Abstract
Background:
Glaucoma is most common irreversible cause of blindness in India. First line management of open-angle glaucoma is either beta blockers or prostaglandin analogs monotherapy. Monotherapy rarely achieves target intraocular pressure within 2 years and patients are shifted to combination medications, usually fixed-dose combination.
Objective:
To compare travoprost monotherapy and timolol/brinzolamide fixed-dose combination for their intraocular pressure lowering efficacy, their effects on hemodynamic parameters and cup disc ratio reversibility in newly diagnosed drug-naïve open-angle glaucoma patients.
Material and methods:
In a 12-week, prospective, randomised, single-blind study, patients were randomised to receive twice daily 0.5% timolol and 0.2% brinzolamide fixed-dose combination (n = 52) or once daily travoprost 0.004% (n = 52). Intraocular pressure, blood pressure, pulse rate and cup disc ratio were compared across treatment groups over 3 months.
Results:
Significant reduction (p < 0.001) in intraocular pressure by 27.99% and 30.49% at 12th-week visit as compared with baseline was observed in monotherapy and fixed-dose combination group, respectively. Significant changes in pulse rate (9 beats/min) and systolic blood pressure (2.35 mmHg) was observed in fixed-dose combination group. No cup disc ratio reversibility was observed at the end of study. Conjunctival hyperaemia (n = 14) and transient blurring of vision (n = 16) were most commonly reported adverse drug reaction in monotherapy and fixed-dose combination, respectively.
Conclusion:
The 0.5% timolol and 0.2% brinzolamide fixed-dose combination produced greater reduction in intraocular pressure than those produced by 0.004% travoprost alone in drug-naïve open-angle glaucoma patients.
Keywords: cup disc ratio, FDC, hemodynamic changes, intaocular pressure, OAG
Introduction
Glaucoma is a group of progressive optic neuropathies characterised by degeneration of retinal ganglion cells and retinal nerve fibre layer resulting in developments in the optic nerve head and functional visual field deficits. It is most common irreversible cause of blindness in India (prevalence 0.5%–1.5%)1 and second most frequent cause of blindness (overall) in world2 (prevalence 3.54% in age group 40–80 years).3 Risk factors such as ageing, male gender, family history, raised intraocular pressure (IOP), myopia,4 and increased cup disc ratio (CDR)5 have been attributed for development of glaucoma. CDR a critical diagnostic criterion6 is elevated because of variations in axon layer secondary to elevated IOP.7 Several studies have established the reversibility of CDR in young individuals owing to greater elasticity of sclera.8 But reversibility of CDR in adults is inconsisitent.9 IOP has been established as the most important modifiable risk factor.10 Hence, contemporary medical management aims at maintaining IOP within normal range, by either curtailing the aqueous humour production, enhancing its drainage or both.
Several classes of drugs like β blockers (BB), carbonic anhydrase inhibitors (CAI), prostaglandin analogs (PGAs) and α2 agonist (AA) have been recommended for decreasing IOP by different mechanisms.11 As the first line management to treat open-angle glaucoma (OAG), we introduce either BB or PGA monotherapy depending on target IOP, efficacy, compliance, safety or affordability.12 A 7-year prospective study concluded that initial IOP reduction because of BB is 20.6 ± 0.3 mmHg from an initial average of 27.8 ± 0.3 mmHg and timolol fares better than other BB.13 A meta-analysis of topical PGA reported 30% and 26.5% reduction of IOP from baseline by travoprost and latanoprost, respectively.14 A practical guide to medical management of glaucoma suggests use of BB to reduce IOP by 20% and PGAs for a reduction of IOP by 30%.12 If however ⩾ 30% lowering of IOP is recommended and a PGA does not accomplish this or there is a cost issue, a combination of BB with AA or CAI may suffice.12
Monotherapy rarely achieves target IOP within 2 years in about 50% patients.15 Multiple studies like Normal Tension Glaucoma Treatment Study,16 the Collaborative Initial Glaucoma Treatment Study17 and the Ocular Hypertension Treatment Study (OHTS)18 have demonstrated that to attain the target IOP, it is often imperative to use multiple drugs. This can be achieved by either instillation of various medication concurrently or fixed-dose combination (FDC). Concurrent instillation of multiple drugs increases the overall cost, exposure to preservative, and is cumbersome, therefore contributing to nonadherence and failure of therapy.19 FDC offer simpler regimen, less preservatives, reduced washout effect and better adherence and is comparatively economical.19 Thus, FDC are preferred agents for OAG when monotherapy fails. The Early-Manifest Glaucoma Treatment Study established that IOP reduction by each 1 mm reduced progression by 10%.20
Topical medication may enter the systemic circulation via the nasolacrimal ducts, where it can be absorbed through the nasal, oropharyngeal, and gastrointestinal mucosa. Although blood levels of topical medication are not as high as those detectable after oral administration, small amounts of systemically absorbed beta blockers can produce significant adverse events in predisposed patients. Thus, topical beta blockade may cause bradycardia and heart block in patients with underlying conduction system disease. Case reports in the literature relate the use of topical b-blockers to syncope, bradycardia, systemic hypotension, palpitation, arrhythmia and heart block.21
Ocular instillation of timolol has been correlated with exacerbation of obstructive pulmonary disease and altered heart rate; thus, its use is restricted in glaucoma patients with cardiopulmonary comorbidities.22 Previous studies on ophthalmic timolol suggest its effects on blood pressure (BP) and heart rate in spite of low plasma concentration mainly during night time.23 The FP receptor is abundantly expressed in the kidney and plays a role in water and electrolyte homeostasis. In addition, PGF2α stimulates renin release from juxtaglomerular granular cells through the FP receptor (Prostaglandin F receptor), causing an increase in BP by activating the renin–angiotensin–aldosterone system. PGAs too have been implicated to uncommonly cause palpitation, irregular or decreased heart rate.24 Increasing age has been associated consistently with increasing risk of cardiopulmonary diseases.25 Studies like OHTS18 and EGPS26 conclude that the magnitude of risk for OAG consistently multiplies with increasing age. The geriatric population will rise to many folds in coming decades in countries like China and India.27 This will lead to a spurt of elderly glaucoma patients and will not be an ideal case to receive medications which alter hemodynamic parameters.
