Abstract
Purpose:
The aim of this study is to determine the effects of saccharide-containing excipients on the surface composition of spray-dried protein formulations and their matrix heterogeneity.
Methods:
Spray-dried formulations of myoglobin or bovine serum albumin (BSA) were prepared without excipient or with sucrose, trehalose, or dextrans. Samples were characterized by solid-state Fourier-transform infrared spectroscopy (ssFTIR), differential scanning calorimetry (DSC), size exclusion chromatography (SEC) and scanning electron microscopy (SEM). Protein surface coverage was determined by X-ray photoelectron spectroscopy (XPS), while conformational differences were determined by solid-state hydrogen/deuterium exchange with mass spectrometry (ssHDX-MS).
Results:
Structural differences were exhibited with the inclusion of different excipients, with dextran formulations indicating perturbation of secondary structure. XPS indicated sucrose and trehalose reduced protein surface concentration better than dextran-containing formulations. Using ssHDX-MS, the amount of deuterium incorporation and populations present were the largest in the samples processed with dextrans. Linear correlation was found between protein surface coverage and ssHDX-MS peak area (R2=0.853) for all formulations with saccharide-containing excipients.
Conclusions:
Lower molecular weight species of saccharides tend to enrich the particle surface and reduce protein concentration at the air-liquid interface, resulting in reduced population heterogeneity and improved physical stability, as identified by ssHDX-MS.
Keywords: Spray-drying, protein structure, surface chemistry, solid formulation, solid-state hydrogen/deuterium exchange with mass spectrometric analysis (ssHDX-MS)
Introduction
To address stability concerns for protein formulations in solution, additional drying steps are often used to better stabilize proteins in the solid state. The most common drying approach in the pharmaceutical industry is lyophilization, which freezes the solution, followed by primary and secondary drying under reduced pressure to remove water from the protein and produce a solid product.(1) Although this technique has been widely employed in pharmaceutical product development, it is a time-consuming batch process with low energy-usage efficiency, and may expose sensitive biologics to freezing-related stresses.(2) Consequently, alternative drying techniques have been explored for solid formulation development of pharmaceutical proteins.
Spray-drying is a processing method that has been widely used in the food industry(3, 4), and has increasingly attracted interest for biopharmaceutical manufacturing.(5) This technique has been successfully used to produce marketed biological products, such as Raplixa and Exubera.(6, 7) During spray-drying, a solution is fed into a spray nozzle, where liquid is atomized into droplets that are briefly exposed to a drying gas at a set temperature. These droplets are rapidly dried into solid particles by evaporative diffusion of water, and then collected by a cyclone. As with lyophilization, proteins are subjected to additional stresses during spray-drying, which include high temperature, dehydration, and air-liquid interfacial stresses, among others.(8)
Exposing proteins to these stressors can lead to deleterious conformational changes which affect drug product stability, depending on the extent and length of exposure to each stress. One of the most problematic stresses in spray-drying is exposure of the protein to the air-liquid interface(9). The amphiphilic nature of the protein can lead to adsorption at the surface, leading to higher concentrations of protein at the interface than dispersed in solution.(10, 11) Protein migration to the surface can cause exposure of the hydrophobic core in order to align at the interface. This unfolding leads to increased denaturation and protein damage, which increases the tendency for aggregation to occur during drying.(10)
To reduce this stress, excipients, such as surfactants, can be used to directly compete with the protein for adsorption at the surface.(12) Less explored are the effects of hydrogen-bonding excipients, such as sugars, and the effects of excipients with increasing molecular weight, on reducing the protein surface composition, as well as their effects on formulation heterogeneity. Previously, solid-state hydrogen/deuterium exchange with mass spectrometry (ssHDX-MS) has been used to demonstrate the effects of drying methods and formulation composition on population heterogeneity(13), which was found to correspond to physical stability.(14) In ssHDX-MS, a sample is stored in a desiccator containing a saturated salt solution in deuterium oxide (D2O). Exposed hydrogen atoms along the protein amide backbone are exchanged with deuterium, which can be monitored by mass spectrometry. This exposure and protein sensitivity to exchange provides information on the hydrogen bonding interactions in the formulation matrix, which may be related to the conformational stability of the protein and its physical stability.(15)
In the work reported here, the model proteins bovine serum albumin (BSA) and myoglobin were formulated with sugar excipients of low and high molecular weight to examine their impact on particle surface composition and protein conformational heterogeneity, following spray drying. The excipients selected were sucrose, trehalose, dextran of molecular weight 20,000 or 70,000 g/mol (listed as dextran 20K or 70K, respectively) based on their differences in size.(16, 17) The samples were characterized regarding secondary structure using solid-state Fourier-transform infrared spectroscopy (ssFTIR), the levels of formed aggregates by size exclusion chromatography (SEC), and glass transition temperature (Tg) by differential scanning calorimetry (DSC). In addition, surface composition was determined by X-ray photoelectron spectroscopy (XPS), and the population heterogeneity was determined by ssHDX-MS. Using these techniques, a correlation between surface composition and population heterogeneity was determined for the spray-dried proteins studied.
