Abstract
Mastectomy often leads to a complete desensitization of the chest, which in turn can give rise to diminished sexual function and to disembodiment of the breasts. One approach to mitigate the sensory consequences of mastectomy is to leverage technology that has been developed for the restoration of sensation in bionic hands. Specifically, sensors embedded under the skin of the nipple-areolar complex can be used to detect touches. The output of the sensors then drives electrical stimulation of the residual intercostal nerves, delivered through chronically implanted electrode arrays, thereby eliciting tactile sensations experienced on the nipple–areolar complex. The hope is that the bionic breast will restore a woman’s sense that her breast belongs to her body so she can experience the pleasure of an embrace and derive the benefit of the sensual touch of her partner.
Keywords: embodiment, tactile feedback, bionics, breast, mastectomy, sensation
Introduction
“…one breast was removed and reconstructed, with nipple sparing, and the other was changed to ‘match it’…No one told me that I would lose erotic sensation, and I waited a long, sad time to realize that the sensation that was, for me, dominant in my sexual arousal would never return.”
Cheryl Lilienstein, breast cancer survivor, commenting online to a New York Times article,1/29/2017
More than 2 million women were diagnosed with breast cancer globally in 2018, one in eight of these cases in the United States (Bray et al., 2018; Siegel et al., 2018). Of more than 3.8 million female breast cancer survivors in the United States today, about a third have undergone mastectomy and hundreds of thousands have had breast reconstruction procedures (Morrow et al., 2014; Kummerow et al., 2015; Wong et al., 2016). More than 100,000 women have one or both breasts removed each year. Since passage of the 1998 U.S. Women’s Health and Cancer Rights Act (WHCRA), which mandates insurance coverage for breast reconstruction after mastectomy, the number of women with mastectomy who choose to undergo breast reconstruction procedures has increased threefold (Kummerow et al., 2015). Additionally, 80% of a growing number of women in North America and Europe without breast cancer, but at elevated risk, elect reconstruction after prophylactic mastectomy (Cemal et al., 2013; Semple et al., 2013). Rates of breast reconstruction after mastectomy are high among women living in United States, Canada, and many European countries (Semple et al., 2013). Although rates are lower in low- and middle-income countries due to resource and access constraints, breast reconstruction procedures after mastectomy are performed around the globe (Anderson et al., 2008).
The Role of Breast Sensation in Female Sexual Function
Loss of breast sensation after mastectomy is a prevalent, well-established, and distressing outcome for women and their partners that—despite best practice guidelines—is rarely addressed in the course of breast cancer care (Snell et al., 2010; Marshall and Galea, 2015; Flitcroft et al., 2017). Advances in breast preservation and reconstruction (including skin-sparing, nipple-sparing, deep inferior epigastric artery perforate, and neurotization techniques) aim to better preserve breast sensation to touch. However, these procedures typically have strict eligibility criteria, sensation outcomes are highly unpredictable, and normal breast sensation is rarely preserved (Magarakis et al., 2013; Zhou et al., 2018). Although surgical innovation continues to evolve (Peled and Peled, 2019), preservation of the distal branches of the intercostal nerves supplying the breast is typically infeasible for women with breast cancer due to concern about oncologic safety (Liebig et al., 2009). Breast transplantation has not been documented. However, transplantation requires immunosuppression, an unacceptable risk for a breast cancer patient, and would likely yield a poor sensory outcome. Although concern for sensory outcomes after mastectomy is growing, the majority of research paradigms in breast reconstruction after mastectomy have focused on aesthetic or cosmetic rather than functional outcomes.
