Abstract
Background
Chemokine receptor CXCR4 has been found to be associated with spinal neuron and glial cell activation during bone cancer pain. However, the underlying mechanism remains unknown. Furthermore, the RhoA/ROCK2 pathway serves as a downstream pathway activated by CXCR4 during bone cancer pain. We first validated the increase in the expressions of CXCR4, p-RhoA, and p-ROCK2 in the spinal dorsal horn of a well-characterized tumor cell implantation-induced cancer pain rat model and how these expressions contributed to the pain behavior in tumor cell implantation rats. We hypothesized that spinal blockade of the CXCR4-RhoA/ROCK2 pathway is a potential analgesic therapy for cancer pain management.
Methods
Adult female Sprague–Dawley rats (body weight of 180–220 g) and six- to seven-week old female Sprague–Dawley rats (body weight of 80–90 g) were taken. Ascitic cancer cells were extracted from the rats (body weight of 80–90 g) with intraperitoneally implanted Walker 256 mammary gland carcinoma cells. Walker 256 rat mammary gland carcinoma cells were then injected (tumor cell implantation) into the intramedullary space of the tibia to establish a rat model of bone cancer pain.
Results
We found increased expressions of CXCR4, p-RhoA, and p-ROCK2 in the neurons in the spinal cord. p-RhoA and p-ROCK2 were co-expressed in the neurons and promoted by overexpressed CXCR4. Intrathecal delivery of CXCR4 inhibitor Plerixafor (AMD3100) or ROCK2 inhibitor Fasudil abrogated tumor cell implantation-induced pain hypersensitivity and tumor cell implantation-induced increase in p-RhoA and p-ROCK2 expressions. Intrathecal injection of stromal-derived factor-1, the principal ligand for CXCR4, accelerated p-RhoA expression in naive rats, which was prevented by postadministration of CXCR4 inhibitor Plerixafor (AMD3100) or ROCK2 inhibitor Fasudil.
Conclusions
Collectively, the spinal RhoA/ROCK2 pathway could be a critical downstream target for CXCR4-mediated neuronal sensitization and pain hypersensitivity in bone cancer pain, and it may serve as a potent therapeutic target for pain treatment.
Keywords: CXCR4, RhoA, ROCK2, Fasudil, bone cancer pain, spinal cord, neurons
Background
Bone cancer pain (BCP) is one of the most common types of chronic pain caused by primary or metastatic bone marrow tumors.1 Recent epidemiologic study have shown that 75% to 90% of patients with bone metastasis or advanced cancer suffer from moderate to severe bone cancer-related pain daily, and their quality of life is severely affected.2 As there is a lack of understanding on the specific pathogenesis of BCP, it is difficult to effectively manage pain using traditional analgesic drugs and analgesic therapies such as radiotherapy and chemotherapy.3–5 Although there are ongoing intensive studies on the mechanisms of BCP and the discovery of novel analgesic targets in basic and clinical research communities, molecular and cellular mechanisms underlying BCP should be clearly elucidated.
The chemokine C–X–C motif receptor 4 (CXCR4) belongs to the G protein-coupled receptor (GPCR) superfamily of proteins. CXCR4 is the primary receptor of stromal-derived factor-1 (SDF-1, also known as CXCL12, which promotes cancer metastasis6). Recent studies have demonstrated that CXCR4 chemokine signaling contributes to the development and maintenance of chronic pain, both of which are characterized by mechanical allodynia and heat hyperalgesia, respectively.7–10 The underlying cellular mechanism is closely related to neuronal sensitization and glial activation because CXCR4 is located in both spinal neurons and glial cells.7–10 Our previous results showed that spinal CXCR4 mediates BCP generation and CaMKII upregulation in the spinal cord.11,12 However, the detailed intracellular molecular mechanism of CXCR4 underlying BCP is unclear.
Data suggest that Rho GTPase is a master regulator controlling cytoskeleton organization in multiple contexts such as tumor cell migration, adhesion, and cytokinesis.13 RhoA, a key member of the Rho family with a protein size of 2 to 25 kDa, plays important roles in regulating the formation of actin stress fibers and focal adhesion complexes in fibroblasts.14 Related studies have shown that RhoA is involved in the regulation of inflammatory pain and neuropathic pain through the activation of Rho kinase (ROCK).15,16 ROCK is a major mediator of RhoA function that contains ROCK1 and ROCK2. ROCK2 is mainly expressed in the central nervous system and skeletal muscle, whereas ROCK1 is mainly expressed in the liver, spleen, kidney, and testis. ROCK inhibitor Fasudil can significantly reduce the development of hyperalgesia in rats with neuropathic pain.15,17 ROCK may be involved in the regulation of synaptic plasticity changes in the central nervous system through active LIM kinases (LIMK phosphorylates Cofilin and inhibits the activity of depolymerized microfilaments). Studies have shown that SDF-1 modulates the migration and adhesion of breast cancer cells by controlling the expression and activation of Rho GTPases. In esophageal squamous cell carcinoma cells.18,19 the CXCR4 expression was significantly higher than that in human esophageal epithelial cells. CXCR4-CXCL12/AKT axis regulates RhoA, Rac-1, and Cdc42 to modulate cell invasion and tumor metastasis.20 Invasion and metastasis of tumor cells are closely related to pain. Therefore, inhibiting RhoA/ROCK signaling using Fasudil could be useful in inhibiting the migration of cancer cells in the treatment of cancer metastasis and the development of hyperalgesia. Hence, we reasonably hypothesized that CXCR4 facilitates BCP progression by activating RhoA/ROCK2 signaling in spinal neurons.
In this study, we first validated the increase in the expressions of CXCR4, p-RhoA, and p-ROCK2 in the spinal dorsal horn of a well-characterized tumor cell implantation (TCI)-induced cancer pain rat model and how these expressions contributed to the pain behavior in TCI rats. Then, we examined the ability of SDF-1 in triggering hyperalgesia in naive rats through the activation of CXCR4-mediated RhoA/ROCK2 pathway. We hypothesized that spinal blockade of the CXCR4-RhoA/ROCK2 pathway is a potential analgesic therapy for cancer pain management.
