Abstract
With the widespread adoption of multi-modality treatment, 5-year survival of children diagnosed with cancer has improved dramatically in the past several decades from approximately 60% in 1970 to greater than 85% currently. As a result, there are an estimated nearly half a million long-term survivors of childhood cancer living in the United States today. However, survivors have, on average, significantly greater serious medical and psychosocial late effects compared with the general population. In this review, we will discuss the current epidemiology of childhood cancer survivorship, including new methods to estimate the burden of late effects and genetic susceptibility towards late effects. We also will review the development of surveillance guidelines for childhood cancer survivors and early toxicity signals from novel agents now being tested and used increasingly to treat pediatric and adult cancers. We conclude with an overview of current models of survivorship care and areas for future research.
Introduction
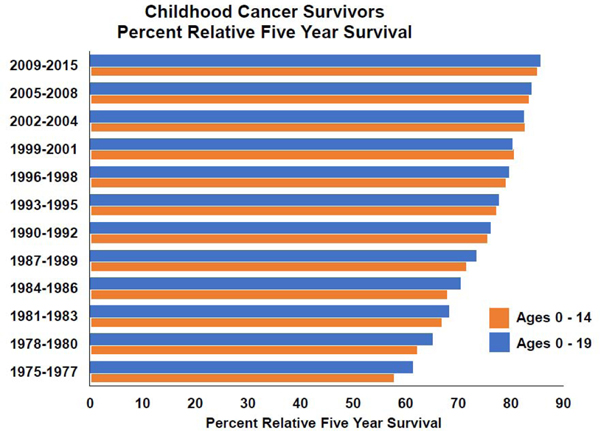
Over the past 50 years, multi-institutional cooperative group clinical trials have led progressive advancements in risk-stratified, multimodality cancer therapy and hospital care for children with cancer, resulting in substantial improvement in long-term survival. Data from the United States Surveillance Epidemiology and End Results program demonstrated a 5-year survival of 61.5% among children diagnosed before 20 years of age from 1975–1977 compared with 85.7% among those diagnosed from 2009–2015 (Figure 1).1 As a result, there are nearly 500,000 survivors of childhood cancer living in the United States today.2 Unfortunately, cure is not without consequences, and long-term survivors, on average, experience significantly greater medical and psychosocial late effects compared with the general population. In this review we provide an overview of: 1) the epidemiology of childhood cancer survivorship, including new methods to estimate the burden of late effects; 2) the current knowledge regarding genetic susceptibility to late effects; 3) the development of surveillance guidelines; 4) novel agents and their potential impact on survivorship issues; and 5) models of survivorship care. We conclude with some proposed future directions for research in this field.
FIGURE 1.
Childhood cancer survivors percent relative five year survival.
Epidemiology of Childhood Cancer Survivorship
Childhood cancer survivors are at risk for early mortality, secondary malignant neoplasms, and treatment-related organ damage. These impairments impact physical, cognitive and emotional health, and influence optimal participation in life roles at home, at school, at work, and in the community. The burden of chronic disease is high, with more than 50% of survivors experiencing at least one, severe, disabling, life-threatening, or fatal chronic health condition by age 50 years, a rate nearly five times greater than expected when compared with siblings (Table 1).3 Cognitive impairment is also prevalent at a higher than expected rate (22.8% among survivor versus 10% among siblings),4 and emotional distress is not uncommon (15% vs. 10% expected).5 These documented late effects can often be attributed to cancer and treatment related risk factors (Tables 2 and 3). As a result, survivors have been more likely to utilize special education services at school and less likely to graduate from college,6 more likely to be unemployed,7 and on average, have lower incomes,8–10 and more likely to receive public benefits to supplement their incomes than the general population (Table 4).11,12 Overall, moderate or severe financial hardship has been reported by half of all survivors.13 In addition, survivors are twice as likely as siblings to live dependently,14 and significant proportions (15–20%) report long-term poor physical, mental, or general health.15 However, for many groups of long-term survivors, those treated in more recent eras appear to be experiencing reduced late mortality16 and serious chronic conditions,17 although these gains have not been uniform, and have not necessarily corresponded with improved self-reported health status.18
TABLE 1.
Health burdens faced by childhood cancer survivors.
| Reference | Methods | Data | Author Conclusion |
|---|---|---|---|
| Armstrong et al3 | ■ 14,359 5-year survivors from the Childhood Cancer Survivor Study ■ 1st diagnosed when ≤21 years old ■ 5,604 ≤35 years old (range, 3562 years) at last follow-up ■ Follow-up = median 24.5 years after diagnosis (range, 5–39.3 years) ■ 4,301 siblings. ■ Severe, disabling, life-threatening, and fatal health conditions >5 years from diagnosis classified using CTCAE (version 4.0), grades 3–5 |
■ By age 50 years, the cumulative incidence of a severe, disabling, life-threatening, or fatal health conditions was greater among survivors than siblings (53.6%, 95%CI 51.5–55.6; v 19.8%, 95%CI 17.0–22.7). ■ Comparing survivors with siblings, hazard ratios (HR) for severe, disabling, life-threatening, or fatal health conditions were significantly increased within: – Age group 5–19 years (HR 6.8, 95%CI 5.5–8.3) – Age group of 20–34 years (HR 3.8, 95%CI 3.2–4.5) – Age group ≥35 years group (HR 5.0, 95%CI 4.1–6.1) ■ HR for severe, disabling, life-threatening, or fatal health conditions significantly higher among those ≥35 years versus those 20–34 years old (P=.03). ■ 25.9% of survivors who reached age 35 years without a previous grade 3/4 condition, experienced a subsequent grade 3–5 condition within 10 years, compared with 6.0% of siblings (P<.001) |
■ After fourth decade of life the elevated risk for morbidity and mortality among cancer survivors increases further |
| Cheung et al4 | • 5507 adult survivors in the Childhood Cancer Survivor Study who completed a self-report measure of neurocognitive function • 47.1% male • Mean [SD] age at evaluation = 31.8[7.6] years • Mean [SD] years postdiagnosis 23.1 [4.5] years. • Cardiac, pulmonary, and endocrine chronic health conditions were graded using NCI CTCAE (v 4.03) |
■ 1/3 of survivors with ≥ grade 2 chronic condition reported impairments in task efficiency and memory ■ “Direct effects” on impaired task efficiency seen with – Cranial radiation – Cardiopulmonary conditions (β=0.10, P=.002; RR=1.27, 95%CI = 1.12–1.44) – Endocrine conditions (β = 0.07, P=.04; RR=1.14, 95%CI = 1.02–1.28) ■ Effects on memory and emotional regulations seen with – Cardiopulmonary conditions [memory (P=.01) and emotional regulation (P=.01). ■ Through endocrine morbidity, thoracic radiation was associated with – Impaired task efficiency (P=.01) – Impaired emotional regulation (P=.01) |
■ Non-neurotoxic exposures, such as thoracic radiation, can adversely impact survivors’ neurocognitive function through chronic conditions ■ Management of chronic diseases may mitigate neurocognitive outcomes among aging survivors of childhood cancer |
| Oancea et al5 | ■ 1863 adult survivors of childhood cancer ■ Median age at follow up = of 32 years ■ Completed comprehensive medical evaluations ■ Clinically relevant emotional distress assessed using the Brief Symptom Inventory 18 and defined as T-scores ≥63. ■ Path analysis used to examine associations among identified risk factors |
■ 15.1% of survivors reported elevated global distress ■ Cancer-related pain associated with elevated distress (OR 8.72; 95%CI, 5.32–14.31) ■ Compared to survivors who reported no learning or memory problems, survivors who reported moderate learning or memory problems more likely to have elevated distress (OR 3.27; 95%CI, 2.17–4.93) ■ Path analysis implied cancer-related pain has direct effect on distress symptoms and indirect effect through socioeconomic status and learning or memory problems |
Childhood cancer-related morbidities including pain and learning or memory problems appear to be directly and indirectly associated with elevated distress symptoms decades after treatment |
Abbreviations: CI, confidence interval; CTCAE, Common Terminology Criteria for Adverse Events; HR, hazard ratio; NCI, National Cancer Institute; OR, odds ratio; RR, relative risk
TABLE 2.
Common or serious late effects by organ system and therapeutic exposure
| Organ system/domain (late effect) | Therapeutic exposure* |
|---|---|
| Brain (neurocognitive deficits) | Methotrexate (intrathecal, high dose intravenous), cytarabine (high dose intravenous) |
| Eye (cataract) | Busulfan, glucocorticoids (e.g., dexamethasone, prednisone) |
| Ear (hearing loss) | Carboplatin (myeloablative doses), cisplatin |
| Peripheral nervous system (neuropathy) | Carboplatin, cisplatin, vinblastine, vincristine |
| Dental (aplasia, dysplasia, hypoplasia) | Any chemotherapy |
| Heart (cardiomyopathy) | Anthracyclincs (i.e., doxorubicin, daunorubicin, epirubicin, idarubicin), anthraquinone (mitoxantrone) |
| Lung (interstitial pneumonitis, fibrosis) | Bleomycin, busulfan, carmustine, lomustine |
| Liver (hepatic dysfunction) | Antimetabolites (i.e., mercaptopurine, methotrexate, thioguanine) |
| Kidney (renal insufficiency) | Ifosfamide |
| Gonads (primary hypogonadism) | Alkylating agents (e.g., cyclophosphamide, ifosfamide, busulfan, carmustine, lomustine, melphalan, procarbazine, thiotepa) |
| Bone (osteopenia) | Glucocorticoids, methotrexate |
| Secondary malignancy (acute myeloid leukemia, myelodysplasia) | Anthracyclines, alkylating agents, epipodophyllotoxins (i.e., etoposide, teniposide) |
| Mental health (anxiety, depression, post-traumatic stress) | Any cancer experience |
Radiation to a given area is associated with late effects to the affected organ system, including endocrinopathies (e.g., hypothyroidism, hypogonadism, diabetes), growth and development (e.g., fibrosis, hypoplasia, neurocognitive impairment), premature aging (e.g., atherosclerosis), and second cancers.
TABLE 3.
Profile of late effects risks by common pediatric malignancies
| Pediatric malignancy | Contemporary frontline therapeutic modalities* |
Potential late effects† |
|---|---|---|
| Acute lymphoblastic leukemia & Non-Hodgkin lymphoma |
Glucocorticoids Vinca alkaloids Antimetabolites Anthracyclines Alkylating agents |
Bone mineral density deficits Peripheral and/or sensory neuropathy Cognitive deficits Cardiomyopathy Gonadal dysfunction/infertility |
| Acute myeloid leukemia | Anthracyclines Antimetabolites Epipodophyllotoxins |
Cardiomyopathy |
| Ewing sarcoma | Surgical resection Epipodophyllotoxins Alkylating Agents Anthracyclines Vinca alkaloids Primary/metastatic site irradiation |
Functional deficits Secondary leukemia Gonadal dysfunction/infertility Cardiomyopathy Peripheral and/or sensory neuropathy Subsequent neoplasms |
| Hodgkin lymphoma | Glucocorticoids Vinca alkaloids Anthracyclines Alkylating agents Involved node radiation |
Bone mineral density deficits Peripheral and/or sensory neuropathy Cardiomyopathy Pulmonary fibrosis Gonadal dysfunction/infertility Hypothyroidism Subsequent neoplasms |
| Medulloblastoma | Surgical resection Vinca alkaloids Alkylating agents Heavy metals Craniospinal irradiation |
Neurologic deficits Peripheral and/or sensory neuropathy Neuroendocrine dysfunction Gonadal dysfunction/infertility Hearing loss Renal dysfunction Cognitive deficits Vasculopathy/stroke |
| Neuroblastoma | Surgical resection Epipodophyllotoxins Alkylating agents Anthracyclines Heavy metals Immunotherapy |
Secondary leukemia Gonadal dysfunction/infertility Hearing loss Renal toxicity Cardiomyopathy Subsequent neoplasms |
| Osteosarcoma | Surgical resection Antimetabolites Alkylating agents Heavy metals |
Functional deficits Renal dysfunction Gonadal dysfunction/infertility Cardiomyopathy Hearing loss |
| Rhabdomyosarcoma | Surgical resection Vinca alkaloids Anthracyclines Involved site radiation |
Peripheral and/or sensory neuropathy Cardiomyopathy Subsequent neoplasms |
| Wilms tumor | Nephrectomy Vinca alkaloids Anthracyclines Primary/metastatic site irradiation |
Renal dysfunction Kyphoscoliosis Peripheral and/or sensory neuropathy Gonadal dysfunction/infertility Cardiomyopathy Subsequent neoplasms |
Exposures listed may vary as therapy is stratified based on clinical and pathological features of pediatric malignancy and early response to therapy.
