Abstract
Background
In patients treated with dimethyl fumarate, absolute lymphocyte count decline typically occurs during the first year and then plateaus; early drops have been associated with the development of severe prolonged lymphopenia.
Objective
We investigated the effect of dimethyl fumarate on absolute lymphocyte counts and CD4+/CD8+ T cells in patients with relapsing–remitting multiple sclerosis treated with dimethyl fumarate in routine practice.
Methods
Lymphocyte data were collected via medical chart abstraction. Primary endpoint: change from baseline in absolute lymphocyte count and CD4+/CD8+ counts at 6‐month intervals following dimethyl fumarate initiation.
Results
Charts of 483 patients were abstracted and 476 patients included in the analysis. Mean baseline absolute lymphocyte count (2.23 × 109/l) decreased by ∼39% (95% confidence interval: –41.1 to –37.2) by month 6 and 44% (95% confidence interval: –46.6 to –42.1) by month 12. CD4+ and CD8+ T-cell subsets strongly correlated with absolute lymphocyte count, with greater decreases from baseline to 6 months vs 6–12 months, and in CD8+ vs CD4+ T cells. Prior natalizumab was not a risk factor for lymphopenia.
Conclusion
Dimethyl fumarate-associated decline in absolute lymphocyte count in the first 12 months correlated with decline in CD4+ and CD8+ T cells and was independent of prior natalizumab. Absolute lymphocyte count monitoring continues to be an effective strategy to identify patients at risk of prolonged lymphopenia.
Keywords: Multiple sclerosis, dimethyl fumarate, absolute lymphocyte count, T-cell subsets, natalizumab
Introduction
Delayed-release dimethyl fumarate (DMF) is approved for patients with relapsing–remitting multiple sclerosis (RRMS).1 As of January 31, 2020, >445,000 patients have been treated with DMF, representing >875,000 patient-years of exposure. Of these, 6,335 patients (14,241 patient-years) were from clinical trials. In patients treated with DMF, absolute lymphocyte count (ALC) decline typically occurs during the first year and then plateaus.2 Early drops in ALC are not indicative of clinical response2–4 but have been associated with subsequent development of severe prolonged lymphopenia.2
The main objective of this study was to evaluate changes in ALC and CD4+/CD8+ T-cell subtypes in patients with RRMS receiving DMF in routine practice. A third of patients enrolled previously received natalizumab (NAT); NAT’s mechanism of action typically leads to an increase in ALC, so we investigated the impact of prior NAT treatment on ALC and CD4+/CD8+ T-cell counts. We also investigated the impact of age (≤50 vs >50 years) on DMF-associated changes in ALC and CD4+/CD8+ cell count, given that immunosenescence may lead to lymphopenia.
Materials and methods
Study design
REALIZE (NCT02519413) was a retrospective, observational study conducted at nine sites in the USA, collecting data via medical chart abstraction at a single timepoint.
Patients and exclusion/inclusion criteria
Eligible patients initiated DMF treatment on/after 27 March 2013 and received ≥6 months’ continuous DMF treatment. Other inclusion criteria: aged ≥18 years; diagnosis of relapsing multiple sclerosis (MS); measurement for ALC, CD4+ count, and CD8+ count at baseline; and ≥1 subsequent timepoint while receiving DMF for ≥6 months. Key exclusion criteria: diagnosis of human immunodeficiency virus/acquired immunodeficiency syndrome and participation in DMF phase 3 studies.3,4
Endpoints
The primary endpoint was estimated adjusted change from baseline in ALC, CD4+ count, and CD8+ count at 6-month intervals within 12 months of DMF initiation. Secondary endpoints assessed at 6 and 12 months following DMF initiation were absolute counts; change from baseline and percentage change from baseline for lymphocytes and CD4+:CD8+ ratio; time to predetermined lymphocyte counts; and potential predictors of low lymphocyte counts.
Exploratory endpoints included ALC, CD4+ and CD8+ counts, and CD4+: CD8+ ratio; incidence of opportunistic and serious infections; reasons for DMF discontinuation; and average time to recovery to baseline values among patients with ALC, CD4+ count, or CD8+ count less than baseline count, and less than the lower limit of normal (LLN) after DMF cessation.
Safety endpoints
Data on serious and opportunistic infections were captured by the healthcare provider or designated personnel performing chart abstraction. For opportunistic infections, a list of potential opportunistic infections was provided or direction was given remotely. For serious infections, instructions were given to sites to review the medical record and capture the infection if it was noted as serious (e.g. requiring extensive treatment or denoted as severe). The study management and pharmacovigilance teams reviewed infection data and queried any information necessary for proper assessment of type of infection.
