Abstract
Convection-enhanced delivery (CED) for the treatment of malignant gliomas is a technique that can deliver chemotherapeutic agents directly into the tumor and the surrounding interstitium through sustained, low-grade positive-pressure infusion. This allows for high local concentrations of drug within the tumor while minimizing systemic levels that often lead to dose-limiting toxicity. Diffuse intrinsic pontine gliomas (DIPGs) are universally fatal childhood tumors for which there is currently no effective treatment. In this report the authors describe CED of the topoisomerase inhibitor topotecan for the treatment of DIPG in 2 children.
As part of a pilot feasibility study, the authors treated 2 pediatric patients with DIPG. Stereotactic biopsy with frozen section confirmation of glial tumor was followed by placement of bilateral catheters for CED of topotecan during the same procedure. The first patient underwent CED 210 days after initial diagnosis, after radiation therapy and at the time of tumor recurrence, with a total dose of 0.403 mg in 6.04 ml over 100 hours. Her Karnofsky Performance Status (KPS) score was 60 before CED and 50 posttreatment. Serial MRI initially demonstrated a modest reduction in tumor size and edema, but the tumor progressed and the patient died 49 days after treatment. The second patient was treated 24 days after the initial diagnosis prior to radiation with a total dose of 0.284 mg in 5.30 ml over 100 hours. Her KPS score was 70 before CED and 50 posttreatment. Serial MRI similarly demonstrated an initial modest reduction in tumor size. The patient subsequently underwent fractionated radiation therapy, but the tumor progressed and she died 120 days after treatment.
Topotecan delivered by prolonged CED into the brainstem in children with DIPG is technically feasible. In both patients, high infusion rates (> 0.12 ml/hr) and high infusion volumes (> 2.8 ml) resulted in new neurological deficits and reduction in the KPS score, but lower infusion rates (< 0.04 ml/hr) were well tolerated. While serial MRI showed moderate treatment effect, CED did not prolong survival in these 2 patients. More studies are needed to improve patient selection and determine the optimal flow rates for CED of chemotherapeutic agents into DIPG to maximize safety and efficacy. Clinical trial registration no.: NCT00324844.
Keywords: convection-enhanced delivery, topotecan, pediatric neurosurgery, brainstem tumor, pontine glioma, oncology
Nearly 300 cases of brainstem gliomas are diagnosed annually in the US, 60%–75% of which are DIPGs.33 Despite extensive collaborative research studies over the last 20 years, the overall prognosis for these tumors remains dismal, with a median survival of about 1 year and less than 20% of children alive at 2 years.6,13 Diffuse intrinsic pontine gliomas now account for the majority of brain tumor–related deaths in children.10
Current therapies for children with DIPG are palliative. Surgical excision is not possible because of the eloquent location and infiltrative nature of these tumors.2,24 Conventional radiation therapy at a total dose of 54–59 Gy is the only treatment that has consistently shown clinical and radiological improvement for children with DIPG.1 Unfortunately, results are short lived, as no study using radiotherapy has demonstrated a long-standing benefit alone or in combination with chemotherapeutic agents.13 Results with chemotherapy have been equally disappointing. Despite many clinical trials investigating multiple chemotherapeutic agents known to have efficacy against malignant gliomas, no single chemotherapeutic agent alone or in combination, either in a neoadjuvant setting or an adjuvant setting, has proven to prolong survival in these patients.13
Newer, more effective therapeutic modalities are needed for children with DIPG. Currently available techniques for brainstem drug delivery include systemic and intrathecal administration, both of which have significant limitations.15,22 Systemic toxicity and the inability to cross the blood-brain barrier limit systemic delivery of most chemotherapeutic agents, while penetration into brainstem tumors from intrathecal administration relies on diffusion, which severely constrains tissue distribution and produces heterogeneous dispersion.20 Convectionenhanced delivery is a strategy developed by Oldfield and colleagues to deliver agents directly into tumors and surrounding brain through the interstitial space.3 Stereotactically placed catheters connected to pumps provide a continuous low positive-pressure microinfusion that distributes chemotherapeutic agents by bulk flow, providing high local concentrations of drugs in the tumor while avoiding systemic toxicity. Extensive mathematical and experimental models have demonstrated the advantages of bulk flow over simple diffusion methods, including systemic chemotherapy and intrathecal adminstration.9,18 Clinical evidence in support of CED with topotecan recently became available as a Phase Ib clinical trial safely demonstrated regression of recurrent supratentorial malignant gliomas in adults using CED.5 In this report, we discuss the technique and outcomes after topotecan administration via CED into the brainstem of 2 pediatric patients with DIPGs.
Case Reports
Methods
Patient Selection and Study Design.
Both patients were treated at the Morgan Stanley Children’s Hospital and Columbia University Medical Center under the auspices of an Institutional Review Board study after an investigational new drug application was approved and informed consent was obtained. Eligibility criteria included: 1) clinical and radiological evidence of a DIPG, 2) a KPS score of at least 60, and 3) age less than 18 years. Tumors with large cysts or extreme expansion of the pons on initial MRI were excluded for CED. Study patients were treated as part of a larger prospective Phase Ib open-label, non-randomized, dose escalation study of adult patients with recurrent supratentorial malignant glioma (clinical trial registration no. NCT00324844). The study was designed for patients to receive an infusion of topotecan via CED at 0.0667 mg/ml, which was the midlevel dose of the adult dose escalation study and one-third the concentration that led to complete tumor regression without toxicity in a rat glioma model.12 Because of the location of tumors in the brainstem and the smaller head size of pediatric patients, 2 modifications were made to the rate and total volume of infusion compared with those used for adult patients: 1) The flow rate and total infusion volume were reduced to 50% of the adult parameters. 2) Further reduction was done after estimating the individual brain volume using head circumference. The ratio of the actual brain volume divided by the average adult brain volume was used to reduce the flow rate proportionally. Infusions were conducted for a total of 100 hours.
Study Procedure.