The risk of specific late effects varies in magnitude based on specific agents and modalities and dose; survivors with favorable and responsive malignancies may have substantially lower risks for specific late effects due to limited exposures of the agents and modalities listed.
TABLE 4.
Socio-economic burdens faced by childhood cancer survivors.
| Reference | ■ Methods | ■ Data | ■ Author Conclusion |
|---|---|---|---|
| Saatci et al6 | ■ 26 studies ■28,434 CCS; 17 814 matched controls [6,582 siblings and 6 population studies from 11 high-income countries, which have similar access to education and years of mandatory schooling] |
■ more likely to remain at compulsory level (OR 1.36, 95%CI 1.26–1.43) ■ CCS less likely to complete secondary (OR 0.93, 95%CI 0.87–1.0) and tertiary level education (OR 0.87, 95%CI 0.78–0.98) ■ CCS more likely to require special educational needs (OR 2.47, 95%CI 1.91–3.20). ■ At secondary level: Irrespective of CNS involvement, compared with cancer–free peers CCS less likely to progress onto secondary (OR 1.77, 95%CI 1.46 to 2.15; OR 1.19, 95%CI 1.00 to 1.42, respectively) ■ At tertiary level: Those with CNS involvement continued to perform worse (OR 0.61, 95%CI 0.55–0.68) but those without appeared to perform similarly to their peers (OR 1.12, 95%CI 1.0–1.25) |
■ Compared with controls, found significant differences in educational attainment in CCS ■ Deficiencies were sustained across different countries, making it an international issue ■ CNS involvement plays a key role in educational achievement ■ Clinicians, teachers and policymakers should advocate for early educational support for survivors |
| Mader et asl7 | ■ Update a systematic review from 2006 assessing unemployment in adult CCS ■ 56 studies, including 27 controlled studies |
■ ~1/6th of CCS unemployed. ■ Overall meta–analysis of controlled studies: CCS more likely to be unemployed than controls (OR=1.48, 95%CI 1.14–1.93 ■ Elevated OR found in CCS in the US and Canada (OR=1.86, 95%CI 1.26–2.75), as well as in Europe (OR=1.39, 95%CI 0.97–1.97) ■ CCS of brain tumors were more likely to be unemployed (OR=4.62, 95%CI 2.56–8.31) ■ Predictors of unemployment: younger age at study and diagnosis, female sex, radiotherapy, and physical late effects |
■ CCS are at considerable risk of unemployment in adulthood ■ CSS may benefit from psychosocial care services along the cancer trajectory to support labor market integration |
| Boman et al8 | ■ National cohort of 1.46 million Swedish residents ■ 1716 CCS diagnosed before 16th birthday, followed up in registries at >25 years of age |
■ CCS of non–CNS cancers had similar education, employment, and income as the general population in adjusted models ■ CCS of CNS tumors: – More often had no more than basic (≤9 years) education (RR 1.80 (95%CI, 1.45–2.23) – Less often attained education beyond secondary school (RR 0.69 95%CI, 0.58–0.81) – Less often were employed (RR 0.85 [95% CI, 0.77–0.94) – Had a predicted net income from work that was lower (P<.001) than in the general population, even after excluding individuals who received economic disability compensation |
■ CNS tumor survivors had poorer social outcomes compared with the general population ■ Outcomes for survivors of other childhood cancers were similar to the general population ■ Highlights importance of improved, safer pediatric CNS tumor treatment protocols |
| Gunnes et al9 | ■ 1,212,013 individuals born in Norway during 1965 through 1985 ■ 5440 had cancer diagnosis before age 25 years ■ Follow-up was through 2007 |
■ Compared with those in the noncancer group CCS had increased probability of: – Receiving governmental financial assistance (men: HR 1.4; 95%CI, 1.3–1.5; women: HR 1.5; 95%CI, 1.3–1.6) – Not being employed (men: HR 1.4; 95%CI, 1.2–1.7; women: HR 1.4; 95%CI, 1.2–1.6) ■ Income discrepancies particularly pronounced for CCS of CNS tumors ■ No difference in representation in higher skilled occupations |
■ Survivors of cancer had increased risk of being economically dependent and unemployed ■ Evident in several tumor groups and most pronounced in female survivors |
| Wengenroth et al10 | ■ Questionnaire sent to CCS aged ≥18 years, registered in the Swiss Childhood Cancer Registry (SCCR), diagnosed at age <21 years, who had survived ≥5 years after diagnosis of the primary tumor ■ Asked questions about education, profession and income and retrieved clinical data from the SCCR ■ 1,506 survivors and 598 siblings |
■ CCS less likely than siblings to have a high monthly income (>4,500 CHF), even after we adjusted for socio-demographic and educational factors (OR=0.46, p<0.001) ■ Lower income than siblings in survivors of: – Leukemia (OR=0.40, p<0.001) – Lymphoma (OR=0.63, P=0.040) – CNS tumors (OR=0.22, P<0.001) – Bone tumors (OR=0.24, P=0.003) ■ Survivors who had cranial irradiation, had lower income than survivors who did not have cranial irradiation (OR=0.48, p=0.006) |
■ CCS of various diagnostic groups have lower incomes than siblings even after adjusting for socio-demographic characteristics, education and working hours |
| Font-Gonzalez et al11 | • Medical record linkage of 1283 adult CCS (diagnosed 1966–2001) from a single–centre and two national registers (1999–2011) • 25,082 reference persons matched on gender and year of birth • Calculated odds (ratios) of specified social outcomes in both groups using multivariable logistic regression • Risk factors for the social outcomes analyzed within survivors |
• Compared with reference persons CCS had higher odds of: – Not being married (OR 1.2, 95%CI 1.07–1.42) – Not living independently (OR 1.7. 95%CI, 1.41–2.00) – Using social benefits (OR 2.3, 95%CI, 1.98–2.69) • Factors that negatively influenced all social outcomes in CCS: – Radiotherapy to head and/or neck – Original CNS tumor diagnosis |
• National register data was able to show differences between social outcomes in CCS and the general population • Differences especially noted for survivors treated with radiotherapy to head and/or neck and those originally diagnosed with CNS tumors • Recommended development and implementation of support strategies to improve social outcomes of CCS |
| Kirchkoof et al12 | Assessed enrollment in supplemental security income (SSI) and social security disability insurance (DI) in 698 long–term CCS vs a comparison group of 210 adults without cancer All had completed a health insurance survey. | • 13.5% and 10.0% of CCS had ever been enrolled on SSI or DI, respectively, compared with 2.6% and 5.4% of the comparison group • Compared with those with mild/moderate or no health conditions the likelihood of receiving assistance increased with: – ≥25 Gy cranial radiation doses [RR of current SSI enrollment. = 3.93, 95%CI 2.05–7.56 and RR of current DI enrollment = 3.65, 95%CI 1.65–8.06] – Severe/life–threatening conditions [RR of current SSI enrollment = 3.77, 95%CI, 2.04–6.96 and RR of current DI enrollment = 2.73, 95%CI, 1.45–5.14] |
• Evidence of disability-related financial challenges in CCS • More likely to receive public benefits to supplement their incomes than the general population |
| Huang et al13 | • Examined financial hardship, determinants, and consequences in 2811 long-term CCS • Mean age at evaluation = 31.8 years; years postdiagnosis = 23.6 years • Financial hardship measured according to (i) material, (ii) psychological, and (iii) coping/behavioral domains. • Outcomes included health and life insurance affordability, retirement planning, symptoms, and HRQOL • ORs were estimated; all statistical tests were two-sided |
• Frequency of hardship: – (i) Material = 22.4%, 95%CI 20.8%–24.0% – (ii) Psychological = 51.1%, 95%CI 49.2%–52.9% – (iii) Coping/behavioral = 33.0%, 95%CI 31.1%–34.6%) • Risk factors across hardship domains included annual household income ≤$39 999 vs ≥$80 000 – (i) Material OR 3.04, 95%CI 2.08–4.46 – (ii) Psychological OR 3.64, 95%CI 2.76–4.80 – (iii) Coping/behavioral OR 4.95, 95%CI 3.57–6.86 • than high school attainment vs college graduate or above: – (i) Material OR 2.22, 95%CI = 1.45–3.42 – (ii) Psychological OR 1.75, 95%CI, 1.18–2.62 – (iii) Coping/behavioral OR 2.05, 95%CI = 1.38–3.06 • Association with higher material hardship (all p<.05) – Myocardial infarction – Peripheral neuropathy – Subsequent neoplasm – Seizure, stroke – Reproductive disorders – Amputation – Upper gastrointestinal disease • Hardship across three domains associated with – Somatization, anxiety and depression (all P<.001) – Suicidal ideation (all P<.05) – Difficulty in retirement planning (all P<.001) – Survivors with hardship had – Statistically significantly lower HRQOL (all P<.001) – Sensation abnormality (all P<.001) – Pulmonary symptoms (all P< 05) – Cardiac symptoms (all P<.05) |
• Substantial proportion of CCS experience financial hardship. Vulnerable sociodemographic status and late effects associated with hardship • CCS with financial hardship had an increased risk of symptom prevalence and impaired HRQOL |
| Kunin-Baston et al14 | • Long–term follow–up questionnaire of 6.047 adult CCS and 2,326 siblings all ≥25 years of age • Assessed adaptive, neurocognitive, and psychological functioning, as well as demographic and health status • Multivariable logistic regression analyses and structural equation modeling (SEM) used to identify predictors of independent living |
• CCS (n = 1063; 17.7%) more than twice as likely to live dependently than siblings (n = 206, 8.7%), survivors were (OR 2.07, 95%CI 1.77–2.42) • CCS who had CNS tumors significantly less likely to live independently than those who had Hodgkin lymphoma (OR 0.13, 95%CI 0.10–0.18) or leukemia (OR 0.29, 95%CI 0.23–0.27) • Other risk factors for reduced independent living: – Cranial radiation (≤24Gy OR 0.76, 95%CI 0.62–0.93; >24Gy OR 0.31, 95%CI 0.24–0.41) – Use of neuroleptic, anticonvulsant, or psychostimulant medication (OR 0.32, 95%CI 0.24–0.43) – Attention and processing speed problems (OR 0.58, 95%CI 0.47–0.71) – Poor physical functioning (OR 0.49, 95%CI 0.38–0.63) – depression (OR 0.68, 95%CI 0.53–0.88) – Racial/ethnic minority status (OR 0.39, 95% CI 0.30–0.51) • SEM demonstrated that neurocognitive functioning had both direct effects on independent living status, and indirect effects through use of neurologically directed medication, depression, and poor mental health |
• Adult CCS are less likely to live independently as adults especially those who experience neurocognitive, psychological, or physical late effects |
| Ness et al15 | • Demographic information used to classify social roles • Medical Outcomes Survey 36–Item ShortForm Health Survey to ascertain HRQOL |
• Deficits reported amongst CCS – 18.1% deficits in physical performance – 10.5% deficits in emotional health – 14.0% deficits in executive function • CCS with physical performance, executive function, or emotional health deficits were less likely to be employed, married, or have incomes >$20,000/year than those who reported no limitations • Limitations in executive function or emotional health associated with no health insurance • Limitations in any activity domain associated with poor HRQOL Emotional health limitations had the most impact – Physical performance summary: OR 3.18 – Mental health: OR 25.8 |
• CSS report long-term poor physical, mental, or general health that negatively impact role attainment and HRQOL |
Abbreviations: CCS, childhood cancer survivors; CI, confidence interval; CNS, central nervous system; CTCAE, Common Terminology Criteria for Adverse Events; HR, hazard ratio; HRQOL, health-related quality of life; NCI, National Cancer Institute; OR, odds ratio; RR, relative risk
However, until recently, traditional measures such as frequencies, incidence and prevalence were used to describe the burden of late effects associated with surviving childhood cancer. However, since these measures report simple counts or proportions with time to a first event, investigators have had to limit analyses of outcomes to single exposures, primary cancer subtypes or few late effects of interest. Although several studies have described long-term morbidities across entire survivorship cohorts, these efforts rarely describe recurrent events and multiple chronic conditions.3,19–21 In a population where substantive multimorbidity (two or more concurrently existing chronic health conditions) and excess early mortality are observed, a comprehensive perspective of disease burden in this population was lacking.16,22
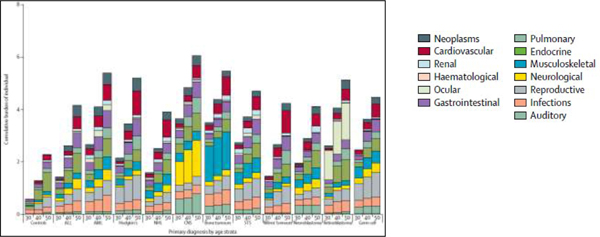
The mean cumulative count (MCC) and cumulative burden approaches both provide analytic options that allow investigators to account for multimorbidity by describing both the magnitude and diversity of chronic health conditions in a cohort of individuals (Table 5).23–25 Both measures account for competing risk and censoring and thus differ from incidence and prevalence or traditional count statistics. While the MCC accounts for each event regardless of type, the cumulative burden applies a structured clinical ruleset based on the pathophysiology and recurrent nature of each measured chronic health condition thus allowing for a more clinically appropriate perspective. Using the cumulative burden metric, a recent analysis from the St. Jude Lifetime Cohort Study found that by age 50, a survivor experienced, on average, nearly 5 chronic health events graded as severe/disabling, life-threatening or fatal, a number nearly twice that observed in matched community-controls.25 Additionally, the analysis highlighted that the survivorship population is not a monolithic population but is quite heterogenous in its composition in relation to observed late effect patterns (Figure 2).