It is acknowledged that neurobiological changes can alter clinical performance. These can be explained by a variety of psychological mechanisms, such as conditioning, expectations, reward and anxiety, and can be modulated by desire, motivation and memory of a person. Most of these factors fall under the concept of conscious, associative or social learning.28 Drug-naïve patients were thus included in the study to mitigate neurobiological conditioning and to keep the study independent of these factors. In view of CDR as a risk factor for glaucoma and effect of various ocular drugs on hemodynamic parameters, this 12 week, prospective, single-blind, parallel study was done to compare travoprost monotherapy and timolol/brinzolamide FDC for their IOP lowering efficacy, their effects on hemodynamic parameters and CDR reversibility in newly diagnosed drug-naïve OAG patients.
Material and methods
Patients of either gender aged ⩾ 40 years with raised IOP ⩾ 21 mmHg on at least two readings, characteristic glaucomatous visual field defect, increased CDR ⩾ 0.4 or asymmetry of ⩾ 0.2 between both eyes and open angle on gonioscopy in at least one eye were recruited from outpatient department of ophthalmology department, Uttar Pradesh University of Medical Sciences, Saifai, India. Central corneal thickness (CCT) and retinal nerve fibre layer thickness (RNFL) was assessed at baseline using spectral-domain optical coherence tomography (SD-OCT). Mean deviation visual field (VF-MD) and pattern standard deviation visual field (VF-PSD) was assessed at baseline by Humphrey automated perimetry (Swedish interactive thresholding algorithm 30-2 programme) by ophthalmologists. Patients with any form of glaucoma other than OAG, concurrent infection or ocular disease, history of ocular surgery, obstructive pulmonary disease, history of hypersensitivity to any component under study, pregnant or nursing women, unstable or uncontrolled cardiovascular disease and those using any topical/systemic corticosteroid were excluded from the study. The ethical approval for the study was obtained from the Ethics committee of our research institute, Uttar Pradesh University of Medical Sciences, Saifai, Etawah, Uttar Pradesh, India (Project ID: 2017/132, approval February 2017). The study was conducted jointly by department of Clinical pharmacology and therapeutics and Ophthalmology of Uttar Pradesh University of Medical Sciences, Saifai, according to the Declaration of Helsinki ethical principles on human research.
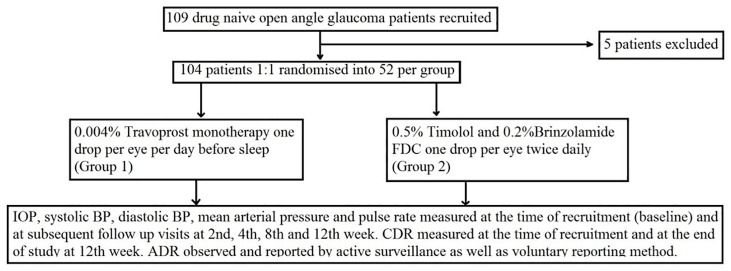
109 patients were recruited, and 104 completed the study. All subjects were asked to sign a written informed consent after explaining the nature and possible consequences of their participation in the study. Patients (52 each group) were randomised to receive 0.004% travoprost monotherapy one drop per day in evening (group 1) and 0.5% timolol/0.2% brinzolamide FDC one drop twice daily per day (group 2), respectively (Figure 1). The study drugs were chosen based on their near similar dimensions of drop-tainers which helped in blinding, easy availability in institution funded pharmacy and they being most commonly prescribed (based on questionnaires response by institution’s ophthalmologists).
Figure 1.
Flowchart for distribution of patients.
IOP, pulse rate, systolic BP, diastolic BP and mean arterial pressure were measured at the time of recruitment and at follow-up visit at 2nd, 4th, 8th and 12th week. We assessed CDR using SD-OCT at the time of recruitment and at end of treatment at 12th week by same operator and equipment. IOP measurements were performed for both eyes using Goldmann applanation tonometer. Between both eyes, we selected the eye with higher IOP value at recruitment as study eye. The fluorescein dye and anaesthetic agent (paracaine) remained constant throughout the study, and the same operator performed all IOP measurements on same tonometer between 10 a.m. and 12 p.m. to mitigate circadian variation.
Hemodynamic parameters were assessed in the sitting position by the principal investigator using same equipments in a separate room. Reading was taken after 5-min rest. Three consecutive readings (5 min apart) were obtained and minimum value was noted.
