Abstract
OBJECTIVE
Intracerebral convection-enhanced delivery (CED) has been limited to short durations due to a reliance on externalized catheters. Preclinical studies investigating topotecan (TPT) CED for glioma have suggested that prolonged infusion improves survival. Internalized pump-catheter systems may facilitate chronic infusion. The authors describe the safety and utility of long-term TPT CED in a porcine model and correlation of drug distribution through coinfusion of gadolinium.
METHODS
Fully internalized CED pump-catheter systems were implanted in 12 pigs. Infusion algorithms featuring variable infusion schedules, flow rates, and concentrations of a mixture of TPT and gadolinium were characterized over increasing intervals from 4 to 32 days. Therapy distribution was measured using gadolinium signal on MRI as a surrogate. A 9-point neurobehavioral scale (NBS) was used to identify side effects.
RESULTS
All animals tolerated infusion without serious adverse events. The average NBS score was 8.99. The average maximum volume of distribution (Vdmax) in chronically infused animals was 11.30 mL and represented 32.73% of the ipsilateral cerebral hemispheric volume. Vdmax was achieved early during infusions and remained relatively stable despite a slight decline as the infusion reached steady state. Novel tissue TPT concentrations measured by liquid chromatography mass spectroscopy correlated with gadolinium signal intensity on MRI (p = 0.0078).
CONCLUSIONS
Prolonged TPT-gadolinium CED via an internalized system is safe and well tolerated and can achieve a large Vdmax, as well as maintain a stable Vd for up to 32 days. Gadolinium provides an identifiable surrogate for measuring drug distribution. Extended CED is potentially a broadly applicable and safe therapeutic option in select patients.
Keywords: convection-enhanced delivery, glioblastoma, malignant gliomas, topotecan, drug delivery, blood-brain barrier, central nervous system, oncology
Convection-enhanced delivery (CED) is a bulk flow-driven method of local-regional drug infusion to distribute high concentrations of therapeutic compounds directly into the brain. Because it circumvents blood-brain barrier limitations and avoids systemic toxicity, CED has inherent advantages over common diffusion-driven systemic methods such as oral or intravenous delivery.24 Multiple studies have demonstrated the safety and efficacy of CED for a variety of CNS diseases.3,13,17,21,24,28,29,34,37,40
The advantages of CED are attractive for the treatment of malignant gliomas where recurrence from invasive tumor cells commonly occurs within centimeters of the original tumor.2,16 In these clinical scenarios, CED allows for uniform delivery of a wide range of compounds to the tumor and peritumoral region; unlike diffusive therapies, uniform delivery is not limited by concentration gradients.3,17,21,24,29,34,40 Consequently, several clinical trials and small case series investigating a wide range of therapeutics have demonstrated the potential efficacy of CED for malignant gliomas, with promising results in phase I, II, and III clinical trials.1,4,7,12,18–20,26,33,39,42 Interestingly, while most clinical series published following the widespread adoption of the Stupp protocol36 have reported excellent survival after CED regardless of the drug infused or pathology, the specific benefits or durability of responses for any particular agents remain poorly described (Table 1).4,7,12,18,19 Furthermore, progression outside the areas of infusion suggests a need for longer-term infusions or modifiable catheter positions in response to tumor progression.
TABLE 1.
Summary of clinical trials of CED for malignant gliomas following adoption of the Stupp protocol in 2005
| Authors & Year | Trial Phase | Agent Infused | WHO Grade & Type (no.) | Flow Rate (mL/hr) | Vol Infused (mL) | Duration | Catheter No. | PFS (wks) | Median OS (wks) |
|---|---|---|---|---|---|---|---|---|---|
| Vogelbaum et al., 2007 | I | Cintredekin besudotox | III AO (1); IV GBM (21) | 0.75 | 3 | 4 days | 2–4 | 5–78 | 44 (5–113) |
| Kunwar et al., 2007 | I | Cintredekin besudotox | III AA (3), AO (2); IV GBM (46) | 0.75 | 3 | 4–6 days | 1–3 | NA | 45.9 |
| Kunwar et al., 2010 | III | Cintredekin besudotox | IV GBM (296) | 0.75 | 3 | 4 days | 2–4 | NA | 45.3 |
| Bogdahn et al., 2011 | IIB | Trabedersen | III AA (42); IV GBM (103) | 0.24 | 40 | 7 days | 1 | NA | 66 (GBM 29.2, AA 158) |
| Bruce et al., 2011 | IB | Topotecan | III high-grade gliomas* (6); IV GBM (10) | 0.4 | 40 | 4.17 days | 2 | 22 | 58 |
| Desjardins et al., 2018 | I | PVSRIPO | IV GBM (61) | 0.5 | 3.25 | 6.5 hrs | 1 | NA | 50 |
AA = anaplastic astrocytoma; AO = anaplastic oligodendroglioma; NA = not available.
Including anaplastic astrocytomas, anaplastic oligodendrogliomas, and anaplastic ependymomas.
Topotecan (TPT), a camptothecin analog and inhibitor of topoisomerase I, has potent antitumor activity with minimal consequence to normal brain in preclinical glioma models.6,8,15,22,23 While systemic TPT administration results in poorly tolerated, dose-limited toxicities without appreciable antitumor effects,14 a recent phase I trial using externalized catheter-pump systems demonstrated that short-term TPT CED is safe and efficacious in patients with recurrent malignant glioma.7 Because preclinical studies showed increased survival with prolonged TPT infusion,22 an implantable, internalized system for prolonged CED was developed and recently tested in a large animal model, establishing the safety and feasibility of up to 10-day infusions.35
Here, we present validation for targeted, prolonged TPT CED utilizing an implantable pump-catheter system that is adaptable for human trials. We evaluate the effects of flow-rate modulation on the achievable volume of distribution (Vd) of infused chemotherapy in a large animal model and show that high flow rates are well tolerated and capable of achieving a large and stable Vd for the duration of treatment. Finally, we demonstrate that the coadministration of gadolinium with TPT provides a reliable noninvasive method to assess Vd.
Methods
Topotecan and Gadolinium Interactions
To examine the effect of gadolinium on TPT activity, U87 human glioma cells were plated in triplicate at 5 × 104 cells/well. Cells were incubated for 48 hours in nutrient-rich media (D10) containing 146 μM TPT, both with and without 1:100 gadolinium. Control experiments were performed with cells cultured in D10 alone—without TPT or gadolinium—and in media containing gadolinium without TPT. MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assays were performed, and absorbance at 490 nm (OD490) was measured as a surrogate for cell density of each sample.