Statistical analysis
Primary and secondary endpoint analyses were performed on the full analysis population, including all eligible enrolled patients with an approved waiver of informed consent. For the primary analysis of ALC, CD4+ count, and CD8+ count, the change from baseline at months 6 and 12 was estimated using a repeated-measures mixed-effects model (RMMM); model covariates included baseline ALC, study day (log transformed), and baseline value by study day interaction. Study day was included as a random effect. Percentage change from baseline was estimated at months 6 and 12, as prespecified, and at 15 months after DMF initiation.
For secondary endpoint analysis, absolute counts, change from baseline, and percentage change from baseline to months 6 and 12 were summarized for total lymphocytes, CD4+ and CD8+ cells, and CD4+: CD8+ ratio. Assessments were only included in the 6 and 12 month tabulation if they were obtained ±30 days from the target timepoint (180 days from DMF initiation for month 6, 360 days from DMF initiation for month 12).
Logistic regression assessed whether age at baseline (≤50 vs >50 years), baseline ALC, and study site were associated with lymphopenia at months 6 and 12. Association between baseline predictors, percentage change, and absolute change from baseline ALC at months 6 and 12 was assessed by an analysis of covariance model (rank-transformed).
Post-hoc analyses explored trends in patients with/without prior NAT exposure, defined as NAT infusion within 180 days of DMF initiation. Using a RMMM specified for the primary analysis, ALC changes were estimated for patients with/without prior NAT exposure. Kaplan-Meier analyses of time to ALC <0.8 × 109/l or <0.5 × 109/l were conducted by prior NAT exposure in patients with a baseline ALC of ≥0.8 × 109/l (n = 459) or ≥0.5 ×109/l (n = 468), respectively. The Cox proportional hazards model assessed whether prior NAT exposure was associated with an increased risk of low ALC (<0.8 × 109/l or <0.5 × 109/l), adjusting for age at DMF initiation and baseline ALC.
The Pearson correlation statistic assessed association between ALC and CD4+/CD8+ counts by age (≤50 vs >50 years).
Safety analysis
Safety data analyses summarized the incidence of serious and opportunistic infections using descriptive statistics.
Results
Patient demographics
In total, 483 patient charts were abstracted; 476 patients were included in the full analysis population (Table 1). Seven patients were excluded post-hoc: five owing to missing data on DMF discontinuation, and two owing to inconsistencies in reported laboratory values. Most patients were female (73%) and white (73%). On average, patients were aged ∼49 years, almost a decade older than patients in pivotal trials.3,4 Of 476 patients with ≥1 treatment period value, 475, 291, 233, and 173 patients had an assessment ≥6, ≥12, >15, and ≥18 months, respectively, after DMF initiation.
Table 1.
Patient baseline demographics.
| Characteristic | Total patients(n = 476) | Patients with prior NAT exposure(n = 132) | Patients without prior NAT exposure(n = 344) |
|---|---|---|---|
| Female, n (%) | 349 (73) | 92 (70) | 257 (75) |
| Age, mean (SD), years | 49 (11.0) | 50.2 (10.6) | 48.4 (11.4) |
| 18–29, n (%) | 17 (4) | 2 (2) | 15 (4) |
| 30–39, n (%) | 91 (19) | 23 (17) | 68 (20) |
| 40–49, n (%) | 135 (28) | 39 (30) | 96 (28) |
| 50–59, n (%) | 149 (31) | 42 (32) | 107 (31) |
| ≥60, n (%) | 84 (18) | 26 (20) | 58 (17) |
| Race, n (%) | |||
| White | 346 (73) | 104 (79) | 242 (70) |
| Black or African American | 35 (7) | 16 (12) | 19 (6) |
| Other | 21 (4) | 6 (5) | 15 (4) |
| Not reported owing to confidentiality | 74 (16) | 6 (5) | 68 (20) |
| Prior MS treatment, n (%) | 344 (72) | 132 (100) | 212 (62) |
| Prior MS treatment type, n (%) | |||
| NATa | 138 (29) | 132 (100) | 6 (2) |
| Glatiramer acetate | 66 (14) | 4 (3) | 62 (18) |
| Methylprednisolone | 60 (13) | 18 (14) | 42 (12) |
| Interferon | 83 (17) | 3 (2) | 80 (23) |
| Methylprednisolone sodium succinate | 27 (6) | 2 (2) | 25 (7) |
| Teriflunomide | 13 (3) | 1 (<1) | 12 (3) |
| Fingolimod | 13 (3) | 0 | 13 (4) |
| Other | 31 (7) | 3 (2) | 28 (8) |
MS: multiple sclerosis; NAT: natalizumab; SD: standard deviation.
aPrior MS treatment included at any treatment prior to initiating dimethyl fumarate; patients were categorized as “prior NAT” or “no prior NAT” based on NAT exposure within 6 months of initiating dimethyl fumarate.