Breast sensation is an important aspect of breast function for two reasons. First, the sense of touch is essential to embodiment or a person’s feeling that a body part belongs to her (Botvinick and Cohen, 1998). Patients seen at the University of Chicago Program in Integrative Sexual Medicine have said about their breasts after reconstruction: “They look great in a sweater, but they are dead to me” and “Aesthetically, they look awesome, but they do nothing for me.” These statements corroborate zoologist Stephen Wainwright’s maxim, especially apt for sense of touch: “Structure without function is a corpse” (Wainwright, 1988). The disconnect between body appearance and function can have deleterious consequences on overall physical, psychic, and social function. Second, breasts play an important role in female sexual function (Levin and Meston, 2006). From a patient with numb breasts after reconstruction: “When you don’t feel something and you know someone is touching them, it’s a turn-off.” Some women describe aversion to and avoidance of sex or even a feeling of anger, disgust, or dissociation during sexual contact with their numb breasts after mastectomy. Nipple–areolar complex sensation is an essential component of arousal and orgasm physiology for many people and their partners (Levin, 2006). Sexual dysfunction affects as many as three quarters of women with breast cancer and is at least partially attributable to loss of breast sensation (Ganz et al., 1999; Fobair et al., 2006; American Psychiatric Association, 2013; Raggio et al., 2014; Rojas et al., 2017).
Preservation and Restoration of Sexual Function in Men With Cancer
In contrast to breast cancer care, medical decision-making for men with prostate cancer is routinely informed by evidence about sexual function outcomes associated with treatment options (American Urology Association, 2007). Preservation and restoration of sexual function in men with prostate cancer has been identified as an important patient-centered outcome. The effort to improve sexual function outcomes for men after prostate cancer (American Urology Association, 2007; Grondhuis Palacios et al., 2017; Elliott and Matthew, 2018; Girodet et al., 2019) has driven substantial surgical innovation over the last two decades, supported in part by the U.S. National Institutes of Health, and has produced significant improvements (Willis et al., 2012). In 2016, a man with penile cancer (a cancer type affecting < 0.001% of men) underwent a successful penile transplant. Cosmesis was not the only outcome of interest—the measure of success in this and a subsequent case also required restoration of urinary, reproductive, and sexual function (Grady, 2016; Merwe et al., 2017). Between 2014 and 2018 at least four successful penile transplants have been accomplished worldwide. These breakthrough efforts to preserve male sexual function, including in the context of penile cancer, both inspire and legitimize innovation to preserve breast function for the large and growing population of women with or at elevated risk of breast cancer (Grady, 2016; Merwe et al., 2017; McDaniels, 2018).
Toward a Bionic Breast
One approach to restore function to reconstructed breasts is to apply bionic technologies that have been successful in sensitizing prosthetic hands. Sensitization of the prosthetic hand is achieved by electrical stimulation of residual nerves delivered through chronically implanted arrays of electrodes, which evokes sensations that are referred to the phantom hand (Saal and Bensmaia, 2015). The referred sensations are highly localized and repeatable, and their magnitude can be manipulated by modulating the amplitude or frequency of microstimulation (Graczyk et al., 2016). These phenomena can be exploited by connecting pressure sensors on the bionic hand to electrodes with somatotopically appropriate projection fields. For example, the pressure sensor on the index fingertip of the bionic hand drives stimulation though an electrode that evokes sensations on the index fingertip. Bionic hands endowed with artificial tactile feedback confer greater dexterity to users than do insensate ones (Ortiz-Catalan et al., 2014; Valle et al., 2018; George et al., 2019). Furthermore, the incorporation of artificial touch leads to a greater embodiment of the hand (Marasco et al., 2011; Schiefer et al., 2016; Page et al., 2018; Rognini et al., 2018; Valle et al., 2018) and restores some of the key psychosocial components of manual touch, including the ability to experience pleasure from touching a loved one (Graczyk et al., 2018, 2019; Cuberovic et al., 2019; George et al., 2019).