Surgical Procedures
Implantation and Chemotherapy Refill
All procedures were approved by the Institutional Animal Care and Use Committee at Columbia University Medical Center and were compliant with standard animal research practices. Twelve male pigs (> 15 kg) underwent subcutaneous implantation of a pump-catheter system consisting of a single proximal tapered SmartFlow Flex ventricular catheter (0.5-mm internal diameter, MRI Intervention, Inc.) connected via a silastic lumbar catheter (model INS-5010, Integra) to a Synchromed II pump (model 8637–20, Medtronic).
Briefly, anesthetized animals underwent preoperative volumetric MRI. The posterior centrum semiovale was targeted for infusion using Brainlab neuronavigation software. Trajectories maximized catheter depth within brain parenchyma to ensure approximately 2 cm of intracranial catheter. Animals were rigidly immobilized prone, and standard registration with the Brainlab frameless stereotactic system (Kolibri Navigation System, Brainlab AG) was performed (Fig. 1). A single cranial burr hole was made, and the proximal catheter was stereotactically guided to its target using the VarioGuide system (Brainlab AG). The pump was implanted and connected as described previously.35 The animals were recovered and maintained postoperatively in an intensive care setting. Microinfusion pumps were aspirated and refilled every 4–5 days according to standard protocol.
FIG. 1. A:

Animals were sedated, intubated, and placed prone with their heads secured with rigid fixation where subsequent scalp registration with the Brainlab frameless stereotactic system was performed. B: The proximal catheter was stereotactically guided to its target using the VarioGuide neuronavigation system. C: A microinfusion pump was implanted subcutaneously in the ipsilateral flank, and a silastic lumbar catheter was primed with infusate and tunneled from the flank to the cranium. D: The pump-catheter system, consisting of a single proximal tapered SmartFlow Flex ventricular catheter (0.5-mm internal diameter) connected via a silastic lumbar catheter to a Synchromed II pump. Figure is available in color online only.
Study Design
Short Term
Five consecutive animals underwent implantation and infusion of 136 μM TPT and 1:100 gadolinium for a 3-to 4-day duration according to facility availability (Table 2). Two animals began infusions at a rate of 4 mL/day—slightly lower than the rate used in our previously published human trial (4.8 mL/day) to accommodate the smaller volume of the porcine brain.7 Three animals began infusions at a rate of 2 mL/day with a subsequent increase in the infusion rate to 4 mL/day for the remainder of the study. Infusions were stopped if loss of convection occurred due to significant intraventricular or subarachnoid extension of infusate, as identified on serial MRI.
TABLE 2.
Characteristics of the short-term cohort
| Pig No. | Catheter Location | Intraventricular Extension | Infusion Rate | Infusion Duration | FVi (mL) |
Vdmax (mL) | FVd (mL) |
FVd/FVi |
|---|---|---|---|---|---|---|---|---|
| 1 | Posterior CSO | Yes | 4 mL/day ×3 days | 3 days | 12.0 | 4.59 | 4.59 | 0.38 |
| 2 | Posterior CSO | Yes | 4 mL/day ×4 days | 4 days | 16.0 | 4.78 | 4.78 | 0.30 |
| 3 | Posterior CSO | No | 2 mL/day ×2 days; 4 mL/day ×2 days | 4 days | 12.0 | 11.12 | 10.8 | 0.90 |
| 4 | Posterior CSO | No | 2 mL/day ×1 day; 4 mL/day ×3 days | 4 days | 14.0 | 15.64 | 15.6 | 1.14 |
| 5 | Hippocampus | No | 2 mL/day ×1 day; 4 mL/day ×3 days | 4 days | 14.0 | 1.98 | 1.98 | 0.14 |
CSO = centrum semiovale.
Long Term
Seven additional animals were subsequently implanted and underwent prolonged CED over increasing intervals from 11 to 32 days (Table 3). One animal underwent infusion with a mixture of low-concentration TPT (43.6 μM) and 1:100 gadolinium for 11 days. Four animals underwent infusion with a mixture of TPT (136 μM) and 1:100 gadolinium for 15 days (n = 1), 25 days (n = 2), and 32 days (n = 1). One animal underwent infusion with saline and 1:100 gadolinium alone for 29 days as a control. An additional animal underwent infusion with a mixture of TPT (136 μM) and 1:100 gadolinium for 30 days on a 7-day intermittent bolus schedule consisting of 2 days of infusion at 4 mL/day followed by a 5-day break and repeating for 5 cycles. All continuous infusions were initiated at a rate of 2 mL/day and subsequently increased to 4 mL/day. The timing of the rate increases and final time points varied with postoperative MRI scheduling and facility availability to capture increasing intervals between 11 and 32 days.
TABLE 3.
Characteristics of the long-term cohort
| Pig No. | Catheter Location | Intraventricular Extension | TPT Concentration (μM) | Infusion Rate | Infusion Duration | FVi (mL) | Vdmax (mL) | FVd (mL) |
|---|---|---|---|---|---|---|---|---|
| 6 | Posterior CSO | No | 43.68 | 2 mL/day ×1 day; 4 mL/day ×10 days | 11 days | 42 | 10.97 | 4.48 |
| 7 | Posterior CSO | No | 136 | 2 mL/day ×1 day; 4 mL/day ×14 days | 15 days | 58 | 12.45 | 4.96 |
| 8 | Posterior CSO | Yes | 136 | 2 mL/day ×7 days; 4 mL/day ×18 days | 25 days | 86 | 8.32 | 6.38 |
| 9 | Posterior CSO | No | 136 | 2 mL/day ×2 days; 4 mL/day ×23 days | 25 days | 96 | 10.52 | 10.52 |
| 10 | Posterior CSO | No | 136 | 2 mL/day ×1 day; 4 mL/day ×31 days | 32 days | 126 | 13.96 | 9.59 |
| 11 | Posterior CSO | No | 0* | 2 mL/day ×1 day; 4 mL/day ×28 days | 29 days | 114 | 11.55 | 5.77 |
| 12 | Posterior CSO | No | 136 | 5 cycles: 4 mL/day ×2 days + 0 mL/day ×5 days | 30 days | 40 | 11.01 | 11.01 |
Saline infusion.