The participants were encouraged to take the picture (on mobile phone) and report any adverse drug event at the time of next follow-up visit. Adverse drug reactions (ADRs) were reported on suspected ADR form (version 1.2) and submitted to ADR monitoring centre, Uttar Pradesh University of Medical Sciences, Saifai, Etawah.
Statistical analysis
All the patients were assessed on intention-to-treat principle. Shapiro–Wilk test was used as a test for normality of data. The test showed that data are normally distributed for IOP; W = 0.97, p = 0.24 and very close to normal for pulse rate, systolic and diastolic BP. For larger sample sizes, the sampling distribution of the mean is always normal, regardless how values are distributed in the population, we performed parametric test for analysis. Data analysis for IOP, pulse rate, systolic BP, diastolic BP, mean arterial pressure and CDR was performed by repeated measure analysis of variance and unpaired student’s t test for intragroup and intergroup comparisons, respectively. Post hoc analysis was conducted by Dunnett’s test. We conducted the analysis using SPSS 17.0 statistical software (SPSS, Inc., Chicago, IL, USA). A p value < 0.05 was considered statistically significant.
Results
The mean age of the study population was 51.49 ± 7.00 years (range: 40–67 years), mean age of patients was 51.13 ± 7.32 and 51.83 ± 6.75 years in groups 1 and 2, respectively. Sixty-six patients (63.5%) were male. The baseline demographic characteristics, CCT, average RNFL, VF-MD and VF-PSD were comparable among both groups (Table 1).
Table 1.
Baseline characteristics of patients in two treatment groups of newly diagnosed drug-naïve open-angle glaucoma patients.
| Travoprost (n = 52) | Timolol/brinzolamide (n = 52) | p value | |
|---|---|---|---|
| Mean age ± SD (range) | 51.13 ± 7.32 (40–65) | 51.83 ± 6.75 (41–67) | 0.198 |
| Female (%) | 21 (40.4%) | 17 (32.7%) | 0.415 |
| Male (%) | 31 (59.6%) | 35 (67.3%) | |
| IOP (mean ± SD) | 23.40 ± 1.83 | 23.35 ± 1.55 | 0.862 |
| Pulse rate (mean ± SD) | 81.23 ± 9.66 | 80.50 ± 10.29 | 0.710 |
| Systolic BP (mean ± SD) | 129.69 ± 13.57 | 128.96 ± 14.67 | 0.793 |
| Diastolic BP (mean ± SD) | 82.92 ± 6.43 | 82.08 ± 6.50 | 0.506 |
| Mean arterial pressure (mean ± SD) | 98.51 ± 8.33 | 97.71 ± 8.92 | 0.634 |
| CDR (mean ± SD) | 0.65 ± 0.13 | 0.62 ± 0.14 | 0.606 |
| Average RNFL (mean ± SD) | 81.27 ± 7.28 | 83.17 ± 6.16 | 0.418 |
| CCT (mean ± SD) | 524 ± 36.11 | 512.8 ± 39.23 | 0.059 |
| VF-MD (Mean ± SD) | –10 ± 7.5 | –9.8 ± 8.7 | 0.127 |
| VF-PSD (mean ± SD) | 8.1 ± 3.9 | 7.9 ± 3.4 | 0.213 |
BP, blood pressure; CCT, central corneal thickness; CDR, cup disc ratio; IOP, intraocular pressure; RNFL, retinal nerve fibre layer thickness; VF-MD, mean deviation visual field; VF-PSD, pattern standard deviation visual field.
Primary outcome: IOP reduction
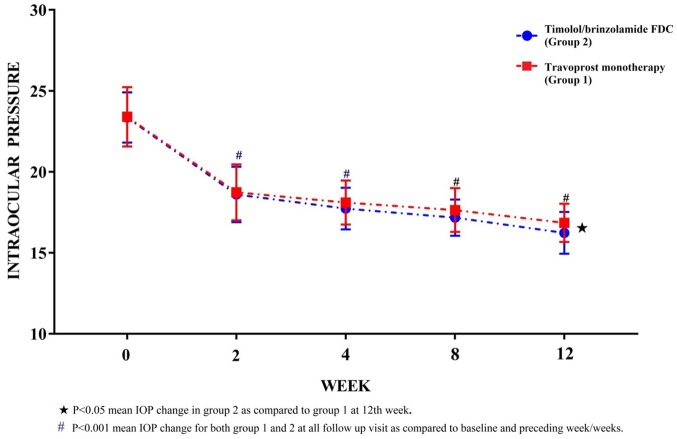
In travoprost group, there is a significant reduction (p < 0.001) in IOP by 6.56 mmHg (27.99%) at 12th-week visit as compared with baseline. IOP was reduced significantly (p < 0.001) by 7.12 mmHg (30.49%) as compared with baseline in FDC group at 12th week. The changes in IOP are more in FDC group as compared with travoprost but comparable up to 8th week. By the end of study, the reduction in IOP of FDC arm patients was significantly more (p < 0.05) as compared with the travoprost patients (Table 2, Figure 2).
Table 2.
