Abstract
Introduction:
177Lutetium-[DOTA°,Tyr3]octreotate (177Lu-DOTATATE) is a type of peptide receptor radionuclide therapy that garnered FDA approval in January 2018 for the treatment of somatostatin receptor-positive gastroenteropancreatic (GEP) neuroendocrine tumor (NET) patients. The therapy approval was based on findings from the randomized international phase III NETTER-1 trial as well as outcome data from a large European registry. The mechanism of the drug stems directly from its structure: a somatostatin analog (octreotate) selectively binding to somatostatin receptor expressing cells and being internalized, along with a chelated beta-emitting isotope 177Lu.
Areas Covered:
Herein we describe the pharmacology, clinical efficacy and adverse event data from prospective and retrospective studies with 177Lu-DOTATATE. We discuss the role of 177Lu-DOTATATE within the current treatment landscape for GEP NET patients.
Expert Opinion:
177Lu-DOTATATE represents a unique addition to the treatment armamentarium for GEP NETs because of its potential to elicit tumor cytoreduction, which is rare among other existing treatment options, and prolonged disease control. Where 177Lu-DOTATATE fits into the treatment sequence for GEP NET patients remains an area of active investigation.
Keywords: Peptide receptor radionuclide therapy, 177Lu-DOTATATE, gastroenteropancreatic neuroendocrine tumor patients, somatostatin receptor agonist, somatostatin receptor antagonist
1. Introduction
The incidence of neuroendocrine tumors (NETs) has risen more than sixfold in the past three decades and more than 170,000 patients are living with the disease in the United States [1]. Gastroenteropancreatic (GEP) NETs represent the most common subtype of well-differentiated NETs, comprising more than 70% of these neoplasms [2]. Somatostatin receptors are expressed in >80% of well-differentiated NETs, with somatostatin receptor subtype 2 being the most prevalent subtype [3]. In patients with unresectable metastatic disease, or those with hormonal over-production leading to syndromes, long-acting somatostatin agonists (SSAs) have been the cornerstone of first-line therapy [4,5]. Radiolabeled SSAs or antagonists have been developed over the past three decades for the management of somatostatin receptor-expressing well-differentiated neuroendocrine tumors which have progressed on SSAs. This form of systemic radiotherapy belongs to a larger category of treatment called peptide receptor radionuclide therapy (PRRT).
PRRT consists of a radioisotope linked via a chelating molecule to a peptide that targets peptide receptors present on the cancer cell surface, thus allowing for targeted delivery of radiation. In the case of PRRT for advanced NET, the peptide can be an SSA or somatostatin antagonist, which binds to the somatostatin receptor. Since the late 1990s, most studies of PRRT have investigated the β-emitting isotopes Yttrium-90 (90Y) and Lutetium-177 (177Lu). PRRT has represented a significant treatment advance for patients with GEP NETs because of its cytoreductive potential and ability to elicit prolonged disease-progression free periods. Response rates with the therapy have been reported in the 15-40% range, which is significantly higher than response rates with targeted treatments in the post-SSA setting including everolimus or sunitinib [6–8]. Despite the benefit of PRRT in patients with progressive well-differentiated GEP NETs, salient questions pertaining to its use remain. Several of these include when should it be sequenced in relation to other available treatments, who are the patients that derive the most benefit from the therapy and should it be used as a monotherapy or in combination with other treatments? Ongoing studies such as COMPETE and CONTROL NETs are aimed at finding some of these answers [NCT03049189, NCT02358356].
2. Overview of the market
Although 177Lu-DOTATATE is the only PRRT therapy approved for GEP NET patients in North America and Europe, other PRRT compounds are available. 111In-DOTATOC and 90Y-DOTATOC were developed prior to 177Lu-based PRRT [9–11]. 111In primarily emits γ rays and conversion electrons and is associated with symptomatic disease control and minimal cytotoxicity, but has a low rate of partial remissions, which is likely due to the small range and limited tissue penetration [12]. 90Y-DOTATOC, in comparison to 177Lu-based PRRT, has a longer-range higher-energy β-emission which theoretically allows for greater efficacy in larger size metastases. Concomitantly, it may also result in more collateral toxicity than 177Lu, particularly nephrotoxicity [13].
The treatment landscape of GEP-NETs is expanding. The SSAs octreotide and lanreotide are typically prescribed in the first-line setting for patients with metastatic somatostatin receptor expressive and/or hormonally active tumors. 177Lu-DOTATATE represents a treatment option for most somatostatin receptor expressing GEP-NETs after progression on an SSA. Everolimus is a mammalian target of rapamycin (mTOR) inhibitor with proven cytostatic activity in pancreatic NETs and nonfunctioning GI and lung NETs [6,7]. Sunitinib, an inhibitor of the vascular endothelial growth factor receptor (VEGFR) has proven cytostatic activity for pancreatic NETs only [8]. Capecitabine and temozolomide, which represents an oral cytotoxic treatment regimen, are primarily active in pancreatic NETs, although small studies have also shown activity in lung and thymic NETs [14–21].
3. Introduction to the drug
3.1. Chemistry
177Lutetium-[DOTA°,Tyr3]octreotate, otherwise known as 177Lu-DOTATATE, consists of an SSA (octreotate), a chelating molecule (DOTA) and a beta-emitting radioisotope (177Lu) (Figure 1). Octreotate [D-Phe-c(Cys-Tyr-D-Trp-Lys-Thr-Cys)-Thr] is a slightly modified form of the commercially available SSA octreotide in which the C-terminal threoninol is replaced with threonine, resulting in an enhanced affinity to somatostatin receptor subtype 2 [22]. In one study, the use of octreotate resulted in higher intra-tumoral radioactivity compared to octreotide, with comparable uptake in normal organs including kidneys, spleen and liver [23]. The radioisotope, 177Lu, is a medium-energy β-emitter with a maximum energy of 0.5 MeV, half-life of 6.7 days, and maximal tissue penetration of 2 mm. 177Lu also emits low-energy γ rays which allows for SPECT imaging and the potential for dosimetry [24]. The chelating molecule DOTA consists of a central 12-membered tetraaza ring which envelops the radioisotope and links it to the SSA.
Figure 1.
