Abstract
In leather industries and tanneries, large amount of wastes has been disposed; which polluting water, soil, and atmosphere and causing serious human health problems. In particular, chemical dehairing process of leather industries produces fair amount of toxic wastes. It is, thus, urgently needed to use alternative processes free from pollution. As more than 90% of keratin is contained in feather, it is desirable to develop bioremediation process using keratinolytic microorganisms. In the present investigation, therefore, we first identified Bacillus cereus and Pseudomonas sp. to be able to produce keratinase. Then, the optimization was performed to maximize the keratinase activity with respect to cultivation temperature, pH, and incubation time. Moreover, the effects of metal ions and various substrates on keratinase activity were also investigated. The result indicates that keratinase activity became maximum at 50°C for both strains, whereas the optimal pH was 10.0 for B. cereus and 7.0 for Pseudomonas sp. The highest keratinase activity of 74.66 ± 1.52 U/mL was attained by B. cereus, whereas 57.66 ± 2.52 U/mL was attained by Pseudomonas sp. Enzymatic dehairing efficiency of leathers was also compared with chemical dehairing (Na2S and CaO), where complete dehairing was achieved by treating them with crude keratinase. Partial enzyme purification was performed by acetone precipitation. Batch cultivation of B. cereus using 1 L fermentor indicates a potential candidate for large-scale keratinase production. Thus, keratinase enzyme by degrading poultry wastes (feather) can be an alternative approach to chemical dehairing in leather industries, thus preventing environmental pollution through bioremediation.
Keywords: Bacillus cereus, Pseudomonas sp., keratinase, fermentation, leather dehairing, bioremediation
Introduction
Although leather industries and tanneries involve highly polluting processes, they offer diverse amenities for manufacturing capacity as well as export potential,1 which require several steps including dehairing, deliming, bating, degreasing, and finally pickling in a cascade manner for converting raw hide into long-lasting finished materials which are suitable for a variety of uses such as shoes, skirts, trousers, hats, jackets, belts, as well as book binding, furniture covering, and so on. Chemical dehairing process requires more than 75 chemicals including lime (CaO) and sulfide (Na2S/NaSH) to turn raw leathers into semi-fabricated leather,2 which gives around 90% of total pollution in leather industries and also produces noxious gases and solid wastes.3 Previous report estimated that nearly 850 kg out of 1000 solid wastes is generated during leather processing, and approximately 40 to 45 L of water is used per kilogram of raw hide/skin processing to get finished leathers.4 This causes many difficulties including availability of good water, treatment of effluent, and sulfide emissions during dehairing. Along with solid wastes, biochemical oxygen demand (BOD) and chemical oxygen demand (COD) as well as total dissolved solid give almost 70% of pollution from pre-tanning, tanning, and re-tanning processes.5 Thus, exposure of waste from tanneries not only pollutes water, soil, and air of respected area but also causes serious health difficulties including asthma, hepatic, dermatitis, various malignancies, and neurological disorders to the tannery workers as well as the people living nearby areas.
To overcome the above problems caused by chemicals and wastes in leather industry, keratinase shows promising nature due to significant advantages like reduced processing time, biodegradable action, better product quality, low energy input, lower cost, nontoxic and eco-friendly characteristics.6 Extracellular keratinase, a versatile and often used enzyme, can be an alternative means of chemical dehairing for minimizing the toxic effects of chemicals in dehairing process of leather industries. Keratinase (EC 3.4.21/24/99.11) is classified as protease7 and can be broadly used in several industries such as leather and detergent, textiles, medicine, cosmetics, fertilizers, waste bioconversion, as well as drug delivery8 by hydrolyzing keratins.
Keratin is an insoluble (in nature) and structural protein in epithelial cells of vertebrates and represents the major constituents of skin and its attachments such as hair, feathers, nail, wool, hooves, beaks, and stratum corneum,9 which have high mechanical stability and resistance to proteolytic degradation.10 Feather is constituted of 91% of keratin protein, 8% water, and 1% of lipid,11 and it is generated in large amounts as waste by-product in poultry processing industries, propagating millions of tons worldwide. As poultry feathers are rich in keratin protein (90%< of crude protein),12 and they are not easily broken down by usual proteolytic enzymes like trypsin, pepsin, and so on,13 keratin-rich feather waste degradation by keratinolytic microorganism is important, not only for removing feather wastes but also for providing the worthy protein, which can be used in animal feed, agriculture, and cosmetic industries as nutrient additives,14 as well as nitrogen fertilizer for organic farming.15 Keratin-degrading bacteria include the genus Bacillus16 and also gram-negative bacteria such as Pseudomonas species.17 Keratinase from Bacillus sp. (Bacillus licheniformis and Bacillus subtilis) have been extensively studied due to their effectiveness on feather degradation.18,19
As chemical treatment of keratin waste is regarded as an eco-destructive means,20 playing negative roles on environment, it is highly desired to consider the industrial application of keratinase.21 In the present investigation, therefore, environment friendly enzymatic dehairing by crude keratinase was considered in comparison with 2 traditionally used chemicals (CaO, Na2S). The reaction condition was optimized with respect to various operating parameters in shake-flask culture. Moreover, an attempt was made for partial purification of crude keratinase activity. In addition, fermentor-scale production of crude keratinase through batch culture with optimized condition was performed in 1 L fermentor to show the possible industrial applications.
Material and Methods
Sampling, isolation, and screening of keratinase-producing bacteria
A total of 50 soil samples were collected aseptically into sterile container from 15 different poultry farms of Chittagong city (Supplementary File 1) and preserved them into the refrigerator at 4°C until analysis.
Serial dilution (10−1-10−9) was conducted by using spread plate techniques,22 and then bacterial pure culture was isolated by streak plate method on nutrient agar media (pH 7.0). Selected pure isolates were then subcultured on their respective media, purified, and stored in the laboratory (−80°C) in glycerol stock (50%) solution for further studies.
Isolates were then cultured and incubated at 37°C for 48 hours on skim milk agar for primary screening,23 where clear zone–forming isolates were selected as proteolytic bacteria.24 Then the isolates were further identified as keratinolytic bacteria by using feather meal at 37°C for 24 to 48 hours according to Raju and Divakar.25
Phenotypic and biochemical characterization of keratinolytic bacteria
Selected keratinolytic bacteria were then characterized by gram staining based on cultural, morphological, as well as biochemical characteristics.26 For the activities of oxidase, catalase, citrate utilization, indole production, methyl-red (MR), Voges-Proskauer (VP), urease production, and carbohydrate utilization, isolates were biochemically analyzed27,28 and provisionally identified according to Bergey’s Manual of Systemic Bacteriology.29
Molecular identification of bacteria
Genomic DNA was extracted30 and stored at −20°C. DNA concentration was measured by Thermo Scientific, NanoDrop 2000 spectrophotometer (Thermo Scientific, USA) following standard protocol. Polymerase chain reaction (PCR) detection of bacteria was performed using previously published primers and targeted gene.31,32 Primer specificity was determined by searching for similar sequences in microbial genome using the Basic Local Alignment Search Tool (BLAST). In each experiment, positive and negative control was carried out. A total composition of target gene, primer sequences, cycling parameters, PCR master mixture, and amplicon size (bp) used for PCR amplifications in a thermal cycler (Nyx Technik) are shown in Table 1.