TABLE 5.
Comparison of the cumulative incidence versus mean cumulative count method
| Cumulative incidence | Mean cumulative count |
|---|---|
| Considers only the first occurrence of the “event of interest” for each individual in the analysis | Provides a summarization of all “events of interest” that occur in the population by a given time, not just the first event for each subject |
| Because subsequent occurrences of the same event are not included, this metric does not describe the total burden of events in a population | Because this metric considers all events during the period of observation, it can be a more relevant measure of overall disease burden of the “event of interest” in a population “at risk” |
| Considered reasonable if the first occurrence of the “event of interest” changes underlying risk and/or biology of any subsequent event | Assumes the first occurrence of the “event of interest” does not meaningfully change the underlying risk and/or biology of any subsequent event |
| Cumulative probability of the first event of interest depends on survival free of both the event of interest and the competing-risk event | Survival probability depends only on survival free of a competing-risk event. |
| A probability – ranges from 0–1 | Not a probability. Not confined by 0–1 range, it can be any positive number |
| Estimates the proportion of individuals who experience the event of interest | Estimates the average number of events per person in the population |
FIGURE 2.
Distribution of cumulative burden of grades 3 to 5 chronic health conditions in the St. Jude Lifetime Cohort Study of childhood cancer survivors and community controls. X-axis shows age in years. Abbreviations: ALL, acute lymphoblastic leukemia; AML acute myeloid leukemia; CNS, central nervous system tumors; NHL, non-Hodgkin lymphoma; STS, soft tissue sarcomas. From Bhakta et al., Lancet 2017.
While prospective follow-up data from large cohort studies can yield important insights, simulation modeling can provide complementary insight on long-term health outcomes. By extrapolating data beyond the period of observation and reflecting age-related competing mortality risks, model-based estimates of life expectancy among 5-year survivors of childhood cancer project that on average, cumulative excess mortality risks associated with late effects may reduce survivor life expectancy by more than 10 years.26 Comparable to estimates for the general population (or other disease populations), summary measures of population health, such as life expectancy or quality-adjusted life expectancy, can quantify the impact of treatment-related late mortality risks on length and quality of life, as well as serve as benchmarks for tracking improvements in survivor health over time. For example, survivors diagnosed in the 1990s are projected to live longer into adulthood than those diagnosed in the 1970s, suggesting evolving treatment approaches have led to improved life expectancy after treatment for childhood cancer.27
Additionally, by synthesizing data from multiple sources, including randomized controlled trials, observational studies, meta-analyses and expert opinion, projecting long-term outcomes, decision modeling provides a valuable analytic framework for simulating the health outcomes associated with various follow-up care strategies for survivors.28 Previous studies have evaluated the Children’s Oncology Group (COG) long-term follow-up recommendations to prevent congestive heart failure,29,30 and more recently, secondary breast cancer among female survivors with a history of chest radiation.31 Decision modeling is increasingly used by policy makers developing guidelines to provide important insight on the tradeoffs between clinical benefits and harms associated with screening.32,33 Given the sample size and follow-up time needed, randomized clinical trials testing different screening strategies in survivors are unlikely. In this context, simulation modeling may be particularly useful for informing screening guidelines for at-risk survivors. As genetic markers of susceptibility for late-effects emerge, decision modeling provides an analytic framework to evaluate how this information can be used to refine and inform screening guidelines for at-risk survivors.
Genetic Susceptibility of Late Effects
Despite the strong and unambiguous relationship between therapeutic exposure and late effects in cancer survivors, there is considerable interindividual variability in risk for any given dose, suggesting the role for genetic susceptibility in possibly influencing individual risk.34,35 Considerable efforts have been expended attempting to identify genetic variants associated with late effects, to determine if the genetic variants can shed light on the underlying disease mechanisms and to incorporate the genetic variants to identify those at highest risk for developing these outcomes. In this section, we highlight our current understanding of the genetic modifiers of exposure-related late effects, such as anthracycline-related cardiomyopathy, subsequent malignant neoplasms, reproductive health issues, and neuropsychological impairment.
Anthracycline-related cardiomyopathy (Table 6)
TABLE 6.
Genetic susceptibility to anthracycline cardiomyopathy/cardiotoxicity (ACT)
| Gene / Variant | Methods | Results | Comment / Conclusion |
|---|---|---|---|
|
RARG36
The retinoic acid receptor (RAR) is a nuclear receptor that can also act as a transcription factor. RAR is activated by both all-trans retinoic acid and 9-cis retinoic acid. There are three RARs: RARα, RARβ, and RARγ, encoded by the RARA RARB and RARG genes, respectively. |
• Genome-wide association study in 280 CCS of European ancestry with independent replication in similarly treated cohorts of 96 European and 80 non-European CCS |
• Identified a nonsynonymous variant (rs2229774, p.Ser427Leu) in RARG highly associated with ACT [P=5.9 × 10−8, OR 4.7 (95%CI 2.7–8.3)]. | • RARG may confer susceptibility to ACT in CCSs. • Variant alters RARG function, leading to derepression of the key ACT genetic determinant Top2b |
|
UGT1A6 and SLC28A337
• UGT1A6: UDP-glucuronosyltransferase 1–6 is an enzyme of the glucuronidation pathway that transforms small lipophilic molecules into watersoluble, excretable metabolites. This gene is part of a complex locus that encodes several UDP-glucuronosyltransferases. UDP-glucuronosyltransferase is also responsible for the inactivation of drugs. • SLC28A3: The human concentrative nucleoside transporter CNT3 (SLC28A3) plays an important role in mediating the cellular entry of a broad array of physiologic nucleosides and synthetic anticancer nucleoside analog drugs |
• 23 variants tested for association with ACT in an independent cohort of 218 patients | • Confirmed association of rs17863783 in UGT1A6 and ACT (P = 0.0062, OR 7.98). • Additional evidence for association of rs7853758 (P = 0.058, OR 0.46) and rs885004 (P = 0.058, OR 0.42) in SLC28A3 was found (combined P=1.6 × 10−5 and P=3.0 × 10−5, respectively). |
• Validation of variants in SLC28A3 and UGT1A6 as genetic markers predictive of ACT in CCSs • An improved prediction model using replicated genetic variants as well as clinical factors discriminated significantly better between cases and controls than clinical factors alone |
|
SLC22A17 and SLC22A738
• SLC22A17: Polyspecific organic cation transporter in liver, kidney, intestine, and other organs. Critical for eliminating endogenous small organic cations and wide array of drugs and environmental toxins. One of three similar cation transporter genes located in a cluster on chromosome 6. • SLC22A7: Involved in sodiumindependent transport and excretion of organic anions some potentially toxic. It’s a membrane protein localized to basolateral membrane of kidney. Alternatively, spliced transcript variants encoding different isoforms exist |
• Two cohorts treated for childhood cancer (n = 344 and 218, respectively) were genotyped for 4578 SNPs in drug ADME and toxicity genes | • Significant associations identified in SLC22A17 (rs4982753; P=0.0078) and SLC22A7 (rs4149178; P=0.0034), with replication in the second cohort (P=0.0071 and 0.047, respectively) | • Genetic variants in SLC22A17 and SLC22A7 were associated with ACT in CSSs • SLC22A17 and SLC22A7 variants improved a genotype-guided risk prediction model, which could improve patient risk stratification |
|
CELF439
• CUGBP Elav-like family member 4 (CELF4) also known as bruno-like protein 4 (BRUNOL4) is encoded by the CELF4 • Members of this protein family regulate pre-mRNA alternative splicing and may also be involved in mRNA editing, and translation. |
• Genome-wide association study in CCSs with/without cardiomyopathy. SNPs surpassing a prespecified threshold for statistical significance were independently replicated | • No SNP was marginally associated with ACT • SNP rs1786814 on CELF4 gene passed significance cutoff for gene-environment interaction (Pge=1.14 × 10−5). In patients with A allele, cardiomyopathy infrequent and not dose related • If exposed to >300 mg/m2 anthracyclines, rs1786814 GG genotype conferred 10.2-fold (95%CI, 3.8- to 27.3-fold; P <.001) cardiomyopathy risk compared with GA/AA genotypes and exposure ≤300 mg/m2 • Gene-environment interaction successfully replicated in independent set of ACT cases |
• Modifying effect of a polymorphism of CELF4 (rs1786814) on the dose-dependent association between anthracyclines and cardiomyopathy; possibly through pathway involving expression of abnormally spliced TNNT2 variants • Coexistence of ≥1 cTnT variant results in temporally split myofilament response to calcium, and decreased contractility • Analysis of TNNT2 splicing variants in healthy human hearts suggested an association between the rs1786814 GG genotype and coexistence of ≥1 TNNT2 splicing variant (90.5% GG v 41.7% GA/AA; P=0.005) |
|
HAS340 HAS3 (Hyaluronan 3): Encodes a protein involved in synthesis of hyaluronic acid, a major constituent of the extracellular matrix. This gene is a member of the NODC/HAS gene family. Compared to the proteins encoded by other members of this gene family, this protein appears to be more of a regulator of hyaluronan synthesis. |
• Used two-stage design, to investigate host susceptibility to ACT by using the ITMAT/Broad CARe cardiovascular SNP array to profile common SNPs in 2,100 genes considered relevant to de novo cardiovascular disease | • Using matched case-control design (93 cases, 194 controls), identified a common SNP, rs2232228, in HAS3 gene that exerts a modifying effect on anthracycline dose-dependent cardiomyopathy risk (P=5.3 × 10−7) • Among individuals with rs2232228 GG genotype, cardiomyopathy infrequent and not dose related • In individuals exposed to >250 mg/m2 anthracyclines, rs2232228 AA genotype conferred 8.9-fold (95%CI, 2.137.5-fold; P=0.003) increased risk of cardiomyopathy compared with GG genotype • HAS3 mRNA levels in healthy hearts lower among individuals with AA compared with GA genotypes (P=0.09) |