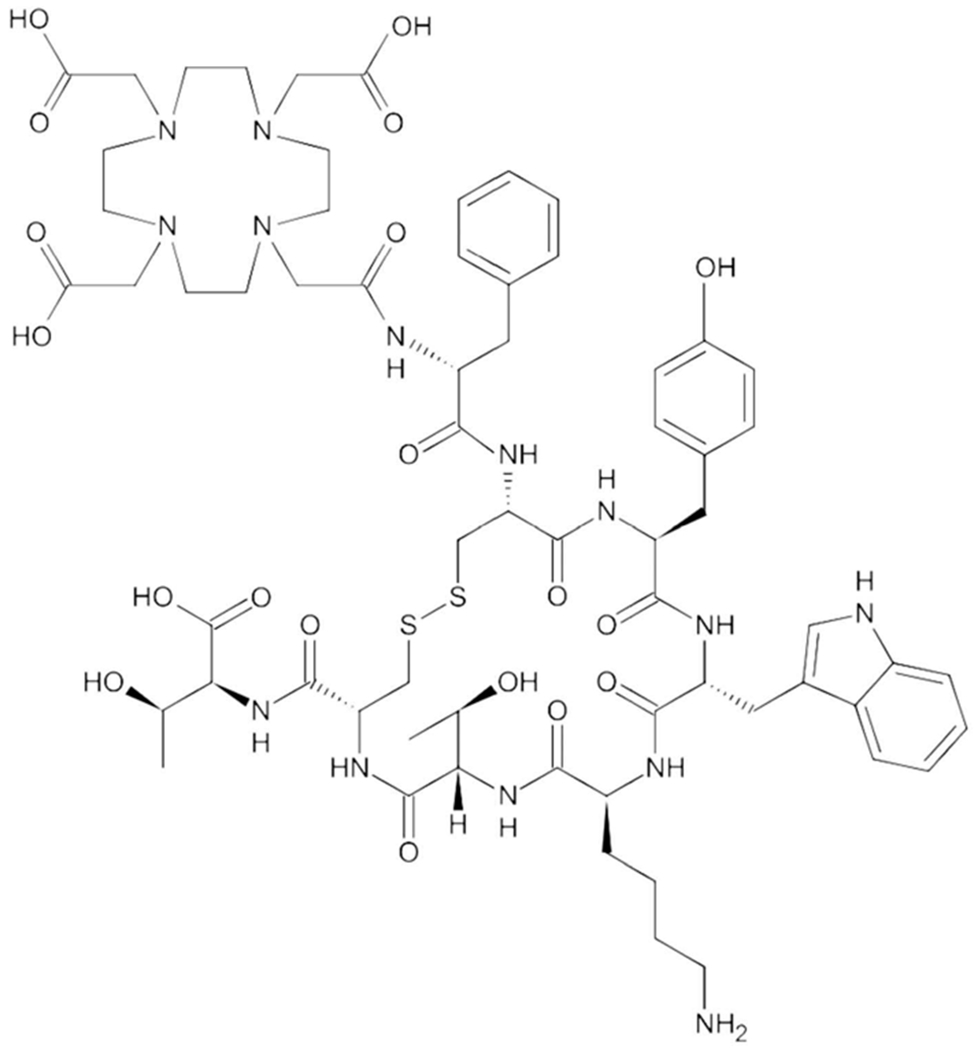
The chemical structure of DOTATATE.
Radiolabeled SSAs are primarily excreted by the kidneys, and reabsorption of the drug in the proximal tubule can result in nephrotoxicity. This risk has been shown to be substantially higher with radiolabeled SSAs utilizing 90Y, which has a longer particle range than 177Lu. Co-administration of amino acids solutions consisting of positively charged amino acids has been found to competitively inhibit this process resulting in reduced nephrotoxicity [25]. One common-compounded formulation consists of 2.5% arginine and 2.5% lysine, administered in 1 l of normal saline over 4 h, starting 30 min prior to administration of the radiopharmaceutical [26]. Commercially available amino acid solutions approved by the Food and Drug Administration (FDA) and developed for total parenteral nutrition (TPN) consist of roughly 18–20 essential and nonessential amino acids, and deliver a much larger osmolar load, resulting in substantially worse nausea during administration.
3.2. Pharmacodynamics
On its FDA label, 177Lu-DOTATATE exposure–response relationships and the time course of its pharmacodynamic response are cited as being unknown.
3.3. Pharmacokinetics
The pharmacokinetics of 177Lu-DOTATATE have been characterized in patients with progressive, somatostatin receptor expressing neuroendocrine tumors [27]. The mean blood exposure (AUC) of 177Lu-DOTATATE at the recommended dose is 41 ng/mL (coefficient of variation [CV] 36%). The mean maximum blood concentration (Cmax) for 177Lu-DOTATATE is 10 ng/mL (CV 50%), which generally is the level at the end of the infusion.
Distribution:
The mean volume of distribution for 177Lu-DOTATATE is 4601 (L) (CV 54%). Within 4 h of administration, 177Lu-DOTATATE distributes throughout the kidneys, tumor lesions, liver, spleen, and occasionally, the pituitary and thyroid glands. Co-administering amino acids reduces the median radiation dose to the kidneys by 47% (34% to 59%) and increases the blood clearance of 177Lu-DOTATATE by 36%. The non-radioactive form of the compound is 43% bound to human plasma proteins [27].
Elimination:
The mean clearance for 177Lu-DOTATATE is 4.5 L/h (CV 31%). The mean (± standard deviation) effective blood elimination half-life is 3.5 (±1.4) hours and the mean terminal blood half-life is 71 (± 28) hours.
Metabolism:
177Lu-DOTATATE does not undergo hepatic metabolism.
Excretion:
177Lu-DOTATATE is primarily renally eliminated with a cumulative excretion of 44% within 5 h, 58% within 24 h and 65% within 48 h following 177Lu-DOTATATE administration. Based on the half-life of 177Lu-DOTATATE, more than 99% will be eliminated within 14 days.
4. Precursor compounds
Early phase I/II studies with radiolabeled SSAs in Europe tested 111In-DOTATOC and 90Y-DOTATOC [9–11]. 111In emits Auger electrons with limited cytotoxicity. 111In-based PRRT improved patient symptoms but demonstrated limited cytoreduction in comparison to 90Y, a beta-emitting isotope with a maximum particle range of roughly 12 mm [12]. Octreotate combined with the chelator DOTA (DOTATATE) was developed based on pre-clinical data suggesting that DOTATATE had a greater affinity for somatostatin receptor 2 than DOTATOC and dosimetry studies suggesting greater intra-tumoral residence time of radioactivity with DOTATATE. Moreover, 177Lu was found to have substantially reduced nephrotoxicity compared to 90Y, likely due to its shorter particle range [23,28–30]. For example, in a large cohort of 1109 NET patients treated with 90Y-DOTATOC, 9.2% experienced permanent nephrotoxicity (grade ≥ 4) [29]. In another large cohort analysis of 807 patients treated at IEO-Milan between 1997 and 2013, 33% of patients treated with 90Y-DOTATOC experienced any grade nephrotoxicity compared to 13.4% treated with 177Lu-DOTATATE [30].
5. Clinical efficacy
5.1. Phase I and phase II trials
In one early study, 35 patients with unresectable GEP NET tumors were treated with 177Lu-DOTATATE [31]. Of these patients, 46% had progressive disease in the year prior to the therapy while 40% had been previously treated with octreotide. Treatment doses were 100 millicurie (mCi) in 7 patients, 150 mCi in 14 patients and 200 mCi in 14 patients; 86% of patients received 600–800 mCi with the other 14% either not completing therapy or reaching their maximum cumulative dose. With regards to the adverse events, 30% and 14% of patients experienced nausea and vomiting within the first 24 h after the administration, respectively. Mild hair loss was noted in 49% of the patients although this was reversible in all patients by the 6-month follow-up period. Grade 3 leukopenia and thrombocytopenia occurred in 1% and 4% of patients, respectively. On 3-month restaging scans after treatment completion, 37% of patients achieved a response (1 complete response, all others partial) and 41% achieved stable disease.