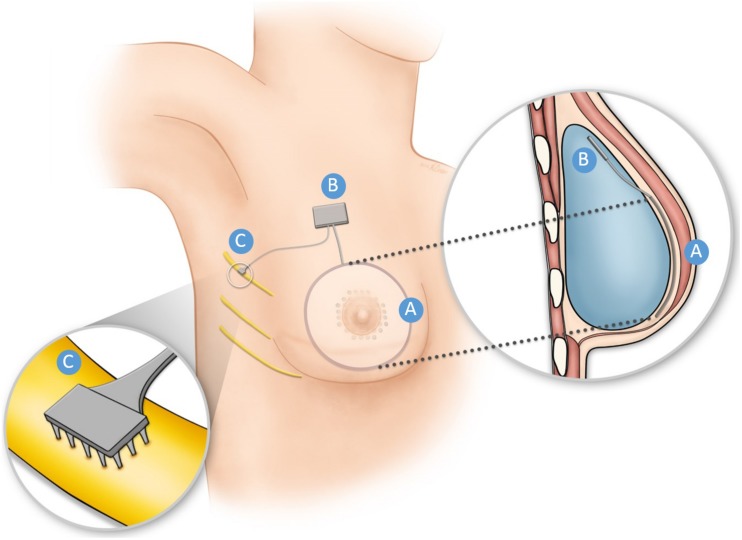
This strategy can be straightforwardly applied to reconstructed breast. An array of sensors would be implanted under the skin of the breast centered on the nipple (Figure 1), an application for which recent advances in flexible sensory technology can be leveraged (Boutry et al., 2019; Niu et al., 2019; Ruth et al., 2019). The output of the sensors would drive electrical stimulation through arrays implanted in residual intercostal nerves III–VI, which carry sensory signals from the breast. Various technologies have been developed to provide electrical interfaces with the peripheral nerves, any one of which would be appropriate for this application (Saal and Bensmaia, 2015). The sensory encoding algorithms—which convert patterns of sensor output into trains of electrical stimulation—would be implemented in a hermetically sealed electronic circuit that would be lodged in the implant or just under the skin. Such an implantable circuit—with the appropriate inputs and outputs—has already been developed for bionic hands (Peterson et al., 2016). The spatial extent of the sensor array under the reconstructed breast will depend on which intercostal nerves are implanted with stimulating arrays. Minimally, the array will span the nipple–areolar complex, but may cover the entire breast if all four intercostal nerves are accessed. Given the current state of technology, the principal bottleneck in the spatial resolution of artificial touch is set by the electrical interface with the nerve, not the sensors, so sensor pitch will not be a major design specification for the sensor sheets.
FIGURE 1.
Schematic illustration of the Bionic Breast concept for a breast reconstructed using an implant. An array of pressure sensors (A) is placed under the reconstructed or tattooed nipple and the surrounding region. The output of the sensors triggered by pressure applied to the skin is converted into electrical stimulation pulse trains via a hermetically sealed electronic circuit (B), shown here to be encased in the breast implant. The electrical stimulation pulse trains are delivered through one or more electrode arrays (C) implanted around or in the residual intercostal nerve(s) that innervated the nipple areolar complex before mastectomy. Nerve stimulation results in a sensation projected to the nipple areolar region. Much of this technology has already been developed to restore sensation to bionic hands.
The current consensus is that the tactile sensibility of the breast is similar to that of the hand, so sensory encoding models developed for the hand (Saal et al., 2017; Okorokova et al., 2018) can likely be applied to the breast, accounting for differences in the relative densities of the different subtypes of tactile nerve fibers. A limitation of this approach is that thermal sensation—key to affective and sexual touch—cannot be restored through electrical stimulation because thermoreceptive nerve fibers are small and unmylienated and thus insusceptible to electrical stimulation. Similarly, nerve fibers implicated in affective touch—c-tactile fibers—will not be activated by the electrical interface. With these caveats, restoration of tactile sensation alone is liable to have a major impact on the sexual and psychosocial function of the breast, based on its documented impact on manual function.
The U.S. National Cancer Institute has invested in the Bionic Breast Project, a new, interdisciplinary program of research that is laying the foundation of basic knowledge—including development of subjective and objective measures of female breast function—to apply bionic technologies to restoration of breast function after mastectomy. In addition to improving outcomes for women with breast cancer, this work could hold promise for improved outcomes after reconstruction for traumatic breast injury and for people undergoing elective breast surgery, for example, for gender affirmation.