Methods
Animals
Adult female Sprague–Dawley (SD) rats (body weight, 180–220 g) and six to seven-week-old female SD rats (body weight, 80–90 g) were purchased from Shandong Jinan Peng yue Experimental Animal breeding Co., Ltd, China. Rats were housed under a constant 12-h light/dark cycle (lights on from 08:00 to 20:00) at 24°C ± 1°C, with food and water available ad libitum. The animals were given a minimum acclimation period of three days before commencing any experiment. The Institutional Animal Care and Use Committee of Xuzhou Medical University approved all research activities involving animals, which were designed to minimize the number of animals used and the suffering of animals. The experimental protocols were consistent with those of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the International Association for the Study of Pains guidelines for pain research.21
Established model of BCP
Ascitic cancer cells were extracted from the rats (body weight, 80–90 g) with intraperitoneally implanted Walker 256 mammary gland carcinoma cells (Institute for Biomedical Research of Shanghai, Shanghai, China). Ascitic tumor cells (2 × 107 cells/mL, 0.5 mL) were injected into the abdominal cavity of the SD rats weighing 80 to 90 g. After five to six days, cancerous ascites was extracted in a sterile manner, and the tumor cells were subsequently washed with sterile normal saline (NS) three times by centrifugation for 5 min at 1000 r/min. Then, the pellets were resuspended with NS and adjusted to an appropriate concentration (1 × 105 cells/µL). Until the cells were injected into the rats’ body, the cell suspension remained on the ice. As described in previous studies,22,23 the rats (body weight, 180–220 g) were anesthetized with sodium pentobarbital (50 mg/kg i.p.). The right hind leg of each rat was shaved and disinfected with 75% (v/v) ethanol, and an incision along the patellar ligament was made to expose the proximal tibia with minimal wound. Subsequently, Walker 256 mammary gland carcinoma cells (1 × 105 cells/µL, 5 µL) were slowly injected into the tibial cavity using a 10 µL microinjection syringe. The syringe needle was retained in place for an additional minute to prevent the tumor cells from leaking out along the injection track, and the injection site was sealed using bone wax. Sham surgery (for control) used a similar procedure by injecting 5 µL of high temperature-inactivated tumor cells into the right tibia.
Drugs and administration
ROCK2 inhibitor (downstream of RhoA) was purchased from Proteintech (Chicago, IL, USA). CXCR4 inhibitor Plerixafor (AMD3100) was purchased from GenePharma (Suzhou, China). Anti-p-RhoA antibody and anti-p-ROCK2 antibody were purchased from Abcam (Cambridge, MA, USA). Rat recombinant SDF-1 (CXCL12) was purchased from Sigma (St. Louis, MI, USA). Drugs were dissolved in sterilized phosphate-buffered saline (PBS) and delivered at a volume of 10 µL into the cerebral spinal fluid via lumbar puncture. Intrathecal (i.t.) injection was performed using a Hamilton 25 µL syringe with a 27-gage needle into the L5 to L6 interspace to deliver the drug. A brisk tail and/or paw flick confirmed a correct subarachnoid injection and complete drug delivery. The injection needle was left for 10 s after each injection. Any animals with signs of motor dysfunction were excluded from the experiments. AMD3100 was administered as described previously.10–12 ROCK inhibitor Y27632 (Fasudil, molecular weight: 320.26, 1–10 nmol i.t. injection) attenuated thermal hyperalgesia and mechanical allodynia in diabetic mice24; 20 µg i.t. dose had a minimal effect on the movement and mind of rats in our preliminary experiment. The dose of each drug and time points of treatment is stated in the figure legends.
Behavioral testing
The mechanical paw withdrawal threshold (PWT) of rats was estimated using von Frey filaments (Aesthesio®, Danmic Global, San Jose, CA, USA).25 An ascending series of filaments (0.4, 0.6, 1.4, 2, 4, 6, 8, and 15 g) were applied to the midplantar surface of rat hind paw with a sustaining pressure to bend the filament for 5 to 6 s or induce a paw withdrawal reflex within 5 s. The 50% PWT was determined using Dixon’s up–down method. Thermal hyperalgesia was assessed via the measurement of the paw thermal withdrawal latency (PWL) in response to radiant heat stimulation generated using a Plantar Analgesia Meter (IITC Life Science, CA, USA).26 The radiant heat source was delivered and focused onto the glabrous surface of the paw through the glass floor. The radiant heat was terminated when the rat withdrew its hind paw or automatically at a 20-s cutoff to prevent tissue damage. The thermal stimulus was delivered three times to each hind paw at 10-min intervals. The intensity of the heat stimulus was maintained constantly throughout this study. The behavioral testing was performed by an investigator blinded to the drug treatment. The experimenter did not participate in behavioral testing and data statistics. After the behavior testing was completed and the corresponding grouping data statistics were obtained, the blind was broken.
Western blot
The L4 to L6 segments of the lumbar spinal cord were quickly isolated from deeply anesthetized rats, and then, the tissue samples were dissected and stored in liquid nitrogen. The tissues were homogenized in radio immunoprecipitation assay lysis buffer containing 1% phenylmethylsulfonyl fluoride and protease/phosphatase inhibitor cocktail (#5872; Cell Signaling Technology, Danvers, MA, USA). Bicinchoninic acid protein assay kit (Thermo Scientific) was used to determine the protein concentration; 20 μg (2 μg/μL) of protein per lane was loaded and separated using 12.5% or 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The separated proteins were transferred onto a 0.22-μm polyvinylidene fluoride membrane. The membranes were placed in a blocking solution that contained Tris-buffered saline with 0.02% Tween (TBST) and 5% dried skim milk for 2 h at room temperature and then incubated with primary antibodies overnight at 4°C. Membranes were incubated with specific horseradish peroxidase-conjugated secondary antibodies. The following primary antibodies were used: goat anti-CXCR4 (1:200, NB100-716; Novus Biologicals, Littleton, CO, USA), rabbit antiphospho-RhoA antibody (1:500, ab125275; Abcam, Cambridge, MA, USA), rabbit antiphospho-ROCK2 antibody (1:500; Abcam, Cambridge, MA, USA), and mouse antiglyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:10,000; Proteintech, Rosemont, IL, USA). Horseradish-peroxidase-conjugated secondary antibodies were applied on the membrane, and the bound secondary antibody was detected using enhanced chemiluminescence reagents (Pierce, Rockford, IL, USA). Band intensity was quantified using Quantity One Analysis Software (Version 4.6.5; Bio-Rad Laboratories, Hercules, CA, USA). Fold changes of target proteins were obtained by normalizing the relative protein expression to that of the internal control, GAPDH, and β-actin. The fold change of the control group was set as 1 for quantification.
Next, five days after injection with cells (rat mammary gland carcinoma cells), the bone showed signs of small radiolucent lesions in the proximal epiphysis, close to the site of the injection. Histological and radiological evaluation of the tumor and bone showed the progressive extension of the tumor and significant destruction and rebuilding of the bone by day 15 after injection. Glial fibrillary acidic protein (GFAP) staining of the spinal cord showed increased staining in the ipsilateral spinal cord 17 days after inoculation into the tibia.22,23 Rats treated with cells displayed a significant decrease in PWT to Von Frey hair stimulation from day 4.22 In the present study, the target proteins showed by Western blot were obtained from the spinal cord of rats 7 days after TCI, while immunofluorescence results were obtained from the spinal cord of rats 14 days after TCI.