Materials and Methods
Materials
Bovine serum albumin, myoglobin from equine skeletal muscle, and sucrose (Bioextra, ≥95%, GC) were purchased from Sigma Aldrich (St. Louis, MO). D-(+)-trehalose dehydrate was purchased from Fisher Scientific (Fair Lawn, NJ). Dextrans of molecular weight 20,000 and 70,000 were procured from Afla Aesar (Ward Hill, MA) and Tokyo Chemical Industry Company (Portland, OR), respectively. Protein-containing solutions were dialyzed with a 2.5 mM phosphate buffer solution at 4°C for 24 hours using Slide-A-Lyzer™ dialysis cassettes from Thermo Scientific (Rockford, IL). Phosphoric acid was used when necessary to adjust solution pH to 6.8. Excipient-containing solutions in the same buffer conditions were prepared separately, and then diluted with the dialyzed solutions for a final concentration of 10 mg/mL protein and 10 mg/mL excipient.
Spray-drying
Samples were spray-dried using a Büchi Mini Spray Dryer B290 (New Castle, DE) with an inlet temperature of 100°C and outlet of 50–55°C. Solutions were atomized by an air volumetric flow rate of 600 L/h with a liquid feed rate of 2 mL/min. Dried powders were collected and distributed into 2R borosilicate glass vials (~4 mg per vial) and further dried in a vacuum oven at 30°C and 100 mTorr for 24 hours to reduce moisture content to ~2–3%. Additional drying was performed to reduce the effects that varying moisture may have on protein structure and stability.
Karl Fischer Titration for Moisture Content Analysis
Moisture content was determined by coulometric titration using an 831 KF Coulometer (Metrohm, Riverview, FL). Samples were reconstituted with 1 mL of methanol (anhydrous, septum sealed bottle DriSolv®, Sigma Aldrich, St. Louis, MO). The suspension was then injected into the cell and titrated with Riedel-de Haën Hydranal® Coulomat reagent (Hoechst Celanese Corp., Germany) for 5 min to reach end point for moisture determination. Samples were measured in triplicate.
X-ray Powder Diffraction
A Rigaku Smart Lab X-ray diffractometer (The Woodlands, TX) equipped with a Cu Kα X-ray source and Bragg-Brentano geometry was used to determine the solid form of the dried samples. Powders were pulverized onto a glass slide and loaded onto a slide holder for analysis. The diffraction intensity was measured between 5 and 40 degrees as a function of 2θ with a step size of 0.02° and scan rate of 5°/min.
Solid-state Fourier Transform Infrared Spectroscopy (ssFTIR)
Measurements were conducted with a Thermo Scientific Nicolet Nexus spectrometer (Waltham, MA) in the attenuated total reflectance mode. Samples were loaded onto a Smart iTR™ accessory and compressed against the diamond by a metal anvil. Spectra were collected in absorbance mode with 120 scans in the range of 800 to 4000 cm−1 with a 4 cm−1 resolution. Results were processed with OPUS 6.5 analysis software (Bruker, Billerica, MA) using baseline correction, smoothing, normalization, and second derivatization.
Modulated Differential Scanning Calorimetry (mDSC)
Dried powders (2–4 mg) were loaded into hermetic aluminum pans and sealed in a nitrogen-purged glovebox. Samples were loaded into a Discovery Series DSC 25 differential scanning calorimeter (TA Instruments, New Castle, DE), with an empty pan as reference. Powders were heated from 25°C to 225°C with a modulation of ±1°C every 120 s. Glass transition temperatures (Tg) were determined using the TRIOS software (v4.3.0, TA Instruments, New Castle, DE).
