Abstract
Background
Endovascular therapy (EVT) is increasingly used to improve cerebral reperfusion after moderate-to-severe acute ischemic stroke (AIS). However, the influence of hemodynamic factors on clinical outcome is still unclear after EVT. Dynamic cerebral autoregulation (dCA) is an important brain reserve mechanism and is impaired after AIS. This study aimed to explore the role of dCA in predicting the outcome of AIS patients after EVT.
Methods
AIS patients with severe stenosis/occlusion of unilateral middle cerebral artery (MCA) or internal carotid and treatment with EVT were enrolled to receive dCA examinations at the 24 h, 72 h and 7th day after stroke onset. Healthy volunteers were also recruited as controls. DCA was recorded from spontaneous fluctuations of blood pressure and MCA flow velocity. Transfer function analysis was used to derive dCA parameters, including phase difference (PD) and coherence in the low-frequency range (0.06–0.12 Hz). The clinical outcome was measured using the modified Rankin Scale (mRS) at 90 days after onset. Multivariate logistic regression was performed to reveal the correlation between dCA and clinical outcomes. The receiver operation characteristics (ROC) curve was performed to determine the cut-off point of PD.
Results
A total of 62 AIS patients and 77 healthy controls were included. Compared with controls, dCA were impaired bilaterally till to 7th day after onset in patients, presenting as much lower PD value on the ipsilateral side. During follow-up, we found that PD on the ipsilateral side at 24 h after onset was significantly lower in patients with unfavourable outcome (n = 41) than those with favourable outcome (n = 21), even after adjustment of confounding factors (p = 0.009). ROC curve analysis revealed that PD < 26.93° was an independent predictor of unfavourable-outcome.
Conclusion
In AIS patients after EVT, dCA was impaired on both sides over the first 7 days. PD on the ipsilateral side at 24 h after onset is an independent unfavourable-outcome predictor for AIS after EVT.
Keywords: Dynamic cerebral autoregulation, Risk factors, Acute ischemic stroke, Endovascular therapy, Outcome, Predictor
Background
Acute ischemic stroke (AIS) is a leading cause of death and adult disability worldwide [1]. In Asian populations, large intracranial artery occlusive disease is the most common cause of ischemic stroke [2, 3]. Endovascular therapy (EVT) has been repeatedly proved to improve cerebral reperfusion for severe AIS caused by the occlusion of large arteries in several randomized clinical trials since 2015 [4–8]. EVT can achieve a complete revascularization ratio of 67–88% [4–8]. However, about 56% of patients remain to experience poor outcome even after endovascular treatment [9]. The causes for the lack of improvement in those cases remain incompletely understood [10].
Dynamic cerebral autoregulation (dCA) is a pivotal mechanism to maintain stable cerebral hemodynamics [11]. It is regarded as an intrinsic protective mechanism of the brain, which ensures relatively constant cerebral blood flow (CBF) despite fluctuations in arterial blood pressure (ABP) or cerebral perfusion pressure [12]. However, dCA may be impaired or even vanish after ischemic stroke [13]. That means under pathological conditions that dCA was dysfunctional, CBF tends to passively vary with changes in ABP, leading to brain edema, intracranial hypertension, and consequential deterioration of neurological functions and clinical outcomes [14]. Since EVT mainly changes cerebral hemodynamics in AIS, we speculated that the integrity of dCA might affect the prognosis of patients after EVT through regulating cerebral hemodynamics. If the correlation between dCA and outcome is established, dCA may be used as an early hemodynamic marker to guide early management in patients who received EVT.
In this study, we sought to assess the dysfunctional pattern of dCA in AIS patients who received EVT. Then we aimed to explore the correlation between dCA and outcome for AIS patients treated with EVT, to find out whether that dCA is an independent outcome predictor and to determine the cut-off point to provide a novel prognostic tool.
Methods
Participants
The study was approved by the Medical Ethics Committee of Nanfang Hospital. Written informed consent was obtained from all participants. During October 2017 to February 2019, AIS patients were recruited if they: (1) aged between 18 and 85 years; (2) had a baseline National Institutes of Health Stroke Scale (NIHSS) scores of 4 to 24; (3) had an acute, unilateral side severe stenosis/occlusion of middle cerebral artery (MCA) or internal carotid; (4) underwent EVT within 24 h after the onset of symptoms [15]; (5) had a Thrombolysis In Cerebral Infarction (TICI) score [16] of ≥2b after EVT; (6) had a sufficient bilateral temporal bone window for insonation of MCA. Patients who were diagnosed as cancer or mental diseases were excluded. Patients received intravenous t-PA treatment before EVT were also regarded as eligible if the t-PA treatment was in a standard dose (0.9 mg/kg body weight) and given with 10% as a bolus and the remainder infused over 1 h (maximum dose, 90 mg). EVT decisions were based on the NIHSS score, occlusion site according to magnetic resonance angiography (MRA), and Arterial Spin Labeling (ASL) mismatch. EVT was performed by interventional neurologists using Solitaire (Solitaire, Covidien/ev3, Dublin, Ireland; FR revascularization device). The angiographic procedure had to begin and be completed within 24 h after the onset of stroke. If the patient had not received intravenous tPA, heparin infusion was started intravenously with a 2000-unit bolus, followed by an infusion of 450 units per hour during EVT, and was discontinued at the end of the procedure [17, 18]. We set up a healthy volunteers’ database who attended the annual physical examination in Nanfang Hospital from March 2017 to May 2017. In this study, we recruited healthy controls who were age-matched with AIS patients from the healthy volunteers’ database. They were without cerebrovascular risk factors and also should meet the following inclusion criteria: (1) without intracranial and extracranial vascular stenosis by transcranial Doppler sonography (TCD), carotid artery color Doppler (CD) examination; (2) had a sufficient bilateral temporal bone window for insonation of MCAs; (3) the absence of atrial fibrillation, hyperlipidemia, hypertension, diabetes mellitus and cerebral vascular disease history; (4) without a history of chronic physical or mental diseases, without an infectious disease in the past month, without a history of smoking or heavy drinking, no being pregnant or lactating.
