84 | 15/21 | Iohexol | Benazepril 10 mg qd | N | 3b |
| Oguzhan, 201322 |
90 | Turkey | Y | Scr<2.1 mg/dl | 66.38/62.07 | 19/18 | Iopromide | Valsartan 160 mg qd | N | 4b |
| Rim, 201223 |
2644 | Korea | N | No dialysis | 61.87/61.62 | 135/128 | Iodixanol, iopromide, Iopamidol |
– | Y | 6 starsa |
| Rosenstock, 200824 |
176 | USA | Y | GFR 15–60 ml/min/1.73 m2 | 71.8/68.5 | 61/19 | Iso-osmolar contrast | – | Y | 4b |
| Zheng, 2013(a)25 |
240 | China | Y | GFR⩾90 ml/min/1.73 m2 | 61.2/63 | 20/22 | Iopamidol | Perindopril 4 mg qd | Y | 3b |
| Zheng, 2013(b)25 |
94 | China | Y | GFR 60–90 ml/min/1.73 m2 | 66.3/65.4 | 13/11 | Iopamidol | Perindopril 4 mg qd | Y | 3b |
| Liao, 201126 |
206 | China | N | DM; Scr<176.8 μmol/l |
63.47/65.01 | – | Iohexol | Irbesartan 150 mg qd | N | 6 starsa |
| Hu, 201227 |
146 | China | Y | GFR⩾60 ml/min/1.73 m2 | 60.81/60.03 | 14/12 | Iopamidol | Enalapri 5–10 mg bid | N | 3b |
| Chen, 201528 |
100 | China | N | No DM; GFR 30–90 ml/min/1.73 m2 |
61/59 | 0/0 | Iohexol | – | N | 6 starsa |
| Li, 201029 |
119 | China | Y | Scr<264 μmol/l | 62.5/61.8 | 16/20 | Iohexol | Benazepril 10 mg qd | N | 3b |
| Fan, 2012(a)30 |
146 | China | Y | Scr<264 μmol/l | 61.01/59.69 | 18/17 | Iopamidol | Losartan 50 mg qd | N | 5b |
| Fan, 2012(b)30 |
147 | China | Y | Scr<264 μmol/l | 60.12/59.69 | 20/17 | Iopamidol | Losartan 100 mg qd | N | 5b |
–: no information available; ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin II receptor blocker; C: control group; CM: contrast media; DM: diabetes mellitus; GFR: glomerular filtration rate; RCT: randomized controlled trial; Scr: serum creatinine; T: treatment group; bid: twice a day; qd: once a day.
The quality of observational studies was assessed by using the Newcastle-Ottawa Quality Assessment Scale (NOS).
The quality of RCTs was assessed by using the Jadad Scale.
The overall effect of RAAS blockers on CIN incidence
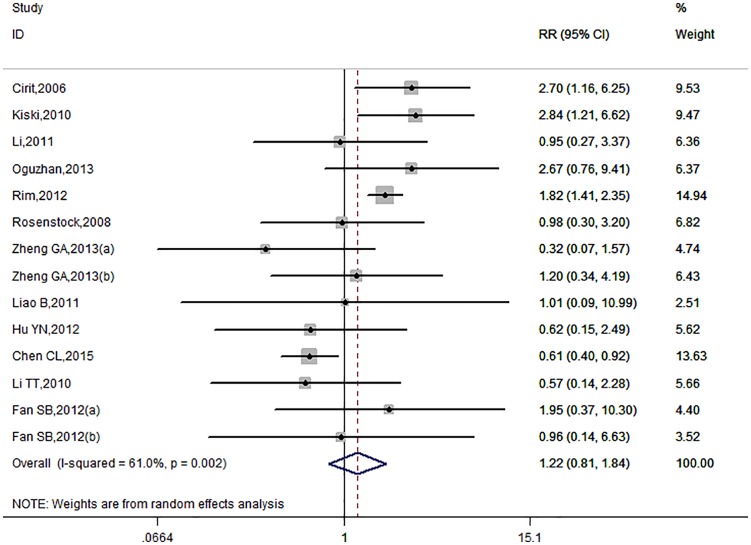
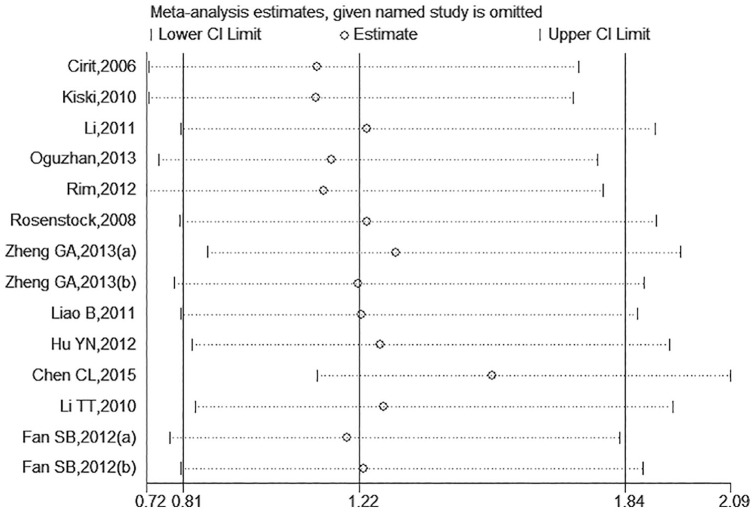