Estimated change in ALC, CD4+ count, and CD8+ count from baseline
Estimated means for ALC, CD4+ count, and CD8+ count declined over the study following DMF initiation from baseline to months 6 and 12. The largest declines were observed during the first 6 months, with slower rates of decline through month 15. Mean baseline ALC (2.23×109/l) decreased by ∼39% (95% confidence interval (CI): –41.1 to –37.2) by month 6 and 44% (95% CI: –46.6 to –42.1) by month 12 (Table 2). Mean baseline CD4+ count (1.06×109/l) decreased by ∼37% (95% CI: –39.3 to –35.1) by month 6 and 42% (95% CI: –44.5 to –39.8) by month 12. Mean baseline CD8+ count (0.50×109/l) decreased by ∼47% (95% CI: –49.0 to –44.1) by month 6 and 53% (95% CI: –55.5 to –49.9) by month 12 (Table 2).
Table 2.
Percentage change in absolute lymphocyte count (ALC), CD4+ count, and CD8+ count.
| Mean, ×109/la (n = 476) | Mean % changefrom baselinea | |
|---|---|---|
| ALC | ||
| Baseline | 2.23 | |
| Month 6 | 1.36 (1.31–1.40) | –39 (–41.1– –37.2) |
| Month 12 | 1.24 (1.19–1.29) | –44 (–46.6– –42.1) |
| Month 15 | 1.20 (1.15–1.25) | –46 (–48.3– –43.7) |
| CD4+ | ||
| Baseline | 1.06 | |
| Month 6 | 0.67 (0.64–0.69) | –37 (–39.3– –35.1) |
| Month 12 | 0.61 (0.59–0.64) | –42 (–44.5– –39.8) |
| Month 15 | 0.60 (0.57–0.62) | –44 (–46.2– –41.3) |
| CD8+ | ||
| Baseline | 0.50 | |
| Month 6 | 0.27 (0.25–0.38) | –47 (–49.0– –44.1) |
| Month 12 | 0.24 (0.22–0.25) | –53 (–55.5– –49.9) |
| Month 15 | 0.23 (0.21–0.24) | –55 (–57.6– –51.8) |
Mean values at months 6, 12, and 15 were estimated using a repeated-measures mixed-effects model with baseline ALC, time (log-transformed), and baseline ALC×time interaction effect. Time (log-transformed) was a random effect. Percentage change was estimated from baseline and the estimated mean change.
aValues in parentheses represent mean 95% confidence intervals.
Changes in ALC, and CD4+/CD8+ T-cell subsets assessed using raw data, were consistent with estimates from the RMMM (Figure 1(a)–(c)). ALC positively correlated with CD4+ and CD8+ at baseline and at months 9–15 in both younger (≤50) and older (>50) patients (r>0.744 for all correlations, p<0.0001). Patients with low ALC also had low CD4+ and CD8+ counts. Among 46 patients with the lowest post-baseline ALC of <0.5 × 109/l during treatment, all had the lowest post-baseline CD4+ and CD8+ counts of <0.4 × 109/l and <0.2 × 109/l, respectively.
Figure 1.
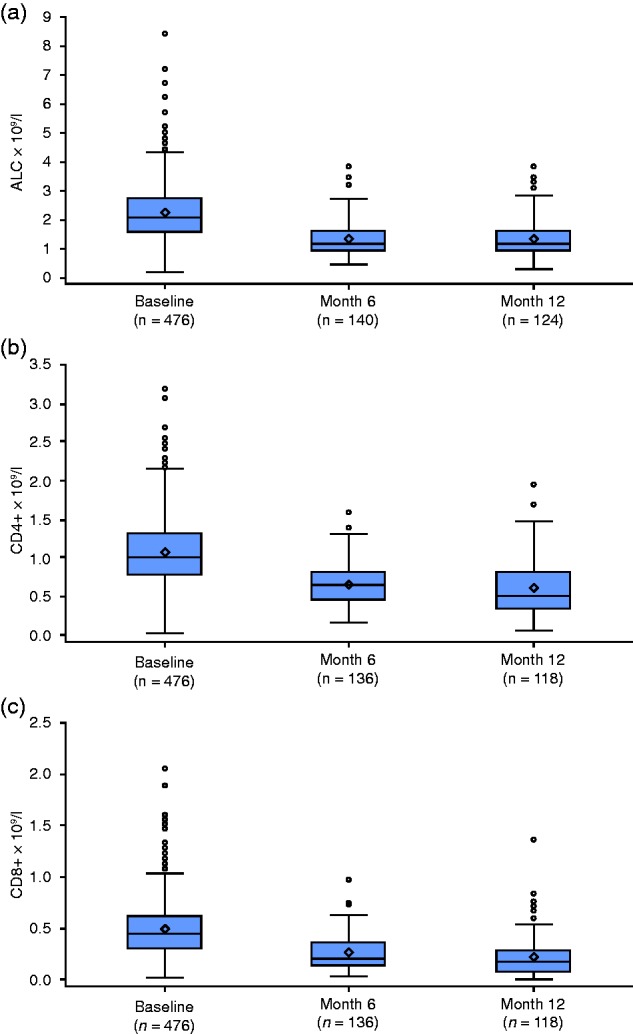
Observed (a) absolute lymphocyte count (ALC), (b) CD4+ count, and (c) CD8+ count over time. Change from baseline ALC was estimated using repeated-measures mixed-effects model analysis.