The clinical prognosis was assessed with the modified Rankin Scale (mRS) [19], with mRS of 2 or less defined as a favourable outcome at 90 days. All mRS assessments at 90 days after stroke onset were performed by two investigators who were unaware of the study protocol.
Dynamic cerebral autoregulation (dCA) protocol
The dCA examination protocol was performed according to the white paper from the International Cerebral Autoregulation Research Network [20]. All healthy control subjects were asked to avoid nicotine, caffeine, alcohol, and all kinds of sleep medicines for at least 24 h before the dCA examination. The examination was performed bedside with minimal surrounding stimuli. The control subjects and the patients rested in a supine position with uncrossed legs for more than 15 min before the examination. First, the baseline arterial blood pressure (ABP) was measured at the brachial artery using an automatic blood pressure monitor (Omron 711). Second, we simultaneously recorded continuous spontaneous ABP via a servo-controlled plethysmograph placed around the left middle finger held at the level of the heart (Finometer Pro, Netherlands) and continuous MCA blood flow velocity (BFV) at a depth of 45 mm to 60 mm with 2 MHz probes attached to a customized head frame (EMS-9 PB, Shenzhen, China). Meanwhile, the PaCO2 level was also monitored, maintaining in stable rang. Data were recorded for 15 min for further data examination analysis. The artifacts were manually removed after recording.
The dCA analysis was performed using the multimodal real-time analysis software ICM+ invented by Brain Physics Lab of Cambridge University. According to the continuous ABP signal and bilateral MCA blood flow recordings, autoregulation indices including phase difference (PD) and coherence between the two signal components in the specific frequency domain range (0.06–0.12 Hz) was calculated by the transfer function. We used coherence as a data quality control parameter, and only when the coherence was greater than 0.4, the data were included in the subsequent statistical analysis.
Statistical evaluation
Continuous variables with normal distribution were presented as mean ± standard deviation, and non-normally distributed continuous variables were presented as median (interquartile range, IQR). Kolmogorov–Smirnov analysis was used to test the normality of data distribution. Frequencies (percentages) were measured for categorical variables. Dynamic CA data of healthy controls were analyzed based on the mean value of the left and right cerebral sides. To assess intergroup differences, we used Student’s t-test (normally distributed), Wilcoxon test (not normally distributed) and Chi-square or Fisher’s exact test as appropriate. To determine the relationship between studied variables, analyses of correlation was used. A multivariate logistic regression model was constructed. We derived crude and adjusted odds ratios of the magnitude of PD and an unfavourable clinical outcome from logistic regression. Odds ratios were adjusted for confounding variables, which were different between the 2 subgroups in univariate analysis (p < 0.1), including Fast blood glucose, triglyceride (TG), fast blood glue, body mass index (BMI), C-reactive protein (CRP), and NIHSS on admission. P values < 0.05 were considered statistically significant. The receiver operation characteristics (ROC) curve was used to get the cutoff point of PD to predict the unfavourable outcome after EVT. All statistical calculations were performed using SPSS 19 (SPSS, Chicago, IL, USA).
Results
Participant characteristics
In 71 AIS patients, 2 cases were EVT failure, 2 cases were diagnosed as cancer, and 5 cases refused to receive EVT. A total of 62 AIS patients (55.6 ± 14.5 years; 73% males) who underwent EVT were enrolled in the study (Table 1). Of them, 23 (37%) received t-PA treatment before EVT. All the patients had severe stenosis/occlusion of a unilateral side of MCA (n = 41) or internal carotid (n = 21). We also recruited 77 healthy volunteers as controls (Table 1). There were no significant differences in gender, age, BMI and TG between the AIS patients and controls. However, AIS patients were associated with higher systolic blood pressure (SBP), diastolic blood pressure (DBP), fast blood glucose, heart rate and C-Reactive protein (CRP) compared with healthy controls (all p < 0.05).
Table 1.