In another phase I/II study, 51 patients with unresectable or metastatic NET patients received escalating doses of 177Lu-DOTATATE [32]. Eighty-four percent possessed tumors of GEP NET origin while 16% of patients had bronchial NETS or tumors of unknown primary. In the patients with grading and Ki-67% available, 95% had well-differentiated NETs. Each of the first five patients received a median of six treatment cycles of 177Lu-DOTATATE at 3.7 gigabecquerel [GBq] (equivalent to 100 mCi). Since no major toxicities were observed in this treatment group, subsequent groups were treated with escalating doses of 177Lu-DOTATATE. After data in the literature emerged about the tolerability of the 7.4 Gbq (200 mCi) dose, the protocol was amended to allow this dose level along with intra-patient dose escalation. Repeat treatments were administered 6 weeks apart with a cumulative activity of 22–30 Gbq considered as maximal based upon existing dosimetry data. With regards to adverse events, grade 1/2 leukopenia, anemia and thrombocytopenia were experienced by 61%, 78% or 27% of patients, respectively. Median creatinine clearance decreased by 23.9% in patients 1-year postcompletion of PRRT. Response rates were 29% in all treated patients and 59% achieved stable disease. The median time-to-progression (TTP) was 36 months while median overall survival (OS) was not reached in the entire cohort. At the 36-month mark, 68% of patients were still alive.
The first US phase II experience with 177Lu-DOTATATE was reported in 2014 [33]. In this study, 37 patients with advanced grade 1 or 2 GEP NETs were treated with up to 4 cycles of 177Lu-DOTATATE to a total of 800 mCi. Of 32 evaluable patients who had received at least 2 treatment cycles, 9.4% and 12.5% of patients developed grade 2 and 3 hematologic toxicity, respectively. Grades 1/2 and grade 3 hepatic toxicity were experienced in 6.2% and 9.4% of patients, respectively. Response rates were 28% in the entire cohort while the stable disease was achieved in 41% of patients. The median progression-free survival (PFS) for all patients and those who received all four treatment cycles were 16.1 months and 16.5 months, respectively. Non-statistically significant differences were observed in PFS outcomes between patients with <50% liver involvement and those with >50% liver involvement. In the study, 34 patients had undergone a pretreatment 18F-FDG PET/CT; 24 patients had a positive PET while 10 patients had negative PET scans. Patients with FDG avid PET scans had a higher likelihood of death compared with those with non-FDG avid PET scans (p = .03 by the Chi-squared test).
A summary of the reported phase I/II studies of 177Lu-DOTATATE in patients with GEP NETs is presented in Table 1.
Table 1.
Completed phase I and phase II studies of 177Lu-DOTATATE.
| Study Reference | Number of Patients | Treatment Dose | Outcomes | Adverse Events |
|---|---|---|---|---|
| [25] | 35 | 86% of patients received 600–800 mCi | 37% response rate and 41% stable disease | Mild hair loss (49%), WHO G3 leukopenia (1%) and thrombocytopenia (4%) |
| [26] | 51 (43 with GEP NETs) | 22-30 Gbq | 29.4% response rate and 59% stable disease | G1/G2 leukopenia (61%), anemia (78%) and thrombocytopenia (27%). |
| [27] | 37 | 800 mCi | 28% response rate, 41% stable disease and median PFS of 16.1 months | G2 hematologic toxicity (9.4%), G3 hematologic toxicity (12.5%) and G3 hepatic toxicity (9.4%) |
Abbreviations: GEP NETs, gastroenteropancreatic neuroendocrine tumors; Gbq, gigabecquerel; mCi, millicurie; G, grade; PFS, progression-free survival
Several ongoing phase II trials are exploring the utility of 177Lu-DOTATATE monotherapy or in combination with additional treatments in patients with other diseases. These are highlighted in Table 2.
Table 2.
Ongoing phase I and phase II studies of 177Lu-DOTATATE.
| Study | Phase | Treatment | Patient Population | Outcome(s) |
|---|---|---|---|---|
| NCT03206060 | II | 177Lu-DOTATATE | Inoperable Paragangliomas and Pheochromocytomas | PFS (primary) and assess safety profile (secondary) |
| NCT03325816 | I/II | 177Lu-DOTATATE plus nivolumab | Inoperable Lung NETs or Refractory Extensive-Stage Small Cell Lung Cancer | RP2D (primary in phase I) and PFS (primary in phase II). Secondary outcomes include assessing safety profile (both phases), OS (phase II), DCR (phase II) and ORR (phase II) |
| NCT03971461 | II | 177Lu-DOTATATE | G1-G3 Progressive or High-Risk Meningioma | 6-month PFS (primary). Secondary outcomes include ORR, 12-month OS, PFS and OS. |
| NCT01456078 | II | 177Lu-DOTATATE | G1-G2 Progressive NETs | Objective tumor response after cumulative kidney BED of 27 ± 2 Gy. Secondary outcome, objective tumor response after receiving a cumulative BED to the kidneys of 40 ± 2 Gy per RECIST 1.1 |
| NCT03454763 | II | 177Lu-DOTATATE | NETs of Any Stage | PFS and treatment emergent AEs (primary); 5-year DCR and OS (secondary) |
| NCT04029428 | II | 177Lu-DOTATATE ± 90Y-DOTATATE | G1-G3 GEP-NETs, Lung NETs, Pheochromocytoma and Paraganglioma, NET-CUP – with overexpression of somatostatin receptors | PFS (primary) and OS, performance status, cancer related symptoms, Hormonal overproduction symptoms, ORR, safety assessments, vital signs, BMI, and ECG analysis (secondary) |
| NCT03590119 | II | 177Lu-DOTATATE (intra-arterial) | G1/G2 NETs | Difference in post-treatment tumor to non-tumor concentration between intra-arterial and intravenous treated liver lobe at 24 hours (primary); difference in post-treatment tumor at 3 and 6 months, toxicity, uptake of 177Lu-DOTATATE in extrahepatic lesions at 24 hours (secondary) |
| NCT02230176 | II | 177Lu-DOTATATE vs sunitinib | Well-differentiated PNETs | 12-month PFS (primary) and OS, best responses per RECIST 1.1–48 months (secondary) |
Abbreviations: NETS, neuroendocrine tumors; PNET, pancreatic neuroendocrine tumor; G, grade; NET-CUP, neuroendocrine tumor-carcinoma of unknown primary; PFS, progression-free survival, RP2D, recommended phase 2 dose; OS, overall survival; DCR, disease control rate; ORR, objective response rate; AEs, adverse events; BED, biologic effective dose; BMI, body mass index; vs, versus; 177 Lu, lutetium-177; 90Y, yttrium-90; Gy, gray
5.2. Phase III trial
The NETTER-1 study was a randomized phase III trial in patients with progressive metastatic or unresectable well-differentiated midgut neuroendocrine tumors [34]. Two hundred and thirty-one patients were randomized to four treatments of 177Lu-DOTATATE every 8 weeks (each treatment followed by 30 mg of octreotide LAR with maintenance octreotide LAR 30 mg every 4 weeks after completion of PRRT) or 60 mg of octreotide every 4 weeks. The primary endpoint was PFS. Of the enrolled patients, 97% had small intestinal primary tumors while 3% had appendiceal or colonic primary tumors. Median progression-free survival (PFS) was not reached in the 177Lu-DOTATATE arm compared to 8.4 months in the high-dose octreotide arm (hazard ratio [HR] for disease progression or death 0.21, 95% confidence interval (CI), 0.13–0.33; p < 0.001). A planned interim analysis for OS was also conducted and the HR for death in the 177Lu-DOTATATE arm compared to the control arm was 0.4 (p = 0.004). While encouraging, this p-value did not meet the prespecified threshold for statistical significance at interim analysis (0.000085), and final OS data is awaited 5 years after the last patient randomization (or after 158 deaths). The data were not sufficiently mature to provide a median OS estimate in either treatment group. Response rates were 18% in the PRRT arm compared to 3% in the octreotide alone arm (p < 0.001). The most common adverse events in the 177Lu-DOTATATE treated patients were nausea (59%) and vomiting (47%) which were attributed in more than 65% of cases to the pre-treatment amino acids. Rates of grade 3 or 4 adverse events were similar between the two groups however hematologic events only occurred in the PRRT treated group. Grade 3/4 lymphopenia, thrombocytopenia and anemia occurred in 9%, 2% and 1% of patients, respectively.