Observed value (mean ± SD) of intraocular pressure (mmHg), pulse rate (per minute), systolic blood pressure (mmHg), diastolic blood pressure (mmHg) and mean arterial pressure (mmHg) in groups 1 and 2, and their respective mean difference (for each parameter) between both treatment groups at baseline and follow-up visits.
| Time | IOP |
PR |
SBP |
DBP |
MAP |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | G2 | Δ | G1 | G2 | Δ | G1 | G2 | Δ | G1 | G2 | Δ | G1 | G2 | Δ | |
| 0 W | 23.40 ± 1.83 | 23.35 ± 1.55 | 0.06 | 81.23 ± 9.66 | 80.50 ± 10.29 | 0.73 | 129.69 ± 13.57 | 128.96 ± 14.67 | 0.73 | 82.92 ± 6.43 | 82.08 ± 6.50 | 0.85 | 98.51 ± 8.33 | 97.71 ± 8.92 | 0.80 |
| 2 W | 18.73 ± 1.73 | 18.60 ± 1.71 | 0.13 | 79.48 ± 7.36 | 74.79 ± 6.22 | 4.69** | 128.69 ± 12.58 | 125.65 ± 12.81 | 3.04 | 82.65 ± 7.51 | 81.73 ± 7.43 | 0.92 | 98.00 ± 8.12 | 96.37 ± 8.58 | 1.63 |
| 4 W | 18.10v1.36 | 17.73 ± 1.29 | 0.37 | 79.96 ± 7.19 | 74.15 ± 6.65 | 5.81** | 127.88 ± 11.69 | 126.73 ± 10.24 | 1.15 | 82.96 ± 8.20 | 81.58 ± 7.75 | 1.39 | 97.94 ± 8.36 | 96.63 ± 7.98 | 1.31 |
| 8 W | 17.64 ± 1.35 | 17.17 ± 1.12 | 0.47 | 79.83 ± 7.26 | 72.62 ± 6.32 | 7.21** | 127.08 ± 9.07 | 126.12 ± 11.95 | 0.96 | 82.92 ± 8.20 | 81.69 ± 7.24 | 1.23 | 97.64 ± 7.88 | 96.50 ± 8.13 | 1.14 |
| 12W | 16.85 ± 1.18 | 16.23 ± 1.29 | 0.62* | 80.37 ± 7.01 | 71.29 ± 5.08 | 9.08** | 127.81 ± 10.20 | 126.62 ± 11.59 | 1.19 | 83.54 ± 8.00 | 81.54 ± 7.38 | 2.00 | 98.29 ± 7.92 | 96.56 ± 8.27 | 1.73 |
DBP, diastolic blood pressure; FDC, fixed-dose combination; G1, travoprost monotherapy; G2, timolol/brinzolamide; IOP, intraocular pressure; MAP, mean arterial; pressure; PR, pulse rate; SBP, systolic blood pressure; W, week; Δ, mean difference in respective parameter between groups 1 and 2.
p < 0.05; **p < 0.001 mean difference in parameter between two groups.
Figure 2.
Observed (mean ± SD) intraocular pressure (mmHg) in groups 1 and 2 at baseline (0 week), 2nd, 4th, 8th and 12th week.
Secondary outcome
Change in pulse rate
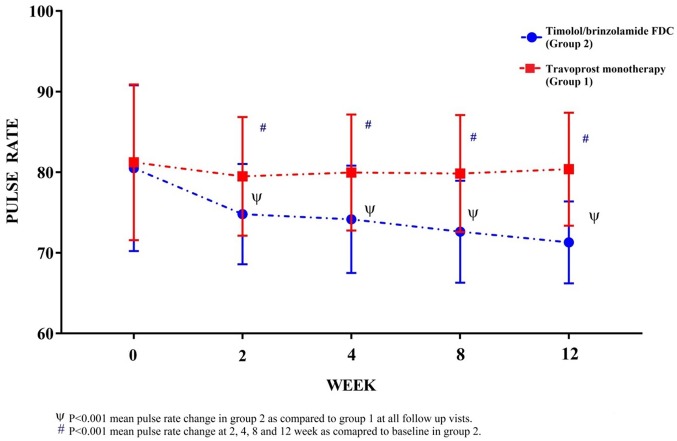
Mean pulse rate per minute (mean ± SD) 81.23 ± 9.66 and 80.50 ± 10.29 in groups 1 and 2 are comparable at baseline (Table 1). In group 2, a significant reduction (p < 0.001) in pulse rate was observed at all follow-up visit as compared with baseline (Table 2). Reduction of pulse rate in group 2 is comparatively higher as compared with group 1 value at all follow-up visits (Figure 3).
Figure 3.
Observed (mean ± SD) pulse rate per minute in groups 1 and 2 at baseline (0 week), 2nd, 4th, 8th and 12th week.
Change in BP
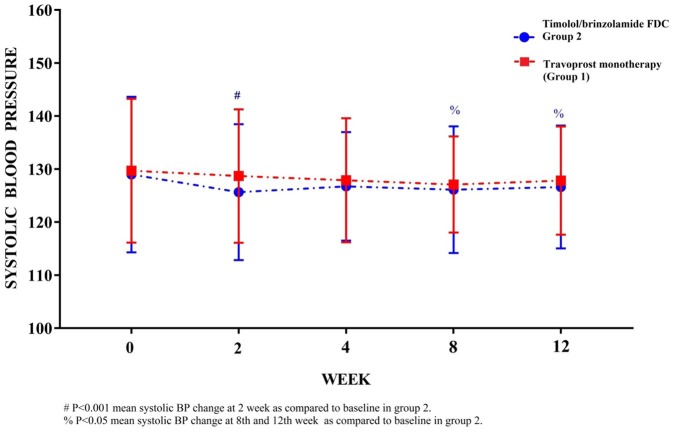
Mean systolic BP (129.69 ± 13.57 and 128.96 ± 14.67 mmHg), mean diastolic BP (82.92 ± 6.43 and 82.08 ± 6.50 mmHg) and mean arterial pressures (98.51 ± 8.33 and 97.71 ± 8.92 mmHg) at baseline are comparable in groups 1 and 2, respectively (Table 1). Intergroup changes are comparable at all respective follow-up visit in groups 1 and 2. A significant reduction in systolic BP is observed at 2nd week visit (p < 0.001), 8th and 12th week visit (p < 0.05) as compared with baseline values in group 2 but similar significant variation in systolic BP is not observed in group 2 (Figure 4).