Steroid and antibiotic agents were administered during the infusion period. After induction of general endotracheal anesthesia, a stereotactic head frame was placed. Patients were positioned prone and a trajectory down the cerebellar peduncle was used (Fig. 1). A standard MRI- and/or CT-guided stereotactic biopsy was performed to confirm histologically the presence of DIPG. Following confirmation of tumor, a Silastic infusion catheter with a single hole at the tip (2.5-mm outer diameter, CSF-peritoneal catheter; Integra) was stereotactically placed down the same track directly into the tumor at a site chosen to maximize coverage of the tumor and adjacent infiltrated brainstem based on a presumed spherical distribution (Fig. 1). A second catheter was subsequently placed stereotactically down the contralateral cerebellar peduncle into the tumor. Care was taken to avoid catheter tip placement within several millimeters of apparently necrotic tumor, cystic regions, fourth ventricle, or the cortical surface/subarachnoid space to prevent loss of drug to nonviable tissue or CSF. After CT or MRI confirmation of catheter location, infusion of topotecan was initiated via a Medfusion 2010 syringe pump (Medex, Inc). To compensate for smaller brain volumes in pediatric patients and the reduced interstitial spaces in the brainstem, infusion volumes were reduced from those used in adults treated with supratentorial CED (see above). After treatment for a total of 100 hours, infusions were stopped, an immediate posttreatment MRI was obtained, and the catheters were removed at the bedside.
FIG. 1.
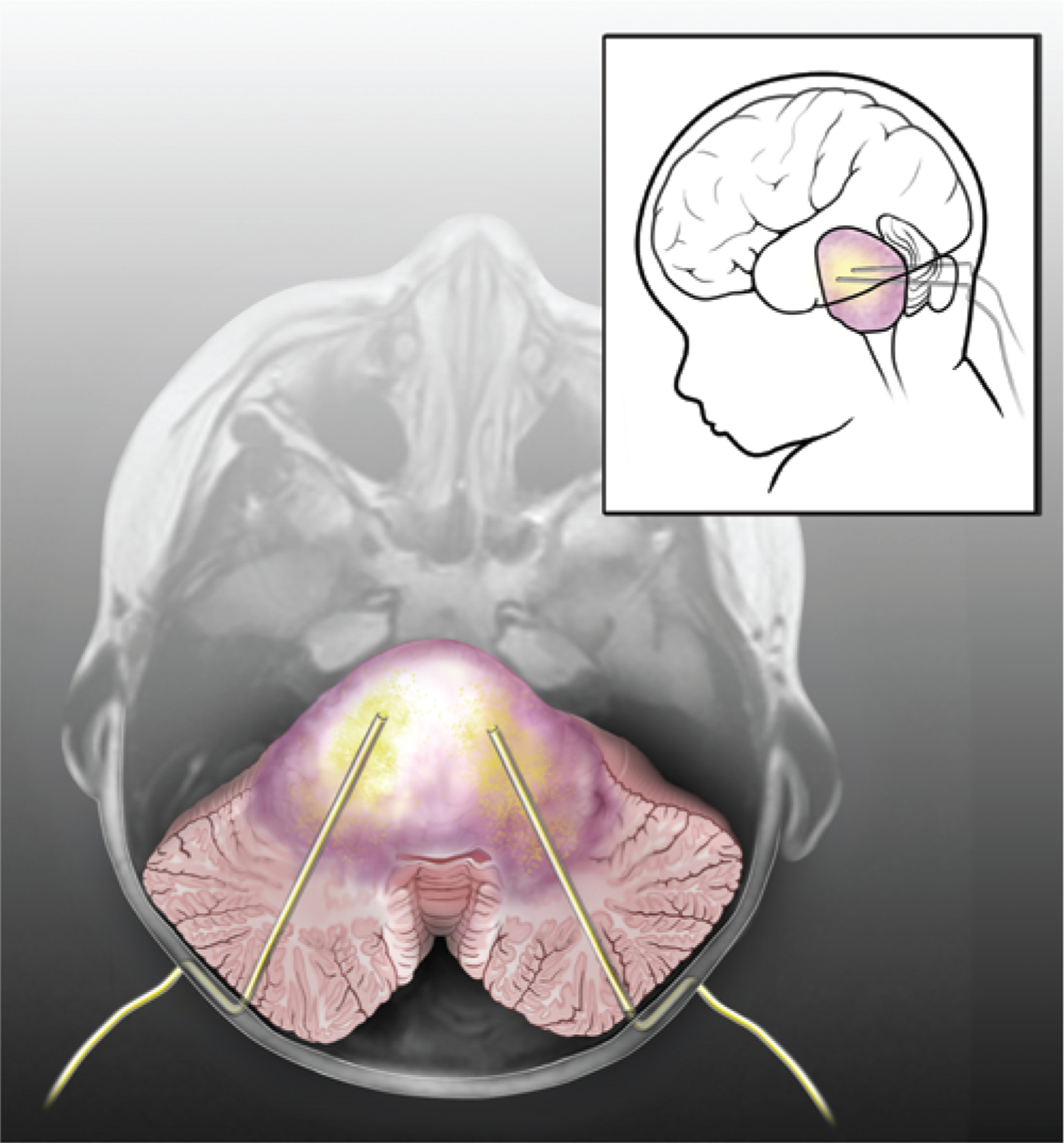
Artist’s rendering of CED. Drawing superimposed on an axial T1-weighted MR image showing bilateral catheters stereotactically placed through the cerebellar peduncles into the brainstem of a patient with a DIPG. Catheters are placed into the tumor with the tips approximately 1 cm apart, avoiding the fourth ventricle to minimize loss of drug to CSF. Catheters are tunneled subcutaneously to prevent migration and CSF leakage. Drug is distributed throughout the tumor via convection (yellow shading). Inset depicts sagittal orientation of patient with catheters in place.
Clinical and Radiological Assessment.
Prior to treatment, patients underwent baseline physical and neurological examinations. Vital signs and neurological status were monitored throughout the infusions. Patients returned for follow-up evaluation on an outpatient basis approximately every 2 weeks unless there were clinical or radiological indications for more frequent monitoring. Magnetic resonance imaging studies were performed at Columbia University Medical Center within 1 week prior to topotecan infusion and at 4- and 8-week intervals following the infusion. All MRI studies were performed using T2-weighted spin echo, T2-weighted FLAIR, and T1-weighted multiplanar scans prior to and following intravenous infusion of a gadolinium contrast agent.