In the first provided update on OS and PFS from the NETTER-1 study population, median OS in the high-dose octreotide arm was 27.4 months while still had not been reached in the 177Lu-DOTATATE arm [35]. The HR for PFS was unchanged from the HR presented in the initial publication.
A quality of life (QOL) analysis of the NETTER-1 trial was published separately [36]. In this study, QOL outcomes were measured using the QOL questionnaires QLQ C-30 and G.I. NET-21. Patients completed the questionnaires at baseline and every 12 weeks until progression. The primary endpoint of the study was time-to-QOL deterioration (TTD) which was counted if a patient experienced a ≥ 10-point reduction in QOL score. TTD was significantly prolonged in the 177Lu-DOTATATE arm compared to patients in the high-dose octreotide arm with regards to global health status (HR 0.41; p < .001), physical functioning (HR 0.52; p < .015), diarrhea (HR 0.47; p = .011), and fatigue (HR 0.62; p = 0 .03). In no QOL domains did the 177Lu-DOTATATE arm fare worse.
5.3. Cohort studies
Several large cohort studies of patients treated with 177Lu-DOTATATE have been reported which add more data for the treatment in GEP NET patients.
In one cohort analysis, 610 Dutch patients with midgut, foregut, hindgut and unknown primary NETs (51% had GEP NETs) received treatment with 177Lu-DOTATATE [37]. For the safety analysis and efficacy analysis, 582 and 443 patients had data available, respectively. Patients included in the safety analysis had received at least 100 mCi of 177Lu-DOTATATE. Grade 3/4 hematologic toxicities were experienced by 10% of patients; 5% experienced grade 3/4 thrombocytopenia, 5% experienced grade 3/4 leukopenia while 4% experienced grade 3 anemia. Grade 3/4 transaminase elevations were observed in 3% of patients while 0.4% of patients experienced grade 3/4 creatinine increases. Myelodysplastic syndrome and acute leukemia occurred in 1.5% and 0.7% of patients, respectively. In patients included in the efficacy analysis, the median PFS was 28 months, median TTP was 36 months and median OS was 63 months. The response rate in the entire patient group was 39% while the stable disease was achieved in 43% of patients. For the subgroup of patients with progressive disease at baseline (54% of efficacy cohort), median PFS was 30 months, median TTP was 36 months and median OS was 58 months.
A German cohort analysis reported on outcomes from 1048 patients who underwent PRRT with either 177Lu-DOTATATE, 90Y-DOTATOC or both therapies in an alternating fashion [38]. Of these patients, 74% had GEP NET primaries. In this analysis, 378 patients received monotherapy with 177Lu-DOTATATE. The average total treatment dose the patients received was 18.8 GBq. Median OS in the 177Lu-DOTATATE treated patients was 44 months while median PFS was 17 months. Patients treated with alternating radiolabeled SSAs experienced a median OS of 64 months with a median PFS of 24 months.
A smaller recent cohort study reported on the experience of 69 (66.7% PNET) well-differentiated G3 NET patients who received PRRT with either 177Lu-DOTATATE or 90Y-DOTATOC [39]. Of these patients, eight received therapy in the first-line setting, 25 in the second-line setting and 36 in the third-line setting. Time to event outcomes were stratified in these patients by Ki-67 >55% or ≤55%. Median PFS and median OS were 9.6 months and 19.9 months, respectively. Patients with tumors with a Ki-67 ≤55% experienced a median PFS of 11 months and median OS of 22 months. Patients with tumors with a Ki-67 >55% experienced a medians PFS of 4 months and a median OS of 7 months. In patients with tumors with strong somatostatin receptor avidity (SUV > 15) on pretreatment 68Ga-dotatate scans, the response rate was more pronounced. None of the treated patients experienced grade 3 or 4 hematologic toxicity or renal dysfunction.
Investigators have also sought to identify whether genomic signatures can identify the patients who will benefit from PRRT versus those who will not. A PRRT predictive quotient (PPQ) stratifying responders from non-responders was validated in 178 NET patients treated with 177Lu-DOTATATE across three cohorts [40]. The PPQ was developed in a cohort of Italian WD NET patients and validated in German and Dutch patient cohorts. The components of the PPQ include a blood-based NET transcript (which measures genes involved in metabolism and growth factor expression) along with a tumor immunohistochemistry score including Ki-67% and grade; the ultimate readout is interpreted as a binary score (PPQ + or PPQ −). The PPQ accurately predicted PRRT responders at both initial and final follow-up (100% at each timepoint). PRRT non-responders were predicted in initial (65%) and final (94%) follow up. In aggregate, at the final follow up, PRRT response was correctly predicted in 93% of patients. Median PFS differences between PPQ + and PPQ − patients were highly statistically significant (not reached versus 8 months, HR 36.4; p < .001). No differences were seen in PFS between PPQ + and PPQ − patients who did not receive PRRT.
6. Administration
Based on FDA’s product label, the recommended dose for 177Lu-DOTATATE is 7.4 GBq (200 mCi) given every 8 weeks for a total of four doses. The dose of 177Lu-DOTATATE can be reduced and/or the time interval between cycles can be extended to 16 weeks based on the patient’s tolerance [27]. There is currently limited evidence about the efficacy of additional PRRT treatments beyond the four doses. In small studies, PRRT retreatment (beyond the initial four cycles) was associated with lower tumor response rate and PFS in comparison to the initial treatment, but appears to be safe, especially if done with 177Lu-DOTATATE instead of 90Y-DOTATOC, and if a cumulative activity does not exceed 45 GBq [41–43]. The patients who are considered for additional PRRT must have tolerated the initial four doses and shown a durable response of at least 1 year following initial treatment.