Discussion
Breakthroughs in restoration of hand and penile function, including for intimate and sexual function, have laid the scientific and moral foundations for the preservation and restoration of breast function in the millions of women affected by breast cancer worldwide. Clinical guidelines in the United States, United Kingdom, and Australia recommend women undergoing mastectomy be offered the option of breast reconstruction (National Breast Cancer Centre, 2001; Harnett et al., 2009). Advocating to the United States Congress about the WHCRA in 1998, a senator expressed his outrage at the medical director of an insurance company who argued that, because the breast “is not a bodily function,” its replacement is not medically necessary (Senate, 1998). Sensitization of the reconstructed breast will promote its embodiment and mitigate the sensory component of mastectomy-induced sexual dysfunction. Preservation and restoration of breast sensation, along with other aspects of breast function are attainable, imperative, and well within the coverage bounds for breast reconstruction mandated by U.S. WHCRA (Centers for Medicare & Medicaid Services, 2013).
Author Contributions
SL conceived of the idea. SB contributed technically to the concept. SL and SB wrote and edited the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The University of Chicago has filed a provisional patent related to the work described here.
Acknowledgments
We would like to acknowledge El Pinkerton, Katie Long, Aneesha Suresh, and Kristine McLellan for their help developing this project and Kenzie Green for the illustration.
Footnotes
Funding. This work was supported by the National Cancer Institute (Grant No. 1R21CA226726) and pilot funding from the Janet D. Rowley Discovery Fund at the University of Chicago Comprehensive Cancer Center. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
References
- American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th Edn Washington, DC: APA. [Google Scholar]
- American Urology Association (2007). Prostate Cancer: Guideline for the Management of Clinically Localized Prostate Cancer: 2007 Update. Linthicum: American Urology Association. [Google Scholar]
- Anderson B. O., Yip C.-H., Smith R. A., Shyyan R., Sener S. F., Eniu A., et al. (2008). Guideline implementation for breast healthcare in low-income and middle-income countries: overview of the Breast Health Global Initiative Global Summit 2007. Cancer 113 2221–2243. 10.1002/cncr.23844 [DOI] [PubMed] [Google Scholar]
- Botvinick M., Cohen J. (1998). Rubber hands “feel” touch that eyes see. Nature 391:756. 10.1038/35784 [DOI] [PubMed] [Google Scholar]
- Boutry C. M., Beker L., Kaizawa Y., Vassos C., Tran H., Hinckley A. C., et al. (2019). Biodegradable and flexible arterial-pulse sensor for the wireless monitoring of blood flow. Nat. Biomed. Eng. 3 47–57. 10.1038/s41551-018-0336-5 [DOI] [PubMed] [Google Scholar]
- Bray F., Ferlay J., Soerjomataram I., Siegel R. L., Torre L. A., Jemal A. (2018). Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68 394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- Cemal Y., Albornoz C. R., Disa J. J., McCarthy C. M., Mehrara B. J., Pusic A. L., et al. (2013). A paradigm shift in U.S. breast reconstruction: Part 2. The influence of changing mastectomy patterns on reconstructive rate and method. Plast. Reconstr. Surg. 131 320e–326e. 10.1097/PRS.0b013e31827cf576 [DOI] [PubMed] [Google Scholar]
- Centers for Medicare & Medicaid Services (2013). WHCRA Factsheet. Available online at: https://www.cms.gov/CCIIO/Programs-and-Initiatives/Other-Insurance-Protections/whcra_factsheet.html (accessed August 29, 2019). [Google Scholar]