Case Summaries
Case 1.
This young girl was brought to the emergency department at almost 5 years of age with a 1-month history of progressive gait difficulty, poor feeding, double vision, and slurred speech. Physical examination demonstrated bilateral CN VI palsy, diffuse hyperreflexia, and ataxic gait. A CT scan and MRI showed a diffusely enlarged pons, consistent with DIPG (Fig. 2A and B).
FIG. 2.
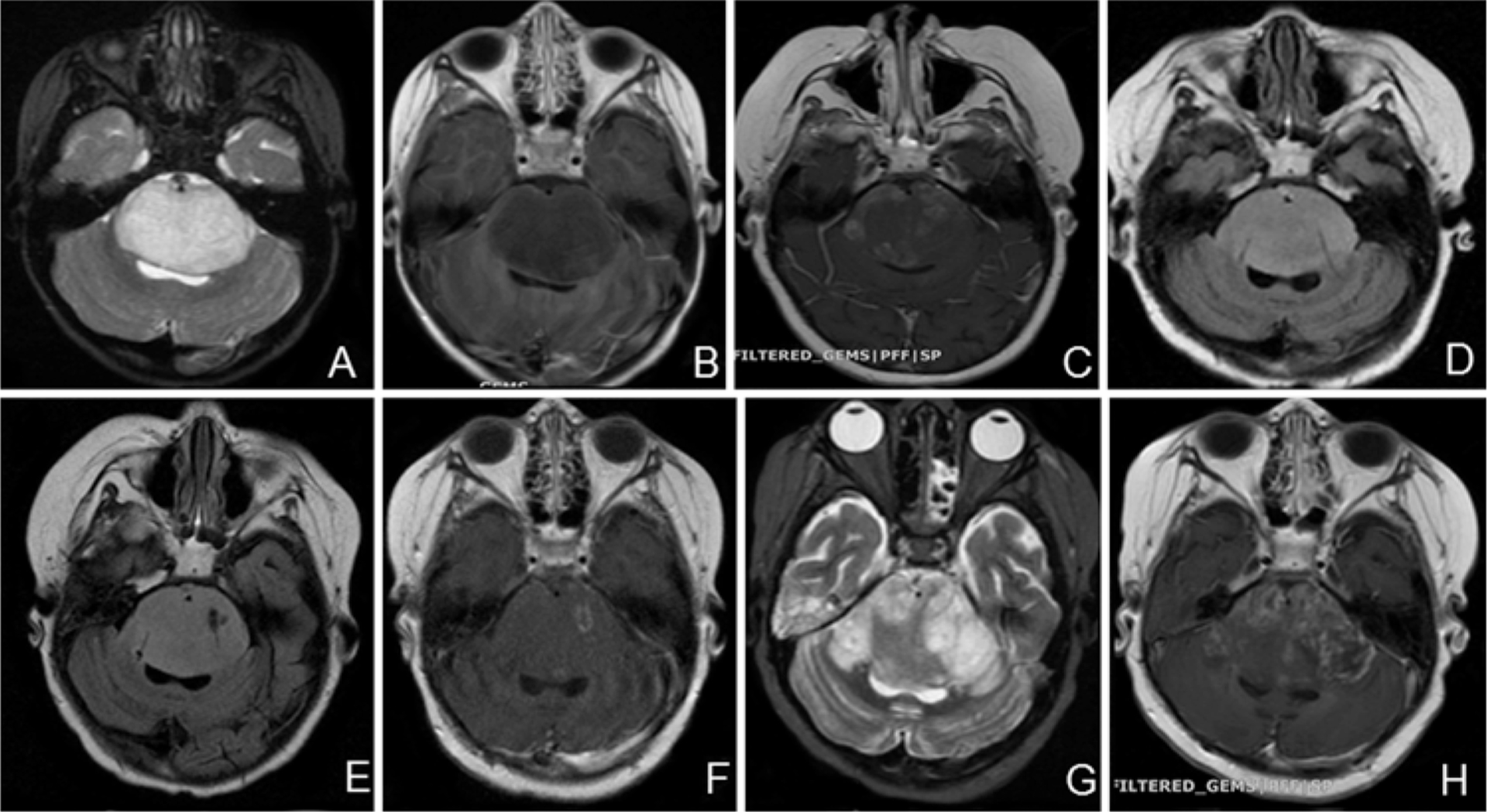
Case 1. Axial MR images. A and B: T2-weighted (A) and contrast-enhanced T1-weighted (B) images obtained at the time of diagnosis showing enlargement of the pons without contrast enhancement. C: Contrast-enhanced T1-weighted image obtained after radiation treatment at the time of recurrence showing new patchy enhancement. D: FLAIR image obtained immediately after surgery demonstrating bilateral catheters within the tumor. E and F: FLAIR (E) and contrast-enhanced T1-weighted (F) images obtained after treatment (6.04 ml infusion volume) showing effects of drug infusion within the tumor. G and H: T2-weighted (G) and contrast-enhanced T1-weighted (H) images obtained 1 month posttreatment demonstrating tumor progression.
She underwent 6 weeks of radiation therapy (total dose 54 Gy) with improvements in her gait and swallowing. Approximately 4 months later, she developed worsening gait and speech. Physical examination again demonstrated gait ataxia, dysarthria, CN VI palsy, and increased tone bilaterally. Magnetic resonance imaging showed increased tumor size with new areas of enhancement as well as new-onset hydrocephalus (Fig. 2C). She underwent an uncomplicated endoscopic third ventriculostomy resulting in some improvement of her gait, but no change in her speech or CN VI palsy. Several days later, with a baseline KPS score of 60, the patient and family elected to proceed with CED.