Since radiolabeled SSAs are reabsorbed by the proximal renal tubules, a concomitant administration of competing positively charged amino acids such as L-arginine and L-lysine is used to decrease renal radiation exposure [25,26].
The intravenous infusion of amino acids should contain between 18 and 24 g of L-arginine and L-lysine, with the most commonly used compounded solution consisting of 2.5% L-arginine and 2.5% L-lysine in 1 L 0.9% NaCl. This infusion starts 30 min prior to the administration of 177Lu-DOTATATE, and is given continuously until delivery of the whole amount, which takes about 4 h to deliver.
The patients are usually premedicated with anti-emetics 30 min before the recommended amino acid solution.
Based on FDA’s on-label use for 177Lu-DOTATATE, the long-acting and short-acting SSAs should not be given within 4 weeks and 24 h from 177Lu-DOTATATE, respectively. SSAs can then be restarted at least 4 h after the administration of 177Lu-DOTATATE.
Holding SSAs prior to PRRT aims at decreasing the risk of competition for the same tumoral somatostatin receptors. However, this is still a subject of investigation as these concerns may not be justifiable due to an increasing body of evidence showing that SSAs might improve the tumor-to-background ratio as seen on 68Ga-DOTATATE PET [44–46].
7. Emerging therapeutics
One of the limitations of 177Lu-DOTATATE and 90Y-DOTATOC is the need to administer multiple doses in order to achieve the anti-tumor effect with tolerable toxicity. Lutetium-177-1, 4, 7, 10-tetra-azacyclododecane-1, 4, 7, 10-tetraacetic acid-Evans blue-Tyr3-octreotate (177Lu-DOTA-EB-TATE) represents a long-lasting SSA which may circumvent this necessity. A phase I Chinese study of 177Lu-DOTA-EB-TATE explored safety and dosimetry of a single dose of the drug [47]. Of the 8 recruited patients, 5 received 177Lu-DOTA-EB-TATE while 3 received 177Lu-DOTATATE; all patients underwent serial whole-body planar and single-photon emission computed tomography (SPECT)-CT scans. Compared to 177Lu-DOTATATE, patients treated with the investigational agent experienced extended blood circulation and a 7.9-fold increase of tumor dose delivery. Patients treated with 177Lu-DOTA-EB-TATE also experienced increased dose delivery to the kidneys (3.2 fold) and bone marrow (18.2 fold) with no noted adverse events.
177Lu-DOTATATE and 90Y-DOTATOC represent radiolabeled somatostatin receptor agonists however radiolabeled somatostatin antagonists are also being developed. 177Lu-OPS201 (also known as 177Lu DOTA-JR11 or Satoreotide tetraxetan) is one of the antagonists that is now being tested in clinical trials [NCT02592707, NCT03773133]. In vitro experiments comparing 177Lu-OPS201 to 177Lu-DOTATATE in osteosarcoma cells transfected with somatostatin receptor 2, demonstrated that the former compound accumulates at cell membrane surfaces rather than being internalized and elicits more double-stranded DNA breaks (as measured by 53BP1 and DAP1) [48]. In vivo experiments in H69 xenografts revealed that mice treated with 177Lu-OPS201 compared to 177Lu-DOTATATE had longer periods of tumor stabilization (45 ± 7 days vs 41 ± 2 days) and longer median survivals (71 days vs 61 days). In a small pilot study utilizing a crossover design, four patients received treatment with both 177Lu-OPS201 and 177Lu-DOTATATE [49]. Patients underwent whole-body imaging and SPECT/CT imaging post-treatment (for pharmacokinetic data) and underwent 68Ga-DOTATATE to assess response. Patients were noted to have increased intra-tumor radioactivity in conjunction with increased intra-tumor residence times during treatment with 177Lu-OPS201 compared to during treatment with 177Lu-DOTATATE; this corresponded to a tumor dose which was 1.7–10.6 times greater with the somatostatin receptor antagonist. These findings led to a phase I study of 177Lu-OPS201 in 20 (45% PNET) refractory well-differentiated NET patients [50]. Patients were treated with 2 cycles of the agent at 3-month intervals. Of these patients, 6 were treated with 1 cycle while 14 were treated with 2 cycles. Grade 4 hematologic toxicity was observed in 4/7 (57.1%) patients after cycle 2, necessitating a protocol amendment to limit total bone marrow exposure to 1 gray and reduce the cycle 2 dose by 50%. Response rates were 45% (1 complete response) and disease control was observed in 85% of patients. Median PFS was 21 months.
Another class of compounds which are currently being studied in clinical trials are miniaturized drug conjugates. One of the compounds that are furthest advanced in development is PEN-221. PEN-221 is a SSA conjugated to the chemotherapeutic agent mertansine (DM1), which is a microtubule inhibitor [51]. Findings from the dose-escalation portion of the phase I/II study of PEN-221 in NET and small cell lung carcinoma (SCLC) patients were presented at ASCO 2018 [52]. Twenty-three patients (22 with NET, 1 with SCLC) were treated and the maximum-tolerated doses (MTD) was established as 18 mg intravenously (IV) every 3 weeks. The most frequent adverse events seen in ≥20% of patients were fatigue, nausea, diarrhea, vomiting and abdominal pain. Among 15 patients who were evaluable for response, 11 achieved stable disease by week 9. In 8 of these 11 patients, stable disease was sustained for 18–45 weeks; in 3 patients, tumor shrinkage was observed. Expansion cohorts in GEP NETs and SCLC are currently enrolling.
A description of the characteristics of 177Lu-DOTATATE and several of its competitor radiolabeled SSAs and somatostatin antagonists are described in Table 3.
Table 3.
Characteristics of 177Lu-DOTATATE and competitor radiolabeled SSAs and somatostatin antagonists.
| Compound | Peptide | Radionuclide | Unique Characteristics |
|---|---|---|---|
| 177Lu-DOTATATE | Octreotate | 177Lu | Compared to 90Y-DOTATOC, it has greater tumor uptake and a reduced risk of toxicity due to the medium energy B-emitting character of 177Lu. This agent has randomized phase III data while no other radiolabeled SRA has such data yet. |
| 90Y-DOTATOC | Octreotide | 90Y | 90Y is a high energy B-emitter with a potential ability to be more effective than 177Lu for larger metastases. This was one of the first radiolabeled SSAs to be trialed in humans. |
| 111In-DOTATOC | Octreotide | 111In | 111In emits γ rays and conversion electrons. It was the initial radiolabeled SSA trialed in humans therapeutically after initially being used at lower doses for diagnostic purposes. |
| 177Lu-DOTA-EB-TATE | Octreotate | 177Lu | The Evans Blue modification of the somatostatin analog enables reversible binding to albumin which increases the circulating half-life of the radiolabeled SSA and increases its tumor exposure. |
| 177Lu-OPS201 | OPS201 | 177Lu | OPS201 is a somatostatin receptor 2 antagonist (which is not internalized) and may result in increased DNA damage than 177Lu-DOTATATE. |
Abbreviations: 177Lu, lutetium-177; 90Y, yttrium-90; SSA, 111In, indium-111; SSA, somatostatin receptor agonist
8. Regulatory affairs
177Lu-DOTATATE was approved by the European Medicines Agency (EMA) on 26 September 2017 for patients with well-differentiated GEP NETs who progressed on first-line SSA therapy. The therapy gained FDA approval for the same indication in GEP NET patients on 26 January 2018. On 7 February 2019 Health Canada approved 177Lu-DOTATATE for the same indication in GEP NET patients.