The demographic and clinical characteristics of healthy controls and patients
| Variable | Healthy controls (n = 77) | Acute ischemic stroke patients (n = 62) | P-value |
|---|---|---|---|
| Gender (male/female) | 53/24 | 45/17 | 0.710 |
| Age (years) | 55.75 ± 11.32 | 55.61 ± 14.53 | 0.949 |
| SBP (mmHg) on admission | 119.54 ± 13.12 | 137.85 ± 22.15 | < 0.001* |
| DBP (mmHg) on admission | 72.22 ± 10.62 | 77.43 ± 16.60 | 0.031* |
| Fast blood glucose (mmol/L) | 5.82 ± 1.55 | 7.29 ± 2.40 | < 0.001* |
| Heart rate (bpm) | 68.09 ± 8.71 | 75.71 ± 17.82 | 0.038* |
| BMI | 24.03 ± 2.65 | 23.49 ± 3.19 | 0.288 |
| TG (mmol/L) | 1.05 ± 0.28 | 1.24 ± 0.67 | 0.083 |
| CRP (mg/L) | 0.60 (0.33,1.20) | 7.89 (2.19, 20.47) | < 0.001* |
| Smoker | 0 (0) | 8 (38.10) | – |
| Drinker | 0 (0) | 4 (19.05) | – |
| Hypertension | 0 (0) | 8 (38.10) | – |
| Diabetes mellitus | 0 (0) | 2 (9.52) | – |
Values are expressed as mean ± SD or numbers (%) or median (inter- quartile range, IQR)
SBP Systolic blood pressure, DBP Diastolic blood pressure, TG Triglyceride, BMI Body Mass Index, CRP Hypersensitive C-Reactive Protein, NA Not applicable
*: significant difference in comparing with control
P-value: p-value of comparing between healthy controls and patients
Dynamic CA in controls and AIS patients
Dynamic CA was assessed at 24 h, 72 h, and 7 d after stroke onset in all AIS patients, but only once in healthy controls. Compared with healthy controls, PD values on bilateral hemispheres were significantly lower in AIS patients at different time points (Table 2). In AIS patients, PD values on the ipsilateral side were significantly lower than that on the contralateral side at 24 h and 7 d after onset. At 72 h, there was a trend towards lower values of PD on the ipsilateral side compared with the contralateral side, although no statistical significance was reached (p = 0.066) (Table 2). Univariate linear regression analysis showed that fast blood glucose on admission was associated with PD on the ipsilateral hemisphere at 24 h after symptom onset, β = − 4.453, p = 0.009.
Table 2.
The cerebral autoregulation value in controls and patients
| Variable | Healthy controls | AIS Patients | P-value | |
|---|---|---|---|---|
| contralateral side | ipsilateral side | |||
| Phase on 24 h after onset | 62.00 ± 18.43 | 40.50 ± 28.11a | 29.07 ± 25.71a | 0.012# |
| Phase on 72 h after onset | 62.00 ± 18.43 | 31.71 ± 23.88a | 26.37 ± 21.86a | 0.066 |
| Phase on 7d after onset | 62.00 ± 18.43 | 37.08 ± 27.06a | 29.13 ± 22.37a | 0.020# |
asignificant difference in comparing between controls and patients
#significant difference in comparing the dynamic cerebral autoregulation value between the contralateral side and the ipsilateral side
P: P-value of comparing between the contralateral side and the ipsilateral side
Dynamic CA in AIS patients with favourable and unfavourable outcomes
During follow up, 21 (34%) patients had favourable outcome and 41 (66%) patients had unfavourable outcome at 90 days after stroke onset (Table 3). Unfavourable outcome patients were more likely to have higher NIHSS, fast blood glucose, BMI, TG and CRP compared with those with favourable outcome (all p < 0.05). However, the ratio of t-PA treatment and ICA stenosis/occlusion, duration from symptoms onset to hospital and the door to needle time (DNT) were all comparable between the 90-day favourable and unfavourable outcome groups (all p > 0.05).
Table 3.
The demographic and clinical characteristics of patients with 90-day favourable outcome (mRS of 0–2) and unfavourable outcome (mRS of 3–6)
| Variable | Favourable outcome (n = 21) | Unfavourable outcome (n = 41) | P-value |
|---|---|---|---|
| Gender (male/female) | 17/4 | 28/13 | 0.375 |
| Age (years) | 51.48 ± 11.23 | 57.73 ± 15.67 | 0.109 |
| SBP (mmHg) on admission | 134.10 ± 20.69 | 139.73 ± 22.87 | 0.358 |
| DBP (mmHg) on admission | 78.05 ± 16.28 | 77.13 ± 15.45 | 0.831 |
| Fast blood glucose (mmol/L) | 6.06 ± 1.88 | 7.92 ± 2.41 | 0.003* |
| Heart rate (bpm) | 74.38 ± 14.50 | 75.71 ± 17.82 | 0.792 |
| BMI | 22.18 ± 3.14 | 24.16 ± 3.03 | 0.019* |
| TG (mmol/L) | 0.98 ± 0.30 | 1.37 ± 0.75 | 0.018* |
| CRP (mg/L) | 3.70 (1.15, 7.13) | 10.96 (4.57, 26.00) | 0.003* |
| Smoker | 8 (38.10) | 19 (46.34) | 0.596 |
| Drinker | 4 (19.05) | 13 (31.71) | 0.375 |
| Hypertension | 8 (38.10) | 18 (43.90) | 0.788 |
| Diabetes mellitus | 2 (9.52) | 11 (26.83) | 0.187 |
| NIHSS on admission | 9 (5, 14) | 15 (11, 20) | < 0.001* |
| Large artery stenosis/occlusion | 0.777 | ||
| MCA | 13 (61.90) | 28 (68.29) | |
| ICA | 8 (38.10) | 13 (31.71) | |
| t-PA treatment | 9 (42.86) | 14 (34.15) | 0.583 |