Clinical Assessment
Veterinary assessments for side effects or evidence of systemic toxicity utilized a 9-point porcine neurobehavioral scale (NBS) evaluating mental status, appetite, and motor function/gait throughout treatment.25,35
Serial Imaging
Serial noncontrast MRI was performed using a 3T Philips magnet. Animals in the short-term cohort were imaged on postoperative days −1, 0, 1, 2, 3, and 4. Animals in the long-term cohort were imaged on postoperative days −1, 0, and 1 and subsequently had between 4 and 7 additional MRI sessions throughout the duration of the infusion based on MRI availability.
Image Analysis
The Vd of gadolinium was quantified as it appeared on T1-weighted MR sequences using a computer-assisted, semiautomated algorithm, as described previously.9 Maximum Vd (Vdmax) and final volume of distribution (FVd) were determined in this fashion and compared with the final volume infused (FVi) using the formula FVd/FVi. MRI pixel intensities were quantified using Brainab software.
Biopsy and Euthanasia
All animals in the short-term cohort underwent stereotactically guided tissue biopsies for liquid chromatography mass spectroscopy (LCMS) analysis. Briefly, using MR images acquired on the final day of infusion, multiple biopsy targets were chosen from areas of varying absolute gadolinium intensity within and around the region of convection. Following MRI, the animals were euthanized, and biopsies were rapidly carried out using the VarioGuide system.
Following the biopsies, the infusion pump and catheter were removed and inspected. Whole brains were harvested from the animals in the long-term cohort (n = 5) for histological analysis. Serum samples were taken from 3 animals in the short-term cohort and 1 animal in the long-term cohort for LCMS analysis to assess systemic levels of infused chemotherapy prior to death.
Tissue Analysis and Histology
LCMS analysis was performed on all tissue samples obtained from regions of varying gadolinium intensity within the final infusion volumes. Briefly, an analytical method for the quantitation of TPT concentrations in tissue using liquid chromatography with tandem spectrometry (LC/MS/MS) was developed. Aliquots of brain homogenate (20 μL of a 10% wt/vol homogenate in water) samples were processed in a single protein precipitation step by adding 3 times the volume of methanol containing 100 ng/mL of the internal standard (camptothecin), and vortexing for 1 minute followed by centrifugation for 5 minutes at 15,000 rpm (21,130g). Aliquots of the resultant supernatant were injected into the LC/MS/MS. The LC system consisted of a C18 guard cartridge and a Luna C18 analytical column with a gradient solvent system that consisted of mobile phases A) 0.5 mM ammonium for-nate (0.1% formic acid, vol/vol) and B) acetonitrile. The optimized mobile phase gradient program was as follows: 0–0.8 minute, 5% acetonitrile; 0.8–1.3 minutes, 5%–100% acetonitrile; 1.3–2.2 minutes, 85% acetonitrile; and 2.2–2.8 minutes, 85%–5% acetonitrile. The mass spectrometer (AB Sciex QTRAP5500) was operated in positive ion scan mode with ESI-SRM (electrospray ionization–selected reaction monitoring) ion transitions of m/z 422.2 → 377.2 for TPT and 349.0 → 305.0 for the internal standard. The analytical methods were specific and sensitive with a lower limit of quantification of 1 ng/mL. The intraday and interday variability was less than 15%. The average run time was about 3 minutes for each sample. Each biopsy’s TPT concentration was compared with its respective absolute gadolinium pixel intensity on MRI and plotted on a logarithmic scale to determine whether measurement of gadolinium distribution functioned adequately as a surrogate for TPT distribution. LCMS analysis was additionally performed in triplicate on 1-mL serum samples in order to detect systemic concentrations of TPT. Additional histological analysis of H & E–stained tissue was performed on whole brains from 5 animals within the long-term cohort and evaluated by a board-certified pathologist (P.C.).
Statistical Analysis
Statistical analysis was performed using Prism 4 (GraphPad Software); p < 0.05 was considered statistically significant.
Results
Topotecan and Gadolinium Do Not Interact