9. Conclusion
The approval of 177Lu-DOTATATE for the treatment of patients with well-differentiated GEP NETs added a potent treatment option for patients with disease progressive on SSAs. Beyond carrying the potential to elicit meaningful cytoreduction, which is rare among approved treatments for these tumors, the greatest promise of the therapy appears to be its ability to prolong disease progression in patients. The therapy also significantly improves patient QOL outcomes, which is not always the case even for active anti-cancer therapeutics. The short-term hematologic, gastrointestinal, hepatic and cosmetic (alopecia) side effects of the treatment are transient while the long-term risks of myelodysplastic syndrome and acute myeloid leukemia are rarer, occurring in roughly 2% and 0.7% of treated patients, respectively. Where 177Lu-DOTATATE fits in the treatment sequence for patients with well-differentiated GEP NETs, which patients should receive the treatment, and whether it should be administered as monotherapy or in combination with other agents remain active questions for researchers in the field. Given the promise demonstrated by 177Lu-DOTATATE, other radiolabeled SSAs and somatostatin antagonists such as 177Lu-DOTA-EB-TATE and 177Lu-OPS201 are being studied in ongoing clinical trials. These may offer theoretical benefits over 177Lu-DOTATATE with regards to treatment schedule and intra-tumoral uptake, however, it is still too early to tell whether they will improve outcomes for patients.
10. Expert opinion
177Lu-DOTATATE has been a transformative therapy for many patients with progressive, somatostatin-receptor expressing well-differentiated GEP NETs. However, many questions remain regarding optimal timing and sequence of treatment with 177Lu-DOTATATE and other PRRT compounds. The NETTER-2 study will compare 177Lu-DOTATATE in combination with standard-dose octreotide (30 mg) to high-dose octreotide (60 mg) as a first-line treatment in patients with well-differentiated grade 2 and 3 GEP NETs (Ki-67 range from 10%-55%) (NCT03972488). The COMPETE study is randomizing patients with progressive nonfunctioning GEP-NETs to receive 177Lu-DOTATOC versus everolimus. Other questions raised by investigators are whether 177Lu-DOTATATE is the most potent type of PRRT and can it be combined with other agents to increase its effectiveness against recalcitrant lesions. Different radiolabeled SSAs and somatostatin antagonists such as 177Lu-DOTA-EB-TATE and 177Lu DOTA-OPS201 are being tested in current clinical trials based upon pre-clinical and dosimetry studies suggesting advantages to each compound over 177Lu-DOTATATE. With regards to the former, the albumin-binding moiety of the compound slows peripheral blood clearance and only a single dose of treatment may be necessary to achieve its therapeutic effect. With regards to the latter, somatostatin receptor antagonists may create more DNA damage in NETs compared to SSAs. Further studies such as CONTROL NETS are exploring whether 177Lu-DOTATATE is most effective alone or in combination with cytoreductive treatments such as capecitabine and temozolomide to elicit a maximum anti-tumor response.
Article highlights.
177Lu-DOTATATE is a radiolabeled somatostatin agonist (SSA), which is approved for the treatment of somatostatin receptor positive well-differentiated gastroenteropancreatic (GEP) neuroendocrine tumor (NET) patients in Europe, the United States, and Canada.
177Lu-DOTATATE belongs to a class of therapeutic agents called peptide receptor radionuclide therapy (PRRT). In the context of NETs, each agent includes a peptide, which is an SSA or somatostatin receptor antagonist, a chelator which serves as a linking molecule, and a specific radioisotope.
Radiolabeled SSAs have been trialed in Europe since 1994. The initial radiolabeled SSAs tested in phase I studies were 111In-DOTATOC (very weakly cytotoxic) and 90Y-DOTATOC. 177Lu-based PRRT was shown to have a more favorable therapeutic index than 90Y, particularly with respect to renal toxicity. Octreotate combined with the chelator DOTA (DOTATATE) showed increased somatostatin receptor 2 avidity and longer intra-tumoral radioactivity residence based on preclinical and dosimetry studies, respectively.
The NETTER-1 trial randomized midgut NET patients to four treatments of 177Lu-DOTATATE followed by standard-dose octreotide LAR or high-dose octreotide LAR. It is the only reported randomized phase III study involving any radiolabeled SSA. In the 177Lu-DOTATATE arm, progression-free (PFS) was markedly prolonged compared to the high-dose octreotide arm: hazard ratio [HR] 0.21; p < 0.001.
Quality of life (QOL) was analyzed on the NETTER-1 study using the European Organisation for Research and Treatment of Cancer (EORTC) questionnaires. Time to deterioration was significantly prolonged in the 177Lu-DOTATATE arm compared to patients in the high-dose octreotide arm with regards to global health status, physical functioning, diarrhea and fatigue.
Other radiolabeled SSAs and somatostatin antagonists which carry theoretical advantages over 177Lu-DOTATATE are now being tested in early safety-focused clinical trials. Two notable agents include the SSA 177Lu-DOTA-EB-TATE and the somatostatin receptor antagonist 177Lu-OPS201. 177Lu-DOTA-EB-TATE has demonstrated increased intra-tumoral radioactivity uptake preclinically and a longer plasma half-life due to its albumin binding moiety, compared to 177Lu-DOTATATE. 177Lu-OPS201 has demonstrated an increased capacity to elicit DNA damage preclinically and increased intra-tumor radioactivity uptake in a small pilot study clinically, compared to 177Lu-DOTATATE.
Ongoing studies are exploring whether PRRT is more effective in earlier treatment lines in GEP NET patients, whether additional cytoreductive treatments should be added to PRRT to improve its anti-tumor efficacy and what other patients (such as those with paragangliomas, pheochromocytomas, meningiomas, lung NETs) may benefit clinically from PRRT.
Acknowledgments
Funding
Vanderbilt clinical oncology research career development award: 5K12CA090625-19.
Footnotes
Declaration of interest
S. Das has received honoraria from Targeted Oncology, Lexicon, Medsphere and Clarivate Analytics. J. Strosberg has received honoraria from Novartis, Lexicon and Ipsen. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3(10):1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• An important article which discussed the evolving epidemiology of NETs in the US.
- 2.Oronsky B, Ma PC, Morgensztern D, et al. Nothing but NET: a review of neuroendocrine tumors and carcinomas. Neoplasia. 2017;19 (12):991–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reubi JC. Somatostatin and other peptide receptors as tools for tumor diagnosis and treatment. Neuroendocrinology. 2004;80 (Suppl 1):51–56. [DOI] [PubMed] [Google Scholar]
- 4.Rinke A, Muller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27(28):4656–4663. [DOI] [PubMed] [Google Scholar]; • The study which demonstrated the anti-proliferative effect of octreotide on patients with NET midgut tumors.