| Onset to hospital time (min) | 348.88 ± 280.57 | 288.33 ± 180.33 | 0.372 |
| DNT (min) | 115.06 ± 49.05 | 95.53 ± 58.58 | 0.258 |
| The time intervals between completed EVT and dCA measurement (hour) | 9.33 ± 3.57 | 7.76 ± 3.75 | 0.117 |
Values are expressed as mean ± SD or numbers (%) or median (inter- quartile range, IQR)
SBP Systolic blood pressure, DBP Diastolic blood pressure, TG Triglyceride, BMI Body Mass Index, CRP Hypersensitive C-Reactive Protein, NIHSS National Institutes of Health Stroke Scale, MCA Middle cerebral artery, ICA Internal carotid artery, DNT Door to needle time, dCA dynamic cerebral autoregulation, EVT Endovascular therapy
*significant difference in comparing between favourable-outcome group and unfavourable-outcome group
P-value: p-value of comparing between favourable-outcome group and unfavourable-outcome group
The dCA values in the favourable outcome and unfavourable outcome patients were presented in Table 4. On the contralateral side, the PD value showed no significant difference between these two groups at different time-points (all p > 0.05). On the ipsilateral side, however, the PD value at 24 h and 72 h were significantly lower in patients with unfavourable outcome than those with favourable outcome (all p < 0.05). Conversely, no significant differences were observed in 7d after onset.
Table 4.
The dynamic cerebral autoregulation value in favourable outcome and unfavourable outcome patients
| Variable | Favourable outcome (n = 21) | Unfavourable outcome (n = 41) | P-value |
|---|---|---|---|
| Phase on 24 h after onset | |||
| Phase on contralateral side | 51.97 ± 33.58 | 34.92 ± 23.59a | 0.072 |
| Phase on ipsilateral side | 45.47 ± 27.67a | 20.64 ± 20.32a | 0.001# |
| Phase on 72 h after onset | |||
| Phase on contralateral side | 39.97 ± 28.35a | 26.20 ± 18.93a | 0.066 |
| Phase on ipsilateral side | 34.70 ± 21.06a | 21.09 ± 21.01a | 0.032# |
| Phase on 7d after onset | |||
| Phase on contralateral side | 45.59 ± 28.65a | 31.23 ± 25.14a | 0.180 |
| Phase on ipsilateral side | 39.74 ± 24.57a | 22.49 ± 18.71a | 0.054 |
asignificant difference in comparing with control after adjusting confounding factors
#: significant difference in comparing between favourable-outcome group and unfavourable-outcome group
P: P-value of comparing between favourable-outcome group and unfavourable-outcome group
After adjusting the confounding factors including fast blood glucose, triglyceride (TG), body mass index (BMI), C-reactive protein (CRP), and NIHSS on admission, logistic regression analysis showed that PD value on the ipsilateral hemisphere at 24 h after onset was an independent predictor of clinical outcome, (adjusted OR = 0.889, 95% CI: 0.813–0.971, P = 0.009) (Table 5, Fig. 1). Lower ipsilateral PD value at 24 h after onset was correlated with higher mRS (r = − 0.402, P = 0.007), indicating poorer clinical outcomes. The ROC curve was performed to determine the cutoff point that optimized the sensitivity and specificity associated with clinical outcomes. The optimal cutoff value of the ipsilateral PD for predicting unfavourable outcomes was < 26.93° (sensitivity 76.50%, specificity 69.20%) (Fig. 2). The area under the curve of the ROC curve was 0.781.
Table 5.
Multivariate analysis of clinical characteristics and dynamic cerebral autoregulation for favorable long-term outcome
| Variables | Crude OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value |
|---|---|---|---|---|
| Clinical factors | ||||
| Fast blood glucose (mmol/L) | 1.726 (1.171, 2.544) | 0.006* | 3.523 (1.255, 9.885) | 0.017* |
| BMI | 1.250 (1.027, 1.521) | 0.026* | 0.623 | |
| TG (mmol/L) | 3.401 (0.937, 12.343) | 0.063 | 0.760 | |
| CRP (mg/L) | 1.088 (1.008,0.175) | 0.031* | 0.162 | |
| NIHSS on admission | 1.276 (1.105, 1.474) | 0.001* | 1.825 (1.155, 2.886) | 0.010* |
| Cerebral autoregulation | ||||
| dCA on 24 h after onset | ||||
| Phase on ipsilateral side | 0.955 (0.926, 0.985) | 0.004* | 0.889 (0.813, 0.971) | 0.009* |
| Phase on contralateral side | 0.978 (0.956, 1.000) | 0.048* | 0.184 | |
| dCA on 72 h after onset | ||||
| Phase on ipsilateral side | 0.970 (0.942, 0.998) | 0.039* | 0.205 | |
| Phase on contralateral side | 0.974 (0.948, 1.000) | 0.053 | 0.183 | |
| dCA on 7d after onset | ||||
| Phase on ipsilateral side | 0.962 (0.923, 1.003) | 0.069 | 0.259 | |
| Phase on contralateral side | 0.979 (0.950, 1.010) | 0.180 | ||
TG Triglyceride, BMI Body Mass Index, CRP Hypersensitive C-Reactive Protein, NIHSS National Institutes of Health Stroke Scale
*significant difference in comparing between favourable-outcome group and unfavourable-outcome group, p < 0.05
Fig. 1.