- Cuberovic I., Gill A., Resnik L. J., Tyler D. J., Graczyk E. L. (2019). Learning of artificial sensation through long-term home use of a sensory-enabled prosthesis. Front. Neurosci. 13:853. 10.3389/fnins.2019.00853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott S., Matthew A. (2018). Sexual recovery following prostate cancer: recommendations from 2 established canadian sexual rehabilitation clinics. Sex. Med. Rev. 6 279–294. 10.1016/j.sxmr.2017.09.001 [DOI] [PubMed] [Google Scholar]
- Flitcroft K., Brennan M., Spillane A. (2017). Women’s expectations of breast reconstruction following mastectomy for breast cancer: a systematic review. Support. Care Cancer 25 2631–2661. 10.1007/s00520-017-3712-x [DOI] [PubMed] [Google Scholar]
- Fobair P., Stewart S. L., Chang S., D’Onofrio C., Banks P. J., Bloom J. R. (2006). Body image and sexual problems in young women with breast cancer. Psychooncology 15 579–594. 10.1002/pon.991 [DOI] [PubMed] [Google Scholar]
- Ganz P. A., Desmond K. A., Belin T. R., Meyerowitz B. E., Rowland J. H. (1999). Predictors of sexual health in women after a breast cancer diagnosis. J. Clin. Oncol. 17 2371–2371. 10.1200/JCO.1999.17.8.2371 [DOI] [PubMed] [Google Scholar]
- George J. A., Kluger D. T., Davis T. S., Wendelken S. M., Okorokova E. V., He Q., et al. (2019). Biomimetic sensory feedback through peripheral nerve stimulation improves dexterous use of a bionic hand. Sci. Robot. 4:eaax2352 10.1126/scirobotics.aax2352 [DOI] [PubMed] [Google Scholar]
- Girodet M., Bouhnik A.-D., Mancini J., Peretti-Watel P., Bendiane M.-K., Ray-Coquard I., et al. (2019). Sexual desire of French representative prostate cancer survivors 2 years after diagnosis (the VICAN survey). Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 27 2517–2524. 10.1007/s00520-018-4536-z [DOI] [PubMed] [Google Scholar]
- Graczyk E. L., Gill A., Tyler D. J., Resnik L. J. (2019). The benefits of sensation on the experience of a hand: a qualitative case series. PLoS One 14:e0211469. 10.1371/journal.pone.0211469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graczyk E. L., Resnik L., Schiefer M. A., Schmitt M. S., Tyler D. J. (2018). Home use of a neural-connected sensory prosthesis provides the functional and psychosocial experience of having a hand again. Sci. Rep. 8:9866. 10.1038/s41598-018-26952-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graczyk E. L., Schiefer M. A., Saal H. P., Delhaye B. P., Bensmaia S. J., Tyler D. J. (2016). The neural basis of perceived intensity in natural and artificial touch. Sci. Transl. Med. 8:362ra142. 10.1126/scitranslmed.aaf5187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady D. (2016). Cancer Survivor Receives First Penis Transplant in the United States. Available online at: https://www.nytimes.com/2016/05/17/health/thomas-manning-first-penis-transplant-in-us.html (accessed February 9, 2017). [Google Scholar]
- Grondhuis Palacios L. A., Krouwel E. M., Duijn M., den Oudsten B. L., den Ouden M. E. M., Putter H., et al. (2017). Written information material and availability of sexual health care for men experiencing sexual dysfunction after prostate cancer treatment: an evaluation of Dutch urology and radiotherapy departments. Eur. J. Cancer Care 26:e12629. 10.1111/ecc.12629 [DOI] [PubMed] [Google Scholar]
- Harnett A., Smallwood J., Titshall V., Champion A. Guideline Development and Group (2009). Diagnosis and treatment of early breast cancer, including locally advanced disease–summary of NICE guidance. BMJ 338:b438. 10.1136/bmj.b438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummerow K. L., Du L., Penson D. F., Shyr Y., Hooks M. A. (2015). Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg. 150 9–16. 10.1001/jamasurg.2014.2895 [DOI] [PubMed] [Google Scholar]
- Levin R. (2006). The breast/nipple/areola complex and human sexuality. Sex. Relatsh. Ther. 21 237–249. 10.1080/14681990600674674 [DOI] [Google Scholar]
- Levin R., Meston C. (2006). Nipple/Breast stimulation and sexual arousal in young men and women. J. Sex. Med. 3 450–454. 10.1111/j.1743-6109.2006.00230.x [DOI] [PubMed] [Google Scholar]