Table 1.
Target genes, primer sequences, cyclic condition, PCR master mixture composition, and amplicon size.
| Target gene | Primer sequence (5ʹ-3ʹ) | Cycling parameters | Total composition of PCR master mixture | Amplicon size (bp) | References |
|---|---|---|---|---|---|
| Common bacterial: 16S rDNA | 8F-AGAGTTTGATCCTGGCTCAG 850R-GACTACCAGGGTATCTAAT |
5 min at 95°C, 35 cycles of 95°C for 40 s, 57°C for 50 s, and 72°C for 1 min | For 10 μL: 5 μL master mix, 2 μL template, 1 μLa and 1 μLb, 1 μL water | 800 | Fouad et al31 and Mina et al32 |
Abbreviation: PCR, polymerase chain reaction.
Forward primer.
Reverse primer.
Amplified PCR products were then analyzed by electrophoresis (Micro-Bio-Tech Brand) in 1% (w/v) agarose gel in 1×TAE buffer, stained with ethidium bromide (1%)30 and compared with marker DNA (GeneRuler 1 kb DNA Ladder), during visualized under ultraviolet (UV) trans-illuminator (Benda company) and then photographed. Then PCR products were purified by ATPTM Gel/PCR Fragment DNA Extraction Kit (Catalog No. ADF100/ADF300).
Two (SJ, SA) biochemically identified29 bacterial isolates were sent for sequencing (Macrogen, South Korea). The results were then analyzed by BLAST program of National Center for Biotechnology Information (NCBI) (https://blast.ncbi.nlm.nih.gov)33 and submitted to GenBank by using Bankit submission tool. The phylogenetic tree was deduced by using the Neighbor-Joining method34 and this tree was drawn with the sum of branch length (0.30277880). Replicates (500) were used in the bootstrap test and have been shown to the branches.35 The branch lengths in the tree is drawn by the same units as those of the evolutionary distances used to infer the phylogenetic tree. The p-distance method was used to compute evolutionary distances.36 This study included 20 nucleotide sequences. Codon positions covered were first + second + third + noncoding. All ambiguous positions were removed for each sequence pair (pairwise deletion option). A total of 1476 positions were in the final data set. Evolutionary analyses of this study were conducted in MEGA X.37
Keratinase production in shake flask culture and keratinase assay
Identified two isolates (SJ and SA) were then transferred aseptically to sterile 50 mL of LB (Luria-Bertani) broth (pH 7.0 ± 0.2) and incubated overnight at 37°C in a rotary shaker incubator (HandyLab), at 150 r/min.
After that, a 250-mL Erlenmeyer flask containing 50 mL (w/v) sterile basal medium was prepared according to Williams et al38 and incubated with 1.5 mL of bacterial inoculum which previously cultured for 24 hours at 37°C and 150 r/min for 96 hours in a shaker incubator.
Keratinase activity was assayed according to Cai et al39 where 1.0 mL of crude enzyme properly diluted in Tris-HCl buffer (0.05 mol/L), followed by the incubation with 1.0 mL keratin solution prepared from chicken feathers40 at 50°C for 10 minutes. Then enzyme-substrate reaction was stopped by adding 2.0 mL of 0.4 mol/L trichloroacetic acid (TCA). After centrifugation at 4000 r/min for 30 minutes, the absorbance of the supernatant was determined at 280 nm by UV-Spectrophotometer (Shimadzu, Japan) compared with control that was prepared by incubating the enzyme solution with 2.0 mL TCA without the addition of keratin solution, where 1 unit (U/mL) of keratinase activity was defined as an increase of corrected absorbance of 280 nm (A280)41 with the control for 0.01 per minute under the assay conditions and calculated by the following equation:
where n is the dilution factor, 4 is the final reaction volume (4 mL), and 10 is the incubation time (minutes).
Optimization of culture conditions for maximum keratinase production
The keratinase production was optimized with respect to different culture conditions such as temperature, pH, agitation speed, incubation period, inoculum volume, substrates, C and N sources.24,42 The effects of media temperature, pH, and agitation speed (100-250 r/min) on the cell growth and keratinase activity were studied by examining bacterial growth at different temperatures such as 35°C, 37°C, 39°C, 40°C, and 43°C and various ranges of pH (6.5, 7.5, 8.5, 9.5, and 10.5), during 72, 84, and 96 hours of incubation. The culture extract was centrifuged at 4000 r/min for 30 minutes at 4°C, to be used to estimate keratinase production. To compare relative keratinase production, the control was also measured in each case.
To investigate the effect of inoculum volume on enzyme production, bacteria were cultured with 1%, 2%, 3%, 4%, and 5% of inoculum using different substrates such as chicken feather, human hair, and feather meal separately at previously optimized conditions.
Carbon sources (glucose, fructose, maltose, starch, and lactose) and nitrogen sources (peptone, tryptone, yeast extract, sodium molybdate, and ammonium sulfate) were used separately at 0.1% concentration to observe their effects on keratinase production at the optimized conditions. Controls were performed without addition of carbon and nitrogen sources to compare the production.
Partial characterization of crude keratinase activity (enzyme-substrate reaction)
Effects of various enzyme reaction conditions such as pH, temperature, reaction time, substrates, and metal ions were studied for optimum crude keratinase activity.24,43 The effect of pH on keratinase activity was determined at pH (4.0-11.0) by preparing substrate with acetate buffer (pH 4.0-5.5), phosphate buffer (pH 6.0-7.5), Tris-HCl buffer (pH 8.0-10.0), and sodium bicarbonate-sodium hydroxide (10.0-11.0). The keratinase activity was measured (λ = 280) using standard assay condition.
Besides, the enzymatic reaction was performed at different temperatures (30°C, 40°C, 50°C, 60°C, 70°C) in water bath and assayed (λ = 280) to find out the optimum temperature of the enzyme-substrate reaction.
Reaction mixtures were prepared with phosphate buffer (pH 6.0-7.5) and Tris-HCl buffer (pH 8.0-10.0), and enzymatic reaction was performed at different reaction times (10, 20, 30, 40, and 50 minutes) at the optimized temperature and pH. To study the substrates specificity, human scalp hair, casein, chicken feathers, and gelatin were used. The impact of enzyme inhibitor was also studied with different metal ions (Na+, K+, Co2+, Ca2+, Mg2+, Fe2+, Hg2+, Zn2+).
Protein estimation
Protein content of culture broth in shake-flask culture was assessed by Lowry method44 using BSA as standard during optimized culture conditions.