There were 14 trials included and the overall CIN incidence in ACEI/ARB and control groups was 10.43% (259/2484) and 6.81% (162/2380), respectively. The pooled RR of CIN incidence in the ACEI/ARB group was 1.22 (95% CI: 0.81–1.84, Figure 2) under the random-effects model as a significant heterogeneity existed (I2=61%, p=0.002). The administration of ACEIs/ARBs was not associated with decreased or increased risk of CIN incidence. There was no publication bias (Figure 3; Egger’s test: p=0.437). Given the marked between-study heterogeneity, a sensitivity analysis was conducted to see whether there was a certain study accounting for the heterogeneity. We found that after we omitted the study by Chen et al.,28 the remaining pooled RR changed to 1.53 (95% CI: 1.12–2.09, Figure 4) and the heterogeneity decreased (I2=17%, p=0.273). Other studies were not observed to significantly influence the overall analysis.
Figure 2.
The forest plot evaluating the effect of angiotensin-converting enzyme inhibitor (ACEI)/angiotensin II receptor blocker (ARB) use on the overall incidence of contrast-induced nephropathy (CIN).
CI: confidence interval; RR: relative risk.
Figure 3.
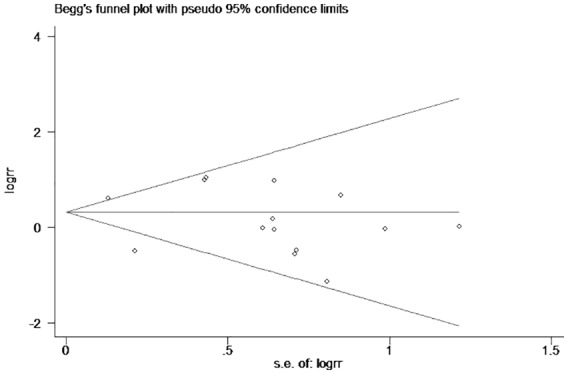
The Begg’s funnel plot of publication bias.
Figure 4.
Sensitivity analysis for the overall estimate on the association between angiotensin-converting enzyme inhibitor (ACEI)/angiotensin II receptor blocker (ARB) use and contrast-induced nephropathy (CIN).
CI: confidence interval.
Subgroup analyses of CIN incidence
The results of subgroup analyses were shown in Table 2. An increased risk of developing to post-procedure CIN in the ACEI/ARB group was observed among older people (mean age⩾65 years), non-Asians, chronic users who continued ACEI/ARB before and after the contrast-using procedure, and studies with larger sample size (population⩾200), and the pooled RR and 95% CI were 2.02 (1.21–3.36), 2.30 (1.41–3.76), 1.69 (1.10–2.59), and 1.83 (1.28–2.63), respectively. Asians, new use of ACEI/ARB, and studies with smaller sample size (population<200) had a slightly decreased risk of CIN incidence, but all of them did not reach significance. Ten trials reported specific usage of ACEI or ARB medications, while we found that the administration of neither ACEI nor ARB were associated with CIN incidence. Similar results were observed in subgroups stratified by study design.
Table 2.