• HAS3 produces hyaluronic acid a ubiquitous component of the extracellular matrix that plays role in tissue remodeling and is known to reduce ROS-induced cardiac injury. • High cardiomyopathy risk associated with AA genotype could be due to inadequate remodeling and/or inadequate protection of the heart from ROSmediated injury after high anthracycline exposure |
|
CBR341
CBR3: Carbonyl reductases (CBRs) catalyze reduction of anthracyclines to cardiotoxic alcohol metabolites. Polymorphisms in CBR1 and CBR3 influence synthesis of these metabolites. |
• 170 CCSs with cardiomyopathy (patient cases) were compared with 317 survivors with no cardiomyopathy (controls; matched on cancer diagnosis, year of diagnosis, length of follow-up, and race/ethnicity) using conditional logistic regression techniques. | • Dose-dependent association observed between cumulative anthracycline exposure and cardiomyopathy risk: – 0 mg/m2: reference; – 1 to 100 mg/m2: OR 1.65 – 101 to 150 mg/m2: OR 3.85; 151 to 200 mg/m2: OR 3.69; – 201 to 250 mg/m2 OR 7.23; – 251 to 300 mg/m2: OR 23.47; – >300 mg/m2: OR 27.59; P(trend) < .001 • No increased risk of cardiomyopathy after exposure to low- to moderate-dose ANTH (1–250 mg/m2) with variant A allele (CBR1:GA/AA and/or CBR3:GA/AA) • Exposure to low- to moderate-dose anthracyclines increased cardiomyopathy risk among individuals with CBR3 V244M homozygous G genotypes (CBR3:GG), when compared with individuals with CBR3:GA/AA genotypes unexposed to anthracyclines (OR 5.48; P=0.003), as well as exposed to low- to moderate-dose anthracyclines (OR 3.30; P=0.006) • <250 mg/m2 anthracyclines associated with increased cardiomyopathy risk, irrespective of CBR genotype status |
• ACT risk increased at doses as low as 101 to 150 mg/m2
• Homozygosis for G allele in CBR3 contributes to increased cardiomyopathy risk with low- to moderate-dose anthracyclines, such that there seems to be no safe dose for patients homozygous for the CBR3 V244M Gallele |
Abbreviations: 95%CI, 95% confidence interval; Anth, anthracycline; ACT, anthracycline-induced cardiotoxicity; OR, odds ratio; ROS, reactive oxygen species; SNPs, single-nucleotide polymorphisms
Overall, the largest amount of activity has focused on anthracycline-related cardiomyopathy. Significant associations have been reported between cardiac compromise and a nonsynonymous coding variant in RARG,36 in the SLC family of drug transporter genes,37,38 and genetic variants in CELF4,39 HAS3,40 and CBR3.41 The underlying mechanism for treatment-related complications has also been largely explored in anthracycline-related cardiomyopathy, with a role for alternative splicing of TNNT2, enzymes responsible for cardiotoxic metabolites, reactive oxygen species and mitochondrial injury.39
Subsequent Malignant Neoplasms (Table 7)
TABLE 7.
Genetic susceptibility to subsequent malignant neoplasms
| Gene / Variant | Methods | Results | Comment / Conclusion |
|---|---|---|---|
|
PRDM142
PRDM1 encodes PR domain zinc finger protein 1 also known as BLIMP-1. Increased Blimp-1 expression in immune system cells leads to proliferation and differentiation of antibody secreting plasma cells. Blimp-1 is also considered a ‘master regulator’ of hematopoietic stem cells. |
• Discovery set was 100 CCSs individuals treated for HL who subsequently developed SMNs and 89 individuals treated for HL who did not develop SMNs. European descent with diagnosis of HL at 10–20 years of age • Treated similarly with 25–44 Gy RT chemotherapy with an alkylating agent • Radiation exposure was to the site at which the subsequent SMN developed • Distribution and frequency of sites exposed to RT similar between cases and controls • Controls from all HL cases without SMN followed for 27 years ≥ treatment for HL |
• Three SNPs achieved genome-wide significance: rs4946728, rs1040411 and rs8083533 • rs4946728 (P=1.09 × 10−8, ORallelic = 4.22; 95%CI = 2.53–7.05).and rs1040411 mapped to chromosome 6q21, between ATG5 and PRDM1. • rs8083533 mapped to 18q11.2, intronic to TAF4B (encoding transcription initiation factor TFIID subunit 4B) (P=4.98 × 10−8, ORallelic 3.78, 95% CI 2.31–6.18) • Gender, age at diagnosis, year of HL diagnosis, gonadal RT (in females) and alkylating chemotherapy exposure, had no effect on the observed associations |
• Survivors of pediatric HL are at risk for RT-induced SMNs • Two variants at chromosome 6q21 associated with SMNs in survivors of HL treated with RT as children but not as adults. The variants comprise a risk locus associated with decreased basal expression of PRDM1 and impaired induction of the PRDM1 after RT • Data suggest gene-exposure interaction that may implicate PRDM1 in the etiology of RT-induced SMNs. |
|
FGFR243
FGFR2 also known as CD332 (cluster of differentiation 332) is a receptor for fibroblast growth factor. Member of fibroblast growth factor receptor family. Extracellular portion interacts with fibroblast growth factors, setting in motion cascade of downstream signals, ultimately influencing mitogenesis and differentiation. FGFR2 is a high-affinity receptor for acidic, basic and/or keratinocyte growth factor. |
• 2 case-control series: • Discovery: 449 women with HL treated with supradiaphragmatic RT in UK at age <36 years: 140 had BC after HL treatment (the “cases”) and 309 had had no solid cancer after HL (the “controls”) • Replication: 244 female Dutch HL patients treated with supradiaphragmatic RT at age <41 years: 92 cases and 152 controls. |
• Genotype frequencies of rs1219648 significantly different between cases vs. controls. • Overrepresentation of the minor, G allele, in HL patients with BC (OR 1.73; P=000273). Association dose-dependent; highest risks if homozygous for G allele • OR > for BC in relation to FGFR2 genotype in the general population (1.26 per allele) • Effect greater if <20 years when first treated (OR 1.70, 95%CI 1.16–2.50) than ≥20 years (OR 1.48, 95%CI 1.09–2.00), and if had not received an alkylating agent or ≥5-Gy pelvic radiotherapy |
• Women treated at young ages with supradiaphragmatic RT for HL have a highly increased risk of BC. • rs1219648, which annotates the FGFR2 gene associated with risk in discovery and replication (combined per-allele OR 1.59, 95%CI 1.26–2.02; P=0.0001) • Evidence genetic variation in FGFR2 influences RT-induced BC risk. |
|
PROX1; TAGLN44
• PROX1: Transcription factor involved in cell fate determination, gene transcriptional regulation and progenitor cell regulation in a number of organs. Plays critical role in embryonic development and functions as a key regulatory protein in neurogenesis and the development of other organs. • TAGLN: Encodes transgelin, a transformation and shapechange sensitive actin crosslinking/gelling protein found in fibroblasts and smooth muscle. Down-regulation of expression may be early and sensitive marker of transformation. Functional role unclear. |
• Genome-wide association study of BC in female CCS, pooling two cohorts with detailed treatment data and systematic, long-term follow-up • 207 survivors who developed breast cancer and 2774 who had not developed any subsequent neoplasm as of last follow-up • 16 958 466 high-quality variants for analysis |
• CCS who received exposure to breast of ≥10 gray, a locus on 1q41 was associated with subsequent BC risk (rs4342822, nearest gene PROX1 , risk allele frequency in control subjects [RAF controls] = 0.46, hazard ratio 1.92, 95%CI 1.49–2.44, P=7.09 × 10−9) • Potentially promising associations for rs74949440, 11q23, TAGLN, RAF controls = 0.02, P=5.84 × 10−8 |
• Strong evidence germline genetics outside high-risk syndromes could modify effect of RT on BC risk in CCSs |
|
HRT2A45 HTR2A encodes one of the receptors for serotonin, a multifunctional neurotransmitter with roles in many physiologic processes such as sleep, hormone secretion, and appetite |
• GWAS of subsequent BCC in European CCS treated with RT • Evaluated genome-wide significant SNPs (P < 5 × 10−8) Discovery cohort (401 and 2,330 control); independent cohort (97 case patients and 1,082 control) |
• Discovery cohort did not identify variants reaching genome-wide significance; however, 14 SNPs on HTR2A showed strongest associations (adjusted HRs ~1.50; P<1 × 10−6) • Further studies showed genome-wide significance for 11/14 HTR2A SNPs • Strongest association for rs633737 (HR = 2.25; P=5.99 × 10−9 , P-value based on 100 million permutations [Pperm] < 1 × 10−8) • High among leukemia and HL survivors • Association of HTR2A with BCC attenuated (HRs 1.30–1.36) and/or not statistically significant (P>0.51) among survivors not in low-risk subgroup/periods (<40 yrs of age; treated when ≥10 yrs) |
• Results suggest HTR2A-BCC association among CCS treated with RT may be more pronounced in individuals <40 years old and those ≥10 years old when treated • Possibly because key nongenetic factors, (RT exposure, years sun exposure, and aging) are less influential relative to the older age of survivors treated at a younger age (≤10 years old). |
| 60 genes associated with autosomal dominant cancer predisposition syndromes with moderate to high penetrance46 | • WGS performed on samples from CCS ≥5 years since initial cancer diagnosis. • Looked for germline mutations in 60 genes known to be associated with autosomal dominant cancer predisposition syndromes with moderate to high penetrance |
• 3,006 survivors (53% male; median age, 35.8 years [range, 7.1 to 69.8 years]; 56% received RT), 1,120 SMNs diagnosed in 439 survivors (14.6%), and 175 P/LP mutations identified in 5.8% (95%CI 5.0–6.7%) • Among survivors who received RT mutations associated with significantly increased rates of; • BC (RR 13.9, 95%CI, 6.0–32.2) • Sarcoma (RR 10.6, 95%CI, 4.3–26.3) • Among survivors who did not received RT mutations associated with significantly increased rates of; • Any SMN (RR 4.7, 95%CI, 2.4–9.3) • BC (RR 7.7, 95%CI, 2.4–24.4) • Nonmelanoma skin cancer (RR 11.0, 95%CI, 2.9–41.4) • ≥2 histologically distinct SMNs (RR 18.6, 95%CI, 3.5–99.3) |
• Findings support referral of all CCSs for potential clinical genetic testing, with priority for survivors who did not receive RT and have any SMN and for those with BC or sarcoma in the field of prior RT |
Abbreviations: 95%CI, 95% confidence interval; BC, breast cancer; BCC, basal cell carcinoma; CCSs, childhood cancer survivors; GWAS, Genome-wide association study; HL, Hodgkin lymphoma; OR, odds ratio; P/LP, pathogenic/likely pathogenic; RT, radiation therapy; SMN, second malignant neoplasms; SNPs, single-nucleotide polymorphisms; WGS, whole genome sequencing
PRDM1 has been implicated in the development of radiation-related malignancies (primarily breast cancer) in Hodgkin lymphoma survivors.42 Other studies have identified genetic variants in FGFR2,43 and in PROX1 and TAGLN,44 to be associated with radiation-related breast cancer. A recent genome-wide association study identified HTR2A to be associated with subsequent basal cell carcinoma.45 Pathogenic or likely pathogenic germline mutations in genes known to be associated with autosomal dominant cancer predisposition syndromes with moderate to high penetrance have also been identified in 5% or more childhood cancer survivors. These mutations were associated with an increased risk for breast cancer and sarcoma among irradiated survivors.46
Reproductive Health (Table 8)
TABLE 8.