Dehairing assay (laboratory trial)
Fresh raw skins of domestic animal (cow) were collected from local slaughter houses of Dewanhat, Chittagong. To remove impurities, they were washed with sodium chloride and water, and then dried at hot air oven (50°C). The skins were then cut into small pieces (5 cm2), kept with 40 mL crude enzyme solution in a petri dish, and incubated at 37°C for 16 hours. Besides, chemical agents like Na2S and CaO were also used to compare dehairing efficiency. Skins treated with distilled water was considered as negative control.45 By gently scraping with blunt scalpel, skins were then withdrawn at regular intervals at 4, 8, 12, and 16 hours to examine better dehairing results.
Partial purification of crude enzyme
The growth extract from shake flasks was partially purified by acetone precipitation method, where keratinase was precipitated by prechilled acetone at 30%, 40%, 50%, 60%, 70%, and 80% fractionation. The acetone was added in ratio 3:1 to the cell free extract and incubated for 60 minutes at −20°C. The mixture was then subjected to centrifugation at 10 000 r/min for 10 minutes. After discarding the supernatant carefully, pellet was dissolved in Tris-acetate buffer (pH 7.0) and used for enzyme assay.43
Keratinase production by the fermentor
Batch cultivations were performed using 1 L of filter sterilized basal medium composed of the following components (per liter of final volume): 0.5 g NH4Cl, 0.5 g NaCl, 0.3 g K2HPO4, 0.4 g KH2PO4, 0.1 g MgCl2.6H2O, 0.1 g yeast extract, including 1.0 g raw chicken feather and made up to 1000 mL with distilled water38 in a 2-L fermenter (Hanil, South Korea) maintained at previously optimized conditions. The pH of the medium was maintained with a pH controller by automatic addition of 2.0 M HCl or 2.0 M NaOH. The aerobic condition was ascertained by controlling the stirring speed with the constant air flow rate of 1 L/min. Triplicate samples were taken after 96 hours to get maximum enzyme production.46
Statistical analysis
Triplicate experiments were done in all the cases during isolation, biochemical analysis, optimization of growth conditions, and partial characterization of keratinase enzyme by the selected isolates. P value of <.05 was considered as statistical significance. The results were expressed as the mean ± SD for triplicates. Data were captured into Microsoft Excel Software, version 2010, which was used to calculate means and SD.
Results and Discussion
Among 50 soil samples, 7 isolates (SA, SH(W), SH(Y), SJ, SK, SN, and SP) were found proteolytic on skim milk agar based on clear zone production (Figure 1A). Then, 2 of them were selected as best keratinolytic isolates (Figure 1B). This observation may be due to the fact that keratinase-producing bacteria can also produce one or more other specific bacterial proteases such as alcalase, pronase, and trypsin.47 This indicates that the isolates which are able to hydrolyze casein might have also the ability to synthesize proteolytic enzymes along with keratinase enzyme.
Figure 1.
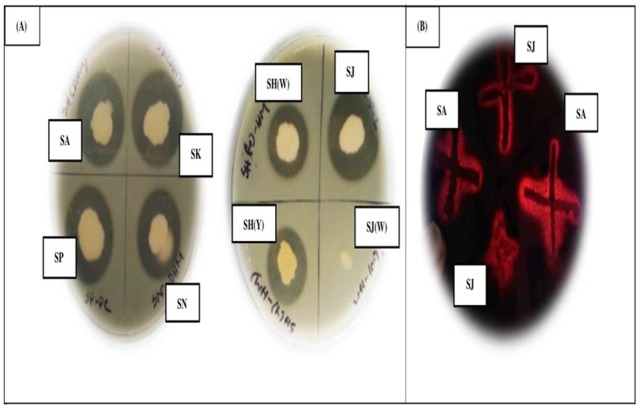
(A) Plate assays of 7 pure isolates. (B) Growth of isolates (SA, SJ) on FMA (Feather Meal Agar).
After two keratinolytic isolates were selected, they were subjected to different biochemical tests. One isolate was identified as gram positive by detecting the presence of peptidoglycan as a thick layer in that bacteria, whereas the other isolate was gram negative.48 Then based on biochemical characterizations, they were provisionally identified26,29 as Bacillus cereus and Pseudomonas sp. (Table 2).
Table 2.
Morphological and biochemical characterization of bacterial isolates.
| Morphological and biochemical characteristics | Bacterial isolate (SJ) | Bacterial isolate (SA) |
|---|---|---|
| Morphological characteristics | ||
| Gram staining | + | − |
| Shape | Rod | Rod |
| Spore formation | + | − |
| Motility | + | + |
| Biochemical test result | ||
| Oxidase | − | + |
| Catalase | + | + |
| Citrate utilization | + | + |
| Indole test | − | − |
| MR test | − | − |
| VP test | + | − |
| Urease production | + | − |
| Carbohydrate utilization | ||
| Glucose | + | + |
| Fructose | + | − |
| Sucrose | + | − |
| Lactose | − | − |
| Starch | + | + |
| Dextrose | + | − |
| Identified strain (provisionally) (Bergey et al29) | Bacillus cereus | Pseudomonas sp. |
Abbreviations: MR, methyl-red; VP, Voges-Proskauer.
+ and − indicates positive and negative result.
The 16S rDNA amplification was conducted through PCR for two keratinolytic49 and biochemically identified bacteria, where extracted genomic DNA showed positive bands in 1% agarose gel (~800 bp), compared with the DNA 1 kb marker (Figure 2). Homology analysis inferred from 16S rDNA sequence comparison clearly shows that the two strains clustered with B. cereus and Pseudomonas sp., respectively, have 100% identity in blast analysis. Finally, all the results were submitted to GenBank, where the accession numbers MH430931 and MH430929 were assigned for B. cereus and Pseudomonas sp., respectively. BLAST similarity and the phylogenetic analysis revealed that the SA and SJ isolates were confirmed to B. cereus and Pseudomonas sp., respectively (Figure 3).
Figure 2.

Electrophoretic (1% agarose) separation of 16S rDNA gene of different isolates. M indicates 1 kb DNA marker; L1-L2, L3-L4 show bands of SJ and SA, respectively; P1, P2 for positive control (SJ and SA); and N for negative control, respectively.
Figure 3.
Phylogenetic tree construction. Phylogenetic tree showing relationship between  Bacillus cereus strain AIMST 4. Ca.8 and
Bacillus cereus strain AIMST 4. Ca.8 and  Pseudomonas sp. B7 16S with other selected members of B. cereus and Pseudomonas sp.
Pseudomonas sp. B7 16S with other selected members of B. cereus and Pseudomonas sp.