Size Exclusion Chromatography (SEC)
The extent of aggregation after spray-drying was determined using size exclusion chromatography (SEC). Solutions and dried powders were diluted to protein concentrations of 1 mg/mL and centrifuged for 10 min at 12,000 rpm and 4°C to remove insoluble aggregates. The supernatant was then removed and used for analysis. A high performance liquid chromatography system (HPLC, 1200 series, Agilent Technologies, Santa Clara, CA) was used for analysis with an isocratic flow of 50 mM sodium phosphate, 100 mM sodium chloride solution at pH 6.8 over 15 min at a 1 mL/min flow rate. Eluted samples were detected at 280 nm by a diode array detector. A TSKgel® G3000SWXL HPLC Column (Sigma Aldrich, St. Louis, MO) was used for size exclusion analysis.
X-ray Photoelectron Spectroscopy (XPS)
X-ray photoelectron spectroscopy (XPS) measurements were performed as described previously.(18) Briefly, the XPS data were collected by an AXIS Ultra DLD spectrometer (Kratos Analytical Inc., Manchester UK) using monochromatic Al Kα radiation (1486.6 eV). The surfaces of these samples are irradiated with an X-ray beam, which causes core-level electrons to be emitted with a specific kinetic energy unique to each element and chemical bonds with other atoms. The kinetic energy of the photoelectrons is measured by an energy analyzer. This technique has been previously used for protein surface coverage for other compounds and formulations.(19, 20)
High resolution and survey XPS spectra were obtained using constant pass energies of 20 and 160 eV, respectively. Data were processed using CasaXPS software. To determine the percentage elemental composition of the formulations, areas of the O 1s, N 1s, and C 1s peaks were calculated following a Shirley background subtraction. Corrections on the corresponding Scofield relative sensitivity factors and inelastic mean free path of the photoelectrons were also applied. The sulfur peaks were not used in these calculations due to its low concentration (<1%) and low relative sensitivity factor. The atomic percentage of nitrogen was used to determine protein surface coverage. Four replicates were measured and the results averaged.
Scanning Electron Microscopy (SEM)
Morphological differences of spray-dried powders were visualized using a Nova NanoSEM 200 system (Fei, Hillsboro, OR). Sample powders were mounted onto a sample holder and sputter-coated with carbon graphite. Particles were then analyzed under vacuum to determine morphological properties.
Solid-State Hydrogen/Deuterium Exchange with Mass Spectrometric Analysis (ssHDX-MS)
Vials containing the dried formulations were stored in a sealed desiccator containing a deuterium oxide (D2O) saturated with lithium chloride to maintain relative humidity at 11% and 25°C. At various time points (4, 12, 24, 48, 120, and 240 h), three vials per formulation were removed and the exchange reaction quenched by rapidly cooling the samples on dry ice. Vials were then stored at −80°C until analysis by mass spectrometry. To produce a fully deuterated control, protein was dissolved in a solution containing 3M guanidine hydrochloride, then aliquoted into a vial containing a 9:1 dilution of D2O:solution. This solution was then stored for 24 hours at 60° before quenching in a 4:1 solution of quench buffer (chilled 0.1% formic acid solution, pH 2.5) and immediately analyzed.
For mass spectrometric analysis to determine the extent of deuterium incorporation, samples were reconstituted in 2 mL of quench buffer, and 10 μL were injected into a protein microtrap (Michrom Bioresources, Inc., Auburn, CA). Using an HPLC system (1200 series, Agilent Technologies, Santa Clara, CA), samples were desalted in the microtrap column for 1.7 min with 90% water, 10% acetonitrile with 0.1% formic acid, followed by elution over 7 min to a gradient of 10% water, 90% acetonitrile with 0.1% formic acid. To minimize back exchange, columns were housed in a custom-built refrigeration unit with the temperature maintained at 4°C. Mass spectra of samples were determined using a 6520 qTOF mass spectrometer (Agilent Technologies, Santa Clara, CA) in the 200–2000 m/z range. Deconvolution of the samples to determine the mass range of the proteins was performed using the Mass Workstation Software (version B.04, Agilent Technologies, Santa Clara, CA). The maximum entropy function was used for this calculation, which converts the mass envelopes of the detected charge states into values that correspond to the different species present in the formulation.