Immunofluorescence
Under deep anesthesia, the rats were intracardially perfused with PBS, followed with 4% paraformaldehyde (PFA). The L4 to L6 spinal segments were dissected and postfixed in 4% PFA overnight at 4°C and then transferred and preserved in 30% sucrose in a phosphate buffer at 4°C for subsequent use. Spinal tissues were transversely cut into 30-μm thick sections on a cryostat and stored in Tris-BufferedSaline (TBS). The sections were blocked in 10% donkey serum for 2 h at room temperature and incubated overnight at 4°C with the following primary antibodies: goat anti-CXCR4 (1:200, NB100-716; Novus Biologicals, Littleton, CO, USA), rabbit antiphospho-RhoA antibody (1:500, ab125275; Abcam, Cambridge, MA, USA), rabbit antiphospho-ROCK2 antibody (1:500, ab125025; Abcam, Cambridge, MA, USA), mouse anti-NeuN (neuronal nuclear marker, 1:1000, MAB377, Millipore), goat anti-GFAP (astrocyte marker, 1:1000; Abcam, Cambridge, MA, USA), and goat anti-ionized calcium binding adapter molecule-1 (anti-IBA-1) (microglia marker, 1:1000, ab5076; Abcam, Cambridge, MA, USA). The specificity for goat anti-CXCR4 (1:200, NB100-716),27 rabbit antiphospho-RhoA antibody (1:500, ab125275, Abcam),28 and rabbit antiphospho-ROCK2 antibody (1:500, ab125025, Abcam)29 has previously been described. The sections were washed three times for 10 min in TBS and then incubated for 1 h at 20°C to 25°C with corresponding secondary antibodies (conjugated to Alexa Fluor 488 or 594; Invitrogen, Carlsbad, CA, USA) overnight at 4°C. Stained sections for double immunostaining were incubated for a second time using the same procedures described above. The immunofluorescent images were captured using a confocal scanning laser microscope (FV1000; Olympus, Tokyo, Japan), and images were shown as merged Z-stack projections consisting of ∼10 optical slices. The mean fluorescence intensity of p-RhoA and p-ROCK2, and the number of p-RhoA immunopositive neurons in the entire superficial dorsal horn included a total of 16 spinal cord sections from four groups of rats (n = 4 per group) and were measured using ImageJ software.
Statistical analyses
GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA) was used to conduct all statistical analyses. Alterations in protein expression were tested using one-way analysis of variance (ANOVA) with repeated measures, followed by the Dunnett multiple comparison test; the changes in behavioral responses to mechanical and thermal stimuli over time among the groups were tested using two-way ANOVA with repeated measures, followed by Bonferroni post hoc test. All data were presented as mean ± standard error of the mean. Statistical results were considered significant if P < 0.05.
Results
Increased expressions of CXCR4, p-RhoA, and p-ROCK2 exacerbated in spinal neurons after TCI
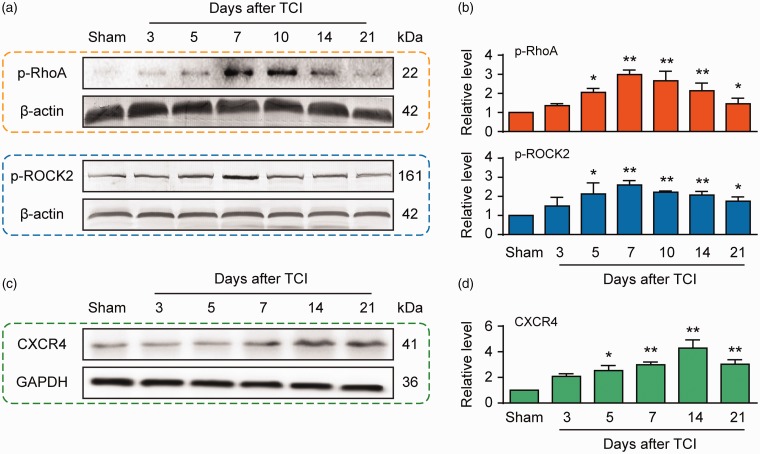
Chemokine changes induced by bone cancer in the spinal dorsal horn or dorsal root ganglion lead to generation of cancer pain.30–32 Here, we examined the CXCR4 protein level in the spinal cord to evaluate whether it could be upregulated in the BCP state. Western blot analysis shows that BCP caused a long-lasting upregulation of CXCR4 protein, which commenced on day 5 and was preserved until day 21 (Figure 1(a) and (b)). These outcomes are in accordance with our previous studies.11,12 As aforementioned, RhoA/ROCK2 signaling may be an important downstream pathway of CXCR4 and it participates in the progress of BCP mediated by CXCR4. Consequently, we further examined the time course expressions of p-RhoA and p-ROCK2 in the spinal cord of TCI and sham-operated rats. The p-RhoA and p-ROCK2 proteins overexpressed in a time-dependent manner in the spinal cord, which began on day 5 after TCI and maintained at a high level until day 21 (Figure 1(a) and (b)). Immunofluorescence results showed that the expressions of CXCR4, p-RhoA, and p-ROCK2 in the dorsal spinal cord increased 14 days after TCI (Figure 1(c)).
Figure 1.
Protein expressions of CXCR4, p-RhoA, and p-ROCK2 in the spinal dorsal horn following TCI treatment in rats. (a to d) Western blot showing the time course of the expressions of p-RhoA (top row), p-ROCK2 (top row), and CXCR4 (bottom row) after TCI. (a and c) Representative bands. (b and d) Quantitative data. *P < 0.05, **P < 0.01 versus sham group; n = 4 for each group. TCI: tumor cell implantation; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; CXCR4: chemokine C–X–C motif receptor 4.
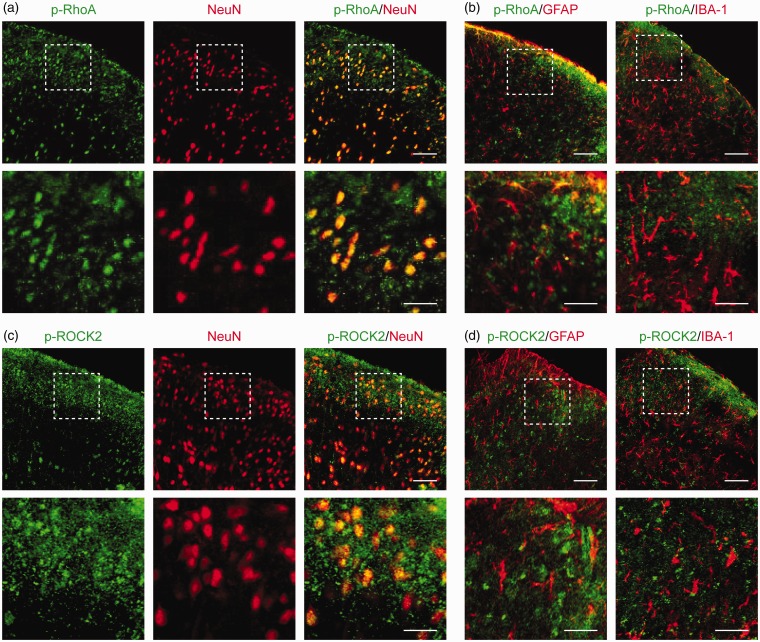
Double immunofluorescence staining showed that p-RhoA (green, Figure 2(a)) and p-ROCK2 (green, Figure 2(b)) were colocalized in the neurons (NeuN, red) in TCI rat spinal cord, but not co-expressed with astrocytes (GFAP, red) or microglial cells (ionized calcium binding adapter molecule-1 (IBA-1), red; Figure 2(c) to (d)). Our previous studies have shown that the increased expression of CXCR4 was colocalized in the neurons of TCI rat spinal cord.11,12 These findings suggest a functional relationship between CXCR4 and RhoA/ROCK2 pathway in the neurons of the spinal cord under the BCP state.
Figure 2.