Genetic susceptibility to reproductive health issues
| Gene / Variant | Methods | Results | Comment / Conclusion |
|---|---|---|---|
|
BRSK147
BRSK1 (BR serine/threonine kinase 1) is a serine/threonine-protein kinase that plays key role in polarization of neurons and centrosome duplication |
• Single-center pilot study of 176 adult female Caucasian CCS with serum AMH levels as a marker of ovarian reserve • Studied SNPs previously reported associated with age at natural menopause: BRSK1 (rs1172822), ARHGEF7 (rs7333181), MCM8 (rs236114), PCSK1 (rs271924), IGF2R (rs9457827) and TNF (rs909253) |
CT genotype of rs1172822 in BRSK1 gene negatively associated with serum AMH levels (OR 3.15, 95%CI 1.35–7.32, P=0.008) and significantly associated with the predicted age at menopause (P=0.04) Other 5 SNPs not associated with serum AMH levels |
Previously identified SNPs associated with age at menopause in healthy women may have an effect on onset of menopause in female CCS. |
|
NPY2R48
NPYR2 (Neuropeptide Receptor 2 gene) belongs to a family of G-protein coupled receptors activated by a group of closely related peptide hormones. These neuropeptide Y receptors control diverse behavioral processes including appetite, circadian rhythm and anxiety. Activated neuropeptide receptors release G1 subunit from the heterotrimeric G protein complex and this in turn inhibits production of the second messenger cAMP |
• GWAS of 779 female CCSs to identify SNPs associated with clinically diagnosed PM (defined as menopause <40 years) Analyses adjusted for cyclophosphamide equivalent dose of alkylating agents and ovarian RT dose • Replication using self-reported PM in 1624 survivors p in the Childhood Cancer Survivor Study (CCSS) |
• PM clinically diagnosed in 30 (3.8%) participants • 13 SNPs upstream of NPY2R associated with prevalence of PM (minimum P=3.3 × 10–7 for rs9999820, all P< 10–5) • Homozygous carrier of a haplotype formed by 4/13 SNPs associated with markedly elevated PM prevalence if exposed to ovarian RT (OR 25.89, 95%CI 6.18–138.31, P=8.2 × 10–6); replicated in the independent second cohort |
• NPY2R haplotype captures majority of clinically diagnosed PM cases • Evidence from bioinformatics suggests the haplotype alters regulation of NPY2R transcription, possibly affecting PM risk through neuroendocrine pathways |
| ERα and ERβ49 | • 51 SNP markers of 12 different haplotype blocks in the AR, ERα and ERβ genes examined in 127 CCS | • Markers of one specific haplotype block of ERα (rs2207396, rs9340958, rs9340978) associated with increased risk of azoospermia • Compared with GG genotype, patients heterozygous for A allele in rs2207396 had significantly increased risk of azoospermia [OR 3.8; 95%CI: 1.5–9.5; P=0.008], and even higher if treated with alkylating agents (OR 8.8; 95%CI 2.1–36; P=0.004) |
• Genetic markers of high risk of post-treatment azoospermia may help identify boys to whom preservation of testicular tissue before cancer therapy should be offered |
95%CI, 95% confidence interval; AR, androgen receptor; CCSs, childhood cancer survivors; ER, estrogen receptor; GWAS, Genome-wide association study; OR, odds ratio; PM, premature menopause; RT, radiation therapy; SNPs, single-nucleotide polymorphisms
Women with a BRSKI gene variant had a significantly increased risk for low serum AMH, though the impact of this genotype on age at menopause was not as strong as the impact of abdominal radiation.47 Using a genome-wide approach, a recent study revealed a risk profile inclusive of four single nucleotide polymorphisms (SNPs) in the regulatory region of neuropeptide receptor 2 (NPY2R) that conferred a 25-fold increase risk for premature menopause among survivors exposed to ovarian radiotherapy, with results that were replicated in an independent cohort.48 SNPs in androgen receptor genes (ERα and ERβ) expressed in the testis have been associated with oligo- and azoospermia; survivors carrying an ERα gene variant were 4-fold more likely to be azoospermic, a risk that increased to 5-fold in survivors exposed to alkylating agents or testicular radiation.49 However, these findings need to be replicated in an independent cohort.
Neuropsychological impairment
Genetic variants in the MTHFR gene, glutathione S transferase (GST), monoamine oxidase (MAOA), methionine synthase (MS, also known as MTR), and nitric oxide synthase (NOS) have been associated with attention deficit disorders, reduced attentiveness and response speed and reduced overall intellectual function in several candidate SNP studies.50–54
Understanding the molecular underpinnings of treatment-related complications serves two purposes: 1) identifying patients at highest (and lowest) risk, such that treatment or follow-up can be tailored; 2) understanding the mechanism of the treatment-related late effects and using this information to inform therapeutic strategies. There is early evidence that genetic variants can be used to identify vulnerable subgroups, and that information from these variants may improve the ability to predict outcomes better than if one only uses clinical and demographic variables. For example, multiple genetic variants in SLC8A3 and other genes allowed the creation of risk prediction models for anthracycline-related cardiomyopathy, such that 75% of the patients in the high-risk group were accurately predicted to develop cardiomyopathy, while 96% of those in the low risk group did not develop cardiomyopathy.55 It has also been possible to identify survivors of childhood cancer at high or low risk for subsequent radiation-related brain tumors on the basis of genetic and clinical information, and the genetic plus clinical model was superior to the clinical model alone (p=0.002).56 Finally, patients in the highest tertile of radiation-interaction polygenic risk scores had a 60% higher risk of radiation-related breast cancer when compared with those in the lowest tertile.57 These findings notwithstanding, a large gap remains between the knowledge gained through research and readiness for clinical application in cancer survivors. The logical next steps would be to incorporate these findings in patients newly diagnosed with cancers as well as cancer survivors in order to personalize the management of those at highest risk.
Development of Surveillance Guidelines
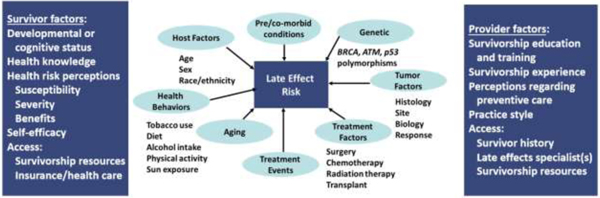
The risk for late therapy-related complications in childhood cancer survivors is the basis for focused screening. These late complications primarily result from therapeutic exposures (e.g., surgery, radiation, chemotherapy) employed during cancer treatment (Tables 2 and 3), although premorbid conditions, genetic predispositions, and treatment events can be impactful. Provision of survivorship care that includes risk-based screening for late complications allows for prevention or timely detection and mitigation of these complications in their early stages.58 Guidelines to direct the care of childhood cancer survivors are predicated on the principle that prevention or early detection of complications will be associated with reduced morbidity. Therefore, screening recommendations are formulated based on risk, taking into consideration the severity of the complications, typical latency from time of exposure to development of the complication, the period during which survivors remain at-risk, the characteristics of the at-risk population (e.g., age at exposure, type of exposure, intensity of exposure), and the cost-effectiveness of the screening modality/schedule.59 Clinicians should also be mindful of the spectrum of clinical factors contributing to risk of late effects as well as survivor and provider factors that can facilitate or challenge access to preventive or remedial resources and services (Figure 3).60
FIGURE 3.
A multitude of factors at both the survivor and healthcare provider level influence the risk of morbidity after cancer. Adapted from Hudson et al., Cancer 2005.
Guidelines to direct the long-term follow-up care of survivors of childhood cancer have been developed by several organizations, including the COG,61 the Dutch Childhood Oncology Group (DCOG LATER),62 the Scottish Intercollegiate Guidelines Network (SIGN),63 and the Late Effects Group of the United Kingdom Children’s Cancer and Leukaemia Group (UKCCLG).64 Guideline development within these groups has been accomplished through the multidisciplinary collaboration of survivorship experts, oncologists (pediatric, medical, radiation), subspecialty providers, primary care practitioners, nurses, patient advocates, and guideline methodologists. Efforts to harmonize and standardize survivorship care recommendations across these organizations and their representative countries have culminated in formation of the International Late Effects of Childhood Cancer Guideline Harmonization Group (IGHG) initiative,65 which is carried out in collaboration with the Pan-European Network for Care of Survivors after Childhood and Adolescent Cancer (PanCare).66
While recommendations for survivorship care may be similar across the guideline development groups, the organization, scope, and content of the guidelines, procedures for guideline updates, and methods for dissemination vary. Most of the European guidelines are organized by organ system (e.g., late effects involving the heart, lungs, etc.), with a focus on the more commonly-occurring late effects (e.g., cardiomyopathy, neurocognitive deficits); whereas the COG Long-Term Follow-Up Guidelines are organized by therapeutic exposure (e.g., alkylating chemotherapy agents, radiation involving specific fields) and in addition to common late effects, also address some of the less common late effects. Methods for guideline dissemination across the groups include website posting, printed copies, presentations at professional meetings, and guideline-related publications aimed at healthcare professionals. In countries with national healthcare systems, guidelines may also be disseminated through governmental healthcare delivery systems. Additionally, some of the groups have developed materials specifically designed for the lay audience of survivors and their families.66–68
The COG, in collaboration with Baylor College of Medicine, has also implemented an electronic, web-based tool that generates personalized survivorship guidelines based on the COG Long-Term Follow-Up Guidelines.69 This tool, known as Passport for Care,70 is available in versions designed for both healthcare providers (which includes detailed guidelines, screening recommendations and rationale, and references), and for patients or caregivers (in lay language).71 A summary of the cancer treatment history (typically part of a Survivorship Care Plan)72 is required to generate these exposure-related guidelines. This summary, which is ideally prepared by the treating oncology center at the end of therapy or at entry into survivorship care, is a key document to which survivors should have ongoing access, in order to assure that they are receiving the recommended guideline-directed follow-up care based on the specific cancer treatment that they received.