Prior NAT exposure
Approximately one-third of the patient population were previously treated with NAT. Mean age was similar in both cohorts. Consistent with NAT’s mechanisms of action, median ALC values for patients with prior NAT exposure were higher at baseline vs patients without (2.90 × 109/l vs 1.97 × 109/l; Table 3). Although relative percentage reduction from initial ALC was greater in patients with prior NAT exposure (decrease of 51.3% at month 6) than in patients without (decrease of 32.1% at month 6), both reached a similar mean at month 6 (1.41 × 109/l vs 1.34 × 109/l, respectively), maintained through month 15 (Table 3). Despite the higher relative reduction, 50% of patients with prior exposure to NAT remained ≥LLN (0.91 × 109/l) during 12 months of DMF treatment.
Table 3.
Percentage change in absolute lymphocyte count (ALC) by prior natalizumab (NAT) exposure.
|
Patients with prior NAT exposure |
Patients without prior NAT exposure |
|||
|---|---|---|---|---|
| Mean,×109/la(n = 132)b | Mean % change from baselinec | Mean,×109/la(n = 344) | Mean % change from baselinec | |
| Baseline | 2.90 | 1.97 | ||
| Month 6 | 1.41 (1.34–1.48) | –51.3 | 1.34 (1.29–1.39) | –32.1 |
| Month 12 | 1.21 (1.13–1.29) | –58.2 | 1.25 (1.19–1.31) | –36.4 |
| Month 15 | 1.15 (1.07–1.23) | –60.4 | 1.22 (1.16–1.29) | –37.8 |
aValues in parentheses represent mean 95% confidence intervals.
bUp to 180 days prior to dimethyl fumarate (DMF), 132 patients received NAT (two patients received NAT >180 days prior to DMF and were excluded from the analysis).
cMean values at months 6, 12, and 15 were estimated using a repeated-measures mixed-effects model with baseline ALC, time (log-transformed), and baseline ALC×time interaction effect. Time (log-transformed) was a random effect. Percentage change was estimated from baseline and the estimated mean change.
Similar to these results for ALC, prior NAT exposure influenced the initial decline of CD4+ and CD8+ counts from baseline to 6 months. Mean CD4+ T-cell counts in patients without prior NAT exposure vs those with prior NAT exposure decreased by 33% vs 46% at month 6 and 37% vs 52% at month 12, respectively. Likewise, mean CD8+ T-cell counts in patients without prior NAT exposure vs those with prior NAT exposure decreased by 40% vs 57% at month 6 and 46% vs 65% at month 12, respectively. Although the relative percentage reductions from initial CD4+ and CD8+ counts were greater in patients with prior NAT exposure than in patients without, both groups demonstrated similar mean CD4+ and CD8+ counts at month 6 (CD4+: 0.68×109/l vs 0.66×109/l, respectively; CD8+: 0.27×109/l vs 0.26×109/l, respectively) and at month 12 (CD4+: 0.60×109/l vs 0.62×109/l, respectively; CD8+: 0.23×109/l vs 0.24×109/l, respectively).
Prior NAT exposure influenced the kinetics of initial ALC decline, but neither increased nor decreased the risk of subsequent development of lymphopenia upon initiation of DMF. In a Cox proportional hazards model adjusted for baseline ALC and age (≤50 vs >50 years), the hazard ratio of patients with and without prior NAT exposure experiencing an ALC <0.8 × 109/l while receiving DMF was 1.180 (95% CI: 0.793 to 1.727; p = 0.4048). Similarly, the hazard ratio for experiencing an ALC <0.5 × 109/l was 1.247 (95% CI: 0.612 to 2.446; p = 0.5304).
Age
At 6 months following initiation of DMF, 25% (18/72) of patients aged ≤50 years had an ALC <LLN and 29% (19/65) of patients aged >50 years had an ALC <LLN (odds ratio (OR) = 1.24; p = 0.7003; Table 4). At this time, there was no difference in the median percentage change from baseline ALC in patients aged ≤50 years vs patients aged >50 years (–40.3% vs –45.5%; p = 0.1253). At month 12, 29% (15/51) of patients aged ≤50 years at baseline had an ALC <LLN compared with 50% (33/66) of patients aged> 50 years (OR = 2.38; p = 0.0366; Table 4). Median percentage change from baseline ALC in patients aged ≤50 years was smaller than in patients aged >50 years (−40.0% vs −55.7%; p = 0.0102) at 12 months.