MTT assays were performed to evaluate TPT efficacy in the setting of gadolinium (Fig. 2). At 48 hours, there was no significant difference in mean OD490 between cells plated in media with or without gadolinium (2.1 vs 2.1, p = 0.69). Addition of TPT (146 μM) resulted in a significant reduction in OD490 at 48 hours in media with and without 1:100 gadolinium (1.3 vs 2.1 and 1.4 vs 2.1, p < 0.01, respectively; data not shown). Conversely, the addition of 1:100 gadolinium to media already containing TPT (146 μM) did not alter the OD490 at 48 hours (1.4 vs 1.3, p = 0.48) confirming the presence of gadolinium does not influence TPT cytotoxicity.
FIG. 2.
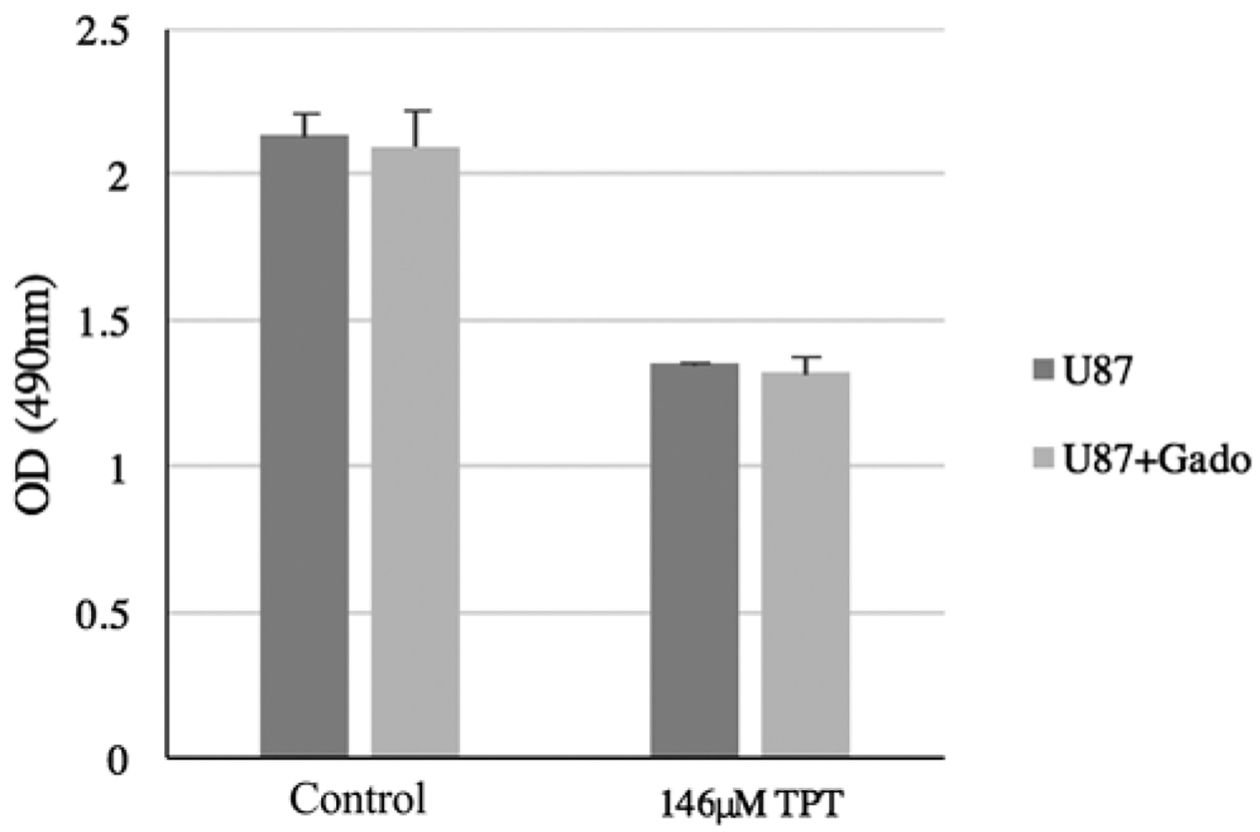
The cytotoxic effects of TPT are not affected by the presence of gadolinium (gado). At 48 hours, there was no difference in OD490 between U87 cells plated in media with or without gadolinium (2.13 vs 2.10, p = 0.69). Addition of TPT (146 μM) resulted in a significant reduction in OD490 at 48 hours in media with and without 1:100 gadolinium (1.28 vs 2.10 and 1.35 vs 2.13, p < 0.01, respectively; data not shown). The addition of 1:100 gadolinium to media already containing TPT (146 μM) did not alter the OD490 at 48 hours (1.35 vs 1.32, p = 0.48). Bars represent the mean and error bars, SD.
Surgical Outcomes and CED Optimization
Short-Term Cohort
To optimize infusion rates for prolonged CED, 5 consecutive pigs underwent pump-catheter implantation (Table 2). Postoperative MRI confirmed catheter tip accuracy in 4 animals (80%). In 1 animal (20%), catheter placement was deep to the preidentified target in the right posterior hippocampus (Fig. 3A). This catheter was left to facilitate comparison of target-tissue factors on infusion dynamics.
FIG. 3. A:

Preoperative (left) and final (right) axial T1-weighted MR images obtained in pig 5, demonstrating infusion volume confined to the right posterior hippocampus. The blue circle on the preoperative image represents the final location of the catheter tip. B: Final axial T1-weighted MR images obtained in pigs 1 and 2, demonstrating extravasation of infusate into the right lateral ventricle. Figure is available in color online only.
Infusion rates and schedules were titrated in consecutive animals to maximize Vd (Table 2). Two animals had infusions at a rate of 4 mL/day with extravasation into the ipsilateral lateral ventricle (Fig. 3B). Intraventricular extravasation resulted in preferential distribution of infusate into the ventricular system and limited the achievable FVd (Table 2).
Two subsequent animals underwent infusions at 2 mL/day (Table 2). In the first animal (pig 3), infusion was maintained at 2 mL/day for 2 days and subsequently increased to 4 mL/day for the remaining 2 days of infusion. In the second animal (pig 4), infusion was maintained at 2 mL/day for 1 day and subsequently increased to 4 mL/day for the remaining 3 days. No extravasation occurred, suggesting a benefit to “priming” the targeted tissue with slow infusion prior to flow-rate increases.
Hippocampal infusion began at 2 mL/day for 1 day and was subsequently increased to 4 mL/day for 3 days. Infusion appeared restricted to the anatomical boundaries of the hippocampus on serial MRI, suggesting that restricted distribution and/or rapid clearance of infusions may occur within the hippocampus (Table 2, Fig. 3A).
Long-Term Cohort
Seven pigs underwent pump-catheter implantation for chronic TPT CED (concentration 0–136 μM; Table 3). In 1 pig (17%), a catheter disconnection was discovered after the first postinfusion MRI study, requiring reoperation and reattachment. Notably, the subcutaneous tissue surrounding the catheter demonstrated no evidence of tissue edema, damage, or necrosis suggestive of TPT toxicity.
Within the long-term cohort, 1 animal (pig 8) demonstrated intraventricular extension on postoperative day 4. Review of the postoperative MRI demonstrated that the catheter tip was closer to the lining of the ipsilateral lateral ventricle than in other implanted animals (data not shown). Table 3 shows the FVi, Vdmax, FVd, and FVd/FVi data for the long-term cohort.
CED Achieves Large and Stable Intracerebral Volumes of Distribution
Gadolinium Is a Surrogate for Drug Distribution
Hyperintense gadolinium signal was identified on T1-weighted sequences on all MR images obtained during active infusion of TPT-gadolinium mixtures. No catheter backflow was identified in any animal. To correlate gadolinium signal intensity to TPT concentration, 92 stereotactic tissue biopsies from 5 animals in the short-term cohort were evaluated by LCMS analysis. Samples were taken from regions of variable gadolinium hyperintensity within and around the infusion volume. Linear regression analysis revealed significant positive correlation between MRI gadolinium signal intensity and TPT concentration (p = 0.008; Fig. 4).
FIG. 4.