Subgroup analyses of contrast-induced nephropathy (CIN) incidence.
| Subgroup | Trials, n | RR (95% CI) | Heterogeneity, I2 |
|---|---|---|---|
| Mean age (years) | |||
| ⩾65 | 4 | 2.02 (1.21–3.36) | 6.10% |
| <65 | 10 | 0.99 (0.58–1.68) | 66.80% |
| Race | |||
| Asian | 10 | 0.92 (0.54–1.54) | 65.00% |
| Non-Asian | 4 | 2.30 (1.41–3.76) | 0.00% |
| Study design | |||
| RCT | 9 | 0.98 (0.62–1.56) | 0.00% |
| Non-RCT | 5 | 1.56 (0.78–3.12) | 84.20% |
| Type of intervention | |||
| chronic use | 6 | 1.69 (1.10–2.59) | 37.30% |
| new use | 8 | 0.75 (0.53–1.05) | 0.00% |
| Type of RAAS blockers | |||
| ACEI | 6 | 0.98 (0.51–1.89) | 39.50% |
| ARB | 4 | 1.81 (0.78–4.17) | 0.00% |
| Sample size | |||
| ⩾200 | 5 | 1.83 (1.12–3.00) | 40.30% |
| <200 | 9 | 0.78 (0.57–1.07) | 0.00% |
ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin II receptor blocker; CI: confidence interval; RAAS: renin–angiotensin–aldosterone system; RCT: randomized controlled trial; RR: relative risk.
The values of Scr after exposure to CM 72 h
There were six trials that reported the values of Scr after exposure to CM 72 h, and the pooled SMD was −0.082 (95% CI: −0.216–0.053) under a fixed-effects model (I2=0.0%, p=0.420). The ACEI/ARB group did not significantly change Scr values after exposure to CM 72 h.
Discussion
In this study we examined the association of ACEI/ARB treatment with the incidence of CIN among patients undergoing CAG or PCI.
CM are traditionally classified by their osmolality: HOCM, >1500 mOsm/kg (i.e. 5–8 times plasma); low-osmolar CM (LOCM), 550–850 mOsm/kg (i.e. 2–3 times plasma); and iso-osmolar CM (IOCM), 290 mOsm/kg (i.e. isotonic to plasma).6,31 Compared with HOCM, decreased risk has been identified with the use of LOCM or IOCM, particularly in high-risk patients.32,33 In our analysis, studies that used HOCM or did not report what kind of CM was used were excluded. Comparing with previous meta-analyses,34,35 the obvious risk factor for CIN was removed in our study.
The overall pooled analysis shows that the administration of ACEIs/ARBs is not associated with decreased or increased risk of CIN incidence. However, if we omitted one study carried out by Chen et al.,28 the remaining pooled RR changed from 1.22 (95% CI: 0.81–1.84) to 1.53 (95% CI: 1.12–2.09) and the heterogeneity decreased. The result indicates that there should be some protective factors in this study. By analyzing it carefully, we found that this study was the only one that excluded patients with DM, while other studies included different proportions of patients with DM. DM is an important risk factor for CIN. Administration of CM will aggravate kidney changes associated with DM, including changes in renal hemodynamics, enhanced tubular transport activity, and ROS generation.36 Removing the factor of DM might indicate the protective issue for the result. Since there were few clinical trials performed without this obvious risk factor, more information could be attained by performing clinical trials excluding patients with DM in the future.
The subgroup analysis was conducted for the background complexity of the study population. The important and interesting finding in our study is that using ACEIs/ARBs increased the risk of developing CIN in non-Asians. Despite the fact that therapeutic and preventive strategies of renal diseases are mainly based on clinical observations, there have been notable advances in genetic studies in recent years.37 Genetic factors, such as angiotensin-converting enzyme (ACE) gene polymorphisms, are complicating factors in the treatment of renal diseases among different racial and ethnic groups.38,39 Meanwhile, genetic background may also determine the responsiveness to ACEI/ARB drugs. For example, Narita et al. found that the apparent lack of therapeutic efficacy of RAAS blockers could be influenced by M235T and A(-20)C genotype of the angiotensinogen gene (AGT) on renal survival in immunoglobulin A nephropathy.40 In addition, with experimental studies showing that inflammatory reaction played a major role in the pathogenesis of CIN, the effect of ARBs on the reduction of inflammatory factors was found to be related to the insertion/deletion (I/D) polymorphism in the ACE gene.41–43 These studies suggest that testing for ACE or AGT gene polymorphism is important for predicting the effect of RAAS blockers in patients with CIN. Our data shows that there is difference between the Asian and non-Asian populations in preventing CIN with ACE-I/ARB usage. This result may give a clue to help explain the conflicting findings in part of previous studies, and research studies with large samples focusing on genotype may give us more accurate information. According to our result, the genetic background should be concerned when evaluating the effect of ACEIs/ARBs on incidence of CIN. Applications of genotype could be a reliable tool to identify patients at risk and those who may benefit the most from therapy with RAAS blockers undergoing CAG or PCI, and to guide individualized strategies.