Figure 4.
Observed (mean ± SD) systolic blood pressure (mmHg) in groups 1 and 2 at baseline (0 week), 2nd, 4th, 8th and 12th week.
No significant variations pertaining to diastolic BP or mean arterial pressure is observed in-between groups 1 and 2 at any follow-up visit (Table 2). The intragroup changes in diastolic BP and mean arterial pressure remain comparable at their entire respective follow-up visit within groups 1 and 2.
Change in CDR
The CDR value (mean ± SD) at the time of recruitment in groups 1 and 2 are 0.65 ± 0.13 and 0.62 ± 0.14 respectively, the values are comparable between two treatment arms (Table 1). At the end of the study, a reduction in CDR is observed, which is insignificant compared with baseline. Reduced CDR is comparable between both the treatment arms.
Adverse drug effects
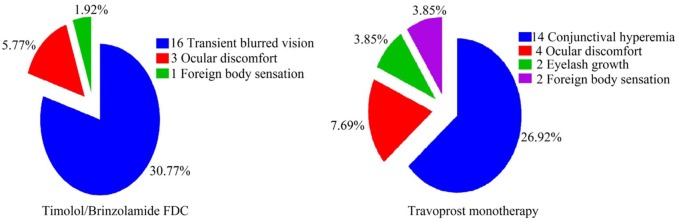
In group 1, 14 patients developed conjunctival hyperaemia between 4th- and 8th-week follow-up, 4 patients complained of ocular discomfort at 2nd week and later were found to have developed conjunctival hyperaemia by week 4. Two different patients complained of foreign body sensation at 2nd follow-up and two complained of difficulty wearing spectacles because of apparent changes in eyelashes at 12th-week follow-up. In group 2, 16 patients complained of transient blurred vision. Ocular discomfort and foreign body sensation were other two complaints noted at 2nd-week follow-up in three and one patients, respectively. Thus a total of 18 (35%) and 20 (38.5%) patients in groups 1 and 2, respectively developed ADR during the 12-week treatment (Figure 5).
Figure 5.
Adverse drug reactions observed in both treatment groups.
Discussion
Both the treatment arms reduced IOP significantly (p < 0.001) with 6.56 mm Hg (28%) and 7.12 mm Hg (30.5%) reductions at 12th week in travoprost monotherapy and timolol/brinzolamide FDC, respectively. Significant reduction (p < 0.001) of IOP in both the arms was appreciable as early as 2nd week appointment. The percentage reduction of IOP at 2nd, 4th, 8th and 12th follow-up appointment (as contrasted to baseline value) was 20%, 22.64%, 24.61%, 28% and 20.34%, 24.06%, 26.46%, 30.5% in travoprost monotherapy and timolol/brinzolamide FDC arms, respectively. The mean IOP reduction was comparable between both treatment arms till 8th week but by 12th week there was significant (p < 0.05) mean IOP reduction (0.62 mmHg) in FDC arm. A meta-analysis on topical PGAs in reducing IOP by Denis and colleagues29 demonstrated a decrease by 30% for travoprost therapy, which was also reflected in our study. Takagi and colleagues30 stated that a 15–20% reduction in IOP by administration of different PGAs. Though the IOP reduction in our investigation was in conformity to those observed by diverse investigators, variation observed could be due to small sample size, strict inclusivity of only drug-naïve OAG patients, and short study period. Another conceivable justification for variation in IOP reduction could be the genetic polymorphism of FP receptors.31 It has been observed that genetic polymorphisms in prostanoid receptors correlate to the difference in the efficacy of latanoprost.31 These polymorphisms may induce the disparities in the 3D-architecture of receptors and affinity of PGAs to those receptors and thus alter their efficacy.
IOP reduction (at 12th week) in 0.5% timolol/0.2% brinzolamide FDC treatment arm was 30.5%; but a significant reduction (p < 0.001) was observed as early as at 2nd-week follow-up. Kaback and colleagues32 reported that brinzolamide 1%/timolol 0.5% FDC produced substantial reductions from baseline ranging from 8.0 to 8.7 mmHg, similar to the 7.12 mmHg reductions observed by timolol/brinzolamide FDC in our study. In a study, Hollo and colleagues33 concluded that a decrease of 30–35% can be expected from timolol/brinzolamide FDC, which confirms the finding of our study of a 30.5% reduction in IOP by FDC.