The patient underwent a frame-based stereotactic biopsy to confirm glioma followed by placement of bilateral catheters as previously described (Fig. 2D). Surgery was uncomplicated and the patient was transported to the pediatric ICU intubated. Infusion of topotecan via CED was started at a rate of 0.08 ml/hr and a concentration of 0.0667 mg/ml per catheter. After 17 hours of infusion (total volume 2.72 ml), she developed increased spasticity and decreased movement of all 4 extremities. The infusion was stopped, her dexamethasone dose was increased, and an MRI study was performed. The MR images showed no change. Her strength improved over the next 48 hours, and by postoperative Day 4 she was nearly back to her neurological baseline but with persistent spasticity. The infusion was restarted at a lower rate of 0.02 ml/hr at the same concentration (0.0667 mg/ml) and continued for another 83 hours until completion. Her examination findings remained stable through the end of the treatment, the catheters were removed, and she was extubated uneventfully. She received a total of 0.403 mg of topotecan in a total volume of 6.04 ml. Posttreatment MRI demonstrated evidence of drug infusion into the tumor, but no changes in the overall tumor size (Fig. 2E and F). Her spasticity remained after cessation of topotecan therapy, and treatment with baclofen was initiated. Her KPS score 3 days after treatment was 50. Her final pathological diagnosis was diffuse infiltrating glioma. Over the next 2 months, her level of consciousness and speech gradually declined. An MRI study performed 1 month after CED demonstrated significant tumor growth (Fig. 2G and H) and she eventually died 49 days after CED. The parents agreed to organ donation and no autopsy was performed.
Case 2.
This young girl was brought to the emergency department at the age of 5 years 10 months with a 2-month history of progressive right leg and arm weakness, right facial weakness, double vision, slurred speech, difficulty with ambulation, and falls. Her main neurological findings included right CN VI and XII palsies, right hemiparesis, and a slow, unbalanced gait that required assistance. She had 3+ bilateral patellar reflexes, 4+ Achilles reflexes, and upgoing toes. Her KPS score at this point was 70. An MRI study revealed diffuse expansion of the pons with T2 signal hyperintensity (Fig. 3A) and patchy heterogeneous enhancement with contrast agent administration (Fig. 3B), consistent with DIPG. She had no hydrocephalus. Dexamethasone therapy was initiated with little improvement. Three weeks later, she was admitted to the Morgan Stanley Children’s Hospital of New York at the Columbia University Medical Center, where a percutaneous endoscopic gastrostomy tube was placed due to swallowing dysfunction. After a brief recovery she elected for CED as previously described.
FIG. 3.
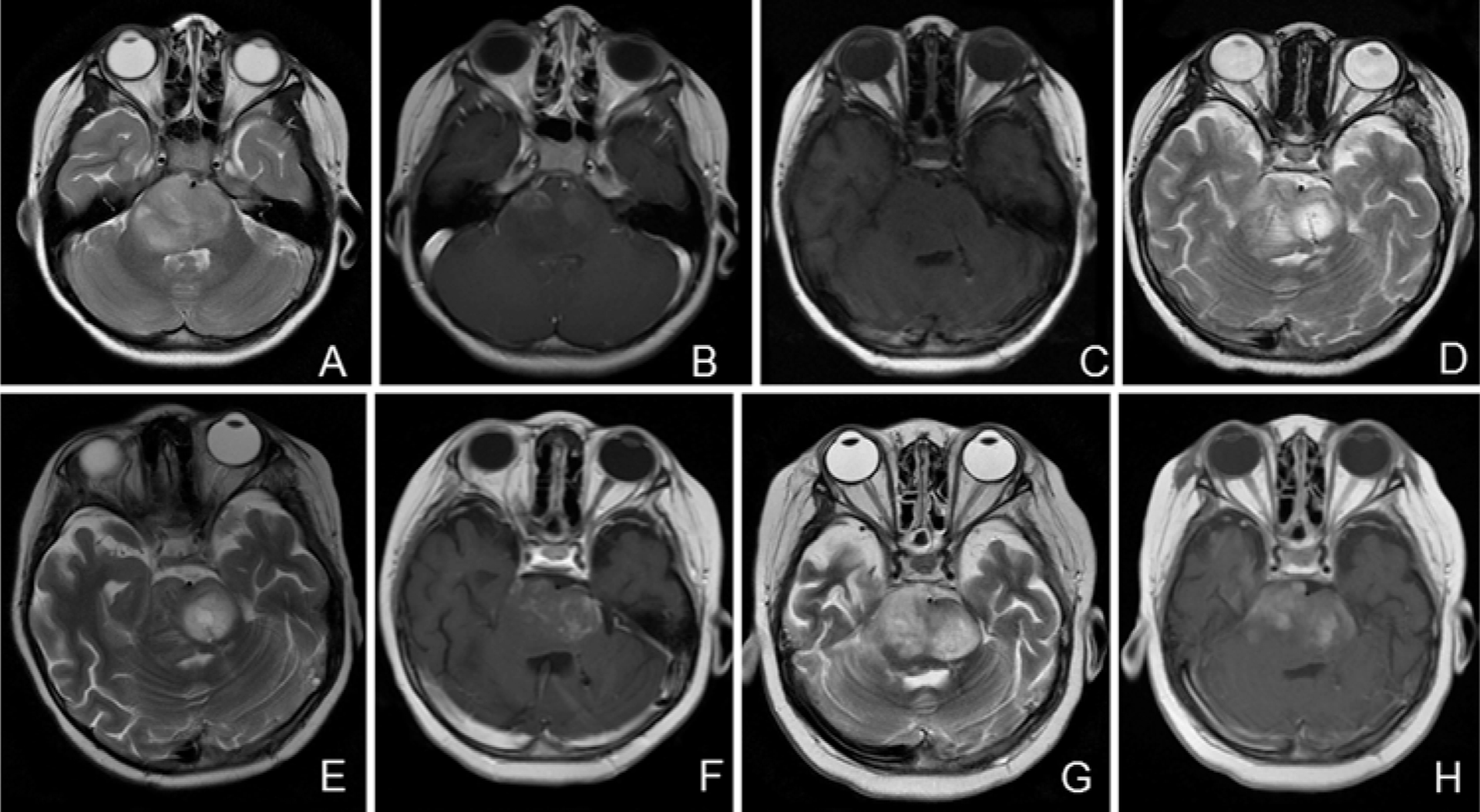
Case 2. Axial MR images. A and B: Preoperative T2-weighted (A) and contrast-enhanced T1-weighted (B) images showing enlargement of the pons with 2 small areas of mild contrast enhancement. C: T1-weighted image obtained immediately after surgery demonstrating bilateral catheters within the tumor. D and E: T2-weighted images obtained mid-treatment (3.09 ml infusion volume [D]) and immediately posttreatment (5.30 ml total infusion volume [E]) showing increasing area of drug distribution within the tumor. F: Contrast-enhanced T1-weighted image obtained 1 month after treatment demonstrating stable tumor. G and H: T2-weighted (G) and contrast-enhanced T1-weighted (H) images obtained nearly 4 months posttreatment demonstrating tumor progression.