Identifying Late Effects of Novel Therapies
Current guidelines, such as those from the COG and other groups has largely focused on conventional cancer therapeutics, since there are now several decades of long-term outcomes data for many patients treated with these modalities and reasonable evidence supporting their associations with various late effects. However, in recent years a better understanding of the biology of many pediatric cancers has led to the development of multiple new agents that offer the promise of more effective and less toxic treatment (Table 9). Specifically, cancer treatments are becoming more personalized with previous histologic diagnoses now being increasingly subclassified based on molecular characteristics, and with subtypes potentially treated with different agents or combinations of agents that target the cancer’s unique molecular or genetic aberration.73 For example, an initial success came with Philadelphia-positive acute lymphoblastic leukemia, where the tyrosine kinase inhibitor (TKI) imatinib plus chemotherapy transformed 3-year event-free survival from <50% to ~80%.74 The addition of other molecularly-targeted agents to conventional chemotherapy is now routine in subsets of acute myeloid leukemia, lymphoma, and sarcoma patients where specific tumor mutations appear amenable to such inhibitors (e.g., FLT3-internal tandem duplications, anaplastic large cell kinase mutations, NTRK fusions, respectively).75–77 The addition of antibody-based therapies to conventional chemotherapy has also improved outcomes for many pediatric malignancies. For example, dinuxtimab, rituximab, brentixumab, and gemtuzumab are already considered standard of care for certain newly diagnosed or relapsed neuroblastomas, lymphomas and leukemias.78–81 Ongoing trials are testing the efficacy of other promising antibodies such as blinatumomab and inotozumab,82,83 and the optimal role of immune checkpoint inhibitors and genetically engineered chimeric antigen receptor (CAR) T cells.84,85 For local control, surgery and radiotherapy also have evolved, becoming less invasive, or featuring new techniques and particles (e.g., protons) that more precisely target the tumor and limit dose to normal tissues.86
TABLE 9.
Molecularly targeted agents being used or under consideration for pediatric cancers (adapted and updated from Chow et al., J Clin Oncol 2018)
| Target(s) | Drug(s) | Notable toxicities* |
|---|---|---|
| ALK/ROS | Ceritinib Crizotinib Ensartinib Lorlatinib |
Arrhythmia Dyslipidemia (lorlatinib) Hyperglycemia (ceritinib) Hallucinations / psychiatric (lorlatinib) Neuropathy/neuromuscular Pulmonary embolism (crizotinib) Respiratory Vision changes |
| BCR/ABL, KIT, PDGFR | Dasatinib Imatinib Nilotinib Ponatinib |
Cardiac dysfunction Edema, effusions Growth & stature* Pulmonary hypertension (dasatinib) Thyroid dysfunction Vascular events, including myocardial ischemia, peripheral arterial occlusion, and stroke (ponatinib) |
| BRAF | Dabrafenib Vemurafenib |
Hyperglycemia (dabrafenib) New acute development of skin cancers QT-prolongation (vemurafenib) Radiation sensitivity |
| CD3 | Blinatumomab | B cell aplasia (CAR T cells)* Cytokine release syndrome Neurotoxicity |
| CD19 | Blinatumomab Chimeric antigen receptor (CAR) T cells |
Same as with CD3-targeted agents above |
| CD20 | Rituximab | B cell aplasia |
| CD30 | Brentuximab vedotin | Neuropathy Progressive multifocal leukoencephalopathy |
| CD33 | Gemtuzumab ozogamicin | Hepatotoxicity, sinusoidal obstruction syndrome |
| CDK/cyclin (cell cycle) | Palbociclib Ribociclib |
QT-prolongation (ribociclib) |
| EZH2 | Tazemetostat | Limited experience to date |
| GD2 | Dinutuximab Hu3F8 Hu14.18K322A |
Capillary leak syndrome Neuropathic pain Reversible posterior leukoencephalopathy |
| HDAC (histone deacetylase) | Entinostat Fimepinostat Panobinostat Romidepsin Vorinostat |
Arrhythmia / myocardial infarction Pulmonary embolus (vorinostat) Limited experience for entinostat, fimepinostat |
| MEK/MAPK | Binimetinib Cobimetinib Selumetinib Trametinib |
Cardiac dysfunction Skin toxicity Vision changes, retinopathy |
| mTOR | Everolimus Sirolimus Temsirolimus ABI-009 (Nab-Rapamycin) LY3023414 |
Dyslipidemia Hyperglycemia |
| PD-1, PDL-1, CTL4 (immune checkpoint) | Atezolizumab Avelimumab Cemiplimab Durvalumab Ipilimumab Nivolumab Pembrolizumab |
Auto-immune/inflammatory, including: Endocrinopathies Myocarditis Neurotoxicity Pneumonitis |
| PI3K | Fimepinostat LY3023414 |
Hyperglycemia |
| TRK | Entrectinib Larotrectinib |
Limited experience to date |
| VEGF, VEGFR, PDGFR, RET | Axitinib Bevacizumab Cabozantinib Lenvantinib Pazopanib Sorafenib Vandetanib |
Cardiac dysfunction Hemorrhage, impaired wound healing Hepatotoxicity (pazopanib) Hypertension, proteinuria Intestinal perforation/fistula (bevacizumab) Thromboembolism Thyroid dysfunction (axitinib, pazopanib, sorafenib) |
Toxicities that may persist well after cessation of therapy, or develop later
Nevertheless, “targeted” agents may have off-target effects.86 For example, some endocrine and immunologic late effects are beginning to emerge among children treated with targeted agents. TKIs have been associated with growth deceleration and alterations in bone mineral and thyroid metabolism. This includes children treated with imatinib for chronic myeloid leukemia (CML) who have since developed varying degrees of growth restriction.87 Although case series in children treated with imatinib have reported normal thyroid function, other TKIs may affect thyroid function. In adult studies, nilotinib, dasatinib, sunitinib, and sorafenib have been associated with de-novo hypothyroidism, variably preceded by hyperthyroidism,88 leading to a general recommendation that children receiving TKIs should also have their thyroid function closely monitored. Immune checkpoint inhibitors have also been associated with hypophysitis and anterior pituitary deficiencies in adults.89 Finally, B cell depletion occurs with agents that target B cell antigens (e.g., rituximab, blinatumomab). Although B cell aplasia is usually short-term, there is a potential for long-term B cell aplasia in patients who have chimeric antigen receptor T cell persistence.85 The health impact of prolonged B cell aplasia is unclear but thought to be minimal so long as affected patients receive ongoing immunoglobulin replacement to minimize infectious risks. However, long-term financial costs may be important as these treatments become more widespread. To date, there have not been any reports of lymphoproliferative disorders or secondary malignancies directly related to CAR T cell products. Finally, the late cardiovascular effects of new targeted agents remain largely unknown in childhood cancer survivors. However, rare but serious toxicities including ischemic events (e.g., ponatinib) and autoimmune myocarditis (e.g., immune checkpoint inhibitors) have been observed in adults, including young adults.90,91
Changes in clinical trial design and protocol development may affect the ability to comprehensively study the potential late effects of these novel agents. For example, the established paradigm of requiring large phase 2 or 3 trials to establish efficacy prior to an agent becoming standard of care may no longer apply in certain situations.92 Given the relative rarity of pediatric cancer, the subclassification of tumors based on genetic or molecular features further increases the heterogeneity of treatment while reducing the numbers of survivors treated similarly, potentially increasing the difficulty of detecting rare but serious late effects in the future. Therefore, long-term comprehensive follow-up of children treated with novel emerging therapies is critical to determining whether these therapies are truly associated with long-term improved outcomes versus historical treatments.
Models of Survivorship Care
One of the key recommendations made by United States National Academy of Medicine (formerly, the Institute of Medicine) regarding the care of cancer survivors was that there is a need for defining standards for systems of comprehensive, multidisciplinary follow-up care that link specialty and primary care providers.93,94 During their childhood and adolescent years, most childhood cancer survivors are transitioned from the acute oncology clinic to a specialized survivor clinic. In a survey of COG institutions, 84% of the 97 responding institutions indicated that they have a clinical program comprised of providers that specifically care for childhood cancer survivors.95 Unfortunately, once these survivors are ready to transition out of the pediatric setting, specialized resources are less accessible. Only 38 of the 97 institutions reported access to specialized survivorship care in an adult care setting. Thus, most adult survivors of childhood cancer likely do not receive care in a survivor clinic or receive follow-up from a health care provider with expertise in survivorship issues.96 Most receive their health care from a primary care physician, many of whom profess discomfort with caring for such survivors independently, have limited knowledge about survivor-specific follow-up guidelines,97,98 and are unlikely to provide recommended surveillance.96
To address this gap, several models have been proposed for the care of adult survivors of childhood cancer, most of which are based on the concept of “shared care” between the cancer center and the primary care provider, with the balance of this care contingent on individual survivors’ existing late effects and risk for future morbidity.99 Successful models of shared survivor care are context specific. For example, the Adult Long-Term Follow-Up Program at Memorial Sloan Kettering Cancer Center is located in an academic medical center and staffed by primary care physicians and nurse practitioners.100 The clinic cares for higher risk survivors, particularly those with multiple morbidities, while lower risk survivors are transitioned back to community-based primary care. In Canada, the Pediatric Oncology Group of Ontario has launched an initiative to recruit family practice teams and academic family practice programs in community hospitals who are willing to care for adult survivors of childhood cancer who have been discharged from cancer-center based survivor care. The goal is to identify geographically diverse primary care practices (particularly those that have multi-disciplinary health care teams that include providers such as psychologists, social workers, dietitians, etc.) that are willing to take on a “critical mass” of survivors with the hope that this will incentivize their developing an expertise in survivorship. These clinics would then be provided with education from the province’s specialized cancer center-based survivor programs, which will remain accessible should specific patient advice be needed or if survivors need referral back into the cancer system.
As more survivors age in older adulthood, and as the size of the population of childhood cancer survivors continues to grow, it is clear that specialized survivor clinics will not have the capacity to care for all survivors. Several recently completed and ongoing clinical trials have evaluated novel approaches for ensuring that survivors receive recommended surveillance during adulthood. The Evaluation of Cardiovascular Health Outcomes Among Survivors (ECHO) trial demonstrated that telephone counseling from an advanced-practice nurse increased the rate of completion of echocardiography in survivors at risk for cardiac dysfunction when added to the provision of a printed survivor care plan.101 A similar benefit to providing a tailored telephone-delivered motivational interview was observed in a study focused on increasing the uptake of screening mammography in survivors at risk for secondary breast cancer.102 Unfortunately, such interventions are resource intensive and there is concern about whether and how these can be scaled to a growing population of survivors. Consequently, new research is investigating the use of mobile health technologies such as smartphone apps to deliver such counseling remotely. For example, a follow-up to the breast cancer screening study above is recruiting patients and their primary care providers to a study that uses a smartphone app that provides interactive 2-way text messages with links to video vignettes to activate survivors and their clinicians (NCT03435380). Further, the emerging capabilities of m-Health technologies that can facilitate communication between survivors and health care providers may overcome some of the geographic and time barriers to the receipt of appropriate survivorship care. Patient-reported outcomes such as pain and anxiety, biometric data such as pulse rate and blood pressure, and elements of the physical examination such as assessment of skin lesions by teledermoscopy,103 can all be accomplished remotely. Similarly, the use of telemedicine, especially for patients who live in rural areas, has the potential to increase access to risk-based survivor care.104
Future Directions
Decades of follow-up have been required to demonstrate improvements in long-term pediatric cancer outcomes to date.17,105 To facilitate this going forward in this era of increasingly personalized cancer medicine, a joint effort by the pharmaceutical industry, government, and non-governmental professional societies to organize infrastructure that enables such long-term follow-up is recommended. Such infrastructure should include, at minimum, the creation of a registry that allows for later linkage and ability to re-contact patients or families for follow-up information, or if possible, a more resource-intensive prospective cohort. Such efforts, in combination with information about individual genetic susceptibility to selected late effects, cost-effectiveness and decision modeling research, will enable the continued refinement of long-term follow-up guidelines. As these guideline refinements are realized, the clinician’s ability to personalize screening and follow-up care for childhood cancer survivors will continue to improve, maximizing screening yield and timely intervention for those at highest risk, while minimizing the need for screening in survivors at low risk for late complications. However, these refinements can only be realized with effective dissemination, including among primary care providers.