- 5.Caplin ME, Pavel M, Cwikla JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371 (3):224–233. [DOI] [PubMed] [Google Scholar]; • The study which demonstrated the anti-proliferative effect of lanreotide in patients with GEP NET tumors.
- 6.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao JC, Fazio N, Singh S, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387(10022):968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):501–513. [DOI] [PubMed] [Google Scholar]
- 9.Waldherr C, Pless M, Maecke HR, et al. The clinical value of [90Y-DOTA]-D-Phe1-Tyr3-octreotide (90Y-DOTATOC) in the treatment of neuroendocrine tumours: a clinical phase II study. Ann Oncol. 2001;12(7):941–945. [DOI] [PubMed] [Google Scholar]
- 10.Otte A, Herrmann R, Heppeler A, et al. Yttrium-90 DOTATOC: first clinical results. Eur J Nucl Med. 1999;26(11):1439–1447. [PubMed] [Google Scholar]; •• The initial phase I study with 90Y-DOTATOC which suggested that the agent could both be safely administered in patients with well-differentiated NETs and that there was an efficacy signal from this type of therapy.
- 11.Valkema R, De Jong M, Bakker WH, et al. Phase I study of peptide receptor radionuclide therapy with [In-DTPA]octreotide: the Rotterdam experience. Semin Nucl Med. 2002;32(2):110–122. [DOI] [PubMed] [Google Scholar]
- 12.Anthony LB, Woltering EA, Espenan GD, et al. Indium-111-pentetreotide prolongs survival in gastroenteropancreatic malignancies. Semin Nucl Med. 2002;32(2):123–132. [DOI] [PubMed] [Google Scholar]; •• One of the initial cohort analyses to suggest the potential PRRT with 111In to prolong overall survival in patients with progressive GEP NET patients.
- 13.Barone R, Borson-Chazot F, Valkema R, et al. Patient-specific dosimetry in predicting renal toxicity with (90)Y-DOTATOC: relevance of kidney volume and dose rate in finding a dose-effect relationship. J Nucl Med. 2005;46(Suppl 1):99S–106S. [PubMed] [Google Scholar]
- 14.Strosberg JR, Fine RL, Choi J, et al. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer. 2011;117(2):268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fine RL, Gulati AP, Krantz BA, et al. Capecitabine and temozolomide (CAPTEM) for metastatic, well-differentiated neuroendocrine cancers: the pancreas center at Columbia University experience. Cancer Chemother Pharmacol. 2013;71(3):663–670. [DOI] [PubMed] [Google Scholar]; • A publication which provided one of the initial signals of activity of capecitabine plus temozolomide in NET tumors (PNET and small bowel NET). This would be a piece of the evidence which would lead to prospective evaluation of the treatment combination.
- 16.Owen DH, Alexander AJ, Konda B, et al. Combination therapy with capecitabine and temozolomide in patients with low and high grade neuroendocrine tumors, with an exploratory analysis of O (6)-methylguanine DNA methyltransferase as a biomarker for response. Oncotarget. 2017;8(61):104046–104056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Toubah T, Morse B, Strosberg J. Capecitabine and temozolomide in advanced lung neuroendocrine neoplasms. Oncologist. 2019. pii: theoncologist.2019-0361. doi: 10.1634/theoncologist.2019-0361. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papaxoinis G, Kordatou Z, McCallum L, et al. Capecitabine and temozolomide in patients with advanced pulmonary carcinoid tumours. Neuroendocrinology. 2019. doi: 10.1159/000502864. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 19.Chatzellis E, Angelousi A, Daskalakis K, et al. Activity and safety of standard and prolonged capecitabine/temozolomide administration in patients with advanced neuroendocrine neoplasms. Neuroendocrinology. 2019. doi: 10.1159/000500135. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 20.Sahu A, Jefford M, Lai-Kwon J, et al. CAPTEM in metastatic well-differentiated intermediate to high grade neuroendocrine tumors: a single centre experience. J Oncol. 2019;2019:9032753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogowski W, Wachula E, Gorzelak A, et al. Capecitabine and temozolomide combination for treatment of high-grade well-differentiated neuroendocrine tumour and poorly-differentiated neuroendocrine carcinoma. Retrospective analysis. Endokrynol Pol. 2019;70:313–317. [DOI] [PubMed] [Google Scholar]
- 22.Kam BL, Teunissen JJ, Krenning EP, et al. Lutetium-labelled peptides for therapy of neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2012;39(Suppl 1):S103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esser JP, Krenning EP, Teunissen JJ, et al. Comparison of [(177) Lu-DOTA(0),Tyr(3)]octreotate and [(177)Lu-DOTA(0),Tyr(3)]octreotide: which peptide is preferable for PRRT? Eur J Nucl Med Mol Imaging. 2006;33(11):1346–1351. [DOI] [PubMed] [Google Scholar]; • A paper which compared dosimetry studies in the same patients after 177Lu-DOTATOC and 177Lu-DOTATATE administration. It demonstrated that the DOTATATE-bound radionuclide had a longer residence times in NETs than the DOTATOC-bound radionuclide.
- 24.Mettler F, Giberteau M. Essentials of nuclear medicine and molecular imaging. 7th ed. Elsevier: Philadelphia (PA); 2019. [Google Scholar]
- 25.Jamar F, Barone R, Mathieu I, et al. 86Y-DOTA0-D-Phe1-Tyr3-octreotide (SMT487)-a phase 1 clinical study: pharmacokinetics, biodistribution and renal protective effect of different regimens of amino acid co-infusion. Eur J Nucl Med Mol Imaging. 2003;30 (4):510–518. [DOI] [PubMed] [Google Scholar]
- 26.Rolleman EJ, Valkema R, de Jong M, et al. Safe and effective inhibition of renal uptake of radiolabelled octreotide by a combination of lysine and arginine. Eur J Nucl Med Mol Imaging. 2003;30(1):9–15. [DOI] [PubMed] [Google Scholar]
- 27.[cited 2019 Aug 20]. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/208700Orig1s000lbll.pdf.; •• The FDA drug label for 177Lu-DOTATATE which provides extensive details about the indications for the drug, pharmacokinetic data and safety plus efficacy findings from pertinent clinical trials.