The optimal growth rates of B. cereus and Pseudomonas sp. were attained at mesophilic temperatures such as 39°C and 37°C, respectively, as expected from other studies on B. subtilis and Bacillus pumilis.50 The medium pH affects the reaction mixture and assists in transporting nutrients across the cell membrane of bacteria.24 Both keratinolytic isolates exhibited optimal keratinase production at alkaline condition (pH 9.50) in our study. This propensity results from the deamination of peptides and amino acid during degradation of keratin leading to the release of ammonium and thus increases the pH. Gupta and Ramnani13 also reported that alkaline pH from 6.0 to 9.0 supports keratinase production in most microorganisms.
In general, higher agitation rates (200-250 r/min) provide good growth of bacteria with possibly low keratinase production due to high dissolved oxygen concentration. On the other hand, Pissuwan and Suntornsuk51 reported that, at low agitation rate (100 r/min), substrates and bacterial cells were not mixed properly and produced heterogeneous formation, thus lowering dissolved oxygen and leading to lower keratinase production. In our study, both isolates produced maximum keratinase (57.33 ± 2.08) U/mL for B. cereus and (38.00 ± 3.00) U/mL for Pseudomonas sp. at 150 r/min.
Although feather degradation can be completely made between 3 to 5 days, it also takes more than 5 days to be degraded. Agrahari and Wadhwa52 as well as Yadav et al53 reported the maximum keratinase production on days 5 and 7 by Bacillus thurengenesis SN2 and Bacillus strain SAA5, respectively. In our study, B. cereus showed the highest keratinase production (60.00 ± 1.00 U/mL) by using chicken feathers at 96 hours (4 days) of incubation, whereas Pseudomonas sp. showed (44.00 ± 1.00 U/mL) at 72 hours (3 days) of incubation.
Several inoculum volumes were also tested, where 3% of inoculum volume was found responsible for the highest keratinase production for both B. cereus and Pseudomonas sp. as also shown by Sivakumar et al.42 As the inoculum volume was further increased, the production of enzyme was gradually decreased which may be due to the rapid growth of bacteria and depletion of essential nutrients by bacteria in the early stages of growth.24 Bacillus cereus and Pseudomonas sp. produced about 2-fold higher keratinase in feather meal compared with raw feather, and human hair was used as substrate due to its inducible nature.54
Carbon and nitrogen sources also affect keratinase production, where B cereus completely hydrolyzed soluble starch, as also shown by Sivakumar et al,42 whereas maltose was totally hydrolyzed by Pseudomonas sp.55 On the other hand, lactose played a suppressive role for keratinase production. In our investigation, we found that yeast extract elevated the keratinase production for both isolates,56 whereas ammonium sulfate played an inhibitory role. After optimization, it has been shown that additional carbon and nitrogen sources have no stimulatory effect on keratinase production.
Overall optimized conditions for maximum keratinase production by B cereus and Pseudomonas sp. are represented in Table 3.
Table 3.
Optimization of culture conditions for production of extracellular keratinase from Bacillus cereus and Pseudomonas sp. in shake-flask cultivation.
| Culture conditions |
Bacillus cereus
|
Pseudomonas sp. |
Culture conditions |
Bacillus cereus
|
Pseudomonas sp. |
||||
|---|---|---|---|---|---|---|---|---|---|
| OD (280 nm) | Enzyme activity (U/mL) | OD (280 nm) | Enzyme activity (U/mL) | OD (280 nm) | Enzyme activity (U/mL) | OD (280 nm) | Enzyme activity (U/mL) | ||
| Incubation temperature (°C) | pH | ||||||||
| 35 | 1.80 | 36.00 ± 1.00 | 1.50 | 30.00 ± 2.00 | 6.5 | 1.70 | 34.00 ± 2.64 | 1.67 | 23.33 ± 3.51 |
| 37 | 2.10 | 42.00 ± 2.64 | 2.05 | 41.00 ± 3.60a | 7.5 | 2.03 | 40.67 ± 1.15 | 1.53 | 30.67 ± 2.51 |
| 39 | 2.85 | 57.00 ± 2.00a | 1.80 | 36.00 ± 1.00 | 8.5 | 2.62 | 52.33 ± 1.15 | 1.70 | 34.00 ± 1.00 |
| 41 | 2.63 | 52.66 ± 2.08 | 1.48 | 29.66 ± 1.53 | 9.5 | 2.82 | 56.33 ± 1.52a | 2.17 | 43.33 ± 1.52a |
| 43 | 2.23 | 44.66 ± 1.53 | 1.10 | 22.00 ± 2.65 | 10.5 | 2.33 | 46.67 ± 4.16 | 1.83 | 36.67 ± 1.52 |
| Inoculum volume (%, mL) | Incubation period (h) | ||||||||
| 1.00 | 1.82 | 36.33 ± 1.52 | 1.05 | 21.00 ± 1.00 | 24 | 0.80 | 16.00 ± 1.00 | 0.35 | 07.00 ± 2.00 |
| 2.00 | 2.18 | 43.67 ± 1.52 | 1.50 | 30.00 ± 2.00 | 48 | 1.35 | 27.00 ± 2.00 | 0.88 | 17.67 ± 2.51 |
| 3.00 | 2.78 | 55.67 ± 2.08a | 1.95 | 39.00 ± 1.00a | 72 | 1.88 | 37.67 ± 2.51 | 2.20 | 44.00 ± 1.00a |
| 4.00 | 2.55 | 51.00 ± 1.00 | 1.25 | 25.00 ± 2.00 | 96 | 3.01 | 60.00 ± 1.00a | 1.63 | 32.67 ± 1.52 |
| 5.00 | 1.98 | 39.67 ± 1.52 | 0.90 | 18.00 ± 1.00 | 120 | 2.07 | 41.33 ± 1.52 | 1.45 | 29.00 ± 1.00 |
| Substrates | Agitation speed (r/min) | ||||||||
| Human hair | 1.12 | 22.33 ± 2.51 | 1.00 | 20.00 ± 1.00 | 100 | 2.42 | 48.33 ± 3.51 | 1.49 | 29.83 ± 3.40 |
| Feather meal | 2.73 | 54.67 ± 1.52a | 2.07 | 41.33 ± 1.52a | 150 | 2.87 | 57.33 ± 2.08a | 1.90 | 38.00 ± 3.00a |
| Raw feather | 2.15 | 43.00 ± 2.00 | 1.65 | 33.00 ± 2.00 | 200 | 1.20 | 39.33 ± 2.36 | 1.62 | 32.33 ± 2.51 |
| 250 | 1.69 | 33.83 ± 3.01 | 1.15 | 23.00 ± 3.00 | |||||
| Carbon sources | Nitrogen sources | ||||||||
| Glucose | 1.58 | 31.67 ± 1.52 | 0.88 | 17.67 ± 3.05 | Peptone | 2.17 | 43.33 ± 1.52 | 1.02 | 20.33 ± 1.52 |
| Fructose | 1.80 | 36.00 ± 1.00 | 1.25 | 25.00 ± 2.00 | Tryptone | 2.45 | 49.00 ± 2.00 | 1.80 | 36.00 ± 1.00 |
| Maltose | 2.17 | 43.33 ± 1.52 | 1.82 | 36.33 ± 2.30a | Yeast extract | 2.80 | 56.00 ± 1.00a | 2.10 | 42.00 ± 2.00a |
| Starch | 2.80 | 56.00 ± 2.00a | 1.50 | 30.00 ± 2.00 | Sodium molybdate | 1.75 | 35.00 ± 2.00 | 1.30 | 26.00 ± 1.00 |
| Lactose | 1.35 | 27.00 ± 2.00 | 1.00 | 20.00 ± 1.00 | Ammonium sulfate | 1.35 | 27.00 ± 2.00 | 0.80 | 16.00 ± 1.00 |
All conditions were conducted on basal medium; results are expressed as mean ± SD for triplicate.