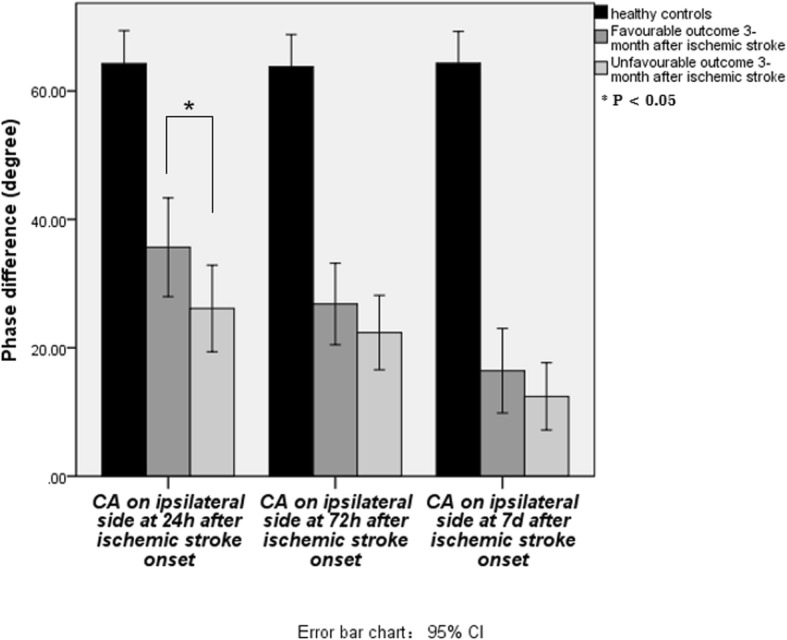
On the ipsilateral side, however, the PD value at 24 h was significantly lower in patients with unfavourable outcome than those with favourable outcome
Fig. 2.
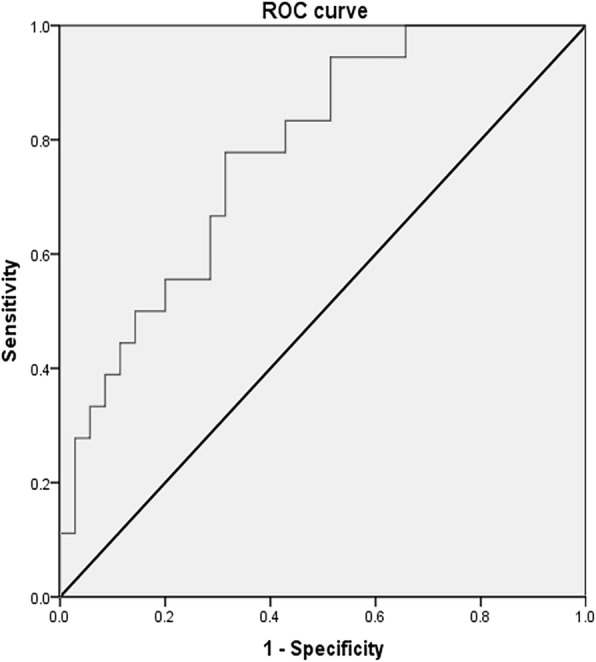
The ROC curve the optimal cutoff value of the ipsilateral PD for predicting unfavourable outcomes was < 26.93° (sensitivity 76.50%, specificity 69.20%)
Discussion
Endovascular therapy is increasingly used to treat patients with occlusions of the large intracranial arteries. However, the effect factors on clinical outcome after EVT are still unclear. In the study, we found that dCA was impaired bilaterally over the first 7 days after symptom onset, even those patients receiving EVT. The impairment of dCA on the ipsilateral side at 24 h after onset was associated with clinical outcome in AIS patients who received EVT. We also determined the optimal cutoff value of the dCA index for favourabe 90-day outcome prediction.
In the study, we enrolled patients who received EVT with or without t-PA treatment previously. We found similar safety outcomes and no significant difference in functional independence with EVT after intravenous t-PA, as compared with without t-PA treatment. That may be attributed to that we determine EVT patients according to the NIHSS score, MRA, and DWI-fluid attenuated inversion recovery mismatch. Further large sample size research is needed.