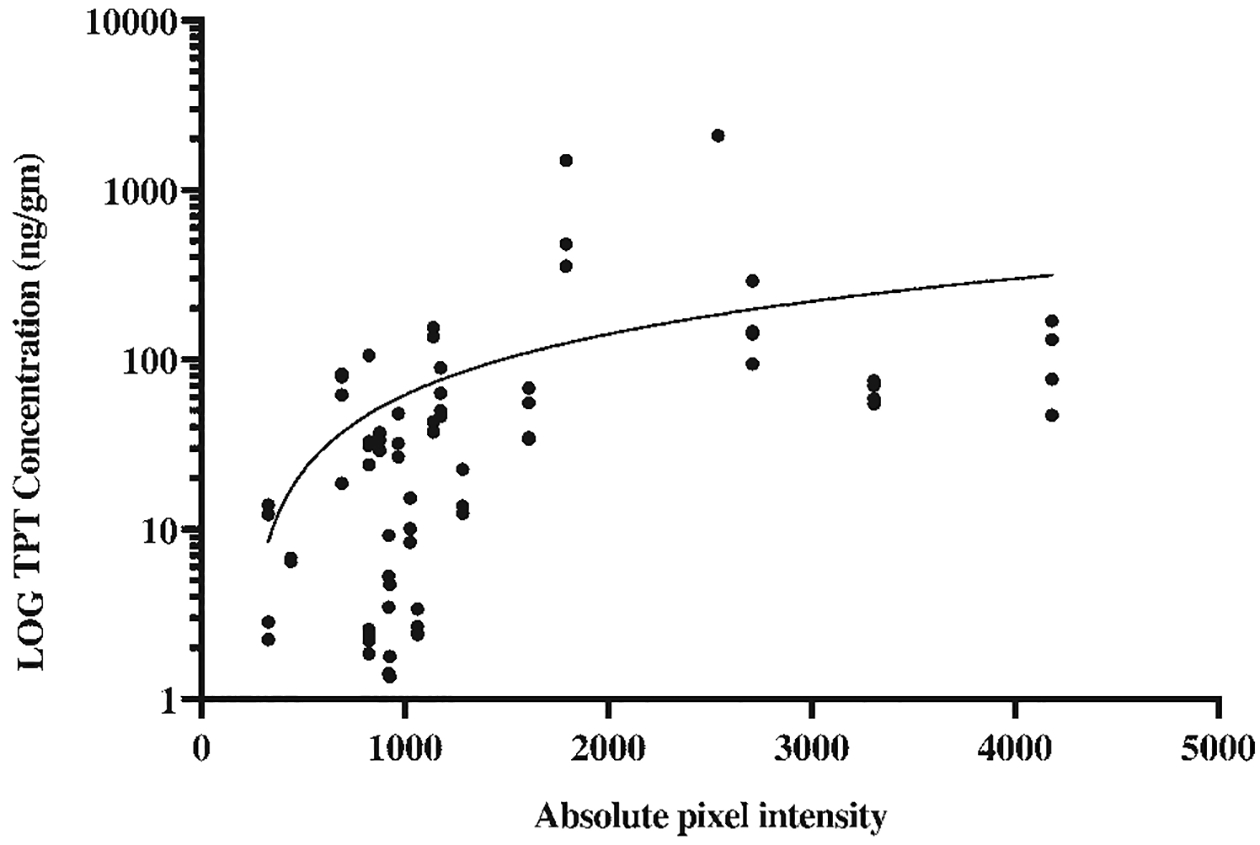
Linear regression of TPT concentration within biopsied tissue as determined by LCMS analysis versus absolute gadolinium signal intensity of the biopsy site as determined by T1-weighted MRI in pigs that underwent short-term CED (p = 0.008). TPT concentration is represented on a logarithmic scale.
CED Achieves Large and Stable Volumes of Distribution
In the short-term cohort, catheter placement within large white matter bundles (pigs 3 and 4) resulted in a mean Vdmax of 13.4 ± 2.3 mL (± SD) and a mean FVd of 13.2 ± 2.4 mL, with infusate reaching 37.5% ± 6.3% and 37.0% ± 6.8% of the ipsilateral hemispheric volume, respectively (Table 2, Fig. 5A). Conversely, animals with intraventricular extravasation (n = 2) and hippocampal infusion (n = 1) combined demonstrated a mean Vdmax and mean FVd of 3.8 ± 1.3 mL, reaching only 10.6% ± 3.6% of the ipsilateral hemispheric volume. Thus, placement of the catheter tip within subcortical white matter significantly increased the achievable Vdmax and FVd compared with instances of loss of convection or infusion into the hippocampus (p = 0.02 and p = 0.02, respectively; Fig. 5B).
FIG. 5. A:

Serial axial T1-weighted MR images obtained in pig 4, demonstrating a progressively increasing volume of distribution with continuous short-term infusion of TPT/gadolinium. Importantly, “priming” with a lower initial infusion rate avoided intraventricular extravasation and facilitated a large Vd. B: Vd as determined by the measurement of the gadolinium signal on T1-weighted MR images on each postoperative day (POD) for individual short-term pigs. Pigs 1 and 2 suffered from intraventricular extravasation of infusate. Pigs 3 and 4 had ideal placement of the catheter tip within the white matter of the posterior centrum semiovale. Pig 5 had placement of the catheter within the posterior hippocampus. C: The ratio of volume of distribution as determined by identification of gadolinium on MRI to volume of drug infused (Vd/Vi) on each POD for pigs that underwent short-term infusions. D: Linear regression of average Vd/Vi versus POD for short-term pigs with optimal catheter placement (pigs 3 and 4, p = 0.01).
The largest gains in Vd relative to Vi were noted to occur within the first 24–48 hours, at which point Vd declined slightly despite continued infusion (Fig. 5C). This trend was most notable in white matter infusions (pigs 3 and 4). Linear regression analysis of Vd/Vi over time for these animals with optimally placed catheters revealed significant anticorrelation (p = 0.01; Fig. 5D), suggesting a decline in the incremental gains in Vd with increasing infusion over time.
Chronic infusion also resulted in a large Vdmax (Table 3, Fig. 6A). In animals undergoing long-term infusions within the posterior white matter, the mean Vdmax was 11.3 ± 1.7 mL, representing 32.7% ± 6.0% of the ipsilateral hemispheric volume, and did not differ significantly from the mean Vdmax achieved in the short-term cohort (p = 0.16). Interestingly, while the average FVd demonstrated a slight decline from the Vdmax to 7.0 ± 2.3 mL with chronic CED, this still represented 19.8% ± 5.5% of the ipsilateral hemispheric volume (Fig. 6B). This decline in the Vd occurred despite a progressive increase in Vi, suggesting that with prolonged infusion, the achievable Vd settles into a steady-state equilibrium between infusion and clearance after 4 days (Fig. 6C). Again, the Vd/Vi was anticorrelated with time (p < 0.01) confirming this steady state between TPT-gadolinium infusion and clearance with chronic CED (Fig. 6D). Notably, in an effort to maximize the possible Vd, a trial of intermittent infusion was carried out and resulted in a similar achievable Vdmax and FVd to continuous infusions, but with decreased Vd/Vi (Table 3, Fig. 6B).