Histological analysis of the biopsy showed a moderately cellular glial neoplasm with prominent nuclear atypia and rare mitotic figures, consistent with anaplastic astrocytoma (WHO Grade III). Two catheters were placed stereotactically with the tips positioned approximately a centimeter apart in the diffusely enlarged pons, and placement was confirmed with an immediate postoperative MRI (Fig. 3C). The patient was extubated and transferred to the pediatric ICU. Infusion of topotecan via CED was begun at a concentration of 0.0667 mg/ml and a rate of 0.06 ml/hr through each catheter.
After 24 hours of infusion (total volume 2.81 ml), the patient became less talkative, but her neurological examination findings, including level of consciousness, remained at baseline. The infusion rate for each catheter was reduced to one-third of the initial rate, 0.02 ml/hr. After 31 hours of infusion, she became more hypophonic with reduced movement of her lower extremities. Her dexamethasone dose was increased and the infusion was stopped.
On postoperative Days 2 and 3, she continued to have lower-extremity weakness and hypophonia and also developed right arm weakness. On postoperative Day 4, an MRI study showed evidence of drug infusion within the tumor with no new hemorrhage or infarction (Fig. 3D). Her neurological examination findings subsequently improved, with increasing strength, and on postoperative Day 5 the infusion was restarted at the same lower rate of 0.02 ml/hr from each catheter, but with half the concentration (at 0.0334 mg/ml). Before the infusion was restarted, she had some mild transient episodes of tachypnea but without oxygen desaturation and was electively intubated to protect her airway. The infusion was continued for another 69 hours with no further changes in her neurological examination findings. She received a total of 0.284 mg of topotecan in a total volume of 5.30 ml.
After a posttreatment MRI demonstrated a larger volume of distribution of topotecan (Fig. 3E), both catheters were removed and she was extubated uneventfully. Her KPS score 3 days after treatment was 50. Her neurological examination findings improved over the next several days in the hospital and during a 10-day course in acute rehabilitation. The final pathological diagnosis was anaplastic astrocytoma (WHO Grade III). Approximately 1 month following treatment, an MRI study showed further enhancement but a slight decrease in overall size of the tumor (Fig. 3F). She then began a 6-week course of fractionated radiation therapy at a dose of 54 Gy. Her examination findings continued to improve during radiation treatment, but she continued to show lower-extremity weakness and never returned to her neurological baseline. At nearly 4 months posttreatment, her clinical condition worsened. An MRI study demonstrated significant growth of the tumor (Fig. 3G and H), and she ultimately died 120 days after CED treatment.
Postmortem analysis of the brain showed a diffusely infiltrating glioma with marked nuclear pleomorphism, including numerous multinucleated tumor cells, frequent mitotic figures, vascular proliferation, and areas of pseudopallisading necrosis, all consistent with glioblastoma (WHO Grade IV) (Fig. 4). Tumor cells had widely infiltrated the brainstem and extended into the cerebellum, thalamus, and cervical spinal cord. There was no hemorrhage or infection at the site of catheter insertion in the pons. Histological findings were consistent with tumor progression as the cause of death, and did not show any adverse effects caused by placement of the catheter or perfusion of the drug.
FIG. 4.
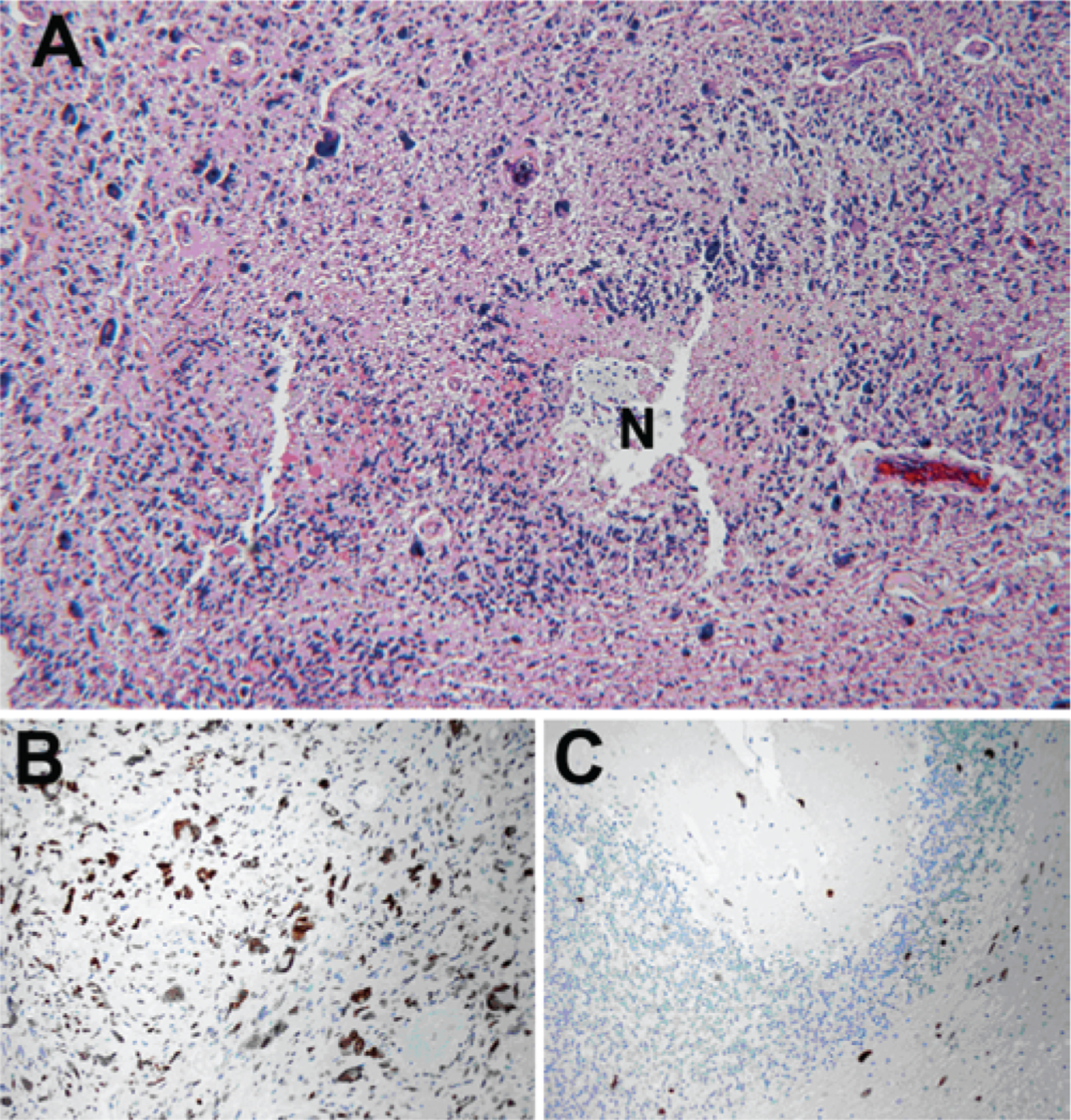
Case 2. Results of histological and immunohistochemical analysis of recurrent tumor at autopsy showing tumor progression. A: Hemotoxylin and eosin staining of the tumor in the pons showing the histological features of glioblastoma, with marked nuclear pleomorphism and areas of pseudopallisading necrosis (N). B and C: Immunoperoxidase staining for p53 showing strong nuclear staining (brown) in a subset of the tumor cells (B) and scattered p53+ tumor cells invading the cerebellar cortex (C). Original magnificatio×100.