Choosing dCA as the marker
Recently, numerous studies emphasize the significant predictive value of cerebrovascular reserve in ischemic stroke [21, 22]. Reduced cerebrovascular reserve indicates that the arteriolar vasodilation activity is not able to maintain stable blood flow properly in the region of cerebral hypoperfusion [21, 23, 24]. DCA is the pivot pathophysiological process to maintain stable cerebrovascular reserve capacity. It was reported that impaired dCA might trigger ischemic brain lesions [14, 25]. The underlying pathophysiological mechanisms might be that the severe stenosis may significantly reduce cerebral perfusion pressure, which could reach its lower limit for autoregulation. Under these conditions, even slight reductions of BP may result in inadequate cerebral perfusion and would not be able to protect the brain from hemodynamic ischemic events [26]. With the development of TCD monitoring, dCA can be evaluated by simultaneous assessment of spontaneous fluctuations in BP and BFV in MCA (assessed by TCD) using different testing modalities in the time and frequency domain [20, 27]. The methodology of such an approach based on the concept that cerebral autoregulatory system functions as a high-pass filter, which means that high-frequency fluctuations of BP are normally passing through BFV unimpededly while low-frequency oscillations are dampened. As a result, slow waves of BFV do not occur simultaneously to similar waves of BP but with a time delay. A PD of 0°indicates total absence of autoregulation, while a large positive PD of 40°-70°can be regarded as intact autoregulation. In our study, the PD obtained from oscillations in ABP and CBFV for MCA in healthy subjects were similar, which is consistent with previous studies. Comparing with healthy controls, we found that dCA on both the ipsilateral hemisphere and the contralateral hemisphere were impaired in AIS patients. Consistently with our study, previous researches also reported bilateral impaired CA in the acute stroke [28, 29]. The mechanism of this trans-hemispheric communication may be diaschisis where there is distant functional depression due to the effects of loss of axons (mainly facilitatory) arising at the site of the lesion and, in the case of the cerebral hemispheres, these may synapse with neurons in the contralateral hemisphere via the corpus callosum [30]. However, further basic research was needed to confirm it. In addition, Dawson et al., have indicated that dCA appeared impaired bilaterally and remained so for at least 1 to 2 weeks over the subacute post-stroke period [31]. In a follow-up study, CA was also abnormal on the affected side > 2 months after stroke onset [32]. All those were closely in keeping with our finding, that the dysautoregulation still existed 7 days after symptom onset, even receiving EVT. That means our results support the theory of an additional secondary autoregulatory failure in acute cerebral ischemia. However, due to time restriction of EVT, we could not assess preoperative dCA, we still do not understand the detail change of postoperative dCA through comparing with preoperative dCA.
Influence factors on clinical outcome after EVT
In our study, we observed significantly lower dCA on the ipsilateral side at 24 h after symptom onset in unfavourable-outcome patients than favourable-outcome patients even after adjusting confounding factors. The t-test showed contralateral dCA with a difference bordering on significance between favourable-outcome and unfavourable-outcome groups. However after adjusting confounding factors, no significance of contralateral dCA was found. Larger sample size research is needed to explore it in future. The ROC showed that the PD value on the ipsilateral side at 24 h lower than 26.93° indicated unfavourable 90-day-outcome in patients who received EVT. In the studies about AIS patients performed by Chi et al. [33] and Castro el al [34], PD values were associated with mRS score > 2 at 3 months in patients with moderate to severe stroke even in the multivariate analysis. Castro et al. suggested PD < 37° as a cutoff to predict unfavourable outcomes, whereas Chi et al. suggested a cutoff of PD < 61°. The difference may be because of the different frequency band selection in transfer function analysis (TFA), that Chi et al. analyze PD in very low frequency. Besides, we noticed that our cut-off PD value was lower than the value reported by previous research. That may indicate that EVT may improve the tolerance to hemodynamic fluctuations. Saur et al. [35] and Reinhard et al. [25] suggested that the development of cerebral dysautoregulation may be particularly critical during the first days of reperfusion in larger infarctions. The first day is probably also the most critical period with regard to functional brain reorganization. It might be explained that in the acute stage after EVT, hemodynamic status changed. Focal dysautoregulation in the ischemic core and the former penumbral area might enhance the reperfusion injury and lead to secondary endothelial dysfunction, losing the protective function for the ischemic penumbra. Our findings indicate that special attention should be paid to the early acute stage of large acute ischemic stroke when secondary autoregulatory failure can evolve mainly in the affected vascular territory. In patients treated with EVT, BP control strategies in large ischemic stroke should be guided by the status of vessel recanalization and autoregulatory capacity, especially at 24 h after ischemic stroke onset. Consistent with our finding although with a different dCA monitoring measurement, a recent research suggested that continuous estimation of autoregulation-based treatment strategies after EVT was feasible and could provide a BP range for individual patients tailored to their own physiology [22]. In future, larger sample size research is needed to observe dCA status when changing BP level.
The present analysis has some limitations. Firstly, we recruited more male subjects than female in our study, since there are more male stroke patients than female in China [36], and female subjects are more likely to have insufficient bilateral temporal bone windows for insonation due to the low density of the temporal bone [37]. Secondly, the sample size of our research is small, further larger sample size research is needed. Thirdly, in our study, we used heparin during EVT, which might be the limitation of the protocol. Further relative research is needed.
Conclusion
In conclusion, dCA are impaired on the ipsilateral hemisphere and contralateral hemisphere during the first 7 days after AIS symptom onset. Dynamic CA on the ipsilateral hemisphere at 24 h after symptom onset is independently associated with clinical outcomes in AIS patients who received EVT. Impaired dCA can be set up as an early hemodynamic marker to guide acute stage management in patients who received EVT. It provides a novel prognostic tool to improve clinical outcomes achieving personalized treatment.