FIG. 6. A:

Representative sequence of serial MR images demonstrating evolving Vd during 32 days of chronic TPT-gadolinium infusion. Axial T1-weighted MR images demonstrated unambiguous hyperintense gadolinium signal during all scans performed during active infusion. The blue circle on the preoperative image represents the target for catheter tip placement within the posterior white matter of the centrum semiovale. B: Vd as determined by identification of gadolinium on MRI on each postoperative day for individual pigs undergoing long-term infusion. C: The ratio of Vd as determined by identification of gadolinium on MRI to volume of drug infused (Vd/Vi) on each POD for pigs undergoing chronic CED. D: Linear regression of average Vd/Vi versus POD for all long-term animals (p < 0.01). Figure is available in color online only.
One animal (pig 11) underwent control infusion without gadolinium alone in the absence of TPT. Interestingly, although the Vdmax was similar to that in animals undergoing comparable infusions (pigs 9 and 10), the FVd was smaller, reflecting either the possibility of volume variability or improved clearance of infusate in the absence of TPT (Table 3).
Topotecan CED Is Well Tolerated
No adverse neurological or behavioral events were encountered in the animals studied, and there were no infections. No infusions were stopped due to clinical side effects, and no premature deaths occurred during the study periods evaluated.
Peripheral blood samples were drawn in 4 pigs and underwent LCMS analysis to determine systemic TPT levels during CED. Three samples were drawn on the final day of infusion in animals within the short-term cohort, and 1 sample was drawn on infusion day 15 from the long-term cohort. All samples were taken from animals during active infusion with TPT (136 μM) and 1:100 gadolinium. Systemic TPT was undetectable in all peripheral blood samples tested. No animal demonstrated clinical signs of systemic toxicity.
Of note, development of T1 hypointensity as seen on MRI occurred around the catheter tip in 5 animals (83%) undergoing long-term infusion. These imaging abnormalities occurred in all animals undergoing chronic CED regardless of the presence of, or concentration of, TPT in the infusate or infusion schedule (continuous vs intermittent) on average around postoperative day 14. The mean final volume of the pericatheter signal abnormality was 0.8 ± 0.5 mL, which represented 14.6% ± 12.2%, 7.1% ± 4.5%, and 2.4% ± 1.2% of the average FVd, Vdmax, and ipsilateral hemispheric volume, respectively (Fig. 7).
FIG. 7.

Comparison of the volume of the ipsilateral cerebral hemisphere (Vh), Vdmax, FVd, and pericatheter signal hypointensity (FVt) for each pig undergoing long-term infusion.
Prolonged CED Is Associated With Histological Changes
Histopathological analysis demonstrated evidence of reactive astrocytes, microglia, and macrophages extending a few hundred microns from the catheter tract consistent with previously described changes.35 These changes were seen in all animals undergoing CED for greater than 10 days regardless of the presence of, or concentration of, TPT or continuous or intermittent infusion schedules.
Discussion
Our previous phase I trial of TPT CED for the treatment of recurrent malignant gliomas demonstrated its safety and efficacy and further promoted CED as a therapeutic option for these treatment-resistant tumors.7 While shorter infusions via externalized pump-catheter systems minimized the risk of infection, preclinical evidence suggests that prolonged TPT infusion may increase survival in gliomas.15,22 As a result, an implantable, internalized system for prolonged CED was developed.35 Here, we present validation of this system for safe, targeted, and prolonged TPT CED. We describe recent advances in pump-catheter design and placement techniques and evaluate the ability of this system to safely maximize TPT delivery. We show that high flow rates are well tolerated, and we characterize the effects of flow-rate modulation on Vd. Finally, we demonstrate that gadolinium-TPT coadministration provides a reliable noninvasive method to assess Vd.
This study expands on the safety and feasibility of intracerebral TPT CED for prolonged periods. We utilized the VarioGuide system to standardize catheter placement within a common anatomical region for all flow-related studies. The VarioGuide stereotactic device permits targeted positioning based on volumetric imaging.5 Importantly, Brainlab neuronavigation and the VarioGuide are specifically designed for human use. Subtle inaccuracies in the registration of pig scalp and fiducials, as well as evolving head fixation techniques, may have diminished the system’s precision and contributed to the inaccurate placement in 2 animals. Furthermore, the acceptable standard error of stereotactic targeting is significantly magnified when utilized within the relatively smaller anatomy of the porcine brain as compared with the human brain.
We have demonstrated the safety and efficacy of the implantable system up to 32 days, as no animal developed clinical side effects as a result of implantation or continuous infusion. This supports previous data demonstrating that CED avoids the risk of systemic toxicities.3,24,40 In particular, while previous studies of systemic TPT for glioblastoma (GBM) were limited by dose-related toxici-ties,14 no animal undergoing prolonged CED demonstrated clinical signs of systemic toxicity from accumulated TPT, and LCMS data failed to demonstrate detectable systemic levels of TPT. This confirms that prolonged CED via an implantable system maintains this therapeutic advantage and suggests that patients could be monitored safely in an outpatient setting.
The therapeutic benefits of CED depend on its ability to reliably achieve a large and stable Vd, and noninvasive means of assessing Vd are essential. Although an imperfect substitute, these data demonstrate that TPT-gadolinium coinfusion provides an acceptable method for estimating the parenchymal TPT distribution and that gadolinium intensity on MRI can be used as a surrogate for TPT concentration. Using gadolinium as a proxy for TPT distribution, we have shown that prolonged infusion does not lead to a constant expansion of Vd.35 Instead, equilibrium between the delivery and clearance of TPT-gadolinium, local anatomical factors (as seen in the limited Vd attained within the hippocampus), and variable and potentially fluctuating intracranial pressure dynamics—as may be seen between tissue types and in the presence of tumors—likely contribute to the establishment of a steady-state volume. Whether manipulation of the achieved steady-state volume is possible with modulation of specific flow parameters remains unknown. However, relative to our previous 10-day infusions, we demonstrated that significantly higher infusion rates are tolerated and capable of infusing large volumes (4 mL/day vs 0.7 mL/day).35 Furthermore, characterization of dynamic fluctuations in the concentration of chemotherapeutic agent delivered also remains unknown. However, this study suggests that prolonged CED is able to maintain stable concentrations of an agent within a focal region over extended periods of time. This characterization of an achievable steady-state volume is important, as the establishment of a sustained drug concentration permits TPT to target neo-plastic cells in various stages of the cell cycle in comparison with the limited efficacy of transient drug delivery. This is particularly important in malignant gliomas, which harbor cell populations that remain relatively quiescent.10,41
These data also suggest that intermittent short-term dosing is feasible, as the Vdmax was noted to occur early and prior to establishment of a steady state. This implies that a pulsatile infusion schedule might improve delivery of chemotherapies to adjacent peritumoral regions and avoid steady state. Interestingly, while our experiment utilizing a pulsatile infusion algorithm did demonstrate a large and stable achievable Vdmax and FVd, two of the intermediate pulses did not achieve the same Vd, suggesting that perhaps brain tissue changes or scarring in the setting of recent chemotherapy infusion somehow changed infusion dynamics (Fig. 6B).