Cellular colocalization of CXCR4 with p-RhoA and p-ROCK2 in rat spinal dorsal neurons. (a to d) Double immunofluorescence staining showing cellular colocalization of p-RhoA/p-ROCK2 and cell markers in TCI rat spinal cord. p-RhoA (green, a) and p-ROCK2 (green, c) were co-localized with neurons (NeuN, red). Meanwhile, p-RhoA (green, b) and p-ROCK2 (green, d) were not co-expressed with astrocytes (GFAP, red) or microglial cells (IBA-1, red). L4 to L6 spinal tissues were taken on day 14 after TCI. Original magnification: 400× (a to d) and 1600× (insets in a to d); scale bar: 50 μm (d to g) and 20 μm (insets in a to d). GFAP: glial fibrillary acidic protein; IBA-1: ionized calcium binding adapter molecule-1.
Inhibiting the activation of CXCR4 in the rat model of BCP attenuates mechanical allodynia and thermal hyperalgesia in BCP rats
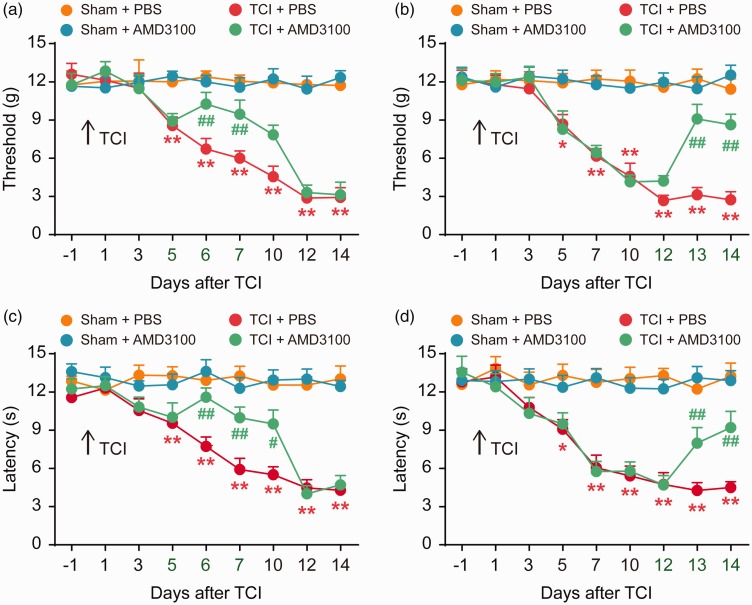
We next sought to explore whether the increased CXCR4 expression in the rat model of BCP could promote the progression of nociceptive behavior. Therefore, we further investigated the role of spinal CXCR4 in pain behavior in rats with BCP by inhibiting the activation of CXCR4 using AMD3100. We examined the analgesic effect of AMD3100 on TCI-induced pain behavior. The results showed that TCI gradually promoted noticeable pain behavior from day 5 to day 14 after TCI, as evidenced by a reduction in PWT and PWL. Compared with the TCI + PBS (10 μL) treatment group, the decrease in PWT and PWL in TCI rats treated with AMD3100 (5 μg/10 μL per i.t. injection, once daily) at the initial stage (five to seven days) was delayed (Figure 3(a) and (c)). Similarly, AMD3100 (5 μg/10 μL per i.t. injection, once a day, from 12 to 14 days) reversed the established PWT and PWL at the late phase of TCI (Figure 3(b) and (d)). There was no significant difference in PWT and PWL between the sham + PBS and sham + AMD3100 groups; so, it was considered that i.t. injection of AMD3100 alone did not affect the rats’ nociceptive behavior. These results indicate that CXCR4 is upregulated in the spinal cord after TCI and plays an important role in the pathological process of TCI.
Figure 3.
Intrathecal injection of CXCR4 inhibitor (AMD3100) attenuates BCP. AMD3100 significantly delayed or attenuated TCI-induced mechanical allodynia (a and b) and thermal hyperalgesia (c and d). AMD3100 (5 μg/10 μL) or PBS (10 μL) was i.t. administered once daily on days 5, 6, and 7 (a and c) or days 12, 13, and 14 (c and d) after TCI. Behavioral tests were performed 4 h after each injection. *P < 0.05, **P < 0.01 versus sham + PBS group; #P < 0.05 vs TCI + PBS group; ##P < 0.01 versus TCI + PBS group; n = 8 for each group. TCI: tumor cell implantation; PBS: phosphate-buffered saline.
Inhibiting the activation of ROCK2 attenuates mechanical allodynia and thermal hyperalgesia in BCP rats
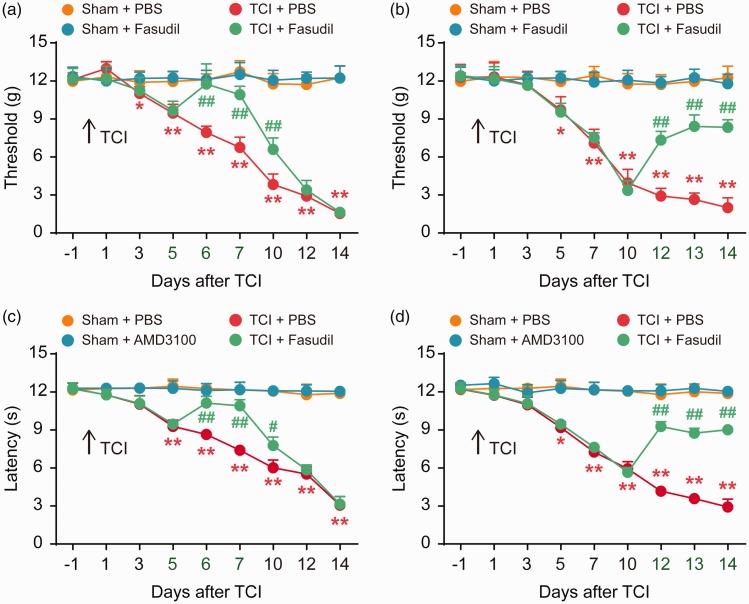
Our further study focused on the role of RhoA/ROCK2 in nociceptive hyperalgesia in rats with BCP. Fasudil is a specific inhibitor of ROCK2. The behavioral results showed that the continuous i.t. injection of Fasudil (20 μg/10 μL per i.t. injection, once daily) at the initial stage (five to seven days) significantly delayed the progression of mechanical allodynia and thermal hyperalgesia after TCI (Figure 4(a) and (c)). Fasudil (20 μg/10 μL per i.t. injection, once daily, from 12 to 14 days) reversed the established PWT and PWL at the late phase of TCI (Figure 4(b) and (d))
Figure 4.
Intrathecal (i.t.) injection of Fasudil attenuates BCP. Fasudil significantly delayed or attenuated TCI-induced mechanical allodynia (a and b) and thermal hyperalgesia (c and d). Fasudil (20 μg/10 μL) or PBS (10 μL) was i.t. administered once daily on days 5, 6, and 7 (a and c) or days 12, 13, and 14 (c and d) after TCI. Behavioral tests were performed 4 h after each injection. *P < 0.05, **P < 0.01 versus sham + PBS group; #P < 0.05 vs TCI + PBS group; ##P < 0.01 versus TCI + PBS group; n = 8 for each group. TCI: tumor cell implantation; PBS: phosphate-buffered saline.