Acknowledgments
The work was funded by the National Natural Science Foundation of China (Grant No. 81801179).
Abbreviations
- EVT
Endovascular therapy
- AIS
Acute ischemic stroke
- dCA
Dynamic cerebral autoregulation
- MCA
Middle cerebral artery
- PD
Phase difference
- mRS
Modified Rankin Scale
- ROC
Receiver operation characteristics
- CBF
Cerebral blood flow
- ABP
Arterial blood pressure
- NIHSS
National Institutes of Health Stroke Scale
- TICI
Thrombolysis In Cerebral Infarction
- MRA
Magnetic resonance angiography
- ASL
Arterial Spin Labeling
- TCD
Transcranial doppler sonography
- CD
Carotid artery color doppler
- IQR
Interquartile range
- TG
Triglyceride
- BMI
Body mass index
- CRP
C-reactive protein
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- TFA
Transfer function analysis
Authors’ contributions
Study concept and design: WYM, PSY, TG. Acquisition of data: TG, JZ, LZZ. Statistical analysis: HKB, LZZ. Drafting of the manuscript: TG, HKB. Critical revision of the manuscript for important intellectual content: TG, WYM, JZ, HKB. Study supervision: WYM, PSY. The authors read and approved the final manuscript.
Funding
The work was funded by the National Natural Science Foundation of China (Grant No. 81801179).
Availability of data and materials
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
Ethics approval and consent to participate
The study was approved by the Medical Ethics Committee of Nanfang Hospital. Written informed consent was obtained from all participants. The data presented were collected for the purpose of quality assurance and, thus, the identity of the individual patients were anonymous.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing financial interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Suyue Pan, Email: pansuyue@126.com.
Yongming Wu, Email: WU_YONGMINGNANFANG@126.com.
References
- 1.Bonita R, Mendis S, Truelsen T, Bogousslavsky J, Toole J, Yatsu F. The global stroke initiative. Lancet Neurol. 2004;3:391–393. doi: 10.1016/S1474-4422(04)00800-2. [DOI] [PubMed] [Google Scholar]
- 2.Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke. 2006;1:158–159. doi: 10.1111/j.1747-4949.2006.00045.x. [DOI] [PubMed] [Google Scholar]
- 3.Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke. 2008;39:2396–2399. doi: 10.1161/STROKEAHA.107.505776. [DOI] [PubMed] [Google Scholar]
- 4.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 5.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 6.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 7.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 8.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. T-PA alone in stroke. N Engl J Med. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 9.Rodrigues FB, Neves JB, Caldeira D, Ferro JM, Ferreira JJ, Costa J. Endovascular treatment versus medical care alone for ischaemic stroke: systematic review and meta-analysis. BMJ. 2016;353:i1754. doi: 10.1136/bmj.i1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabinstein AA, Albers GW, Brinjikji W, Koch S. Factors that may contribute to poor outcome despite good reperfusion after acute endovascular stroke therapy. Int J Stroke. 2018;14(1):23. doi: 10.1177/1747493018799979. [DOI] [PubMed] [Google Scholar]
- 11.Xiong L, Liu X, Shang T, Smielewski P, Donnelly J, Guo ZN, et al. Impaired cerebral autoregulation: measurement and application to stroke. J Neurol Neurosurg Psychiatry. 2017;88:520–531. doi: 10.1136/jnnp-2016-314385. [DOI] [PubMed] [Google Scholar]
- 12.van Beek AH, Claassen JA, Rikkert MG, Jansen RW. Cerebral autoregulation: an overview of current concepts and methodology with special focus on the elderly. J Cereb Blood Flow Metab. 2008;28:1071–1085. doi: 10.1038/jcbfm.2008.13. [DOI] [PubMed] [Google Scholar]
- 13.Immink RV, van Montfrans GA, Stam J, Karemaker JM, Diamant M, van Lieshout JJ. Dynamic cerebral autoregulation in acute lacunar and middle cerebral artery territory ischemic stroke. Stroke. 2005;36:2595–2600. doi: 10.1161/01.STR.0000189624.06836.03. [DOI] [PubMed] [Google Scholar]
- 14.Reinhard M, Rutsch S, Lambeck J, Wihler C, Czosnyka M, Weiller C, et al. Dynamic cerebral autoregulation associates with infarct size and outcome after ischemic stroke. Acta Neurol Scand. 2012;125:156–162. doi: 10.1111/j.1600-0404.2011.01515.x. [DOI] [PubMed] [Google Scholar]
- 15.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the american heart association/American stroke association. Stroke. 2018;49:e46–e110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 16.Tomsick T. TIMI, TIBI, TICI: I came, I saw, I got confused. AJNR Am J Neuroradiol. 2007;28:382–384. [PMC free article] [PubMed] [Google Scholar]