- 28.Kwekkeboom DJ, Bakker WH, Kooij PP, et al. [177Lu-DOTAOTyr3] octreotate: comparison with [111In-DTPAo]octreotide in patients. Eur J Nucl Med. 2001;28(9):1319–1325. [DOI] [PubMed] [Google Scholar]
- 29.Imhof A, Brunner P, Marincek N, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol. 2011;29(17):2416–2423. [DOI] [PubMed] [Google Scholar]
- 30.Bodei L, Kidd M, Paganelli G, et al. The failure of clinical factors to predict long-term toxicity after PRRT in >800 patients - Is a genetic screen needed?. J Nucl Med. 2014;55:1948. [Google Scholar]
- 31.Kwekkeboom DJ, Bakker WH, Kam BL, et al. Treatment of patients with gastro-entero-pancreatic (GEP) tumours with the novel radiolabelled somatostatin analogue [177Lu-DOTA(0),Tyr3]octreotate. Eur J Nucl Med Mol Imaging. 2003;30(3):417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bodei L, Cremonesi M, Grana CM, et al. Peptide receptor radionuclide therapy with (1)(7)(7)Lu-DOTATATE: the IEO phase I-II study. Eur J Nucl Med Mol Imaging. 2011;38(12):2125–2135. [DOI] [PubMed] [Google Scholar]
- 33.Delpassand E, Samarghandi A, Zamanian S, et al. Peptide receptor radionuclide therapy with 177Lu-DOTATATE for patients with somatostatin receptor-expressing neuroendocrine tumors: the first US phase 2 experience. Pancreas. 2014;43(4):518–525. [DOI] [PubMed] [Google Scholar]
- 34.Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 trial of 177Lu-dotatate for midgut neuroendocrine tumors. N Engl J Med. 2017;376(2):125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The PRRT treatment arm demonstrated marked improvements in PFS and OS compared to high dose octreotide arm. The data from this study was used to support the EMA and FDA approvals for 177Lu-DOTATATE.
- 35.Strosberg JR, Wolin EM, Chasen BA, et al. First update on overall survival, progression-free survival, and health-related time-to-deterioration quality of life from the NETTER-1 study: 177Lu-Dotatate vs. high dose octreotide in progressive midgut neuroendocrine tumors. J Clin Oncol. 2018;36(15_suppl):4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strosberg J, Wolin E, Chasen B, et al. Health-related quality of life in patients with progressive midgut neuroendocrine tumors treated with (177)Lu-Dotatate in the phase III NETTER-1 trial. J Clin Oncol. 2018;36(25):2578–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This publication focused on the QOL endpoints examined in the NETTER-1 study. It highlighted the QOL improvements seen with the PRRT arm compared to the high dose octreotide arm across nearly every patient domain tested in the study.
- 37.Brabander T, van der Zwan WA, Teunissen JJM, et al. Long-term efficacy, survival, and safety of [(177)Lu-DOTA(0),Tyr(3)]octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clin Cancer Res. 2017;23(16):4617–4624. [DOI] [PubMed] [Google Scholar]; •• A large (>600 patient) cohort study which highlighted the efficacy and safety of 177Lu-DOTATATE in patients with GEP NETs and bronchial NETs. The study findings were also used to support the EMA and FDA approvals of 177Lu-DOTATATE.
- 38.Baum RP, Kulkarni HR, Singh A, et al. Results and adverse events of personalized peptide receptor radionuclide therapy with (90) Yttrium and (177)Lutetium in 1048 patients with neuroendocrine neoplasms. Oncotarget. 2018;9(24):16932–16950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J, Kulkarni HR, Singh A, et al. Peptide receptor radionuclide therapy (PRRT) in patients with progressive grade 3 neuroendocrine neoplasms (NEN). J Nucl Med. 2019;60(no supplement 1):560. [DOI] [PubMed] [Google Scholar]
- 40.Bodei L, Kidd MS, Singh A, et al. PRRT genomic signature in blood for prediction of (177)Lu-octreotate efficacy. Eur J Nucl Med Mol Imaging. 2018;45(7):1155–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forrer F, Uusijarvi H, Storch D, et al. Treatment with 177Lu-DOTATOC of patients with relapse of neuroendocrine tumors after treatment with 90Y-DOTATOC. J Nucl Med. 2005;46(8):1310–1316. [PubMed] [Google Scholar]
- 42.van Essen M, Krenning EP, Kam BL, et al. Salvage therapy with (177) Lu-octreotate in patients with bronchial and gastroenteropancreatic neuroendocrine tumors. J Nucl Med. 2010;51(3):383–390. [DOI] [PubMed] [Google Scholar]
- 43.Severi S, Sansovini M, Ianniello A, et al. Feasibility and utility of re-treatment with (177)Lu-DOTATATE in GEP-NENs relapsed after treatment with (90)Y-DOTATOC. Eur J Nucl Med Mol Imaging. 2015;42(13):1955–1963. [DOI] [PubMed] [Google Scholar]
- 44.Haug AR, Rominger A, Mustafa M, et al. Treatment with octreotide does not reduce tumor uptake of (68)Ga-DOTATATE as measured by PET/CT in patients with neuroendocrine tumors. J Nucl Med. 2011. ;52(11):1679–1683. [DOI] [PubMed] [Google Scholar]
- 45.Cherk MH, Kong G, Hicks RJ, et al. Changes in biodistribution on (68)Ga-DOTA-Octreotate PET/CT after long acting somatostatin analogue therapy in neuroendocrine tumour patients may result in pseudoprogression. Cancer Imaging. 2018;18(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ayati N, Lee ST, Zakavi R, et al. Long-acting somatostatin analog therapy differentially alters (68)Ga-DOTATATE uptake in normal tissues compared with primary tumors and metastatic lesions. J Nucl Med. 2018;59(2):223–227. [DOI] [PubMed] [Google Scholar]
- 47.Zhang J, Wang H, Jacobson O, et al. Safety, pharmacokinetics, and dosimetry of a long-acting radiolabeled somatostatin analog (177) Lu-DOTA-EB-TATE in patients with advanced metastatic neuroendocrine tumors. J Nucl Med. 2018;59(11):1699–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dalm SU, Nonnekens J, Doeswijk GN, et al. Comparison of the therapeutic response to treatment with a 177Lu-labeled somatostatin receptor agonist and antagonist in preclinical models. J Nucl Med. 2016;57(2):260–265. [DOI] [PubMed] [Google Scholar]; • A paper which highlighted the preclinical rationale for developing radiolabeled SSA antagonists and the potential advantages of utilizing this class of agents over SSA agonists.
- 49.Wild D, Fani M, Fischer R, et al. Comparison of somatostatin receptor agonist and antagonist for peptide receptor radionuclide therapy: a pilot study. J Nucl Med. 2014;55(8):1248–1252. [DOI] [PubMed] [Google Scholar]
- 50.Reidy-Lagunes D, Pandit-Taskar N, O’Donoghue J, et al. Phase I trial of well-differentiated neuroendocrine tumors (NETs)with radiolabeled somatostatin antagonist 177 Lu-Satoreotide Tetraxetan. Clin Cancer Res. 2019. DOI: 10.1158/1078-0432.CCR-19-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White BH, Whalen K, Kriksciukaite K, et al. Discovery of an SSTR2-targeting maytansinoid conjugate (PEN-221) with potent activity in vitro and in vivo. J Med Chem. 2019;62(5):2708–2719. [DOI] [PubMed] [Google Scholar]
- 52.Johnson ML, Meyer T, Halperin DM, et al. First in human phase 1/2a study of PEN-221 somatostatin analog (SSA)-DM1 conjugate for patients (PTS) with advanced neuroendocrine tumor (NET) or small cell lung cancer (SCLC): phase 1 results. J Clin Oncol. 2018;36(15_suppl):4097. [Google Scholar]