Loss of infusate into CSF spaces, in addition to catheter backflow, remains a serious limitation to the establishment of a stable Vd.32 As initiation of flow at a lower rate avoided intraventricular extension in animals with catheters placed in similar anatomical locations, we hypothesize that initial low flow rates may permit priming of the tissue for higher flow rates and associated interstitial pressures. These data also reinforce that catheter tips should be placed within adequate parenchyma and as far as possible from sulcal or ventricular surfaces. Interestingly, while the absence of backflow of infusate might reflect the use of the stepped catheter which has been demonstrated in previous studies to prevent backflow at low flow rates,11,24 an implantable system permitting delayed initiation of chemotherapy may allow initial scar formation around the catheter entry site, which may preclude backflow of infusate. This is supported by the histological findings of reactive astrocytes, microglia, and macrophages extending a few hundred microns from the catheter tract. Further study in the presence of tumors is necessary to better define practice patterns to maximize and ensure stable volumes while limiting the loss of infusate.
The relevance of T1 hypointense signal abnormalities around the catheter tip remains unknown. As this phenomenon occurred in all infusions regardless of the presence or concentration of TPT, it is possible that placement of a rigid catheter into the brain in the setting of a chronic fluid infusion and an imperfect method of catheter immobilization may have resulted in catheter-associated microtrauma to adjacent tissues. Fortunately, the relative volumes of these regions were small and did not significantly alter the achievable Vd (Fig. 7). Further study using the pulsatile infusion schedule with periods of infusion followed by periods of washout may limit any potential pericatheter tissue damage. Otherwise, improved methods of catheter fixation or the use of softer catheter tips requires further study.
This study expands on the safety and feasibility of prolonged intracerebral TPT CED. These data provide proof-of-principle for the use of an implanted infusion system as a means of prolonging CED and thus achieving constant local/regional infusion of agents into the surrounding brain parenchyma over extended periods of time. It also serves as the foundation for the study of prolonged infusion of TPT for malignant gliomas. However, optimization of prolonged CED in the human brain is still necessary and ultimately may involve the use of multiple catheters or varied infusion rates and schedules. This study additionally validates the utility of gadolinium coinfusion as a method to monitor Vd. The absent systemic toxicity of TPT CED combined with programmable subcutaneous pumps permits monitoring and refilling of chemotherapies in the outpatient setting, modifiable dosing schedules, and the ability to provide high-dose, stable concentrations of effective chemotherapies to targeted regions of CNS disease. Prolonged CED has therapeutic implications for patients with a variety of neurological disease, including those with malignant brain tumors, Huntington’s disease, epilepsy, or Parkinson’s disease.27,30,31,38
Conclusions
Prolonged TPT-gadolinium CED via an internalized system is safe and well tolerated, and it can achieve a large maximal Vd, as well as maintain a stable Vd for up to 32 days. Gadolinium provides an identifiable surrogate for measuring drug distribution. Extended CED is a broadly applicable and safe therapeutic option in select patients.
Acknowledgments
We acknowledge James M. Gallo, PharmD, PhD, and his team for his contribution to the LCMS analyses.
Grant support to J. N. Bruce was provided by NIH grant no. R011CA16140.
ABBREVIATIONS
- CED
convection-enhanced delivery
- FVd
final volume of distribution
- FVi
final volume infused
- GBM
glioblastoma
- LCMS
liquid chromatography mass spectroscopy
- NBS
neurobehavioral scale
- TPT
topotecan
- Vd
volume of distribution
- Vdmax
maximal volume of distribution
- Vi
volume of infusion
Footnotes
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Anderson RC, Kennedy B, Yanes CL, Garvin J, Needle M, Canoll P, et al. : Convection-enhanced delivery of topotecan into diffuse intrinsic brainstem tumors in children. J Neurosurg Pediatr 11:289–295, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker FG II, Chang SM, Gutin PH, Malec MK, McDermott MW, Prados MD, et al. : Survival and functional status after resection of recurrent glioblastoma multiforme. Neurosurgery 42:709–723, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH: Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A 91:2076–2080, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogdahn U, Hau P, Stockhammer G, Venkataramana NK, Mahapatra AK, Suri A, et al. : Targeted therapy for high-grade glioma with the TGF-β2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro Oncol 13:132–142, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradac O, Steklacova A, Nebrenska K, Vrana J, de Lacy P, Benes V: Accuracy of VarioGuide frameless stereotactic system against frame-based stereotaxy: prospective, randomized, single-center study. World Neurosurg 104:831–840, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Bruce JN, Falavigna A, Johnson JP, Hall JS, Birch BD, Yoon JT, et al. : Intracerebral clysis in a rat glioma model. Neurosurgery 46:683–691, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Bruce JN, Fine RL, Canoll P, Yun J, Kennedy BC, Rosenfeld SS, et al. : Regression of recurrent malignant gliomas with convection-enhanced delivery of topotecan. Neurosurgery 69:1272–1280, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burris HA III: Topotecan: incorporating it into the treatment of solid tumors. Oncologist 3:1–3, 1998 [PubMed] [Google Scholar]
- 9.Chow DS, Qi J, Guo X, Miloushev VZ, Iwamoto FM, Bruce JN, et al. : Semiautomated volumetric measurement on post-contrast MR imaging for analysis of recurrent and residual disease in glioblastoma multiforme. AJNR Am J Neuroradiol 35:498–503, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Das S, Srikanth M, Kessler JA: Cancer stem cells and glioma. Nat Clin Pract Neurol 4:427–435, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Debinski W, Tatter SB: Convection-enhanced delivery for the treatment of brain tumors. Expert Rev Neurother 9:1519–1527, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desjardins A, Gromeier M, Herndon JE II, Beaubier N, Bolognesi DP, Friedman AH, et al. : Recurrent glioblastoma treated with recombinant poliovirus. N Engl J Med 379:150–161, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dickinson PJ, LeCouteur RA, Higgins RJ, Bringas JR, Roberts B, Larson RF, et al. : Canine model of convection-enhanced delivery of liposomes containing CPT-11 monitored with real-time magnetic resonance imaging: laboratory investigation. J Neurosurg 108:989–998, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Friedman HS, Kerby T, Fields S, Zilisch JE, Graden D, McLendon RE, et al. : Topotecan treatment of adults with primary malignant glioma. Cancer 85:1160–1165, 1999 [PubMed] [Google Scholar]