Best result.
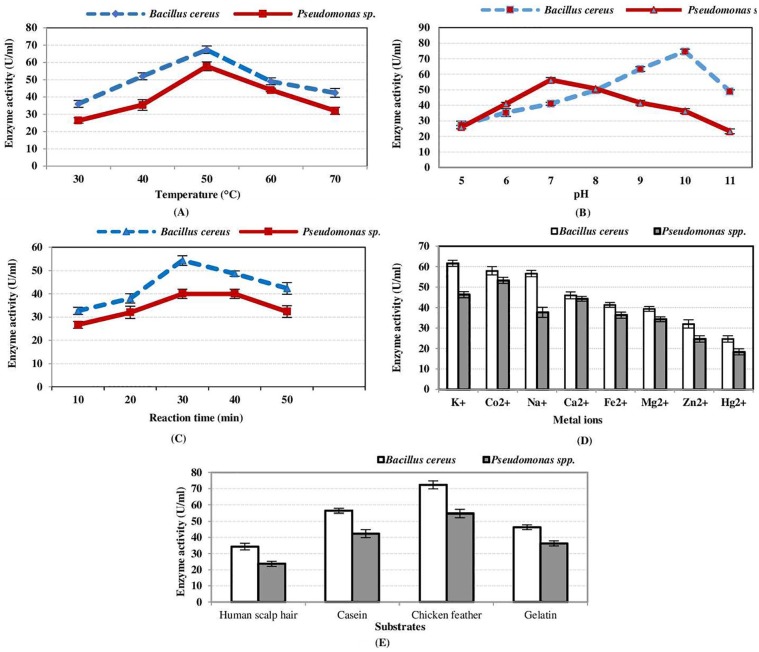
Figure 4 shows the effects of culture condition on the keratinase activity, where Figure 4A indicates that the optimal temperature of keratinase from B. cereus and Pseudomonas sp. is 50°C, as most keratinase shows thermoactive nature in the range of 50°C to 60°C. It has been reported that B cereus LAU shows the highest keratinase activity at 50°C and pH 7.0,10,43 whereas the maximum keratinase activity found for Microbacterium sp. at 55°C and pH 7.0.19 The elevated keratinase activity was attained at pH 10.0 and 7.00 for B cereus and Pseudomonas sp., respectively, in our study (Figure 4B).
Figure 4.
Partial characterization of crude keratinase activity (enzyme-substrate reaction). Effects of (A) temperature, (B) pH, (C) reaction time, (D) metal ions, and (E) substrates.
The effects of reaction time (10-50 minutes) on extracellular keratinase enzyme activity shows that the highest activity was attained at 30 minutes (Figure 4C) for both species as Gupta et al,57 stated that incubation at 70°C for 30 minutes enhanced keratinase activity (21%) of B. subtilis RSE163.
The effects of metal ions (Na+, K+, Co2+, Ca2+, Mg2+, Fe2+, Hg2+, and Zn2+) were also studied (Figure 4D) by preincubating them with enzymes. Inhibitory effects of Hg2+ and Zn2+ were reported by Kainoor and Naik56 as well as Lin and Yin58 in case of Bacillus sp. JB 99 and B. licheniformis YJ4, and their results are coincident with our study.
Hydrolysis of complex protein substrate responds to keratinase activity efficiently, which had a broad spectrum of substrate specificity for soluble and insoluble substrates. Practically, keratinase can hydrolyze feather keratin with maximum activity, followed by casein, gelatin, and human scalp hair (Figure 4E) in our study. Similar findings were obtained by Gupta et al57 and Rajput et al,59 where the highest keratinase activity was found on chicken feather substrate by both B. subtilis RSE163 and B. pumilis KS12.
Crude proteins were estimated approximately to be 0.73 and 0.57 mg/mL (Figure 5) from keratinases of B cereus and Pseudomonas sp., respectively, compared with standard concentration of BSA (Table 4) in this study. Deivasigamani and Alagappan60 reported that crude protein was found around 1.44 mg/mL from keratinase-producing microorganisms, previously.
Figure 5.

Standard graph of Folin-Lowry assay.
Table 4.
Folin-Lowry standard assay for BSA.
| Concentration of BSA (mg/mL) | Reading 1 (A660) | Reading 2 (A660) | Reading 3 (A660) | Average (A660) |
|---|---|---|---|---|
| 0.0 | 0.000 | 0.000 | 0.000 | 0.000 ± 0.000 |
| 0.2 | 0.524 | 0.526 | 0.522 | 0.524 ± 0.002 |
| 0.4 | 0.900 | 0.904 | 0.903 | 0.902 ± 0.002 |
| 0.6 | 1.150 | 1.200 | 1.040 | 1.130 ± 0.081 |
| 0.8 | 1.550 | 1.630 | 1.600 | 1.593 ± 0.040 |
Abbreviation: BSA: bovine serum albumin.
Data represent mean ± SD for triplicate.
To study the chicken feather degradation rate (%), 96 hours of incubation was performed for B. cereus at optimized conditions, whereas pH and temperature were 9.5 and 39°C in basal medium (Supplementary File 2), where the maximum degradation was found (80% ± 0.025%) at 96 hours (Table 5).
Table 5.
Visual observation of chicken feather degradation (%) by Bacillus cereus in optimized conditions.
| Name of isolate | Incubation period, h | Visual degradation rate, % |
|---|---|---|
| SJ | 24 | (20 ± 0.015) |
| 48 | (30 ± 0.026) | |
| 72 | (50 ± 0.029) | |
| 96 | (80 ± 0.025)a |
Data represent mean ± SD for triplicate.
Best result.