Involvement of primary care and medical subspecialists is particularly relevant as survivors age. Premature aging and frailty are increasingly documented findings among survivors of childhood cancer.106 As in older adults, frailty in childhood cancer survivors is associated with additional accumulation of chronic disease and with mortality. Thus, understanding when and how frailty develops among childhood cancer survivors, and identifying the pathology responsible for frailty onset, may provide biological targets for development of remediation and early intervention strategies.107 Telehealth and other strategies to disseminate information to survivors and primary care providers will also be critical to develop and test. Overall, survivorship as a field has largely developed as a result of the tremendous progress in curing many cancers, the focus now needs to be directed at preventing or mitigating the long-term consequences of otherwise very successful cancer therapy.
ACKNOWLEDGEMENTS
Funded in part by the US National Institutes of Health (CA180886)
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Howlader N, Noone AM, Krapcho M, et al. : SEER Cancer Statistics Review, 1975–2016, National Cancer Institute; Bethesda, MD, https://seer.cancer.gov/csr/1975_2016/, based on November 2018 SEER data submission, posted to the SEER web site, April 2019. [Google Scholar]
- 2.Robison LL, Hudson MM: Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer 14:6170, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong GT, Kawashima T, Leisenring W, et al. : Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol 32:1218–27, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung YT, Brinkman TM, Li C, et al. : Chronic Health Conditions and Neurocognitive Function in Aging Survivors of Childhood Cancer: A Report from the Childhood Cancer Survivor Study. J Natl Cancer Inst 110:411–419, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oancea SC, Brinkman TM, Ness KK, et al. : Emotional distress among adult survivors of childhood cancer. J Cancer Surviv 8:293303, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saatci D, Thomas A, Botting B, et al. : Educational attainment in childhood cancer survivors: a meta-analysis. Arch Dis Child, 2019 [DOI] [PubMed] [Google Scholar]
- 7.Mader L, Michel G, Roser K: Unemployment Following Childhood Cancer. Dtsch Arztebl Int 114:805–812, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boman KK, Lindblad F, Hjern A: Long-term outcomes of childhood cancer survivors in Sweden: a population-based study of education, employment, and income. Cancer 116:1385–91, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Gunnes MW, Lie RT, Bjorge T, et al. : Economic independence in survivors of cancer diagnosed at a young age: A Norwegian national cohort study. Cancer 122:3873–3882, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wengenroth L, Sommer G, Schindler M, et al. : Income in Adult Survivors of Childhood Cancer. PLoS One 11:e0155546, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Font-Gonzalez A, Feijen EL, Sieswerda E, et al. : Social outcomes in adult survivors of childhood cancer compared to the general population: linkage of a cohort with population registers. Psychooncology 25:933–41, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Kirchhoff AC, Parsons HM, Kuhlthau KA, et al. : Supplemental security income and social security disability insurance coverage among long-term childhood cancer survivors. J Natl Cancer Inst 107:djv057, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang IC, Bhakta N, Brinkman TM, et al. : Determinants and Consequences of Financial Hardship Among Adult Survivors of Childhood Cancer: A Report From the St. Jude Lifetime Cohort Study. J Natl Cancer Inst 111:189–200, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunin-Batson A, Kadan-Lottick N, Zhu L, et al. : Predictors of independent living status in adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer 57:1197–203, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ness KK, Gurney JG, Zeltzer LK, et al. : The impact of limitations in physical, executive, and emotional function on health-related quality of life among adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Arch Phys Med Rehabil 89:128–36, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Armstrong GT, Chen Y, Yasui Y, et al. : Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med 374:833–42, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibson TM, Mostoufi-Moab S, Stratton KL, et al. : Temporal patterns in the risk of chronic health conditions in survivors of childhood cancer diagnosed 1970–99: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol 19:1590–1601, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ness KK, Hudson MM, Jones KE, et al. : Effect of Temporal Changes in Therapeutic Exposure on Self-reported Health Status in Childhood Cancer Survivors. Ann Intern Med 166:89–98, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oeffinger KC, Mertens AC, Sklar CA, et al. : Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 355:1572–82, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Hudson MM, Ness KK, Gurney JG, et al. : Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA 309:2371–81, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geenen MM, Cardous-Ubbink MC, Kremer LC, et al. : Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA 297:2705–15, 2007 [DOI] [PubMed] [Google Scholar]
- 22.The L: Making more of multimorbidity: an emerging priority. Lancet 391:1637, 2018 [DOI] [PubMed] [Google Scholar]
- 23.Dong H, Robison LL, Leisenring WM, et al. : Estimating the burden of recurrent events in the presence of competing risks: the method of mean cumulative count. Am J Epidemiol 181:532–40, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhakta N, Liu Q, Yeo F, et al. : Cumulative burden of cardiovascular morbidity in paediatric, adolescent, and young adult survivors of Hodgkin’s lymphoma: an analysis from the St Jude Lifetime Cohort Study. Lancet Oncology 17:1325–1334, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhakta N, Liu Q, Ness KK, et al. : The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet 390:2569–2582, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeh JM, Nekhlyudov L, Goldie SJ, et al. : A model-based estimate of cumulative excess mortality in survivors of childhood cancer. Ann Intern Med 152:409–17, W131–8, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeh JM, Ward ZJ, Chaudhry A, et al. : Life Expectancy in Adult Survivors of Childhood and Adolescent Cancer: Improvements Over Three Decades. JAMA Oncology, In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunink MG, Weinstein MC, Wittenberg E, et al. : Decision Making in Health and Medicine: Integrating Evidence and Values. Second Edition United Kingdom, Cambridge University Press, 2014 [Google Scholar]
- 29.Yeh JM, Nohria A, Diller L: Routine echocardiography screening for asymptomatic left ventricular dysfunction in childhood cancer survivors: a model-based estimation of the clinical and economic effects. Ann Intern Med 160:661–71, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong FL, Bhatia S, Kurian S, et al. : Efficacy of the Children’s Oncology Group (COG) Long-Term Follow-Up (LTFU) guidelines in reducing the risk of congestive heart failure (CHF) in long-term childhood cancer survivors (CCS) [abstract]. Presented at the 2012 American Society for Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, 2012 [Google Scholar]
- 31.Yeh JM, Lowry KP, Schechter CB, et al. : Clinical outcomes and cost-effectiveness of breast cancer screening for childhood cancer survivors treated with chest radiation: A comparative modeling study. Presented at the 2019 American Society for Clinical Oncology, Chicago, IL, 2019 [Google Scholar]
- 32.Habbema JD, Wilt TJ, Etzioni R, et al. : Models in the development of clinical practice guidelines. Ann Intern Med 161:812–8, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Owens DK, Whitlock EP, Henderson J, et al. : Use of Decision Models in the Development of Evidence-Based Clinical Preventive Services Recommendations: Methods of the U.S. Preventive Services Task Force. Ann Intern Med 165:501–508, 2016 [DOI] [PubMed] [Google Scholar]
- 34.Armenian SH, Bhatia S: Chronic health conditions in childhood cancer survivors: is it all treatment-related--or do genetics play a role? J Gen Intern Med 24 Suppl 2:S395–400, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhatia S: Genetic variation as a modifier of association between therapeutic exposure and subsequent malignant neoplasms in cancer survivors. Cancer 121:648–63, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aminkeng F, Bhavsar AP, Visscher H, et al. : A coding variant in RARG confers susceptibility to anthracycline-induced cardiotoxicity in childhood cancer. Nat Genet 47:1079–84, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Visscher H, Ross CJ, Rassekh SR, et al. : Validation of variants in SLC28A3 and UGT1A6 as genetic markers predictive of anthracycline-induced cardiotoxicity in children. Pediatr Blood Cancer 60:1375–81, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Visscher H, Rassekh SR, Sandor GS, et al. : Genetic variants in SLC22A17 and SLC22A7 are associated with anthracycline-induced cardiotoxicity in children. Pharmacogenomics 16:1065–76, 2015 [DOI] [PubMed] [Google Scholar]
- 39.Wang X, Sun CL, Quinones-Lombrana A, et al. : CELF4 variant and anthracycline-related cardiomyopathy: a Children’s Oncology Group genome-wide association study. J Clin Oncol 34:863–70, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang X, Liu W, Sun CL, et al. : Hyaluronan synthase 3 variant and anthracycline-related cardiomyopathy: a report from the children’s oncology group. J Clin Oncol 32:647–53, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blanco JG, Sun CL, Landier W, et al. : Anthracycline-related cardiomyopathy after childhood cancer: role of polymorphisms in carbonyl reductase genes--a report from the Children’s Oncology Group. J Clin Oncol 30:1415–21, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Best T, Li D, Skol AD, et al. : Variants at 6q21 implicate PRDM1 in the etiology of therapy-induced second malignancies after Hodgkin’s lymphoma. Nat Med 17:941–3, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma YP, van Leeuwen FE, Cooke R, et al. : FGFR2 genotype and risk of radiation-associated breast cancer in Hodgkin lymphoma. Blood 119:1029–31, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Morton LM, Sampson JN, Armstrong GT, et al. : Genome-Wide Association Study to Identify Susceptibility Loci That Modify Radiation-Related Risk for Breast Cancer After Childhood Cancer. J Natl Cancer Inst 109, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sapkota Y, Turcotte LM, Ehrhardt MJ, et al. : Genome-Wide Association Study in Irradiated Childhood Cancer Survivors Identifies HTR2A for Subsequent Basal Cell Carcinoma. J Invest Dermatol 139:2042–2045 e8, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Z, Wilson CL, Easton J, et al. : Genetic Risk for Subsequent Neoplasms Among Long-Term Survivors of Childhood Cancer. J Clin Oncol 36:2078–2087, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Dorp W, van den Heuvel-Eibrink MM, Stolk L, et al. : Genetic variation may modify ovarian reserve in female childhood cancer survivors. Hum Reprod 28:1069–76, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Brooke RJ, Im C, Wilson CL, et al. : A High-risk Haplotype for Premature Menopause in Childhood Cancer Survivors Exposed to Gonadotoxic Therapy. J Natl Cancer Inst 110:895–904, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romerius P, Giwercman A, Moell C, et al. : Estrogen receptor alpha single nucleotide polymorphism modifies the risk of azoospermia in childhood cancer survivors. Pharmacogenet Genomics 21:263–9, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Kamdar KY, Krull KR, El-Zein RA, et al. : Folate pathway polymorphisms predict deficits in attention and processing speed after childhood leukemia therapy. Pediatr Blood Cancer 57:454–60, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krull KR, Brouwers P, Jain N, et al. : Folate pathway genetic polymorphisms are related to attention disorders in childhood leukemia survivors. J Pediatr 152:101–5, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Krull KR, Bhojwani D, Conklin HM, et al. : Genetic mediators of neurocognitive outcomes in survivors of childhood acute lymphoblastic leukemia. J Clin Oncol 31:2182–8, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krajinovic M, Robaey P, Chiasson S, et al. : Polymorphisms of genes controlling homocysteine levels and IQ score following the treatment for childhood ALL. Pharmacogenomics 6:293–302, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Marcoux S, Robaey P, Gahier A, et al. : Role of NOS3 DNA variants in externalizing behavioral problems observed in childhood leukemia survivors. J Pediatr Hematol Oncol 35:e157–62, 2013 [DOI] [PubMed] [Google Scholar]