- 15.Kaiser MG, Parsa AT, Fine RL, Hall JS, Chakrabarti I, Bruce JN: Tissue distribution and antitumor activity of topotecan delivered by intracerebral clysis in a rat glioma model. Neurosurgery 47:1391–1399, 2000 [PubMed] [Google Scholar]
- 16.Kelly PJ, Daumas-Duport C, Kispert DB, Kall BA, Scheithauer BW, Illig JJ: Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg 66:865–874, 1987 [DOI] [PubMed] [Google Scholar]
- 17.Ksendzovsky A, Walbridge S, Saunders RC, Asthagiri AR, Heiss JD, Lonser RR: Convection-enhanced delivery of M13 bacteriophage to the brain. J Neurosurg 117:197–203, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunwar S, Chang S, Westphal M, Vogelbaum M, Sampson J, Barnett G, et al. : Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro Oncol 12:871–881, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunwar S, Prados MD, Chang SM, Berger MS, Lang FF, Piepmeier JM, et al. : Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J Clin Oncol 25:837–844, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Lidar Z, Mardor Y, Jonas T, Pfeffer R, Faibel M, Nass D, et al. : Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: a phase I/II clinical study. J Neurosurg 100:472–479, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Lonser RR, Sarntinoranont M, Morrison PF, Oldfield EH: Convection-enhanced delivery to the central nervous system. J Neurosurg 122:697–706, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Lopez KA, Tannenbaum AM, Assanah MC, Linskey K, Yun J, Kangarlu A, et al. : Convection-enhanced delivery of topotecan into a PDGF-driven model of glioblastoma prolongs survival and ablates both tumor-initiating cells and recruited glial progenitors. Cancer Res 71:3963–3971, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsumoto Y, Fujiwara T, Honjo Y, Sasaoka N, Tsuchida T, Nagao S: Quantitative analysis of DNA topoisomerase I activity in human and rat glioma: characterization and mechanism of resistance to antitopoisomerase chemical, camptothecin-11. J Surg Oncol 53:97–103, 1993 [DOI] [PubMed] [Google Scholar]
- 24.Mehta AM, Sonabend AM, Bruce JN: Convection-enhanced delivery. Neurotherapeutics 14:358–371, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Midulla PS, Gandsas A, Sadeghi AM, Mezrow CK, Yerlioglu ME, Wang W, et al. : Comparison of retrograde cerebral perfusion to antegrade cerebral perfusion and hypothermic circulatory arrest in a chronic porcine model. J Card Surg 9:560–575, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Mueller S, Polley MY, Lee B, Kunwar S, Pedain C, Wembacher-Schröder E, et al. : Effect of imaging and catheter characteristics on clinical outcome for patients in the PRECISE study. J Neurooncol 101:267–277, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parney IF, Kunwar S, McDermott M, Berger M, Prados M, Cha S, et al. : Neuroradiographic changes following convection-enhanced delivery of the recombinant cytotoxin interleukin 13-PE38QQR for recurrent malignant glioma. J Neurosurg 102:267–275, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Raghavan R, Brady ML, Rodríguez-Ponce MI, Hartlep A, Pedain C, Sampson JH: Convection-enhanced delivery of therapeutics for brain disease, and its optimization. Neurosurg Focus 20(4):E12, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Raghavan R, Brady ML, Sampson JH: Delivering therapy to target: improving the odds for successful drug development. Ther Deliv 7:457–481, 2016 [DOI] [PubMed] [Google Scholar]
- 30.Rainov NG, Gorbatyuk K, Heidecke V: Clinical trials with intracerebral convection-enhanced delivery of targeted toxins in malignant glioma. Rev Recent Clin Trials 3:2–9, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Rogawski MA: Convection-enhanced delivery in the treatment of epilepsy. Neurotherapeutics 6:344–351, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sampson JH, Akabani G, Archer GE, Bigner DD, Berger MS, Friedman AH, et al. : Progress report of a Phase I study of the intracerebral microinfusion of a recombinant chimeric protein composed of transforming growth factor (TGF)-alpha and a mutated form of the Pseudomonas exotoxin termed PE-38 (TP-38) for the treatment of malignant brain tumors. J Neurooncol 65:27–35, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Sampson JH, Archer G, Pedain C, Wembacher-Schröder E, Westphal M, Kunwar S, et al. : Poor drug distribution as a possible explanation for the results of the PRECISE trial. J Neurosurg 113:301–309, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Sampson JH, Raghavan R, Brady M, Friedman AH, Bigner D: Convection-enhanced delivery. J Neurosurg 115:463–466, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Sonabend AM, Stuart RM, Yun J, Yanagihara T, Mohajed H, Dashnaw S, et al. : Prolonged intracerebral convection-enhanced delivery of topotecan with a subcutaneously implantable infusion pump. Neuro Oncol 13:886–893, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. : Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Szerlip NJ, Walbridge S, Yang L, Morrison PF, Degen JW, Jarrell ST, et al. : Real-time imaging of convection-enhanced delivery of viruses and virus-sized particles. J Neurosurg 107:560–567, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Vogelbaum MA: Convection enhanced delivery for treating brain tumors and selected neurological disorders: symposium review. J Neurooncol 83:97–109, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Vogelbaum MA, Sampson JH, Kunwar S, Chang SM, Shaf-frey M, Asher AL, et al. : Convection-enhanced delivery of cintredekin besudotox (interleukin-13-PE38QQR) followed by radiation therapy with and without temozolomide in newly diagnosed malignant gliomas: phase 1 study of final safety results. Neurosurgery 61:1031–1038, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Yun J, Rothrock RJ, Canoll P, Bruce JN: Convection-enhanced delivery for targeted delivery of antiglioma agents: the translational experience. J Drug Deliv 2013:107573, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao H, Traganos F, Darzynkiewicz Z: Kinetics of histone H2AX phosphorylation and Chk2 activation in A549 cells treated with topotecan and mitoxantrone in relation to the cell cycle phase. Cytometry A 73:480–489, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Zhou Z, Singh R, Souweidane MM: Convection-enhanced delivery for diffuse intrinsic pontine glioma treatment. Curr Neuropharmacol 15:116–128, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]