- 17.Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368:893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khatri P, Yeatts SD, Mazighi M, Broderick JP, Liebeskind DS, Demchuk AM, et al. Time to angiographic reperfusion and clinical outcome after acute ischaemic stroke: an analysis of data from the interventional Management of Stroke (IMS III) phase 3 trial. Lancet Neurol. 2014;13:567–574. doi: 10.1016/S1474-4422(14)70066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 20.Claassen JA, Meel-van DAA, Simpson DM, Panerai RB. Transfer function analysis of dynamic cerebral autoregulation: a white paper from the international cerebral autoregulation research network. J Cereb Blood Flow Metab. 2016;36:665–680. doi: 10.1177/0271678X15626425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshall RS, Rundek T, Sproule DM, Fitzsimmons BF, Schwartz S, Lazar RM. Monitoring of cerebral vasodilatory capacity with transcranial Doppler carbon dioxide inhalation in patients with severe carotid artery disease. Stroke. 2003;34:945–949. doi: 10.1161/01.STR.0000062351.66804.1C. [DOI] [PubMed] [Google Scholar]
- 22.Petersen NH, Silverman A, Strander SM, Kodali S, Wang A, Sansing LH, et al. Fixed compared with autoregulation-oriented blood pressure thresholds after mechanical thrombectomy for ischemic stroke. Stroke. 2020;51:914–921. doi: 10.1161/STROKEAHA.119.026596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King A, Serena J, Bornstein NM, Markus HS. Does impaired cerebrovascular reactivity predict stroke risk in asymptomatic carotid stenosis? A prospective substudy of the asymptomatic carotid emboli study. Stroke. 2011;42:1550–1555. doi: 10.1161/STROKEAHA.110.609057. [DOI] [PubMed] [Google Scholar]
- 24.Gupta A, Chazen JL, Hartman M, Delgado D, Anumula N, Shao H, et al. Cerebrovascular reserve and stroke risk in patients with carotid stenosis or occlusion: a systematic review and meta-analysis. Stroke. 2012;43:2884–2891. doi: 10.1161/STROKEAHA.112.663716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reinhard M, Wihler C, Roth M, Harloff A, Niesen WD, Timmer J, et al. Cerebral autoregulation dynamics in acute ischemic stroke after rtPA thrombolysis. Cerebrovasc Dis. 2008;26:147–155. doi: 10.1159/000139662. [DOI] [PubMed] [Google Scholar]
- 26.Semenyutin VB, Asaturyan GA, Nikiforova AA, Aliev VA, Panuntsev GK, Iblyaminov VB, et al. Predictive value of dynamic cerebral autoregulation assessment in surgical management of patients with high-grade carotid artery stenosis. Front Physiol. 2017;8:872. doi: 10.3389/fphys.2017.00872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Phys. 1998;274:H233–H241. doi: 10.1152/ajpheart.1998.274.1.h233. [DOI] [PubMed] [Google Scholar]
- 28.Armstead WM. Cerebral blood flow autoregulation and dysautoregulation. Anesthesiol Clin. 2016;34:465–477. doi: 10.1016/j.anclin.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiong L, Tian G, Lin W, Wang W, Wang L, Leung T, et al. Is dynamic cerebral autoregulation bilaterally impaired after unilateral acute ischemic stroke? J Stroke Cerebrovasc Dis. 2017;26:1081–1087. doi: 10.1016/j.jstrokecerebrovasdis.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 30.Eames PJ, Blake MJ, Dawson SL, Panerai RB, Potter JF. Dynamic cerebral autoregulation and beat to beat blood pressure control are impaired in acute ischaemic stroke. J Neurol Neurosurg Psychiatry. 2002;72:467–472. doi: 10.1136/jnnp.72.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dawson SL, Panerai RB, Potter JF. Serial changes in static and dynamic cerebral autoregulation after acute ischaemic stroke. Cerebrovasc Dis. 2003;16:69–75. doi: 10.1159/000070118. [DOI] [PubMed] [Google Scholar]
- 32.Dawson SL, Manktelow BN, Robinson TG, Panerai RB, Potter JF. Which parameters of beat-to-beat blood pressure and variability best predict early outcome after acute ischemic stroke? Stroke. 2000;31:463–468. doi: 10.1161/01.str.31.2.463. [DOI] [PubMed] [Google Scholar]
- 33.Chi NF, Hu HH, Wang CY, Chan L, Peng CK, Novak V, et al. Dynamic cerebral autoregulation is an independent functional outcome predictor of mild acute ischemic stroke. Stroke. 2018;49:2605–2611. doi: 10.1161/STROKEAHA.118.022481. [DOI] [PubMed] [Google Scholar]
- 34.Castro P, Serrador JM, Rocha I, Sorond F, Azevedo E. Efficacy of cerebral autoregulation in early ischemic stroke predicts smaller infarcts and better outcome. Front Neurol. 2017;8:113. doi: 10.3389/fneur.2017.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, et al. Dynamics of language reorganization after stroke. Brain. 2006;129:1371–1384. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- 36.Liu M, Wu B, Wang WZ, Lee LM, Zhang SH, Kong LZ. Stroke in China: epidemiology, prevention, and management strategies. Lancet Neurol. 2007;6:456–464. doi: 10.1016/S1474-4422(07)70004-2. [DOI] [PubMed] [Google Scholar]
- 37.Wijnhoud AD, Franckena M, van der Lugt A, Koudstaal PJ, Dippel ED. Inadequate acoustical temporal bone window in patients with a transient ischemic attack or minor stroke: role of skull thickness and bone density. Ultrasound Med Biol. 2008;34:923–929. doi: 10.1016/j.ultrasmedbio.2007.11.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.