The kinetics of deuterium incorporation was fitted using the mono-exponential model:
| (1) |
where D(t) is the number of deuterons exchanged at time t, Dmax is the maximum number of deuterons that can be incorporated into the sample, and k is the observed rate constant for deuterium incorporation.
Statistical Analysis
The effects of excipient choice on moisture content, surface composition, exchange kinetics, and physical stability were compared statistically using Prism Software (GraphPad, La Jolla, CA). A one-way ANOVA with Tukey’s Test was used for multiple comparisons among formulations.
Results
Moisture Content and Thermal Stability
All formulations had similar moisture content at the end of vacuum drying (Table I). The amorphous nature of these formulations is indicated by the XRPD patterns (Fig. 1).
Table I:
Moisture content and Tg for protein formulations (mean ± SD, n=3)
| Formulation | Moisture Content (%) | Tg (°C) |
|---|---|---|
| Myo Only | 2.5 ± 0.7 | ND* |
| Myo Suc | 2.5 ± 0.6 | 77.2 ± 2.8 |
| Myo Tre | 2.5 ± 0.7 | 112.9 ± 2.1 |
| Myo Dex 20K | 3.7 ± 0.6 | 210.8 ± 0.4 |
| Myo Dex 70K | 3.0 ± 0.3 | 219.2 ± 0.5 |
| BSA Only | 3.1 ± 0.3 | ND* |
| BSA Suc | 2.4 ± 0.1 | 74.7 ± 3.8 |
| BSA Tre | 2.5 ± 0.3 | 105.0 ± 1.3 |
| BSA Dex 20K | 3.9 ± 0.3 | 213.3 ± 0.3 |
| BSA Dex 70K | 2.8 ± 0.1 | 223.2 ± 0.3 |
ND indicates Tg could not be determined for formulation.
Figure 1:
X-ray powder diffraction patterns of BSA (A) and myoglobin (B) formulations. Samples were formulated without excipient or with sucrose, trehalose, dextran 20K, or dextran 70K.
For samples where Tg could be determined, sucrose-containing formulations had the lowest Tg values for both myoglobin and BSA, with values between 74–78°C. These values are consistent with those reported for amorphous sucrose.(21) Samples with trehalose also had Tg values consistent with previous reports (21), and were approximately 40°C higher than sucrose-containing systems. Dextran-containing formulations had the highest Tg values for both myoglobin and BSA, and were consistent with values for dextran alone.(22) Differences in glass transition between protein samples can be due to differences in moisture content, which has been demonstrated to impact glass transition.(23)
ssFTIR Spectroscopy for Secondary Structural Analysis
The amide I region of the ssFTIR spectra (Fig. 2) was evaluated to compare the secondary structure of each protein formulation. For BSA (Fig. 2A), sucrose- and trehalose-containing formulations had bands at 1653 and 1655 cm−1, respectively, which correspond to α-helical structure and is in general agreement with previously reported spectra.(24) For samples of BSA spray-dried without excipient, there is a band at 1653 cm−1, however there is a reduction in band intensity and increased broadening, which suggests structural perturbation.