Since ACEIs and ARBs constitute a major treatment for CKD, DM, and cardiovascular disease, chronic usage of them is very common among patients undergoing CAG or PCI. It is very important to analyze the effect of ACEIs/ARBs on CIN between chronic and new users. By conducting subgroup analysis, we found that ACEI/ARB use was a risk factor for developing CIN in chronic users who continued such drugs before and after the contrast-using procedure. Cirit et al.19 also found that chronic ACEI administration is a risk for developing CIN in elderly patients with renal insufficiency. Although the pathogenesis of CIN is not completely understood, change in renal hemodynamics is one of the primary mechanisms.44 Using ACEIs/ARBs leads to a decrease in glomerular hydrostatic pressure and glomerular filtration, and combined with increased viscosity of CM this may increase the incidence of CIN.
Advanced age has been recognized as an independent risk factor for the development of CIN for years.45,46 Our result is consistent with this, with the increase of age, renal tubular function, renal vascular compliance, and renal blood flow auto-regulation decline which lead to decreased renal blood flow and prolonged excretion of contrast agent. We should master the indications for using contrast agents in elderly patients strictly, and pay much attention to the preventive treatment. An increased risk of developing to CIN in the ACEI/ARB group was observed in studies with larger sample size. A large sample size may have less bias, and thus reduce the impact on the result. This result is consistent with a previous meta-analysis.35
There are still some limitations in our study. For example, the methodological quality of included literature was not high, the brand name and dose of ACEI/ARB drugs were not the same, and the baseline usage of other drugs, such as N-acetylcysteine (N-ACC) were different. The proportion of patients undergoing CAG or PCI and whether magnetic resonance angiography (MRA) was performed were not specified.
Conclusions
In the meta-analysis, we found that administration of RAAS blockers was associated with the increased risk of developing CIN among non-Asians, chronic users, older people, and studies with larger sample size. However, the robustness of our study remains weak for the complexity of the population background, and large-scale trials with strict inclusion criteria are needed to evaluate the effect further in the future.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (Grant No. 81700637) and the Natural Science Foundation of Liaoning Province (Grant No. 20170540993).
ORCID iD: Xiaodan Liu  https://orcid.org/0000-0001-9112-1032
https://orcid.org/0000-0001-9112-1032
References
- 1. Yin WJ, Yi YH, Guan XF, et al. Preprocedural prediction model for contrast-induced nephropathy patients. J Am Heart Assoc 2017; 6: e004498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pandya B, Chalhoub JM, Parikh V, et al. Contrast media use in patients with chronic kidney disease undergoing coronary angiography: A systematic review and meta-analysis of randomized trials. Int J Cardiol 2017; 228: 137–144. [DOI] [PubMed] [Google Scholar]
- 3. Silver SA, Shah PM, Chertow GM, et al. Risk prediction models for contrast-induced nephropathy: Systematic review. BMJ 2015; 351: h4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sun G, Chen P, Wang K, et al. Contrast-induced nephropathy and long-term mortality after percutaneous coronary intervention in patients with acute myocardial infarction. Angiology 2019; 70: 621–626. [DOI] [PubMed] [Google Scholar]
- 5. Lameire N, Kellum JA. KDIGO AKI Guideline Work Group. Contrast-induced acute kidney injury and renal support for acute kidney injury: A KDIGO summary (Part 2). Crit Care 2013; 17: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kelly AM, Dwamena B, Cronin P, et al. Meta-analysis: Effectiveness of drugs for preventing contrast-induced nephropathy. Ann Intern Med 2008; 148: 284–294. [DOI] [PubMed] [Google Scholar]
- 7. Katsiki N, Athyros VG, Karagiannis A, et al. Contrast-induced nephropathy: An “all or none” phenomenon? Angiology 2015; 66: 508–513. [DOI] [PubMed] [Google Scholar]