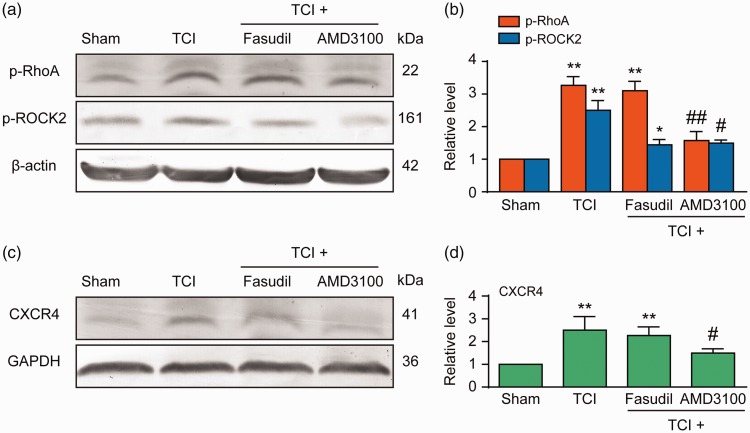
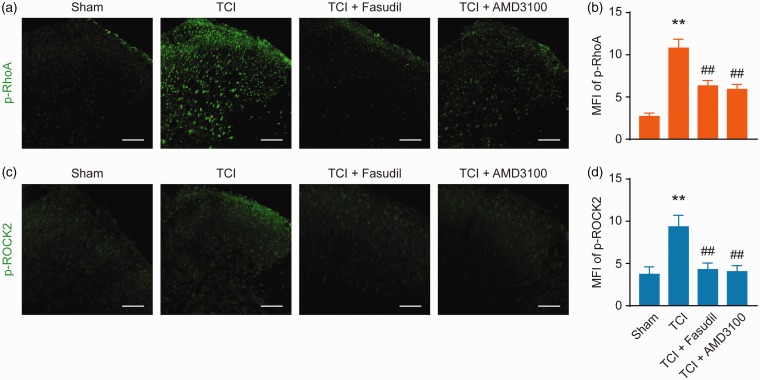
We continued to examine whether the RhoA/ROCK2 pathway is the downstream pathway of CXCR4 in spinal cord neurons. Western blot results showed that the administration of AMD3100 (5 μg/10 μL per i.t. injection, once every five to seven days) significantly attenuated the TCI-induced upregulation of p-ROCK2, p-RhoA, and CXCR4 expressions and that the administration of Fasudil (20 μg/10 μL per i.t. injection, once every five to seven days) significantly attenuated the upregulation of p-ROCK2. No changes were observed on p-RhoA and CXCR4 (Figure 5(a) and (b)). Similar results were obtained from the immunofluorescence experiment, where AMD3100 (5 μg/10 μL per i.t. injection, once every 12–14 days) or Fasudil (20 μg/10 μL per i.t. injection, once every 12–14 days; Figure 6) was administered to the BCP rats. These results clearly indicate that the RhoA/ROCK2 pathway is a functional downstream target of CXCR4 in spinal cord neurons under the condition of BCP.
Figure 5.
Intrathecal (i.t.) injection of Fasudil or AMD3100 attenuates TCI-induced upregulation of p-ROCK2, p-RhoA, and CXCR4 expressions (a and d). (a and c) Representative bands and (b and d) quantitative data. Intrathecal injection of Fasudil attenuates TCI-induced upregulation of p-ROCK2 expression, but not TCI-induced upregulation of p-RhoA and CXCR4. AMD3100 attenuates the TCI-induced upregulation of p-ROCK2, p-RhoA, and CXCR4 expressions. Western blot analysis showing the inhibitory effect of Fasudil and AMD3100 on TCI-induced overexpressions of p-ROCK2, p-RhoA, and CXCR4 on day 7 after TCI. Fasudil (20 μg/10 μL), AMD3100 (5 μg/10 μL), or PBS (10 μL) was i.t. administered once daily on days 5, 6, and 7 (a and b) after TCI. L4 to L6 spinal tissues were collected 12 h after the last injection. *P < 0.05 vs sham group; **P < 0.01 versus sham group; #P < 0.05,##P < 0.01 versus TCI group; n = 4 for each group. TCI: tumor cell implantation; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; CXCR4: chemokine C–X–C motif receptor 4.
Figure 6.
Intrathecal injection of AMD3100 or Fasudil attenuates TCI-induced upregulation of p-RhoA and p-ROCK2 expressions (a to d). Immunofluorescence analysis showing the inhibitory effect of AMD3100 on TCI-induced increase in fluorescence intensity of p-RhoA and p-ROCK2 on day 14 after TCI. Fasudil attenuates TCI-induced upregulation of p-RhoA and p-ROCK2 expression, AMD3100 (5 μg/10 μL) or Fasudil (20 μg/10 μL) was administered once daily from day 12 to 14. L4 to L6 spinal tissues were collected 12 h after the last injection.**P < 0.01 versus sham group; ##P < 0.01 versus TCI group; n = 6 to 10 slices from three rats. Original magnification: 200×; scale bar: 100 μm. TCI: tumor cell implantation; MFI: mean fluorescence intensity.
Intrathecal injection of CXCL12 induces RhoA/ROCK2 activation through CXCR4 in the spinal cord
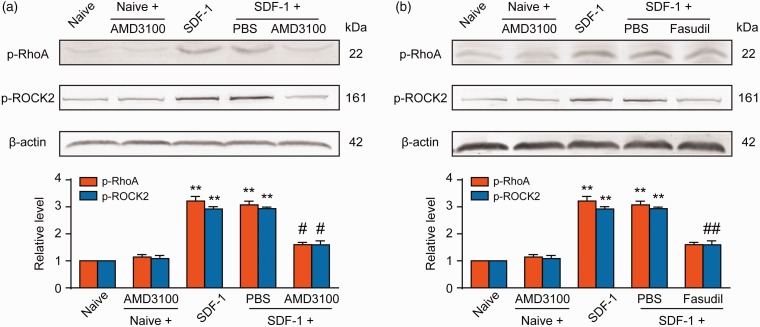
Our previous research shows that spinal injection of CXCL12 (SDF-1) triggers CXCR4-dependent hyperalgesia in naive rats.33 The decrease in PWT and PWL detected within 2 h after i.t. delivery lasted for at least 16 h and returned to basic pain threshold within 24 h. CXCL12 at 2 μg produced the most distinct hyperalgesia compared with lower doses at 0.5 and 1 μg.27 Therefore, we decided to examine the expressions of p-RhoA and p-ROCK2 at spinal cord level after i.t. injection of 2 μg of CXCL12 in rats and also to assess the explicit correlation between CXCR4 and RhoA/ROCK2. Western blot results showed that the expressions of p-RhoA and p-ROCK2 increased 8 h after a single injection of exogenous SDF-1 (2 μg). The high expressions of p-RhoA and p-ROCK2 induced by exogenous SDF-1 were significantly decreased by the injection of CXCR4-specific inhibitor AMD3100 (5 μg/10 μL) 30 min after the first injection (Figure 7(a)). In the subsequent experiments, we injected Fasudil (20 μg/10 μL) 30 min after SDF-1 injection, a specific inhibitor of ROCK2. The results showed that Fasudil (20 μg/10 μL) eliminated the upregulation of p-ROCK2 in the spinal cord induced by SDF-1 without affecting the expression of p-RhoA, indicating that CXCR4 mediates SDF-1-induced RhoA/ROCK2 activation in naive rats (Figure 7(b)).