Table 4.
Association between age and absolute lymphocyte count (ALC) <LLN at months 6 and 12.
| n | Category | n/N (%) <LLN | ORa | Predictor p-valueb | |
|---|---|---|---|---|---|
| Month 6 | |||||
| Baseline ALC | 137 | Continuous predictor | 0.51 | 0.0106 | |
| Age at baseline, years | 137 | ≤50 | 18/72 (25) | 1.24 | 0.7003 |
| >50 | 19/65 (29) | ||||
| Month 12 | |||||
| Baseline ALC | 117 | Continuous predictor | 0.78 | 0.2154 | |
| Age at baseline, years | 117 | ≤50 | 15/51 (29) | 2.38 | 0.0366 |
| >50 | 33/66 (50) |
LLN: lower limit of normal; OR: odds ratio.
Analysis is restricted to patients with an ALC ≥ 0.91 × 109/l at baseline. Exact logistic regression analysis methods applied; p-values are from exact conditional tests. For categorical predictor variables, the first category is the reference category.
aThe OR is the increase in odds of having an event with an increase in baseline ALC of 1.0 × 109/l.
bThe p-value evaluates the association between ALC <LLN at the timepoint and each predictor variable.
Results were similar when evaluating change in ALC. Interpretation of results from both analyses is limited by the small number of patients with measurements 6 and 12 months after treatment initiation.
Baseline ALC
Baseline ALC correlated with an ALC <LLN at month 6 (OR = 0.51, p = 0.0106 with each increase in baseline ALC of 1.0 × 109/l), and the percentage change from baseline at months 6 and 12.
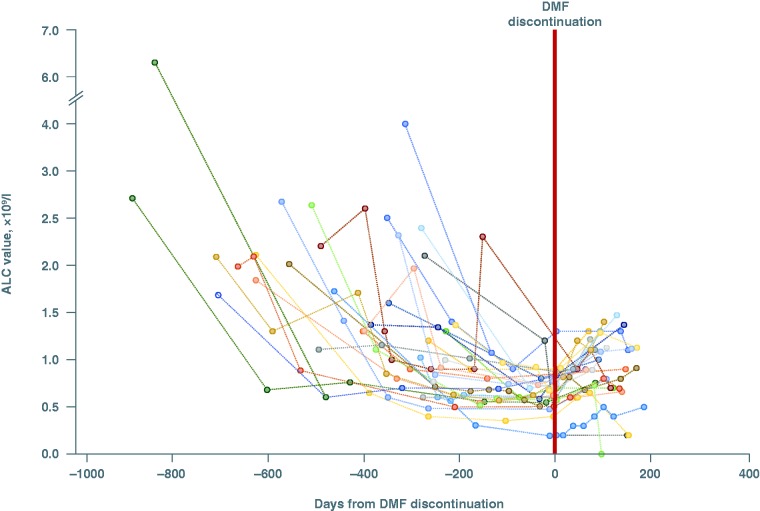
DMF discontinuation and lymphocyte recovery after DMF discontinuation
In total, 114 (24%) patients discontinued DMF after an average of 1.3 years of treatment. Forty patients discontinued for reasons associated with low lymphocyte or blood counts (13 of these were aged ≤50 years and 27 were aged >50 years).
Of 114 patients who discontinued DMF, 29% had ≥1 ALC <0.91 × 109/l while receiving treatment and >2 months after DMF discontinuation. Among these, 55% (16/29) had ALC values ≥0.91 × 109/l after discontinuation, and mean (standard deviation) time for receiving DMF treatment before discontinuation of 414 (198) days. While receiving DMF treatment, 23 of 29 patients experienced an ALC <0.8 × 109/l and six of 29 experienced an ALC <0.5 × 109/l (Figure 2). Of 29 patients with ≥1 ALC <0.91 × 109/l during DMF treatment and >2 months after DMF discontinuation, three initiated NAT and two initiated fingolimod following DMF discontinuation; of 25 patients who did not initiate NAT or fingolimod after DMF discontinuation, 12 had ALC values >0.91 × 109/l.
Figure 2.
Individual absolute lymphocyte count (ALC) values before and after delayed dimethyl fumarate (DMF) discontinuation and regression line. The regression line was calculated by locally estimated scatterplot smoothing with linear interpolation.
Safety: adverse events
Since this was a retrospective chart review, adverse event incidence and severity could not be accurately determined. Commonly reported infections included urinary tract infection, respiratory infections, and varicella zoster. There was no pattern of infection type among the few patients who experienced a possible opportunistic or serious infection (n = 7 (1%) in the 6 months prior to DMF treatment; n = 38 (8%) during DMF treatment; and n = 6 (5%) in the 6 months after DMF treatment). The most common infections during DMF treatment were urinary tract infections (n = 13), upper respiratory infections (n = 9), lower respiratory infections (n = 5), and varicella zoster (n = 4). There were no significant associations between ALC, CD4+ count, CD8+ count, and occurrence of possible opportunistic or serious infections for any time period (prior to, during, or after DMF treatment).