An attempt was made to observe the effectiveness of produced keratinase enzyme over chemicals in dehairing process, as chemicals cause damage and reduce the quality of leather. Leather pieces were soaked with enzymes and chemicals (Na2S, CaO), separately for 16 hours in total. After 12 hours, 100% dehairing with enzyme showed effectiveness over chemicals without any damaging of leather (Figure 6). After 16 hours, Na2S caused damage to leather, while dehairing remain incomplete by CaO. Moreover, pulp of hair exhibited rough texture when chemical treatment was conducted (Supplementary File 3). In this study, the crude keratinase from B. cereus showed the best hide dehairing at 12 hours to dehair the hides, which is comparatively better than previous reports for B. cereus,61 Pseudomonas stutzeri K4,62 B cereus AT,49 Pseudomonas aeroginosa MCM B-327,63 and B. safenis64 which required 24, 20, 18, 16 to 21, and 16 hours, respectively, for total dehairing process.
Figure 6.
Comparative study of dehairing assay (field trial).
Partial purification of crude keratinase contained in the cell free supernatant by acetone precipitation eliminates interfering elements present in that supernatant. Although acetone is a good purifying agent for proteins, due to denaturing tendency, it is not commonly used in purification procedures. Maximum specific activity was observed for B. cereus and Pseudomonas sp. at 70% and 50% saturation level with ~1.35 and ~1.67 times purification fold, respectively (Table 6), which was different from that reported by Thangam and Rajkumar.65
Table 6.
Purification fold of enzymes at different saturation levels of acetone precipitation.
| Purification steps (saturation level of acetone) | Total volume, (mL) | Total enzyme activity, (U) | Total protein concentration, (mg) | Specific activity, (U/mg) | Purification (fold) |
|---|---|---|---|---|---|
| Bacillus cereus | |||||
| Crude enzyme | 100 | 6000 | 73 | 82.19 | 1.00 |
| 30% | 80 | 6800 | 72 | 94.44 | 1.14 |
| 50% | 50 | 6500 | 60 | 108.3 | 1.32 |
| 70% | 30 | 7320 | 66 | 111.0a | 1.35a |
| 80% | 10 | 1700 | 19.3 | 88.0 | 1.07 |
| Pseudomonas sp. | |||||
| Crude enzyme | 100 | 4400 | 57 | 77.19 | 1.00 |
| 30% | 80 | 5840 | 56 | 104.3 | 1.35 |
| 50% | 50 | 7100 | 55 | 129.0a | 1.67a |
| 70% | 30 | 4650 | 45 | 103.3 | 1.34 |
| 80% | 10 | 1020 | 13 | 78.46 | 1.02 |
Best result.
In addition to flask culture, large-scale keratinase production was studied by batch cultivation, while it was found (136.0 ± 0.030) U/mL after 4 days (80 hours) of incubation, 2-fold more than in shake flask culture (60 ± 1.0) U/mL (Table 7).
Table 7.
Data from fermentor for keratinase production by Bacillus cereus.
| Days | Time (hours) | OD (280 nm) | pH | Temperature (°C) | r/min | DO | Enzyme activity (U/mL) |
|---|---|---|---|---|---|---|---|
| 1 | 0 | 0.00 | 9.50 | 0.000 ± 0.000 | |||
| 4 | 0.14 | 9.50 | 2.800 ± 0.010 | ||||
| 8 | 0.75 | 9.50 | 39 | 150 | 25 | 15.00 ± 0.002 | |
| 16 | 1.75 | 9.50 | 35.00 ± 0.049 | ||||
| 24 | 2.30 | 9.50 | 46.00 ± 0.050 | ||||
| 2 | 32 | 3.80 | 9.50 | 76.00 ± 0.041 | |||
| 40 | 4.50 | 9.50 | 39 | 150 | 25 | 90.00 ± 0.068 | |
| 48 | 5.00 | 9.50 | 100.0 ± 0.071 | ||||
| 3 | 56 | 5.80 | 9.50 | 116.0 ± 0.002 | |||
| 64 | 6.00 | 9.50 | 39 | 150 | 25 | 120.0 ± 0.010 | |
| 72 | 6.50 | 9.50 | 130.0 ± 0.049 | ||||
| 4 | 80 | 6.80 | 9.50 | 136.0 ± 0.030a | |||
| 88 | 3.50 | 9.50 | 39 | 150 | 25 | 70.00 ± 0.048 | |
| 96 | 1.87 | 9.50 | 37.40 ± 0.075 |
All conditions were conducted on basal medium.
Abbreviation: DO-Dissolved oxygen
Highest production.
Conclusions
In the present work, it was shown that the keratinase produced from B. cereus can be a prominent leather dehairing agent that exhibited no detrimental effect on leather, whereas chemicals caused noxious effects. Thus, proposed poultry waste–degrading keratinase enzyme provides not only better dehairing but also better leather quality over chemicals. Moreover, enzymatic dehairing minimizes the dependence on harmful chemicals (sulfide, lime and amines) usually used in tanneries of Bangladesh, to save human health and wild life by diminishing water, soil, and overall environment pollution.
Supplemental Material
Supplemental material, Supplementary_file_1_xyz33416b0980c9e for Microbial Bioremediation of Feather Waste for Keratinase Production: An Outstanding Solution for Leather Dehairing in Tanneries by Mursheda Akhter, Lolo Wal Marzan, Yasmin Akter and Kazuyuki Shimizu in Microbiology Insights
Supplemental material, Supplementary_file_2_xyz3341651296bf9 for Microbial Bioremediation of Feather Waste for Keratinase Production: An Outstanding Solution for Leather Dehairing in Tanneries by Mursheda Akhter, Lolo Wal Marzan, Yasmin Akter and Kazuyuki Shimizu in Microbiology Insights
Supplemental material, Supplementary_file_3_xyz334166ba60c36 for Microbial Bioremediation of Feather Waste for Keratinase Production: An Outstanding Solution for Leather Dehairing in Tanneries by Mursheda Akhter, Lolo Wal Marzan, Yasmin Akter and Kazuyuki Shimizu in Microbiology Insights
Acknowledgments
The authors are pleased to mention about the fruitful discussions with Md. Mahbub Hasan, Assistant Professor of Department of Genetic Engineering and Biotechnology, University of Chittagong.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was done in the “Molecular Biology Laboratory” of the Department of Genetic Engineering and Biotechnology, University of Chittagong, Bangladesh and partially funded by the Research & Publication Office of the University of Chittagong (Ref. 6016/2017; fiscal year 2016-2017).
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: LWM and YA contributed to the conceptualization, acquired funding, and assisted in the supervision; MA performed formal analysis and investigation, and wrote the original draft of the manuscript; LWM, YA, and KS wrote, reviewed, and edited the manuscript; and all authors approved the final draft of the manuscript.