Figure 7.
Exogenous SDF-1 induces spinal RhoA/ROCK2 phosphorylation through CXCR4 in naive rats. Intrathecal injection of exogenous SDF-1 (2 μg/10 μL) markedly upregulated the expressions of p-RhoA and p-ROCK2 in the spinal cord. Postadministration of CXCR4 inhibitor AMD3100 (10 μg/10 μL, a) or ROCK2 inhibitor Fasudil (20 μg/10 μL, b) 30 min after SDF-1 injection could notably suppress SDF-1-induced overexpressions of p-RhoA and p-ROCK2. L4 to L6 spinal tissues were collected 8 h after SDF-1 injection. **P < 0.01 versus naive group; #P < 0.05, ##P < 0.01 versus SDF-1 group; n = 4 for each group. PBS: phosphate-buffered saline; SDF-1: stromal-derived factor-1.
Discussion
BCP exists as a combination of background and breakthrough pain, with the latter either being related to events or occurring spontaneously without any obvious precipitating factor.34 Neuronal and glial cell sensitization associated with the activation of multiple signaling pathways at the spinal cord level has been proven to be the most important basic condition for the diversification of pain properties.35,36 Studies have indicated that chemokines in the dorsal horn of the spinal cord or dorsal root ganglion play an important role in the occurrence and development of BCP.30–32 Previous studies have shown that CXCL12/CXCR4 actively participates in nociception via direct stimulation of nociceptive neurons;37 therefore, increased CXCR4 in spinal neurons under BCP may change the nociceptive behavior.11 However, CXCR4 may have multiple neuronal mechanisms during the development and maintenance of BCP. In Li-Hua Hang’s study, i.t. injection of C3 exoenzyme (a RhoA inhibitor, 10 pg) significantly attenuated rat BCP behavior as well as the upregulation of spinal RhoA and ROCK2 protein levels. Its possible upstream pathway has not been further confirmed.38 Therefore, we hypothesized that the role of the RhoA/ROCK signaling pathway during the development and maintenance of BCP has a close relationship with CXCR4.
RhoA/ROCK2 signaling pathway involved in the regulation of pain hyperalgesia in BCP rats may be related to an activation of CXCR4
RhoA with a molecular size of 20 to 25 kD is one of the Rho family members.14 RhoA upregulation is commonly observed in patients with breast cancer, wherein it promotes the proliferation and metastasis of cancer cells.39 The RhoA/ROCK signaling pathway regulates breast cancer cell motility, and it may be involved in the development of BCP. ROCK is a major mediator of RhoA function, which contains ROCK1 and ROCK2 two subtypes. Experiments confirmed that ROCK1, but not ROCK2, acted on the phosphorylation of myosin regulatory light chain, and ROCK2 acted more on the phosphorylation of Cofilin.40 Cofilin is a low molecular-weight actin-modulating protein, which can inhibit its binding to actin after binding with inositol triphosphate (IP). Ample evidence show that a chemokine receptor activates Phospholipases C, catalyzes decomposition of PIP2 (PIP, hosphatidylinositol biphosphate), produces IP3, binds IP3 receptor, and releases intracellular Ca2+ from endoplasmic reticulum. Intracellular Ca2+ release is related to F-actin recombination. Cofilin can improve the stability of F-actin,41 which participates in the plasticity of synapses. Meanwhile, previous studies indicated that the RhoA/ROCK signaling pathway participates in the progression of inflammatory and neuropathic pain.15,16,24,42 Other study have shown that Slit2 and RhoA contribute to BCP by mediating synaptic plasticity,43 but the molecular mechanism is yet to be elucidated. In the present study, we established a rat model of BCP where pain hyperalgesia occurred after TCI, p-RhoA, and p-ROCK2 intensely expressed in spinal cord neurons, and the activation levels of CXCR4 increased. These results are consistent with those of previous studies that demonstrated a role of RhoA/ROCK2 in BCP.38 Intrathecal injection of Fasudil (a specific inhibitor of ROCK2) can prevent and alleviate pain in early and advanced stages of BCP and downregulate p-ROCK2 increment induced by TCI. Immunofluorescence showed that i.t. administration of Fasudil could downregulate the increment p-RhoA, which was different from that of Western blot. In our experiment, the p-RhoA protein began to overexpress in the spinal cord in a time dependent manner on the 5 days after TCI, and reached its peak on the 7 to 10 days, then decreased. In Western blot group, Fasudil was i.t. administered once daily on days 5, 6, and 7, while in immunofluorescence group, Fasudil was i.t on days 12, 13, and 14 .This should be the reason for the difference. Repeated i.t. injection of AMD3100 (CXCR4 inhibitor) could alleviate pain sensitization induced by TCI in rats with BCP and downregulate p-RhoA and p-ROCK2 increment induced by TCI. However, we found that i.t. injection of AMD3100 also decrease spinal CXCR4 expression. It may inhibit the activation of PI3K-AKT signaling pathway and Ras-Raf-Mek-MAPKp42/44 signaling pathway, may inhibit neuronal sensitization and activation of intracellular-related signal pathways, and may inhibit the synthesis of CXCR4 protein.9,33,44 Therefore, these findings suggest that CXCR4 can directly activate the RhoA/ROCK2 signaling pathway in bone cancer-evoked nociception and hyperalgesia. However, it is noteworthy that there is no definite evidence to confirm whether p-RhoA or p-ROCK2 is co-expressed with CXCR4 in the neurons in the spinal cord; hence, further study is warranted.
CXCL12 evokes pain hyperalgesia by sensitizing CXCR4-mediated RhoA/ROCK2 pathways in naive rats
CXCL12, also called stromal cell-derived factor 1 (SDF-1), belongs to the CXC subfamily of chemokines. CXCR4 is the receptor of CXCL12. It belongs to the GPCR of seven transmembranes. Emerging evidence has clarified that the CXCL12/CXCR4 signaling pathway is directly involved in many biological processes such as hematopoiesis, immune response, nerve growth, and organogenesis and plays an important role in tumors and some autoimmune diseases. Recent studies have found that chemokines are widely expressed in the nervous system and that they participate in pain signal transduction and regulation.45–47 A CXCL12/CXCR4 signal in the dorsal horn of the spinal cord is a carcinogenic factor in the bone tumor in rats with tibial metastasis,48 and it promotes formation of chronic pain in the neuro-inflammation state.45 Our previous research observed that i.t. delivery of CXCL12 in naive rats induced profound mechanical allodynia and thermal hyperalgesia, which was maintained for almost 24 h, whereas intraspinal application of AMD3100 for blocking CXCR4 significantly blunted CXCL12-induced pain behavior.11 Furthermore, we also confirmed that the MAPK pathway is the downstream molecular mechanism of CXCL12/CXCR4 in spinal glial cells. However, CXCR4 may have neuronal mechanisms underlying the nociceptive transduction that remain unclear. In various inflammatory and neuropathic pains, the RhoA/ROCK signaling pathway plays an important role, such as mediating p38 MAPK activation. The RhoA/ROCK signaling pathway may be a good candidate in this study. In support of this notion, we reproduced a model of hyperalgesia induced by the i.t. injection of exogenous SDF-1 (2 μg/10 μL) into naive rats. Western blot results showed increased expressions of p-RhoA and p-ROCK2 after a single injection of exogenous SDF-1 (Figure 7(a)). AMD3100 and Fasudil significantly inhibited CXCL12-induced p-ROCK2 upregulation. Therefore, these findings suggest that spinal CXCL12/CXCR4 is directly implicated in the modulation of nociceptive signaling by sensitizing the RhoA/ROCK pathways. These findings are the first to elucidate the role of CXCR4 in pain sensitization in spinal cord neurons through the activation of RhoA/ROCK2 in normal rats and BCP rats.