Discussion
We have demonstrated for the first time that CED of topotecan into the brainstem of children with DIPG is technically feasible. In this limited study, we have also shown that a total volume of infusion greater than 2.7 ml with flow rates greater than 0.12 ml/hr led to new neurological deficits, prompting the need for further studies to determine optimal flow rates, drug concentration, and patient selection.
Convection-enhanced delivery is a method of regional drug delivery pioneered by Oldfield et al.3 with demonstrated safety in several clinical trials of adult patients.14,16, 23,25,26,28,32,34,35 Bulk flow provides a relatively uniform distribution of drug within the treatment volume with a steep drop in drug concentrations outside the volume of distribution, beyond which further distribution ultimately occurs by diffusion. Preclinical studies from our laboratory4,12 and others19,21,29–31,36 have demonstrated that CED in rat and nonhuman primate glioma models can achieve widespread perfusion of drugs throughout the brain and brainstem. For locally invasive and nonresectable tumors like DIPG, this treatment provides elevated concentrations of drug in the peritumoral region with undetectable serum drug concentrations, thereby avoiding systemic toxicity such as myelosuppression. Our previous studies showed that the blood-brain barrier to natural product drugs like topotecan was still partially intact in gliomas but not intact in metastatic brain lesions8 and therefore, while this partially functioning blood-brain barrier could impede the transit of systemically delivered topotecan into brain tumors, it is not an impediment with CED.
Topotecan, a topoisomerase I inhibitor, is an ideal chemotherapeutic agent for CED to malignant gliomas for several reasons: 1) it is cytotoxic to glioma cells and nontoxic to normal brain at clinical doses; 2) topoisomerase I levels are higher in glioma cells and tumor tissue than normal brain; and 3) it is a natural product drug with a high molecular weight and thus should only minimally traverse the blood-brain barrier from the brain to the systemic circulation. While previous clinical trials have shown minimal effects when topotecan is delivered intravenously,7 we have previously shown that CED of topotecan had significant antitumor effects and led to prolonged survival in animal models.4,12 Furthermore, we have recently completed a Phase Ib dose escalation study of topotecan via CED in patients with recurrent supratentorial malignant glioma that demonstrated significant antitumor activity with prolonged survival and minimal drug-associated toxicity.5
Treatment by means of CED has been previously reported in 2 children, one of whom had a DIPG. Lonser and colleagues17 reported CED of the antiglioma cytotoxin IL-13PE directly into the brainstem of a 4-year-old girl with a recurrent DIPG. Infusion was performed through a transfrontal approach at increasing flow rates (0.03–0.3 ml/hr) until a total infusion volume of 1.4 ml was reached. The patient developed mild lethargy and exacerbation of her preoperative bilateral CN VI palsy that resolved after 5 days of steroid treatment. Magnetic resonance imaging demonstrated tumor stability at 4 weeks posttreatment but recurrence at 2 months, and the child ultimately died 4 months posttreatment. In another study, Saito and colleagues27 reported the use of CED of nimustine hydrochloride for treatment of a cerebellar glioblastoma that was resected but recurred with partial extension into the brainstem. Infusion of nimustine hydrochloride at 0.25 mg/ml was performed through a transfrontal approach at increasing flow rates (0.06–0.3 ml/hr) until a total volume of 7.02 ml was achieved after 60 hours. The patient developed diplopia and a hemiparesis that resolved within 1 week. Posttreatment MRI demonstrated good distribution of the drug throughout the tumor and reduction in the enhancing portion of the tumor. The patient remained clinically well until his tumor recurred, and he died 6 months posttreatment.