ORCID iD: Lolo Wal Marzan  https://orcid.org/0000-0002-7426-9841
https://orcid.org/0000-0002-7426-9841
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Luthra YK. Indian leather industry. Leather Age, March, 2006:69-71. [Google Scholar]
- 2. Bondrea DA, Mocanu R. Recovery of trivalent chromium from the waste water used for tanning of hides. Paper presented at: 5th International Conference on Thermal Equipment, Renewable Energy and Rural Development, TE-RE-RD; June 2–4, 2016:472-476; Golden Sands, Bulgaria. [Google Scholar]
- 3. Thanikaivelan P, Rao JR, Nair BU, Ramasami T. Progress and recent trends in biotechnological methods for leather processing. Trends Biotechnol. 2004;22:181-188. [DOI] [PubMed] [Google Scholar]
- 4. Sundar VJ, Ramesh R, Rao PS, et al. Water management in leather industry. J Sci Ind Res. 2001;60:443-450. [Google Scholar]
- 5. Hassen AS, Woldeamanuale TB. Evaluation and characterization of tannery waste water in each process at batu and modjo tannery, Ethiopia. Int J Rural Dev Environ Health Res. 2017;1:17-26. [Google Scholar]
- 6. Singh R, Kumar M, Mittal A, Mehta PK. Microbial enzymes: industrial progress in 21st century. 3 Biotech. 2016;6:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sahoo DK, Thatoi HN, Mitra B, et al. Advances in microbial keratinase and its potential applications. In: Patra J, Vishnuprasad C, Das G, eds. Microbial Biotechnology. Singapore: Springer; 2017:105-133. [Google Scholar]
- 8. Machado JR, Severo EE, de Oliveira JM, et al. One-step ultrafiltration process for separation and purification of a keratinolytic protease produced with feather meal. Int J Chem Eng. 2018;2018:6729490. [Google Scholar]
- 9. Adelere IA, Lateef A. Degradation of keratin biomass by different microorganisms. In: Sharma S, Kumar A, eds. Keratin as a Protein Biopolymer. Cham: Springer; 2019:123-162. [Google Scholar]
- 10. Lateef A, Oloke JK, Kana EG, et al. Keratinolytic activities of a new feather-degrading isolate of Bacillus cereus LAU 08 isolated from Nigerian soil. Int Biodeterior Biodegradation. 2010;64:162-165. [Google Scholar]
- 11. Chittturi CMCK, Peddu J, Lakshmi VV. Microbial keratinases and their applications. Int J Sci Eng Res. 2015;6:173-176. [Google Scholar]
- 12. Jin HS, Park SY, Kim K, et al. Development of a keratinase activity assay using recombinant chicken feather keratin substrates. PLoS ONE. 2017;12:e0172712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gupta R, Ramnani v. Microbial keratinases and their prospective applications: an overview. Appl Microbiol Biotechnol. 2006;70:21-33. [DOI] [PubMed] [Google Scholar]
- 14. Bhange K, Chaturvedi V, Bhatt R. Ameliorating effects of chicken feathers in plant growth promotion activity by a keratinolytic strain of Bacillus subtilis PF1. Bioresour Bioprocess. 2016;3:13. [Google Scholar]
- 15. Nagarajan S, Eswaran P, Masilamani RP, Natarajan H. Chicken feather compost to promote the plant growth activity by using keratinolytic bacteria. Waste Biomass Valori. 2018;9:531-538. [Google Scholar]
- 16. Burtt JEH, Ichida JM. Keratinase produced by Bacillus licheniformis. United States Patent US 5,877,000, 1999. [Google Scholar]
- 17. Tork S, Aly MM, Nawar L. Biochemical and molecular characterization of a new local keratinase producing Pseudomonas sp., MS21. Asian J Biotechnol. 2010;2:1-3. [Google Scholar]
- 18. Manczinger L, Rozs M, Vágvölgyi C, Kevei F. Isolation and characterization of a new keratinolytic Bacillus licheniformis strain. World J Microbiol Biotechnol. 2003;19:35-39. [Google Scholar]
- 19. Thys RC, Lucas FS, Riffel A, Heeb P, Brandelli A. Characterization of a protease of a feather degrading Microbacterium species. Lett Appl Microbiol. 2004;39:181-186. [DOI] [PubMed] [Google Scholar]
- 20. Ghaffar I, Imtiaz A, Hussain A, et al. Microbial production and industrial applications of keratinases: an overview. Int Microbiol. 2018;21:163-174. [DOI] [PubMed] [Google Scholar]
- 21. Gregg R. From feathers to degradable plastic. BRI: end of mad cow’s disease. Triangle Tech J. http://www.triangletechjournal.com. Published 2002.
- 22. Veenayohini K, Sangeetha D. Isolation and identification of keratinolytic bacteria from poultry waste and assessment of its keratinase activity on chicken feathers. Int J Appl Res. 2016;2:396-402. [Google Scholar]
- 23. Allpress JD, Mountain G, Gowland PC. Production, purification and characterization of an extracellular keratinase from Lysobacter NCIMB 9497. Lett Appl Microbiol. 2002;34:337-342. [DOI] [PubMed] [Google Scholar]
- 24. Barman NC, Zohora FT, Das KC, et al. Production, partial optimization and characterization of keratinase enzyme by Arthrobacter sp. NFH5 isolated from soil samples. AMB Express. 2017;7:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Raju EV, Divakar G. Screening and isolation of keratinase producing bacteria from poultry waste. Int J. 2013;2:70-74. [Google Scholar]
- 26. Cowan ST. Cowan and Steel’s Manual for the Identification of Medical Bacteria. 2nd ed. Cambridge, England: Cambridge University Press; 1974:67-83. [Google Scholar]
- 27. Marzan LW, Hossain M, Mina SA, et al. Isolation and biochemical characterization of heavy-metal resistant bacteria from tannery effluent in Chittagong city, Bangladesh: bioremediation viewpoint. Egypt J Aquat Res. 2017;43:65-74. [Google Scholar]
- 28. Hasan MM, Marzan LW, Hosna A, Hakim A, Azad AK. Optimization of some fermentation conditions for the production of extracellular amylases by using Chryseobacterium and Bacillus isolates from organic kitchen wastes. J Genet Eng Biotechnol. 2017;15:59-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bergey DH, Buchanan RE, Gibbons NE. Bergey’s Manual of Determinative Bacteriology. Baltimore, MD: Williams & Wilkins Co.; 1974. [Google Scholar]
- 30. Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. New York, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 31. Fouad AF, Barry J, Caimano M, et al. PCR-based identification of bacteria associated with endodontic infections. J Clin Microbiol. 2002;40:3223-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mina SA, Marzan LW, Sultana T, Akter Y. Quality assessment of commercially supplied drinking jar water in Chittagong City, Bangladesh. Appl Water Sci. 2018;8:24. [Google Scholar]
- 33. https://blast.ncbi.nlm.nih.gov
- 34. Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406-425. [DOI] [PubMed] [Google Scholar]
- 35. Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783-791. [DOI] [PubMed] [Google Scholar]
- 36. Nei M, Kumar S. Molecular Evolution and Phylogenetics. New York, NY: Oxford University Press; 2000. [Google Scholar]