Discussion
The effect of DMF on ALC decline during early treatment has been assessed in several studies.2,5–10 In integrated analyses of phase 2b/3 extension studies, mean ALC decreased during the first year, then plateaued, remaining ≥LLN (0.91 × 109/l).2 Among those treated for ≥6 months (n = 2099), ALCs remained ≥LLN in 84% and 76% of patients after 6 and 12 months, respectively, and were within normal range at all visits in 61% of patients evaluated. Lowest post-baseline lymphopenia was <LLN to ≥0.800 × 109/l in 9%, <0.800 × 109/l to 0.500 ×109/l in 21%, <0.500 × 109/l to 0.200 × 109/l in 7%, and <0.200 × 109/l in <1% of patients overall.
Very rare cases of progressive multifocal leukoencephalopathy have occurred in patients treated with DMF in the clinical trial and post-marketing setting, predominantly in the context of moderate (≥0.5 ×109/l to <0.8 × 109/l) to severe (<0.5 × 109/l) lymphopenia persisting for ≥6 months; one case occurred in a patient with mild lymphopenia.1 Prescribing guidelines vary according to local labels, but all recommend monitoring lymphocyte levels and considering discontinuing DMF for patients with an ALC <0.5 × 109/l persisting for ≥6 months.1 Thus, it is important to characterize ALC profiles in patients with MS during and after treatment with DMF, especially in the real-world setting of routine clinical practice.
We found that ALC dynamics over the first year of DMF treatment were generally consistent with clinical trial observations and other real-world data.2,11 CD4+ and CD8+ T-cell subsets strongly correlated with ALC; decreases from baseline were more pronounced in the first 6 months of treatment than months 6–12. A greater reduction in mean ALC after 12 months of DMF was observed (44%) than reported by Fox et al. (30%).2 This was likely influenced by the difference in the proportion of patients who had received prior NAT treatment, known to increase the number of circulating lymphocytes.12 Patients with prior NAT treatment had on average a higher baseline ALC compared with patients without prior NAT treatment. Over the course of the study, there were no differences in mean ALC values at months 6, 12, and 15 between patients with prior NAT treatment and those without. Previous NAT treatment was not found to be a risk factor for lymphopenia upon subsequent initiation of DMF. In addition, in a recently published study of patients with RRMS who switched from NAT to DMF treatment (STRATEGY), no new major adverse events were reported compared with what is already known about the safety profile of DMF.13
Our study demonstrated a more pronounced decline in CD8+ T cells than in CD4+ T cells. Others have suggested that such decline is driven by CD8+ memory cells rather than naive T cells.5,14 Although the clinical relevance of DMF-associated decline in memory CD8+ T cells remains unclear, activated CD8+ T cells seem to play a role in the demyelination of axons in MS lesions.15 In >2500 patients receiving DMF for a median (range) of 5.7 (0.1–8.3) years in the ENDORSE long-term safety study,16 those with severe prolonged lymphopenia (2% (n = 53)) did not present with an increased incidence of serious infections, herpes infections or with malignancies and continued treatment after becoming severely lymphopenic for a total median (range) of 34 (4–81) months before discontinuing.16
REALIZE also assessed the impact of age on the risk of developing DMF-associated lymphopenia. The pattern of lymphocyte decline was generally similar across age groups; however, older age (≥50 years) was associated with an increase in median percentage decline in ALC at month 12 compared with younger age (<50 years). This association was not observed at month 6. Although the incidence of ALCs <LLN was higher in patients aged ≥50 years, it should be noted that both younger and older patients experienced ALCs <LLN (0.91 × 109/l). Of note, older age in general is associated with immunosenescence, characterized by decreased levels and functionality of B and T lymphocytes.17 The clinical significance of these findings in the context of DMF treatment is unclear; this study was not powered to determine if there was an increased risk of prolonged lymphopenia (>6 months duration) in older patients.