The IOP reduction at each respective follow-up visits was greater in timolol/brinzolamide FDC than travoprost monotherapy. Reduction in IOP was significantly more (p < 0.05) as compared with 0.004% travoprost monotherapy at end of study. This observation could be possible because of an additive effect of timolol and brinzolamide as well as the distinctive mechanism of action. Previous studies32,34,35 studying the effect of FDC and monotherapy for glaucoma have overwhelmingly concluded the superior efficacy of FDCs over single agents, and our finding is in accordance with them.
Interestingly, reports of systemic side effects (post ocular instillation) are not well reported, and with the target population mostly comprising middle-aged and geriatric patients who are themselves at elevated risk of cardio-metabolic disorders, there was a need to analyse the systemic effects of first line glaucoma medications on hemodynamic parameters.
The result of our study reveals that there was a significant reduction in the pulse rate by about 9 beats per minute, and there was a significant reduction in systolic BP (2.34 mm Hg) at 12th week as compared with baseline in 0.5% timolol/0.2% brinzolamide FDC group. We observed no significant changes in diastolic BP and mean arterial pressure in either of treatment arms at any follow-up time interval. Dickstein and colleagues36 demonstrated that timolol solution reduce the heart rate and systolic arterial BP at baseline and during exercise, and the effect on the heart rate was statistically more significant than the effect on systolic arterial BP. Similar to our study, Watson and colleagues37 found that treatment with timolol produced a slight but significant reduction in heart rate from a baseline value of 73.8 ± 11.6 (mean ± SD) beats per minute to 71.8 ± 10.9 beats per minute, with no effect from treatment with latanoprost. Although neither timolol nor latanoprost had a consistent effect on BP, there was a general tendency towards a slight decrease with the use of both agents. Mishima and colleagues38 found that latanoprost was more efficacious than timolol maleate in reducing IOP in patients with OAG and ocular hypertension; the main systemic effect was a slight but statistically significant reduction in mean heart rate in the patients in the timolol group at 4, 8, and 12 weeks (p < .01). Since, studies have suggested that nocturnal hypotension may play a role in the progression of chronic OAG, and systemic hypotension as a risk factor for glaucomatous damage39 therefore careful attention to the systemic effects of topical beta blockers is paramount.
Systemic spill over of brinzolamide post ocular instillation enters the blood circulation and binds preferentially to CA inside the erythrocytes.40 As result, the concentration of free brinzolamide in the plasma is insignificant. The low plasma concentration of brinzolamide and its poor affinity to the other CA isoform explain the paucity of systemic effects during topical use of this molecule. Hence, possible reason for the significant reduction in pulse rate and systolic BP could be β receptor antagonising property of timolol present in timolol/brinzolamide FDC. In a large, multicenter trial comparing dorzolamide with betaxolol and timolol maleate, patients receiving betaxolol had significantly more cardiovascular adverse events, including angina, hypertension, and bradycardia, than did those in the dorzolamide group.41
Under normal conditions, the human tear volume averages 7 µL although the estimated maximum volume that the cul-de-sac can momentarily contain is ≈30 µL.42 Thus, abrupt escalation of large volumes such as those produced by an eye drop instillation (commercial drops range from 50 to 75 µL in volume)43 and this contributes to rapid drainage of about 80% or more of an administered eye drop through the nasolacrimal canal. It bypasses the first pass effect and is accessible for systemic absorption. Thus, an eye drop can mimic intravenous dose. This holds true for brinzolamide and travoprost. But as we argued, brinzolamide because of its extensive internalisation into RBC40 could not have had any significant free plasma concentration. Likewise, travoprost too could have had an effect on the above said hemodynamic parameters, but with an instillation dose of 0.004% once daily and greater selectivity towards FP receptors31 could not have reached significant plasma concentration for the same.
The CDR is an important diagnostic parameter for glaucoma and for evaluating the efficacy of treatment.6 At the end of 12th week, there was a reduction in CDR in both treatment arms. As the reduction was not significant, we cannot comment on the CDR reversal potential of drug. But, we consider that the treatment halted the further derangement in CDR.
Reports from prior studies establish that reversal of CDR is feasible in young humans8 attributable to greater elasticity of sclera and optic nerve head compliance. In adults, the compliance and elasticity are diminished; we expect the decrease in CDR observed in our investigation could be owing to pseudo reversal phenomenon. The optic disc oedema that develops after sudden IOP reduction could have mimicked reversal of cupping, and thus slight improvement was observed. It is believed the phenomenon of pseudo reversal is observed for 2 to 3 months post IOP control;44 hence, a study of larger duration would be fundamental to clarify this query.
Conjunctival hyperaemia and transient blurring of vision was most commonly observed ADR in both drug groups 1 and 2, respectively. The adverse reactions observed in our study were mild and not troublesome for the patients as they seldom reported it without leading questions.
The strength of our study is that it reports the effect of timolol/brinzolamide FDC and travoprost on hemodynamic parameters and CDR on topical instillation of drugs which has been inconsistently reported in previous studies. It reinforces the data pertaining to IOP lowering efficacy of study drugs as well as it also expands our understanding of effects on hemodynamic parameters and CDR reversibility. At the same time, the shortcomings of our study were that our sample size was small and the study duration of 12 weeks was insufficient to draw a conclusion on CDR reversal and few long-term ADRs. The ADRs obtained were not entirely passive, and investigators had to ask leading questions to unearth them; thus, a bias might be present. A longer duration study with a larger sample size would be helpful in covering these shortcomings.