- 8. Harjutsalo V, Thomas MC, Forsblom C, et al. ; FinnDiane Study Group. Risk of coronary artery disease and stroke according to sex and presence of diabetic nephropathy in type 1 diabetes. Diabetes Obes Metab 2018; 20: 2759–2767. [DOI] [PubMed] [Google Scholar]
- 9. Kalyesubula R, Bagasha P, Perazella MA. ACE-I/ARB therapy prior to contrast exposure: What should the clinician do? Biomed Res Int 2014; 2014: 423848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patel K, King CA, Jovin IS. Angiotensin-converting enzyme inhibitors and their effects on contrast-induced nephropathy after cardiac catheterization or percutaneous coronary intervention. Cardiovasc Revasc Med 2011; 12: 90–93. [DOI] [PubMed] [Google Scholar]
- 11. Liang DL, Li XY, Wang L, et al. [Current status and influence factors of ACEI/ARB application in elderly coronary heart disease outpatients complicated with diabetes mellitus in China.] Zhonghua Yi Xue Za Zhi 2016; 96: 2917–2922. [DOI] [PubMed] [Google Scholar]
- 12. Umruddin Z, Moe K, Superdock K. ACE inhibitor or angiotensin II receptor blocker use is a risk factor for contrast-induced nephropathy. J Nephrol 2012; 25: 776–781. [DOI] [PubMed] [Google Scholar]
- 13. Spatz C, Saadulla L, Lapsiwala A, et al. Effect of renin–angiotensin–aldosterone system blockade therapy on incidence of contrast-induced nephropathy in patients with chronic kidney disease. Iran J Kidney Dis 2012; 6: 432–436. [PMC free article] [PubMed] [Google Scholar]
- 14. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials 1996; 17:1–12. [DOI] [PubMed] [Google Scholar]
- 15. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses, http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (2003, accessed 5 May 2012).
- 16. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. [PubMed] [Google Scholar]
- 18. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cirit M, Toprak O, Yesil M, et al. Angiotensin-converting enzyme inhibitors as a risk factor for contrast-induced nephropathy. Nephron Clin Pract 2006; 104: c20–c27. [DOI] [PubMed] [Google Scholar]
- 20. Kiski D, Stepper W, Brand E, et al. Impact of renin–angiotensin–aldosterone blockade by angiotensin-converting enzyme inhibitors or AT-1 blockers on frequency of contrast medium-induced nephropathy: A post-hoc analysis from the Dialysis-versus-Diuresis (DVD) trial. Nephrol Dial Transplant 2010; 25: 759–764. [DOI] [PubMed] [Google Scholar]
- 21. Li XM, Cong HL, Li TT, et al. Impact of benazepril on contrast-induced acute kidney injury for patients with mild to moderate renal insufficiency undergoing percutaneous coronary intervention. Chin Med J (Engl) 2011; 124: 2101–2106. [PubMed] [Google Scholar]
- 22. Oguzhan N, Cilan H, Sipahioglu M, et al. The lack of benefit of a combination of an angiotensin receptor blocker and calcium channel blocker on contrast-induced nephropathy in patients with chronic kidney disease. Ren Fail 2013; 35: 434–439. [DOI] [PubMed] [Google Scholar]
- 23. Rim MY, Ro H, Kang WC, et al. The effect of renin–angiotensin–aldosterone system blockade on contrast-induced acute kidney injury: A propensity-matched study. Am J Kidney Dis 2012; 60: 576–582. [DOI] [PubMed] [Google Scholar]
- 24. Rosenstock JL, Bruno R, Kim JK, et al. The effect of withdrawal of ACE inhibitors or angiotensin receptor blockers prior to coronary angiography on the incidence of contrast-induced nephropathy. Int Urol Nephrol 2008; 40: 749–755. [DOI] [PubMed] [Google Scholar]
- 25. Zheng GA, Xie ZY, Chen JD. [The effect of perindopril on contrast-induced nephropathy in patients undergoing percutaneous coronary intervention.] Chinese Journal of Cardiovascular Research 2013; 11: 497–501. ISSN: 1672-5301. [Google Scholar]
- 26. Liao B. [The effects of irbesartan on renal function of patients with type 2 diabetes mellitus undergoing diagnosis and treatment of percutaneous coronary angiography.] Journal of Youjiang Medical University for Nationalities 2011; 33: 139–140. ISSN: 1001-5817. [Google Scholar]
- 27. Hu YN, Xu HP. [Efficacy of enalapri on renal function in patients undergoing percutaneous coronary intervention.] China Journal of Modern Medicine 2012; 22: 88–91. ISSN:1005-8982. [Google Scholar]