Assessment of ALC recovery kinetics and predictors in patients with lymphopenia who discontinued DMF treatment was challenging, owing to the low number of patients who discontinued the study because of low ALCs (who also had follow-up ALCs in the absence of other disease-modifying therapies). However, among the small number available for evaluation, at ≥2 months after DMF discontinuation ALC values returned to >0.91 ×109/l (<LLN) in nearly half of patients (12/25). Interestingly, a recently published article by Chan et al. reports an analysis of patients from clinical trials and real-world clinical care, showing that the majority of patients who discontinued DMF due to lymphopenia experienced ALC reconstitution within 2–4 months following DMF discontinuation.18
The strengths of this study include the use of observational data from routine clinical care and the availability of baseline ALC assessments; however, there are limitations with retrospective studies. Data collection was limited to routinely recorded information and information on laboratory values, as well as potential confounders, may not have been consistently recorded. Furthermore, although sites were provided with a list of possible opportunistic infections and guidance on how to record serious infections, the decision to record a safety event as a possible opportunistic or serious infection was at the discretion of the healthcare provider. Nevertheless, our results are consistent with previous studies that have evaluated changes in circulating immune cells from 6 months to 2 years.9,10,14,19
In summary, our data from real-world clinical practice support the results of previous clinical trials in demonstrating that DMF-associated decline in ALC in the first 12 months of treatment is closely correlated with decline in CD4+ and CD8+ T cells, independent of prior NAT treatment. ALC monitoring continues to be an effective strategy to identify patients who may be at risk of prolonged lymphopenia over the first year of DMF treatment, irrespective of age. These results may help guide clinicians in managing patients at risk of lymphopenia during DMF treatment.
Acknowledgements
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. Because no identifying data for the patients were collected, informed consent was waived by the institutional review board based on non-personally identifiable retrospective data collection and minimal risk.
Contributor Information
Guy Buckle, Multiple Sclerosis Institute at Shepherd Center, Inc., USA.
Daniel Bandari, Multiple Sclerosis Center of California, USA.
Jeffrey Greenstein, Multiple Sclerosis Research Institute, USA.
Mark Gudesblatt, South Shore Neurologic Associates PC, USA.
Bhupendra Khatri, Center for Neurological Disorders, Wheaton Franciscan Healthcare, USA.
Mariko Kita, Virginia Mason Medical Center, USA.
Pavle Repovic, Swedish Neuroscience Institute, USA.
Emily Riser, Alabama Neurology Associates, USA.
Bianca Weinstock-Guttman, Jacobs Multiple Sclerosis Center, USA.
Sherrill Loring, Multiple Sclerosis Institute at Shepherd Center, Inc., USA.
Conflict of Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: GB has served on speaker bureaus and as a consultant for Biogen, EMD Serono, Genentech, Mallinckrodt, Novartis, Sanofi-Genzyme, and Teva; and has received research support from Biogen for this study. DB has received research support from Biogen, Genentech, MedDay; and has served on speaker bureaus and as a consultant for Acorda, Biogen, Genentech, Genzyme, Mallinckrodt, Serono, and Teva. JG has received research support from Biogen. MG has been a principal investigator for studies sponsored by Acorda, Adamas, Biogen, Novartis, Roche, Sanofi-Genzyme, and Teva; has served on speaker bureaus for Biogen, EMD Serono, Novartis, Sanofi-Genzyme, and Teva; and is a consultant for Biogen, EMD Serono, Novartis, and Sanofi-Genzyme. BK has received compensation from Acorda, Biogen, EMD Serono, Genentech, Genzyme, Mallinckrodt, Novartis, Pfizer, Terumo, and Teva. MK has been a principal investigator for studies sponsored by Acorda, Biogen, Novartis, and Serono; has served on speaker bureaus for Biogen; and has served on advisory boards for Biogen and Novartis. PR has served as a consultant for Biogen, EMD Serono, Novartis, Questcor, Sanofi-Genzyme, and Teva; has served on speaker bureaus for Acorda, Biogen, EMD Serono, Novartis, and Teva; and has received grant support from Novartis. ER has been a principal investigator for studies sponsored by Acorda, Biogen, Mallinckrodt, and Novartis; and has served on speaker bureaus for Biogen, Genzyme, Mallinckrodt, Novartis, and Teva Neuroscience. BW-G. has received grant/research support from Biogen, EMD Serono, Genentech, and Mallinckrodt; and has served on speaker bureaus and as a consultant for Biogen, EMD Serono, Genentech, Novartis, Sanofi-Genzyme, and Teva Neuroscience. BT has served on speaker bureaus and as a consultant for Biogen, EMD Serono, Genentech, Mallinckrodt, Novartis, Sanofi-Genzyme, and Teva; and has received research support from Biogen for this study. SL has served on speaker bureaus and as a consultant for Biogen, EMD Serono, Genentech, Novartis, Sanofi-Genzyme, and Teva. KR, NE, CP, IK, and MM are employees of and hold stock/stock options in Biogen.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Biogen (Cambridge, MA) provided funding for medical writing support in the development of this paper; Katherine Ayling-Rouse, from Excel Scientific Solutions wrote the first draft of the manuscript based on input from authors, and Adie Trout and Miranda Dixon from Excel Medical Affairs copyedited and styled the manuscript per journal requirements. Biogen was involved in the study design, data collection, data analysis, and preparation of the manuscript. Biogen reviewed and provided feedback on the paper. The authors had full editorial control of the paper, and provided their final approval of all content. REALIZE is registered with ClinicalTrials.gov (NCT02519413). The datasets used and/or analyzed during the current study are available upon reasonable request. Requests for data supporting this manuscript should be submitted to the Biogen Clinical Data Request Portal (www.biogenclinicaldatarequest.com). The study name is A Retrospective, Multi-Center, Observational Study to Assess the Effect of Tecfidera® Delayed-Release Capsules on Lymphocyte Subsets in Subjects with Relapsing Forms of Multiple Sclerosis (REALIZE), BIIB number: 109MS419.