Our study differs from these 2 prior studies in several important ways. The most significant difference is the higher complication rate seen in our study. There are several possible reasons for this; most notably, the total volumes of infusion were significantly higher in our patients than in the report of Lonser et al.17 (5.30 ml and 6.04 ml vs 1.4 ml total volume). In both of our patients, symptoms did not occur during CED until after a total volume of more than 2.7 ml was infused. It is also possible that the complications seen in our patients were due to higher initial flow rates than previously used (> 0.12 ml/hr vs 0.03 ml/hr). Although much higher flow rates and volumes of infusion have been well tolerated in supratentorial CED studies, we hypothesize that the reduced extracellular space intrinsic to the brainstem in the setting of a DIPG is likely to limit the tolerated total volume and flow rate of infusate. In the study by Saito et al.,27 a total volume of 7.02 ml was reached, with flow rates up to 0.3 ml/hr. However, it seems likely that these higher volumes and flow rates were achievable because the child did not have a DIPG. Rather, CED was performed for tumor recurrence in the lateral brainstem in the setting of a large resection cavity that could accommodate larger volumes and flow rates.
Our study also differs from previous studies in that our length of infusion was much longer (100 hours vs 4.6 hours17 and 60 hours27). This is less likely to be problematic because the complications seen in our patients occurred within the first 24 hours, with clinical improvement or stabilization after flow rates were reduced. Furthermore, preclinical studies have shown safety with prolonged infusions up to 7 days in rat brainstem models.21
Other differences include the number and trajectory of implanted catheters for CED. We chose to implant 2 catheters rather than a single catheter to try to maximize the volume of drug distribution throughout the tumor. In addition, we chose a posterior fossa approach with 1 catheter down each cerebellar peduncle rather than a transfrontal approach in order to minimize the amount of normal brain tissue traversed with the catheter. It is possible that some of the morbidity seen in our patients was due to the biopsy itself or the use of bilateral catheters rather than a single catheter. However, it is unlikely that these differences contributed to the complications in these patients, as there did not appear to be any morbidity associated with the initial biopsy or implanting of the catheters.
Finally, complications could have been related to the concentration of topotecan initially used (0.0667 mg/ml), although this seems less likely since both preclinical and adult human studies have established safety with higher concentrations of topotecan (0.1 mg/ml).
There are limitations to this study. First, detailed information regarding drug distribution throughout the brainstem cannot be determined because a tracer was not used.11,17,27 Recent studies using tracers with CED in nonhuman primates have demonstrated that the volume of distribution achieved with CED is approximately twice the volume seen on traditional T2-weighted MR images.11 In our patients, the T2 signal changes on MRI were asymmetrical and inconsistent. Although the reasons for this are not known, different tissue densities or anatomical locations of the catheter tips within the tumors may in part explain these differences. Second, prior to this trial, we did not have any clinical evidence that topotecan delivered via CED was effective against malignant gliomas. We chose to use topotecan, because in our preclinical laboratory studies, topotecan had a reasonable safety profile and was most effective against malignant gliomas.12 Furthermore, by targeting proliferative processes such as DNA repair, synthesis, and cell proliferation, topotecan provides a more clinically specific and effective mechanism of action than prior studies using CED with targeted toxins for supratentorial malignant gliomas.14,16,26,28
Although the clinical outcomes for these 2 patients were disappointing, the need for Phase I clinical trials with routine histological sampling along with CED of therapeutic agents in children with DIPG is warranted given the lack of treatment alternatives.13 These 2 patients were treated as part of a Phase Ib trial designed specifically to determine feasibility, not efficacy. Additional Phase I studies of CED utilizing dose escalation with lower infusion volumes and flow rates in combination with tracer are needed to determine optimal safety and efficacy to treat children with DIPG.
Acknowledgment
The authors would like to thank Nancy Heim for her original artwork.
Disclosure
This study was supported by NIH grant 5R01CA89395. This publication was made possible by grant number UL1 RR024156 from the National Center for Research Resources (NCRR), a component of the NIH, and the NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at the NCRR website. Information on Re-engineering the Clinical Research Enter prise can be obtained from the NIH Roadmap website.
Abbreviations used in this paper:
- CED
convection-enhanced delivery
- CN
cranial nerve
- DIPG
diffuse intrinsic pontine glio -ma
- KPS
Karnofsky Performance Status.
References
- 1.Albright AL, Price RA, Guthkelch AN: Brain stem gliomas of children. A clinicopathological study. Cancer 52:2313–2319, 1983 [DOI] [PubMed] [Google Scholar]
- 2.Allen JC, Siffert J: Contemporary chemotherapy issues for children with brainstem gliomas. Pediatr Neurosurg 24:98–102, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH: Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A 91:2076–2080, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruce JN, Falavigna A, Johnson JP, Hall JS, Birch BD, Yoon JT, et al. : Intracerebral clysis in a rat glioma model. Neurosurgery 46:683–691, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Bruce JN, Fine RL, Canoll P, Yun J, Kennedy BC, Rosenfeld SS, et al. : Regression of recurrent malignant gliomas with convection-enhanced delivery of topotecan. Neurosurgery 69: 1272–1280, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frazier JL, Lee J, Thomale UW, Noggle JC, Cohen KJ, Jallo GI: Treatment of diffuse intrinsic brainstem gliomas: failed approaches and future strategies. A review. J Neurosurg Pediatr 3:259–269, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Friedman HS, Kerby T, Fields S, Zilisch JE, Graden D, Mc-Len don RE, et al. : Topotecan treatment of adults with primary ma lignant glioma. Cancer 85:1160–1165, 1999 [PubMed] [Google Scholar]