- 28. Chen CL, Huang Z, Lai WY, et al. [Comparative study on effect of ACEI/ARBs and CCBs in prevention of contrast-induced nephropathy in patients with hypertension associated with chronic renal insufficiency after coronary artery intervention.] Chin Heart J 2015; 27: 703–707. [Google Scholar]
- 29. Li TT, Li XM, Cong HL. [Role of benazepril on contrast-induced nephropathy in patients of coronary heart disease with hypertension undergoing coronary angioplasty.] Chin J Hypertens 2010; 18: 861–864. [Google Scholar]
- 30. Fan SB. [Effect of losartan on contrast-induced acute kidney injury and serum uric acid.] MM Thesis, Tianjing Medical University, CHN, 2012. [Google Scholar]
- 31. Solomon R, Briguori C, Bettmann M. Selection of contrast media. Kidney Int Suppl 2006; 100: S39–S45. [DOI] [PubMed] [Google Scholar]
- 32. Golshahi J, Nasri H, Gharipour M. Contrast-induced nephropathy: A literature review. J Nephropathol 2014; 3: 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Solomon R. The role of osmolality in the incidence of contrast-induced nephropathy: A systematic review of angiographic contrast media in high risk patients. Kidney Int 2005; 68: 2256–2263. [DOI] [PubMed] [Google Scholar]
- 34. Wu Z, Zhang H, Jin W, et al. The effect of renin–angiotensin–aldosterone system blockade medications on contrast-induced nephropathy in patients undergoing coronary angiography: A meta-analysis. PLoS One 2015; 10: e0129747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jo SH, Lee JM, Park J, et al. The impact of renin–angiotensin–aldosterone system blockade on contrast-induced nephropathy: A meta-analysis of 12 studies with 4,493 patients. Cardiology 2015; 130: 4–14. [DOI] [PubMed] [Google Scholar]
- 36. Heyman SN, Rosenberger C, Rosen S, et al. Why is diabetes mellitus a risk factor for contrast-induced nephropathy? Biomed Res Int 2013; 2013: 123589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Padullés A, Rama I, Llaudó I, et al. Developments in renal pharmacogenomics and applications in chronic kidney disease. Pharmgenomics Pers Med 2014; 7: 251–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Satirapoj B, Adler SG. Prevalence and management of diabetic nephropathy in Western countries. Kidney Dis (Basel) 2015; 1: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shah VN, Cheema BS, Sharma R, et al. ACACβ gene (rs2268388) and AGTR 1 gene (rs5186) polymorphism and the risk of nephropathy in Asian Indian patients with type 2 diabetes. Mol Cell Biochem 2013; 372: 191–198. [DOI] [PubMed] [Google Scholar]
- 40. Narita I, Goto S, Saito N, et al. Angiotensinogen gene variation and renoprotective efficacy of renin-angiotensin systemblockade in IgA nephropathy. Kidney Int 2003; 64: 1050–1058. [DOI] [PubMed] [Google Scholar]
- 41. Chang CF, Lu TM, Yang WC, et al. Gene polymorphisms of interleukin-10 and tumor necrosis factor-ɑ are associated with contrast-induced nephropathy. Am J Nephrol 2013; 37: 110–117. [DOI] [PubMed] [Google Scholar]
- 42. Mamoulakis C, Tsarouhas K, Fragkiadoulaki I, et al. Contrast-induced nephropathy: Basic concepts, pathophysiological implications and prevention strategies. Pharmacol Ther 2017; 180: 99–112. [DOI] [PubMed] [Google Scholar]
- 43. Seki N, Hashimoto N, Suzuki Y, et al. Differential effects of RAS inhibitors associated with ACE gene polymorphisms in type 2 diabetic nephropathy. Diabetes Res Clin Pract 2006; 72: 135–141. [DOI] [PubMed] [Google Scholar]
- 44. Zhou L, Duan S. Effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in contrast-induced nephropathy. Kidney Blood Press Res 2013; 38: 165–171. [DOI] [PubMed] [Google Scholar]
- 45. Lian D, Liu Y, Liu YH, et al. Pre-procedural risk score of contrast-induced nephropathy in elderly patients undergoing elective coronary angiography. Int Heart J 2017; 58: 197–204. [DOI] [PubMed] [Google Scholar]
- 46. Sone W, Zhang T, Pu J, et al. Incidence and risk of developing contrast-induced acute kidney injury following intravascular contrast administration in elderly patients. Clin Interv Aging 2014; 9: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]