- Liebig C., Ayala G., Wilks J. A., Berger D. H., Albo D. (2009). Perineural invasion in cancer: a review of the literature. Cancer 115 3379–3391. 10.1002/cncr.24396 [DOI] [PubMed] [Google Scholar]
- Magarakis M., Venkat R., Dellon A. L., Shridharani S. M., Bellamy J., Vaca E. E., et al. (2013). Pilot study of breast sensation after breast reconstruction: evaluating the effects of radiation therapy and perforator flap neurotization on sensory recovery. Microsurgery 33 421–431. 10.1002/micr.22124 [DOI] [PubMed] [Google Scholar]
- Marasco P. D., Kim K., Colgate J. E., Peshkin M. A., Kuiken T. A. (2011). Robotic touch shifts perception of embodiment to a prosthesis in targeted reinnervation amputees. Brain 134 747–758. 10.1093/brain/awq361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall B., Galea S. (2015). Formalizing the role of agent-based modeling in causal inference and epidemiology. Am. J. Epidemiol. 181 92–99. 10.1093/aje/kwu274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniels A. K. (2018). Johns Hopkins performs world’s first “total” penis transplant, on veteran wounded by IED blast. Available online at: https://www.baltimoresun.com/health/bs-hs-hopkins-transplant-surgery-20180423-story.html (accessed August 29, 2019). [Google Scholar]
- Merwe A., van der Graewe F., Zühlke A., Barsdorf N. W., Zarrabi A. D., Viljoen J. T., et al. (2017). Penile allotransplantation for penis amputation following ritual circumcision: a case report with 24 months of follow-up. Lancet 390 1038–1047. 10.1016/S0140-6736(17)31807-X [DOI] [PubMed] [Google Scholar]
- Morrow M., Li Y., Alderman A. K., Jagsi R., Hamilton A. S., Graff J. J., et al. (2014). Access to breast reconstruction after mastectomy and patient perspectives on reconstruction decision making. JAMA Surg. 149 1015–1021. 10.1001/jamasurg.2014.548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Breast Cancer Centre (2001). Clinical Practice Guidelines for the Management of Early Breast Cancer, 2nd Edn Camperdown: National Breast Cancer Centre. [Google Scholar]
- Niu S., Matsuhisa N., Beker L., Li J., Wang S., Wang J., et al. (2019). A wireless body area sensor network based on stretchable passive tags. Nat. Electron. 2 361–368. 10.1038/s41928-019-0286-2 [DOI] [Google Scholar]
- Okorokova E. V., He Q., Bensmaia S. J. (2018). Biomimetic encoding model for restoring touch in bionic hands through a nerve interface. J. Neural Eng. 15:066033. 10.1088/1741-2552/aae398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Catalan M., Håkansson B., Brånemark R. (2014). An osseointegrated human-machine gateway for long-term sensory feedback and motor control of artificial limbs. Sci. Transl. Med. 6:257re6. 10.1126/scitranslmed.3008933 [DOI] [PubMed] [Google Scholar]
- Page D. M., George J. A., Kluger D. T., Duncan C., Wendelken S., Davis T., et al. (2018). Motor control and sensory feedback enhance prosthesis embodiment and reduce phantom pain after long-term hand amputation. Front. Hum. Neurosci. 12:352. 10.3389/fnhum.2018.00352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peled A. W., Peled Z. M. (2019). Nerve preservation and allografting for sensory innervation following immediate implant breast reconstruction. Plast. Reconstr. Surg. Glob. Open 7:e2332. 10.1097/GOX.0000000000002332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. J., Dinsmoor D. A., Tyler D. J., Denison T. J. (2016). “Stimulation artifact rejection in closed-loop, distributed neural interfaces,” in Proceedings of the ESSCIRC Conference 2016: 42nd European Solid-State Circuits Conference, Lausanne, 233–236. [Google Scholar]