There are some limitations to this study. Only female rats were used in our study, the sex function on experiments is indeed worth considering. Of all cases of breast cancers, cases of men with breast cancer are rare, with the annual prevalence of <1%.49,50 Therefore, to closely mimic the human pathophysiology of breast cancer-induced pain, female rats are the most commonly used animal species for this BCP model with Walker 256 cells.51 Furthermore, whether androgen receptor can affect the downstream pathway of CXCR4 signal needs further study.
In conclusion, our results suggest that CXCR4 is an important link in the pathological process of BCP and an effective target for pain treatment. In addition, the RhoA/ROCK2 pathway in the spinal cord may be a key downstream target for CXCR4-mediated neuronal sensitization and hyperalgesia.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (81571066 to Wen Shen),the Key Project of the Natural Science Foundation of Jiangsu Education Department (16KJA320002 to Wen Shen), and the General Project of Social Development Program of Jiangsu Science and Technology Department (BE2015626 to Wen Shen).
ORCID iD
References
- 1.Lozano-Ondoua AN, Symons-Liguori AM, Vanderah TW. Cancer-induced bone pain: mechanisms and models. Neurosci Lett 2013; 557: 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 2006; 12: 6243s–6249s. [DOI] [PubMed] [Google Scholar]
- 3.Goblirsch MJ, Zwolak P, Clohisy DR. Advances in understanding bone cancer pain. J Cell Biochem 2005; 96: 682–688. [DOI] [PubMed] [Google Scholar]
- 4.Mantyh PW, Clohisy DR, Koltzenburg M, Hunt SP. Molecular mechanisms of cancer pain. Nat Rev Cancer 2002; 2: 201–209. [DOI] [PubMed] [Google Scholar]
- 5.Middlemiss T, Laird BJ, Fallon MT. Mechanisms of cancer-induced bone pain. Clin Oncol (R Coll Radiol) 2011; 23: 387–392. [DOI] [PubMed] [Google Scholar]
- 6.Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood 2006; 107: 1761–1767. [DOI] [PubMed] [Google Scholar]
- 7.Luo X, Tai WL, Sun L, Pan Z, Xia Z, Chung SK, Cheung CW. Crosstalk between astrocytic CXCL12 and microglial CXCR4 contributes to the development of neuropathic pain. Mol Pain 2016; 12: 174480691663638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang F, Sun W, Luo W-J, Yang Y, Yang F, Wang X-L, Chen J. SDF1-CXCR4 signaling contributes to the transition from acute to chronic pain state. Mol Neurobiol 2017; 54: 2763–2775. [DOI] [PubMed] [Google Scholar]
- 9.Bai L, Wang X, Li Z, Kong C, Zhao Y, Qian J-L, Kan Q, Zhang W, Xu J-T. Upregulation of chemokine CXCL12 in the dorsal root ganglia and spinal cord contributes to the development and maintenance of neuropathic pain following spared nerve injury in rats. Neurosci Bull 2016; 32: 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie F, Wang Y, Li X, Chao YC, Yue Y. Early repeated administration of CXCR4 antagonist AMD3100 dose-dependently improves neuropathic pain in rats after L5 spinal nerve ligation. Neurochem Res 2016; 41: 2289–2299. [DOI] [PubMed] [Google Scholar]
- 11.Shen W, Hu X-M, Liu Y-N, Han Y, Chen L-P, Wang C-C, Song C. CXCL12 in astrocytes contributes to bone cancer pain through CXCR4-mediated neuronal sensitization and glial activation in rat spinal cord. J Neuroinflammation 2014; 11: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu X-M, Zhang H, Xu H, Zhang H-L, Chen L-P, Cui W-Q, Yang W, Shen W. Chemokine receptor CXCR4 regulates CaMKII/CREB pathway in spinal neurons that underlies cancer-induced bone pain. Sci Rep 2017; 7: 4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thumkeo D, Watanabe S, Narumiya S. Physiological roles of rho and rho effectors in mammals. Eur J Cell Biol 2013; 92: 303–315. [DOI] [PubMed] [Google Scholar]
- 14.Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 1992; 70: 389–399. [DOI] [PubMed] [Google Scholar]
- 15.Tatsumi E, Yamanaka H, Kobayashi K, Yagi H, Sakagami M, Noguchi K. RhoA/ROCK pathway mediates p38 MAPK activation and morphological changes downstream of P2Y12/13 receptors in spinal microglia in neuropathic pain. Glia 2015; 63: 216–228. [DOI] [PubMed] [Google Scholar]
- 16.Wang C, Song S, Zhang Y, Ge Y, Fang X, Huang T, Du J, Gao J. Inhibition of the Rho/Rho kinase pathway prevents lipopolysaccharide-induced hyperalgesia and the release of TNF-α and IL-1β in the mouse spinal cord. Sci Rep 2015; 5: 14553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Julian L, Olson MF. Rho-associated coiled-coil containing kinases (ROCK): structure, regulation, and functions. Small GTPases 2014; 5: e29846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wendel C, Hemping-Bovenkerk A, Krasnyanska J, Mees ST, Kochetkova M, Stoeppeler S, Haier J. CXCR4/CXCL12 participate in extravasation of metastasizing breast cancer cells within the liver in a rat model. PLoS One 2012; 7: e30046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pasquier J, Abu-Kaoud N, Abdesselem H, Madani A, Hoarau-Véchot J, Thawadi HA, Vidal F, Couderc B, Favre G, Rafii A. SDF-1alpha concentration dependent modulation of RhoA and Rac1 modifies breast cancer and stromal cells interaction. BMC Cancer 2015; 15: 569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo J, Yu X, Gu J, Lin Z, Zhao G, Xu F, Lu C, Ge D. Regulation of CXCR4/AKT-signaling-induced cell invasion and tumor metastasis by RhoA, Rac-1, and Cdc42 in human esophageal cancer. Tumour Biol 2016; 37: 6371–6378. [DOI] [PubMed] [Google Scholar]
- 21.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16: 109–110. [DOI] [PubMed] [Google Scholar]
- 22.Mao-Ying QL, Zhao J, Dong ZQ, Wang J, Yu J, Yan MF, Zhang YQ, Wu GC, Wang YQ. A rat model of bone cancer pain induced by intra-tibia inoculation of Walker 256 mammary gland carcinoma cells. Biochem Biophys Res Commun 2006; 345: 1292–1298. [DOI] [PubMed] [Google Scholar]
- 23.Medhurst SJ, Walker K, Bowes M, Kidd BL, Glatt M, Muller M, Hattenberger M, Vaxelaire J, O’Reilly T, Wotherspoon G, Winter J, Green J, Urban L. A rat model of bone cancer pain. Pain 2002; 96: 129–140. [DOI] [PubMed] [Google Scholar]