- 8.Gerstner ER, Fine RL: Increased permeability of the blood-brain barrier to chemotherapy in metastatic brain tumors: establishing a treatment paradigm. J Clin Oncol 25:2306–2312, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Groothuis DR, Benalcazar H, Allen CV, Wise RM, Dills C, Dobrescu C, et al. : Comparison of cytosine arabinoside delivery to rat brain by intravenous, intrathecal, intraventricular and intraparenchymal routes of administration. Brain Res 856:281–290, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Hargrave D, Bartels U, Bouffet E: Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol 7: 241–248, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Iyer RR, Butman JA, Walbridge S, Gai ND, Heiss JD, Lonser RR: Tracking accuracy of T2- and diffusion-weighted magnetic resonance imaging for infusate distribution by convection-enhanced delivery. Laboratory investigation. J Neurosurg 115:474–480, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaiser MG, Parsa AT, Fine RL, Hall JS, Chakrabarti I, Bruce JN: Tissue distribution and antitumor activity of topotecan delivered by intracerebral clysis in a rat glioma model. Neurosurgery 47:1391–1399, 2000 [PubMed] [Google Scholar]
- 13.Khatua S, Moore KR, Vats TS, Kestle JR: Diffuse intrinsic pontine glioma-current status and future strategies. Childs Nerv Syst 27:1391–1397, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Kunwar S: Convection enhanced delivery of IL13-PE38QQR for treatment of recurrent malignant glioma: presentation of interim findings from ongoing phase 1 studies. Acta Neurochir Suppl 88:105–111, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Langer R: New methods of drug delivery. Science 249:1527–1533, 1990 [DOI] [PubMed] [Google Scholar]
- 16.Laske DW, Youle RJ, Oldfield EH: Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat Med 3:1362–1368, 1997 [DOI] [PubMed] [Google Scholar]
- 17.Lonser RR, Warren KE, Butman JA, Quezado Z, Robison RA, Walbridge S, et al. : Real-time image-guided direct convective perfusion of intrinsic brainstem lesions. Technical note. J Neurosurg 107:190–197, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Morrison PF, Chen MY, Chadwick RS, Lonser RR, Oldfield EH: Focal delivery during direct infusion to brain: role of flow rate, catheter diameter, and tissue mechanics. Am J Physiol 277:R1218–R1229, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Murad GJ, Walbridge S, Morrison PF, Garmestani K, Degen JW, Brechbiel MW, et al. : Real-time, image-guided, convection-enhanced delivery of interleukin 13 bound to pseudomonas exotoxin. Clin Cancer Res 12:3145–3151, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Murad GJ, Walbridge S, Morrison PF, Szerlip N, Butman JA, Oldfield EH, et al. : Image-guided convection-enhanced delivery of gemcitabine to the brainstem. J Neurosurg 106:351–356, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Occhiogrosso G, Edgar MA, Sandberg DI, Souweidane MM: Prolonged convection-enhanced delivery into the rat brainstem. Neurosurgery 52:388–394, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Pardridge WM: Drug delivery to the brain. J Cereb Blood Flow Metab 17:713–731, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Patel SJ, Shapiro WR, Laske DW, Jensen RL, Asher AL, Wessels BW, et al. : Safety and feasibility of convection-enhanced delivery of Cotara for the treatment of malignant glioma: initial experience in 51 patients. Neurosurgery 56:1243–1253, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Pierre-Kahn A, Hirsch JF, Vinchon M, Payan C, Sainte-Rose C, Renier D, et al. : Surgical management of brain-stem tumors in children: results and statistical analysis of 75 cases. J Neurosurg 79:845–852, 1993 [DOI] [PubMed] [Google Scholar]
- 25.Pöpperl G, Goldbrunner R, Gildehaus FJ, Kreth FW, Tanner P, Holtmannspötter M, et al. : O-(2-[18F]fluoroethyl)-L-tyrosine PET for monitoring the effects of convection-enhanced delivery of paclitaxel in patients with recurrent glioblastoma. Eur J Nucl Med Mol Imaging 32:1018–1025, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Rand RW, Kreitman RJ, Patronas N, Varricchio F, Pastan I, Puri RK: Intratumoral administration of recombinant circularly permuted interleukin-4-Pseudomonas exotoxin in patients with high-grade glioma. Clin Cancer Res 6:2157–2165, 2000 [PubMed] [Google Scholar]
- 27.Saito R, Sonoda Y, Kumabe T, Nagamatsu K, Watanabe M, Tominaga T: Regression of recurrent glioblastoma infiltrating the brainstem after convection-enhanced delivery of nimustine hydrochloride. Case report. J Neurosurg Pediatr 7: 522–526, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Sampson JH, Akabani G, Archer GE, Bigner DD, Berger MS, Friedman AH, et al. : Progress report of a Phase I study of the intracerebral microinfusion of a recombinant chimeric protein composed of transforming growth factor (TGF)-alpha and a mutated form of the Pseudomonas exotoxin termed PE-38 (TP-38) for the treatment of malignant brain tumors. J Neurooncol 65:27–35, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Sandberg DI, Edgar MA, Souweidane MM: Convection-enhanced delivery into the rat brainstem. J Neurosurg 96:885–891, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Thomale UW, Tyler B, Renard V, Dorfman B, Chacko VP, Carson BS, et al. : Neurological grading, survival, MR imaging, and histological evaluation in the rat brainstem glioma model. Childs Nerv Syst 25:433–441, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Thomale UW, Tyler B, Renard VM, Dorfman B, Guarnieri M, Haberl HE, et al. : Local chemotherapy in the rat brainstem with multiple catheters: a feasibility study. Childs Nerv Syst 25:21–28, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Voges J, Reszka R, Gossmann A, Dittmar C, Richter R, Garlip G, et al. : Imaging-guided convection-enhanced delivery and gene therapy of glioblastoma. Ann Neurol 54:479–487, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Walker DA, Punt JA, Sokal M: Clinical management of brain stem glioma. Arch Dis Child 80:558–564, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber FW, Floeth F, Asher A, Bucholz R, Berger M, Prados M, et al. : Local convection enhanced delivery of IL4-Pseudomonas exotoxin (NBI-3001) for treatment of patients with recurrent malignant glioma. Acta Neurochir Suppl 88:93–103, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Yang W, Barth RF, Wu G, Bandyopadhyaya AK, Thirumamagal BT, Tjarks W, et al. : Boronated epidermal growth factor as a delivery agent for neutron capture therapy of EGF receptor positive gliomas. Appl Radiat Isot 61:981–985, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Yin D, Richardson RM, Fiandaca MS, Bringas J, Forsayeth J, Berger MS, et al. : Cannula placement for effective convection-enhanced delivery in the nonhuman primate thalamus and brainstem: implications for clinical delivery of therapeutics. Laboratory investigation. J Neurosurg 113:240–248, 2010 [DOI] [PubMed] [Google Scholar]