- 55.Visscher H, Ross CJ, Rassekh SR, et al. : Pharmacogenomic prediction of anthracycline-induced cardiotoxicity in children. J Clin Oncol 30:1422–8, 2012 [DOI] [PubMed] [Google Scholar]
- 56.Wang X, Sun CL, Hageman L, et al. : Clinical and genetic risk prediction of subsequent CNS tumors in survivors of childhood cancer: a report from the COG ALTE03N1 study. J Clin Oncol 35:3688–3696, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Opstal-van Winden AWJ, de Haan HG, Hauptmann M, et al. : Genetic susceptibility to radiation-induced breast cancer after Hodgkin lymphoma. Blood 133:1130–1139, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oeffinger KC, Hudson MM: Long-term complications following childhood and adolescent cancer: foundations for providing risk-based health care for survivors. CA Cancer J Clin 54:208–36, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Landier W, Skinner R, Wallace WH, et al. : Surveillance for Late Effects in Childhood Cancer Survivors. J Clin Oncol 36:2216–2222, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hudson MM: A model for care across the cancer continuum. Cancer 104:2638–42, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Landier W, Bhatia S, Eshelman DA, et al. : Development of risk-based guidelines for pediatric cancer survivors: the Children’s Oncology Group Long-Term Follow-Up Guidelines from the Children’s Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol 22:4979–90, 2004 [DOI] [PubMed] [Google Scholar]
- 62.Dutch Childhood Oncology Group: Guidelines for follow-up in survivors of childhood cancer 5 years after diagnosis. Den Haag/Amsterdam, SKION, 2010. pp. Available online: https://www.skion.nl/workspace/uploads/vertaling-richtlijn-LATER-versie-final-okt-2014_2.pdf [Google Scholar]
- 63.Wallace WH, Thompson L, Anderson RA, et al. : Long term follow-up of survivors of childhood cancer: summary of updated SIGN guidance. BMJ 346:f1190, 2013 [DOI] [PubMed] [Google Scholar]
- 64.Wallace WH, Blacklay A, Eiser C, et al. : Developing strategies for long term follow up of survivors of childhood cancer. BMJ 323:271–4, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kremer LC, Mulder RL, Oeffinger KC, et al. : A worldwide collaboration to harmonize guidelines for the long-term follow-up of childhood and young adult cancer survivors: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Pediatr Blood Cancer 60:543–9, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hjorth L, Haupt R, Skinner R, et al. : Survivorship after childhood cancer: PanCare: a European Network to promote optimal long-term care. Eur J Cancer 51:1203–11, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eshelman D, Landier W, Sweeney T, et al. : Facilitating care for childhood cancer survivors: integrating children’s oncology group long-term follow-up guidelines and health links in clinical practice. J Pediatr Oncol Nurs 21:271–80, 2004 [DOI] [PubMed] [Google Scholar]
- 68.Mulder RL, van der Pal HJ, Levitt GA, et al. : Transition guidelines: An important step in the future care for childhood cancer survivors. A comprehensive definition as groundwork. Eur J Cancer 54:64–8, 2016 [DOI] [PubMed] [Google Scholar]
- 69.Children’s Oncology Group: Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers, version 5.0. Monrovia, CA, Children’s Oncology Group, 2018; available: www.survivorshipguidelines.org [Google Scholar]
- 70.Poplack DG, Fordis M, Landier W, et al. : Childhood cancer survivor care: development of the Passport for Care. Nat Rev Clin Oncol 11:740–50, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Passport for Care; available: https://cancersurvivor.passportforcare.org/: Passport for Care Cancer Survivor Website, 2019
- 72.Hewitt ME, Greenfield S, Stovall E: From cancer patient to cancer survivor: lost in transition. Washington, D.C., National Academies Press, 2006 [Google Scholar]
- 73.Seibel NL, Janeway K, Allen CE, et al. : Pediatric oncology enters an era of precision medicine. Curr Probl Cancer 41:194–200, 2017 [DOI] [PubMed] [Google Scholar]
- 74.Schultz KR, Bowman WP, Aledo A, et al. : Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: a children’s oncology group study. J Clin Oncol 27:5175–81, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rollig C, Serve H, Huttmann A, et al. : Addition of sorafenib versus placebo to standard therapy in patients aged 60 years or younger with newly diagnosed acute myeloid leukaemia (SORAML): a multicentre, phase 2, randomised controlled trial. Lancet Oncol 16:1691–9, 2015 [DOI] [PubMed] [Google Scholar]
- 76.Mosse YP, Voss SD, Lim MS, et al. : Targeting ALK With Crizotinib in Pediatric Anaplastic Large Cell Lymphoma and Inflammatory Myofibroblastic Tumor: A Children’s Oncology Group Study. J Clin Oncol 35:3215–3221, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laetsch TWD SG; Nagasubramanian R; Turpin B; Mascarenhas L; Federman N; Reynolds M; Smith S; Cruickshank S; Cox MC; Pappo AS; Hawkins DS: A pediatric phase 1 study of larotrectinib, a highly selective inhibitor of the tropomyosin receptor kinase (TRK) family. J Clin Oncol 35, 2017 [Google Scholar]
- 78.Yu AL, Gilman AL, Ozkaynak MF, et al. : Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 363:1324–34, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Younes A, Gopal AK, Smith SE, et al. : Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol 30:2183–9, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pollard JA, Loken M, Gerbing RB, et al. : CD33 Expression and Its Association With Gemtuzumab Ozogamicin Response: Results From the Randomized Phase III Children’s Oncology Group Trial AAML0531. J Clin Oncol 34:747–55, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Minard-Colin V, Auperin A, Pillon M, et al. : Results of the randomized Intergroup trial Inter-B-NHL Ritux 2010 for children and adolescents with high-risk B-cell non-Hodgkin lymphoma (B-NHL) and mature acute leukemia (B-AL): Evaluation of rituximab (R) efficacy in addition to standard LMB chemotherapy (CT) regimen. J Clin Oncol 34:10507–10507, 2016 [Google Scholar]
- 82.von Stackelberg A, Locatelli F, Zugmaier G, et al. : Phase I/Phase II Study of Blinatumomab in Pediatric Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia. J Clin Oncol 34:4381–4389, 2016 [DOI] [PubMed] [Google Scholar]
- 83.Kantarjian HM, DeAngelo DJ, Stelljes M, et al. : Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N Engl J Med 375:740–53, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pinto N, Park JR, Murphy E, et al. : Patterns of PD-1, PD-L1, and PD-L2 expression in pediatric solid tumors. Pediatr Blood Cancer, 2017 [DOI] [PubMed] [Google Scholar]
- 85.Maude SL, Frey N, Shaw PA, et al. : Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371:150717, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chow EJ, Antal Z, Constine LS, et al. : New agents, emerging late effects, and the development of precision survivorship. J Clin Oncol 36:2231–2240, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shima H, Tokuyama M, Tanizawa A, et al. : Distinct impact of imatinib on growth at prepubertal and pubertal ages of children with chronic myeloid leukemia. J Pediatr 159:676–81, 2011 [DOI] [PubMed] [Google Scholar]
- 88.Illouz F, Braun D, Briet C, et al. : Endocrine side-effects of anti-cancer drugs: thyroid effects of tyrosine kinase inhibitors. Eur J Endocrinol 171:R91–9, 2014 [DOI] [PubMed] [Google Scholar]
- 89.Torino F, Corsello SM, Salvatori R: Endocrinological side-effects of immune checkpoint inhibitors. Curr Opin Oncol 28:278–87, 2016 [DOI] [PubMed] [Google Scholar]
- 90.Moslehi JJ: Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med 375:1457–1467, 2016 [DOI] [PubMed] [Google Scholar]
- 91.Moslehi JJ, Salem JE, Sosman JA, et al. : Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 391:933, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.DuBois SG, Corson LB, Stegmaier K, et al. : Ushering in the next generation of precision trials for pediatric cancer. Science 363:1175–1181, 2019 [DOI] [PubMed] [Google Scholar]
- 93.Hewitt M, Weiner SL, Simone JC: Childhood Cancer Survivorship: Improving Care and Quality of Life. Washington, DC, The National Academies Press, 2003 [PubMed] [Google Scholar]
- 94.Hewitt M, Greenfield S, Stovall E: From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, D.C., National Academies Press, 2005 [Google Scholar]
- 95.Sadak KT, Szalda D, Lindgren BR, et al. : Transitional care practices, services, and delivery in childhood cancer survivor programs: A survey study of U.S. survivorship providers. Pediatr Blood Cancer 66:e27793, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nathan PC, Greenberg ML, Ness KK, et al. : Medical care in long-term survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol 26:4401–9, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nathan PC, Daugherty CK, Wroblewski KE, et al. : Family physician preferences and knowledge gaps regarding the care of adolescent and young adult survivors of childhood cancer. J Cancer Surviv, 2013 [DOI] [PubMed] [Google Scholar]
- 98.Suh E, Daugherty CK, Wroblewski K, et al. : General internists’ preferences and knowledge about the care of adult survivors of childhood cancer: a cross-sectional survey. Ann Intern Med 160:11–7, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Oeffinger KC, McCabe MS: Models for delivering survivorship care. J Clin Oncol 24:5117–24, 2006 [DOI] [PubMed] [Google Scholar]
- 100.Oeffinger KC, Argenbright KE, Levitt GA, et al. : Models of cancer survivorship health care: moving forward. Am Soc Clin Oncol Educ Book:205–13, 2014 [DOI] [PubMed] [Google Scholar]
- 101.Hudson MM, Leisenring W, Stratton KK, et al. : Increasing cardiomyopathy screening in at-risk adult survivors of pediatric malignancies: a randomized controlled trial. J Clin Oncol 32:3974–81, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oeffinger KC, Ford JS, Moskowitz CS, et al. : Promoting Breast Cancer Surveillance: The EMPOWER Study, a Randomized Clinical Trial in the Childhood Cancer Survivor Study. J Clin Oncol 37:2131–2140, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Daniel CL, Armstrong GT, Keske RR, et al. : Advancing Survivors’ Knowledge (ASK) about skin cancer study: study protocol for a randomized controlled trial. Trials 16:109, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Costello AG, Nugent BD, Conover N, et al. : Shared Care of Childhood Cancer Survivors: A Telemedicine Feasibility Study. J Adolesc Young Adult Oncol 6:535–541, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Turcotte LM, Liu Q, Yasui Y, et al. : Temporal Trends in Treatment and Subsequent Neoplasm Risk Among 5-Year Survivors of Childhood Cancer, 1970–2015. JAMA 317:814–824, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ness KK, Krull KR, Jones KE, et al. : Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St Jude Lifetime cohort study. J Clin Oncol 31:4496–503, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ness KK, Armstrong GT, Kundu M, et al. : Frailty in childhood cancer survivors. Cancer 121:1540–7, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]