Conclusion
Our study shows that 0.5% timolol/0.2% brinzolamide FDC was superior to 0.004% travoprost monotherapy in reducing IOP among drug-naïve OAG patients. Timolol/brinzolamide FDC diminished pulse rate and systolic BP while travoprost monotherapy had no such effect. No significant variations were recognised in diastolic BP, mean arterial pressure and CDR by both pharmaceutical groups. Both medications were well tolerated, and no new safety findings were established.
Acknowledgments
The authors extend their appreciation to the patients who participated in the study.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Atul Ashish  https://orcid.org/0000-0001-9410-1686
https://orcid.org/0000-0001-9410-1686
Contributor Information
Alok Dixit, Department of Pharmacology, Uttar Pradesh University of Medical Sciences, Saifai, India.
Atul Ashish, Department of Pharmacology, Uttar Pradesh University of Medical Sciences, Saifai 206130, Etawah, Uttar Pradesh, India.
Reena Sharma, Department of Ophthalmology, Uttar Pradesh University of Medical Sciences, Saifai, India.
References
- 1. Parihar J. Glaucoma: the ‘black hole’ of irreversible blindness. Med J Armed Forces India 2016; 72: 3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WHO. Glaucoma is second leading cause of blindness globally. https://www.who.int/bulletin/volumes/82/11/feature1104/en/ (2019, accessed 6 February 2019). [PMC free article] [PubMed]
- 3. Tham Y, Li X, Wong T, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040. Ophthalmology 2014; 121: 2081–2090. [DOI] [PubMed] [Google Scholar]
- 4. McMonnies C. Glaucoma history and risk factors. J Optom 2017; 10: 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Le A, Mukesh B, McCarty C, et al. Risk factors associated with the incidence of open-angle glaucoma: the visual impairment project. Invest Ophthalmol Vis Sci 2003; 44: 3783–3789. [DOI] [PubMed] [Google Scholar]
- 6. Thomas R, Loibl K, Parikh R. Evaluation of a glaucoma patient. Indian J Ophthalmol 2011; 59: 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weinreb R, Aung T, Medeiros F. The pathophysiology and treatment of glaucoma. JAMA 2014; 311: 1901–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meirelles SH, Mathias CR, Bloise RR, et al. Evaluation of the factors associated with the reversal of the disc cupping after surgical treatment of childhood glaucoma. J Glaucoma 2008; 17: 470–473. [DOI] [PubMed] [Google Scholar]
- 9. Park KH, Kim DM, Youn DH. Short-term change of optic nerve head topography after trabeculectomy in adult glaucoma patients as measured by Heidelberg Retina Tomography. Korean J Ophthalmol 1997; 11: 1–6. [DOI] [PubMed] [Google Scholar]
- 10. Actis A, Versino E, Brogliatti B, et al. Risk factors for primary open angle glaucoma (POAG) progression: a study ruled in Torino. Open Ophthalmol J 2016; 10: 129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dikopf M, Vajaranant T, Edward D. Topical treatment of glaucoma: established and emerging pharmacology. Expert Opin Pharmacother 2017; 18: 885–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parikh R, Parikh S, Navin S, et al. Practical approach to medical management of glaucoma. Indian J Ophthalmol 2008; 56: 223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Watson P. A 7 year prospective comparative study of three topical beta blockers in the management of primary open angle glaucoma. Br J Ophthalmol 2001; 85: 962–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee AJ, McCluskey P. Clinical utility and differential effects of prostaglandin analogs in the management of raised intraocular pressure and ocular hypertension. Clin Ophthalmol 2010; 4: 741–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kobelt-Nguyen G, Gerdtham UG, Alm A. Costs of treating primary open-angle glaucoma and ocular hypertension: a retrospective observational two-year chart review of newly diagnosed patients in Sweden and the United States. J Glaucoma 1998; 7: 95–104. [PubMed] [Google Scholar]
- 16. Anderson D. Collaborative normal tension glaucoma study. Curr Opin Ophthalmol 2003; 14: 86–90. [DOI] [PubMed] [Google Scholar]
- 17. Feiner L, Piltz-Seymour J. Collaborative Initial Glaucoma Treatment Study: a summary of results to date. Curr Opin Ophthalmol 2003; 14: 106–111. [DOI] [PubMed] [Google Scholar]
- 18. Higginbotham E. Ocular hypertension treatment study. Arch Ophthalmol 2009; 127: 213–215. [DOI] [PubMed] [Google Scholar]
- 19. Gautam C, Saha L. Fixed dose drug combinations (FDCs): rational or irrational: a view point. Br J Clin Pharmacol 2008; 65: 795–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leske M, Heijl A, Hussein M. Factors for glaucoma progression and the effect of treatment. Arch Ophthalmol 2003; 121: 48–56. [DOI] [PubMed] [Google Scholar]
- 21. Fraunfelder FT, Meyer SM. Systemic adverse reactions to glaucoma medications. Int Ophthalmol Clin 1989; 29: 143–146. [DOI] [PubMed] [Google Scholar]
- 22. Umetsuki M, Kotegawa T, Nakamura K, et al. Temporal variation in the effects of ophthalmic timolol on cardiovascular and respiratory functions in healthy men. J Clin Pharmacol 1997; 37: 58–63. [DOI] [PubMed] [Google Scholar]
- 23. Nieminen T, Uusitalo H, Turjanmaa V, et al. Association between low plasma levels of ophthalmic timolol and haemodynamics in glaucoma patients. Eur J Clin Pharmacol 2005; 61: 369–374. [DOI] [PubMed] [Google Scholar]
- 24. European Medicines Agency Commission. Travatan, https://www.ema.europa.eu/en/medicines/human/EPAR/travatan (2018, accessed 7 February 2019).