References
- 1.Biogen. Highlights of prescribing information. TECFIDERA® (dimethyl fumarate) delayed-release capsules, for oral use, https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/204063s017lbl.pdf (2017, accessed 11 November 2017).
- 2.Fox RJ, Chan A, Gold R, et al. Characterizing absolute lymphocyte count profiles in dimethyl fumarate–treated patients with MS: Patient management considerations. Neurol Clin Pract 2016; 6: 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012; 367: 1087–1097. [DOI] [PubMed] [Google Scholar]
- 4.Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012; 367: 1098–1107. [DOI] [PubMed] [Google Scholar]
- 5.Longbrake EE, Ramsbottom MJ, Cantoni C, et al. Dimethyl fumarate selectively reduces memory T cells in multiple sclerosis patients. Mult Scler 2016; 22: 1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghadiri M, Rezk A, Li R, et al. Dimethyl fumarate-induced lymphopenia in MS due to differential T-cell subset apoptosis. Neurol Neuroimmunol Neuroinflamm 2017; 4: e340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Q, Wang Q, Mao G, et al. Dimethyl fumarate selectively reduces memory T cells and shifts the balance between Th1/Th17 and Th2 in multiple sclerosis patients. J Immunol 2017; 198: 3069–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zecca C, Antozzi CG, Torri Clerici V, et al. Severe multiple sclerosis reactivation during prolonged lymphopenia after dimethyl fumarate discontinuation. Acta Neurol Scand 2018; 137: 623–625. [DOI] [PubMed] [Google Scholar]
- 9.Spencer CM, Crabtree-Hartman EC, Lehmann-Horn K, et al. Reduction of CD8(+) T lymphocytes in multiple sclerosis patients treated with dimethyl fumarate. Neurol Neuroimmunol Neuroinflamm 2015; 2: e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berkovich R, Weiner LP. Effects of dimethyl fumarate on lymphocyte subsets. Mult Scler Relat Disord 2015; 4: 339–341. [DOI] [PubMed] [Google Scholar]
- 11.Wenten M, Soman T, Lally C, et al. Lymphopenia in patients with multiple sclerosis treated with delayed-release dimethyl fumarate: Analysis of two United States electronic health record databases. Neurology 2016; 86: P2.098. [Google Scholar]
- 12.Biogen Idec. Highlights of prescribing information. TYSABRI (natalizumab) injection, for intravenous use, https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125104s953s955lbl.pdf (2016, accessed 11 November 2017).
- 13.Cohan SL, Moses H, Calkwood J, et al. Clinical outcomes in patients with relapsing–remitting multiple sclerosis who switch from natalizumab to delayed-release dimethyl fumarate: A multicenter retrospective observational study (STRATEGY). Mult Scler Relat Disord 2018; 22: 27–34. [DOI] [PubMed] [Google Scholar]
- 14.Von Hehn C, Mehta D, Prada C, et al. Interim results of an open-label study to assess the effects of delayed-release dimethyl fumarate on lymphocyte subsets and immunoglobulins in patients with relapsing-remitting multiple sclerosis. Neurology 2017; 88: P5–380.. [Google Scholar]
- 15.Pilli D, Zou A, Tea F, et al. Expanding role of T cells in human autoimmune diseases of the central nervous system. Front Immunol 2017; 8: 652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox RJ, Chan A, Gold R, et al. Lymphocyte decline and reconstitution after discontinuation in patients with severe, prolonged lymphopenia treated with delayed-release dimethyl fumarate. Neurology 2018; 90: P5–366.. [Google Scholar]
- 17.Sansoni P, Vescovini R, Fagnoni F, et al. The immune system in extreme longevity. Exp Gerontol 2008; 43: 61–65. [DOI] [PubMed] [Google Scholar]
- 18.Chan A, Rose J Alvarez E,. et al. Lymphocyte reconstitution after DMF discontinuation in clinical trial and real-world MS patients. Neurol Clin Pract 2020; 10: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khatri BO, Garland J, Berger J, et al. The effect of dimethyl fumarate (Tecfidera™) on lymphocyte counts: A potential contributor to progressive multifocal leukoencephalopathy risk. Mult Scler Relat Disord 2015; 4: 377–379. [DOI] [PubMed] [Google Scholar]