- 24.Ohsawa M, Aasato M, Hayashi S-S, Kamei J. RhoA/Rho kinase pathway contributes to the pathogenesis of thermal hyperalgesia in diabetic mice. Pain 2011; 152: 114–122. [DOI] [PubMed] [Google Scholar]
- 25.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 26.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988; 32: 77–88. [DOI] [PubMed] [Google Scholar]
- 27.Ma J, Zhang S, Liu J, Liu F, Du F, Li M, Chen AT, Bao Y, Suh HW, Avery J, Deng G, Zhou Y, Wu P, Sheth K, Wang H, Zhou J. Targeted drug delivery to stroke via chemotactic recruitment of nanoparticles coated with membrane of engineered neural stem cells. Small 2019; 15: e1902011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu J, Zhu X, Wang W, Lu H, Zhang Z, Liu T, Xu H, Fu H, Ma S, Luo Y. 1, 6-di-O-caffeoyl-β-D-glucopyranoside, a natural compound from Callicarpa nudiflora Hook impairs P2Y and thromboxane A receptor-mediated amplification of platelet activation and aggregation. Phytomedicine 2017; 36: 273–282. [DOI] [PubMed] [Google Scholar]
- 29.Han Y, Wang X, Chen J, Sha S-H. Noise-induced cochlear F-actin depolymerization is mediated via ROCK2/p-ERM signaling. J Neurochem 2015; 133: 617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Ni H, Li H, Deng H, Xu LS, Xu S, Zhen Y, Shen H, Pan H, Yao M. Nuclear factor kappa B regulated monocyte chemoattractant protein-1/chemokine CC motif receptor-2 expressing in spinal cord contributes to the maintenance of cancer-induced bone pain in rats. Mol Pain 2018; 14: 1744806918788681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demir IE, Kujundzic K, Pfitzinger PL, Saricaoglu ÖC, Teller S, Kehl T, Reyes CM, Ertl LS, Miao Z, Schall TJ, Tieftrunk E, Haller B, Diakopoulos KN, Kurkowski MU, Lesina M, Krüger A, Algül H, Friess H, Ceyhan GO. Early pancreatic cancer lesions suppress pain through CXCL12-mediated chemoattraction of Schwann cells. Proc Natl Acad Sci USA 2017; 114: E85–E94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Old EA, Nadkarni S, Grist J, Gentry C, Bevan S, Kim K-W, Mogg AJ, Perretti M, Malcangio M. Monocytes expressing CX3CR1 orchestrate the development of vincristine-induced pain. J Clin Invest 2014; 124: 2023–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu X-M, Liu Y-N, Zhang H-L, Cao S-B, Zhang T, Chen L-P, Shen W. CXCL12/CXCR4 chemokine signaling in spinal glia induces pain hypersensitivity through MAPKs-mediated neuroinflammation in bone cancer rats. J Neurochem 2015; 132: 452–463. [DOI] [PubMed] [Google Scholar]
- 34.Laird BJA, Walley J, Murray GD, Clausen E, Colvin LA, Fallon MT. Characterization of cancer-induced bone pain: an exploratory study. Support Care Cancer 2011; 19: 1393–1401. [DOI] [PubMed] [Google Scholar]
- 35.Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nat Rev Neurosci 2001; 2: 83–91. [DOI] [PubMed] [Google Scholar]
- 36.Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci 2010; 11: 823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lazarini F, Tham TN, Casanova P, Arenzana-Seisdedos F, Dubois-Dalcq M. Role of the alpha-chemokine stromal cell-derived factor (SDF-1) in the developing and mature central nervous system. Glia 2003; 42: 139–148. [DOI] [PubMed] [Google Scholar]
- 38.Hang L-H, Shao D-H, Chen Z, Sun W-J. Spinal RhoA/Rho kinase signalling pathway may participate in the development of bone cancer pain. Basic Clin Pharmacol Toxicol 2013; 113: 87–91. [DOI] [PubMed] [Google Scholar]
- 39.Cáceres M, Guerrero J, Martínez J. Overexpression of RhoA-GTP induces activation of the epidermal growth factor receptor, dephosphorylation of focal adhesion kinase and increased motility in breast cancer cells. Exp Cell Res 2005; 309: 229–238. [DOI] [PubMed] [Google Scholar]
- 40.Peng Y, Chen Z, Chen Y. ROCK isoforms differentially modulate cancer cell motility by mechanosensing the substrate stiffness. Acta Biomater 2019; 88: 86–101. [DOI] [PubMed] [Google Scholar]
- 41.Aizawa H, Sutoh K, Yahara I. Overexpression of cofilin stimulates bundling of actin filaments, membrane ruffling, and cell movement in Dictyostelium. J Cell Biol 1996; 132: 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dill J, Patel AR, Yang X-L, Bachoo R, Powell CM, Li S. A molecular mechanism for ibuprofen-mediated RhoA inhibition in neurons. J Neurosci 2010; 30: 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ke C, Gao F, Tian X, Li C, Shi D, He W, Tian Y. Slit2/Robo1 mediation of synaptic plasticity contributes to bone cancer pain. Mol Neurobiol 2017; 54: 295–307. [DOI] [PubMed] [Google Scholar]
- 44.Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf DJ, Zhang J, Ratajczak J, Ratajczak MZ. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol 2004; 35: 233–245. [DOI] [PubMed] [Google Scholar]
- 45.Ji R-R, Xu Z-Z, Gao Y-J. Emerging targets in neuroinflammation driven chronic pain. Nat Rev Drug Discov 2014; 13: 533–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ji RR, Berta T, Nedergaard M. Glia and pain: is chronic pain a gliopathy? Pain 2013; 154: S10–S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacology Ther 2010; 126: 56–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi J, Wei Y, Xia J, Wang S, Wu J, Chen F, Huang G, Chen J. CXCL12-CXCR4 contributes to the implication of bone marrow in cancer metastasis. Future Oncol 2014; 10: 749–759. [DOI] [PubMed] [Google Scholar]
- 49.Fentiman IS, Fourquet A, Hortobagyi GN. Male breast cancer. Lancet 2006; 367: 595–604. [DOI] [PubMed] [Google Scholar]
- 50.Anderson WF, Jatoi I, Tse J, Rosenberg PS. Male breast cancer: a population-based comparison with female breast cancer. J Clin Oncol 2010; 28: 232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shenoy PA, Kuo A, Vetter I, Smith MT. The Walker 256 breast cancer cell-induced bone pain model in rats. Front Pharmacol 2016; 7: 286. [DOI] [PMC free article] [PubMed] [Google Scholar]