- Raggio G. A., Butryn M. L., Arigo D., Mikorski R., Palmer S. C. (2014). Prevalence and correlates of sexual morbidity in long-term breast cancer survivors. Psychol. Health 29 632–650. 10.1080/08870446.2013.879136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognini G., Petrini F. M., Raspopovic S., Valle G., Granata G., Strauss I., et al. (2018). Multisensory bionic limb to achieve prosthesis embodiment and reduce distorted phantom limb perceptions. J. Neurol. Neurosurg. Psychiatry 90 833–836. 10.1136/jnnp-2018-318570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas K., Onstad M., Raker C., Clark M. A., Stuckey A., Gass J. (2017). The impact of mastectomy type on the female sexual function index (FSFI), satisfaction with appearance, and the reconstructed breast’s role in intimacy. Breast Cancer Res. Treat. 163 273–279. 10.1007/s10549-017-4174-z [DOI] [PubMed] [Google Scholar]
- Ruth S. R. A., Beker L., Tran H., Feig V. R., Matsuhisa N., Bao Z. (2019). Rational design of capacitive pressure sensors based on pyramidal microstructures for specialized monitoring of biosignals. Adv. Funct. Mater. 2019:1903100 10.1002/adfm.201903100 [DOI] [Google Scholar]
- Saal H. P., Bensmaia S. J. (2015). Biomimetic approaches to bionic touch through a peripheral nerve interface. Neuropsychologia 79 344–353. 10.1016/j.neuropsychologia.2015.06.010 [DOI] [PubMed] [Google Scholar]
- Saal H. P., Delhaye B. P., Rayhaun B. C., Bensmaia S. J. (2017). Simulating tactile signals from the whole hand with millisecond precision. Proc. Natl. Acad. Sci. U.S.A. 114 E5693–E5702. 10.1073/pnas.1704856114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefer M., Tan D., Sidek S. M., Tyler D. J. (2016). Sensory feedback by peripheral nerve stimulation improves task performance in individuals with upper limb loss using a myoelectric prosthesis. J. Neural Eng. 13:016001. 10.1088/1741-2560/13/1/016001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple J., Metcalfe K. A., Lynch H. T., Kim-Sing C., Senter L., Pal T., et al. (2013). International rates of breast reconstruction after prophylactic mastectomy in BRCA1 and BRCA2 mutation carriers. Ann. Surg. Oncol. 20 3817–3822. 10.1245/s10434-013-3040-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senate (1998). Omnibus appropriations conference report. Cong. Rec. 144 S12810–S12839. [Google Scholar]
- Siegel R. L., Miller K. D., Jemal A. (2018). Cancer statistics, 2018. CA Cancer J. Clin. 68 7–30. 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- Snell L., McCarthy C., Klassen A., Cano S., Rubin L., Hurley K., et al. (2010). Clarifying the expectations of patients undergoing implant breast reconstruction: a qualitative study. Plast. Reconstr. Surg. 126 1825–1830. 10.1097/PRS.0b013e3181f44580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle G., Mazzoni A., Iberite F., D’Anna E., Strauss I., Granata G., et al. (2018). Biomimetic intraneural sensory feedback enhances sensation naturalness, tactile sensitivity, and manual dexterity in a bidirectional prosthesis. Neuron 100 37.e7–45.e7. 10.1016/j.neuron.2018.08.033 [DOI] [PubMed] [Google Scholar]
- Wainwright S. A. (1988). Form and function in organisms. Am. Zool. 28 671–680. [Google Scholar]
- Willis D. L., Gonzalgo M. L., Brotzman M., Feng Z., Trock B., Su L.-M. (2012). Comparison of outcomes between pure laparoscopic vs robot-assisted laparoscopic radical prostatectomy: a study of comparative effectiveness based upon validated quality of life outcomes. BJU Int. 109 898–905. 10.1111/j.1464-410X.2011.10551.x [DOI] [PubMed] [Google Scholar]
- Wong S. M., Freedman R. A., Sagara Y., Aydogan F., Barry W. T., Golshan M. (2016). Growing use of contralateral prophylactic mastectomy despite no improvement in long-term survival for invasive breast cancer. Ann. Surg. 265 581–589. 10.1097/SLA.0000000000001698 [DOI] [PubMed] [Google Scholar]
- Zhou A., Ducic I., Momeni A. (2018). Sensory restoration of breast reconstruction - The search for the ideal approach continues. J. Surg. Oncol. 118 780–792. 10.1002/jso.25223 [DOI] [PubMed] [Google Scholar]