- 37. Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Williams CM, Richter CS, Mackenzie JM, Shih JC. Isolation, identification, and characterization of a feather-degrading bacterium. Appl Environ Microbiol. 1990;56:1509-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cai CG, Lou BG, Zheng XD. Keratinase production and keratin degradation by a mutant strain of Bacillus subtilis. J Zhejiang Univ Sci B. 2008;9:60-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wawrzkiewicz K, Lobarzewski J, Wolski T. Intracellular keratinase of Trichophyton gallinae. J Med Vet Mycol. 1987;25:261-268. [PubMed] [Google Scholar]
- 41. Gradišar H, Friedrich J, Križaj I, Jerala R. Similarities and specificities of fungal keratinolytic proteases: comparison of keratinases of Paecilomyces marquandii and Doratomyces microsporusto some known proteases. Appl Environ Microbiol. 2005;71:3420-3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sivakumar T, Shankar T, Thangapand V, Ramasubram V. Optimization of cultural condition for keratinase production using Bacillus cereus TS1. Insight Microbiol. 2013;3:1-8. [Google Scholar]
- 43. Saibabu V, Niyonzima FN, More SS. Isolation, partial purification and characterization of keratinase from Bacillus megaterium. Int Res J Biol Sci. 2013;2:13-20. [Google Scholar]
- 44. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275. [PubMed] [Google Scholar]
- 45. Sivasubramanian S, Manohar BM, Rajaram A, Puvanakrishnan R. Ecofriendly lime and sulfide free enzymatic dehairing of skins and hides using a bacterial alkaline protease. Chemosphere. 2008;70:1015-1024. [DOI] [PubMed] [Google Scholar]
- 46. Salaheen S, Mamun MA, Khan SN, Hoq MM. Improvement of Bacillus licheniformis MZK05 by mutation for increased production of keratinase. Dhaka Univ J Biol Sci. 2015;24:17-23. [Google Scholar]
- 47. Wakil SM, Dada MT, Onilude AA. Isolation and characterization of keratinase-producing bacteria from poultry waste. J Pure Appl Microbiol. 2011;5:567-580. [Google Scholar]
- 48. Burke BE, Pfister RM. Cadmium transport by a Cd2+-sensitive and a Cd2+-resistant strain of Bacillus subtilis. Can J Microbiol. 1986;32:539-542. [DOI] [PubMed] [Google Scholar]
- 49. Vijayaraghavan P, Lazarus S, Vincent SG. De-hairing protease production by an isolated Bacillus cereus strain AT under solid-state fermentation using cow dung: biosynthesis and properties. Saudi J Biol Sci. 2014;21:27-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim JM, Lim WJ, Suh HJ. Feather-degrading Bacillus species from poultry waste. Process Biochem. 2001;37:287-291. [Google Scholar]
- 51. Pissuwan D, Suntornsuk W. Production of keratinase by Bacillus sp. FK 28 isolated in Thailand. Kasetsart J (Nat Sci). 2001;35:171-178. [Google Scholar]
- 52. Agrahari S, Wadhwa N. Degradation of chicken feather a poultry waste product by keratinolytic bacteria isolated from dumping site at Ghazipur poultry processing plant. Int J Poult Sci. 2010;9:482-489. [Google Scholar]
- 53. Yadav AK, Vardhan S, Yandigeri MS, et al. Optimization of keratin degrading enzyme from thermophillic strain of Streptomyces sclerotialus. Res J Microbiol. 2011;6:693-705. [Google Scholar]
- 54. Mazotto AM, Coelho RR, Cedrola SM, et al. Keratinase production by three Bacillus spp. using feather meal and whole feather as substrate in a submerged fermentation. Enzyme Res. 2011;2011:523780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gowdhaman D, Mary neetha CS, Ponnusami V. Production of keratinase from a new strain of Pseudomonas aeruginosa gmp and its application for the removal of dyed keratin waste. Biotechnol: Indian J. 2014;9:210-217. [Google Scholar]
- 56. Kainoor PS, Naik GR. Production and characterization of feather degrading keratinase from Bacillus sp. JB 99. Indian J Biotechnol. 2010;9:384-390. [Google Scholar]
- 57. Gupta S, Nigam A, Singh R. Purification and characterization of a Bacillus subtilis keratinase and its prospective application in feed industry. Acta Biol Szegediensis. 2015;59:197-204. [Google Scholar]
- 58. Lin HH, Yin LJ. Feather meal and rice husk enhanced keratinases production by Bacillus licheniformis YJ4 and characters of produced keratinases. J Mar Sci Technol Taiwan. 2010;18:458-465. [Google Scholar]
- 59. Rajput R, Sharma R, Gupta R. Biochemical characterization of a thiol-activated, oxidation stable keratinase from Bacillus pumilus KS12. Enzyme Res. 2010;2010:132148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Deivasigamani B, Alagappan KM. Industrial application of keratinase and soluble proteins from feather keratins. J Environ Biol. 2008;29:933-936. [PubMed] [Google Scholar]
- 61. Kazi YF, Kumar P, Soomro IH. Characterization of the keratinolytic activity of indigenous Bacillus subtilis keratinase. J Chem Pharm Res. 2015;7:800-809. [Google Scholar]
- 62. Chaturvedi V, Bhange K, Bhatt R, Verma P. Production of kertinases using chicken feathers as substrate by a novel multifunctional strain of Pseudomonas stutzeri and its dehairing application. Biocatal Agric Biotechnol. 2014;3:167-174. [Google Scholar]
- 63. Zambare V, Nilegaonkar S, Kanekar P. A novel extracellular protease from Pseudomonas aeruginosa MCM B-327: enzyme production and its partial characterization. N Biotechnol. 2011;28:173-181. [DOI] [PubMed] [Google Scholar]
- 64. Lateef A, Adelere IA, Gueguim-Kana EB. Bacillus safensis LAU 13: a new source of keratinase and its multi-functional biocatalytic applications. Biotechnol Equip. 2015;29:54-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Thangam EB, Rajkumar GS. Purification and characterization of alkaline protease from Alcaligenes faecalis. Biotechnol Appl Biochem. 2002;35:149-154. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_file_1_xyz33416b0980c9e for Microbial Bioremediation of Feather Waste for Keratinase Production: An Outstanding Solution for Leather Dehairing in Tanneries by Mursheda Akhter, Lolo Wal Marzan, Yasmin Akter and Kazuyuki Shimizu in Microbiology Insights
Supplemental material, Supplementary_file_2_xyz3341651296bf9 for Microbial Bioremediation of Feather Waste for Keratinase Production: An Outstanding Solution for Leather Dehairing in Tanneries by Mursheda Akhter, Lolo Wal Marzan, Yasmin Akter and Kazuyuki Shimizu in Microbiology Insights
Supplemental material, Supplementary_file_3_xyz334166ba60c36 for Microbial Bioremediation of Feather Waste for Keratinase Production: An Outstanding Solution for Leather Dehairing in Tanneries by Mursheda Akhter, Lolo Wal Marzan, Yasmin Akter and Kazuyuki Shimizu in Microbiology Insights