- 25. Prabhakaran D, Jeemon P, Roy A. Cardiovascular diseases in India. Circulation 2016; 133: 1605–1620. [DOI] [PubMed] [Google Scholar]
- 26. Takwoingi Y, Botello A, Burr J, et al. External validation of the OHTS-EGPS model for predicting the 5-year risk of open-angle glaucoma in ocular hypertensives. Br J Ophthalmol 2013; 98: 309–314. [DOI] [PubMed] [Google Scholar]
- 27. Demographic transition, https://en.wikipedia.org/wiki/Demographic_transition#Asia (2019, accessed 7 February 2019).
- 28. Pollo A, Carlino E, Benedetti F. Placebo mechanisms across different conditions: from the clinical setting to physical performance. Philos Trans R Soc Lond B Biol Sci 2011; 366: 1790–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Denis P, Lafuma A, Khoshnood B, et al. A meta-analysis of topical prostaglandin analogues intra-ocular pressure lowering in glaucoma therapy. Curr Med Res Opin 2007; 23: 601–608. [DOI] [PubMed] [Google Scholar]
- 30. Takagi Y, Santo K, Hashimoto M, et al. Ocular hypotensive effects of prostaglandin analogs in Japanese patients with normal-tension glaucoma: a literature review. Clin Ophthalmol 2018; 12: 1837–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sakurai M, Higashide T, Ohkubo S, et al. Association between genetic polymorphisms of the prostaglandin F2α receptor gene, and response to latanoprost in patients with glaucoma and ocular hypertension. Br J Ophthalmol 2014; 98: 469–473. [DOI] [PubMed] [Google Scholar]
- 32. Kaback M, Scoper S, Arzeno G, et al. Intraocular pressure-lowering efficacy of brinzolamide 1%/timolol 0.5% fixed combination compared with brinzolamide 1% and timolol 0.5%. Ophthalmology 2008; 115: 1728–1734. [DOI] [PubMed] [Google Scholar]
- 33. Holló G, Bozkurt B, Irkec M. Brinzolamide/timolol fixed combination: a new ocular suspension for the treatment of open-angle glaucoma and ocular hypertension. Expert Opin Pharmacother 2009; 10: 2015–2024. [DOI] [PubMed] [Google Scholar]
- 34. Fechtner RD, Realini T. Fixed combinations of topical glaucoma medications. Curr Opin Ophthalmol 2004; 15: 132–135. [DOI] [PubMed] [Google Scholar]
- 35. Sezgin Akçay Bİ, Güney E, Bozkurt K, et al. The safety and efficacy of brinzolamide 1%/timolol 0.5% fixed combination versus dorzolamide 2%/timolol 0.5% in patients with open-angle glaucoma or ocular hypertension. J Ocul Pharmacol Ther 2013; 29: 882–886. [DOI] [PubMed] [Google Scholar]
- 36. Dickstein K, Aarsland T. Comparison of the effects of aqueous and gellan ophthalmic timolol on peak exercise performance in middle-aged men. Am J Ophthalmol 1996; 121: 367–371. [DOI] [PubMed] [Google Scholar]
- 37. Watson P, Stjernschantz J. and the Latanoprost Study Group. A six-month, randomized, double-masked study comparing latanoprost with timolol in open-angle glaucoma and ocular hypertension. Ophthalmology 1996; 103: 126–137. [DOI] [PubMed] [Google Scholar]
- 38. Mishima HK, Masuda K, Kitazawa Y, et al. A comparison of latanoprost and timolol in primary open-angle glaucoma and ocular hypertension: a 12-week study. Arch Ophthalmol 1996; 114: 929–932. [DOI] [PubMed] [Google Scholar]
- 39. Kaiser HJ, Flammer J. Systemic hypotension: a risk factor for glaucomatous damage? Ophthalmologica 1991; 203: 105–108. [DOI] [PubMed] [Google Scholar]
- 40. Iester M. Brinzolamide ophthalmic suspension: a review of its pharmacology and use in the treatment of open angle glaucoma and ocular hypertension. Clin Ophthalmol 2008; 2: 517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Strahlman E, Tipping R, Vogel R. A double-masked, randomized 1-year study comparing dorzolamide (trusopt), timolol, and betaxolol. International Dorzolamide Study Group. Arch Ophthalmol 1995; 113: 1009–1016. [DOI] [PubMed] [Google Scholar]
- 42. Mishima S, Gasset A, Klyce SD, Jr, et al. Determination of tear volume and tear flow. Invest Ophthalmol 1966; 5: 264–276 [PubMed] [Google Scholar]
- 43. Lederer CM, Jr, Harold RE. Drop size of commercial glaucoma medications. Am J Ophthalmol 1986; 101: 691–694. [DOI] [PubMed] [Google Scholar]
- 44. Swinnen S, Stalmans I, Zeyen T. Reversal of optic disc cupping with improvement of visual field and stereometric parameters after trabeculectomy in young adult patients (two case reports). Bull Soc Belge Ophtalmol 2010; 316: 49–57. [PubMed] [Google Scholar]