Figure 2:
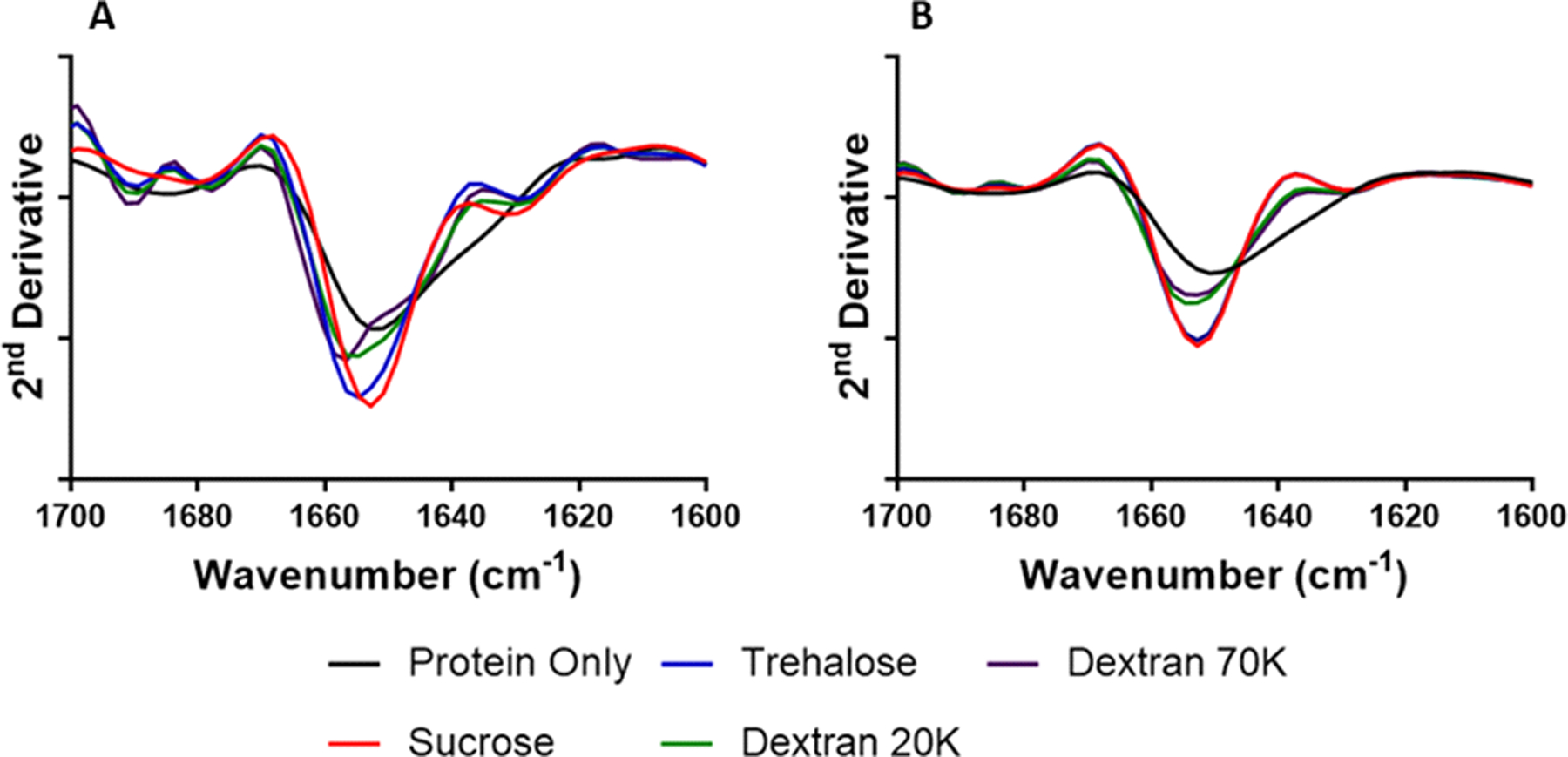
Solid-state FTIR spectra of formulated BSA (A) and myoglobin (B).
For myoglobin formulations (Fig. 2B) containing dextran 20K and 70K, bands were detected at 1656 and 1658 cm−1, respectively, with greater band broadening and intensity reduction than sucrose- and trehalose-containing samples, although less than for pure spray-dried BSA. In myoglobin formulations, sucrose- and trehalose-containing samples both had bands at 1653 cm−1 corresponding to α-helical structure.(25) For dextran-containing formulations, both showed a band shift to 1654 cm−1 and slight broadening relative to formulations containing lower molecular weight excipients. Similar to BSA, this suggests an increase in structural perturbation of the α-helices. For pure spray-dried myoglobin, a broad band at 1651 cm−1 was observed, indicating structural perturbation of the predominantly α-helical structure of the protein.
SEC for Monomer Content Determination Post-Drying
SEC was used to determine the level of aggregates formed after drying (Fig. 3). For BSA, the monomer content for all formulations was similar (~83–86%) with the exception of those spray-dried with dextran 70K (~78%). The presence of higher-order aggregates is expected under these buffering conditions, as BSA exists as a mixture of monomers and dimers at pH 6.8.(26) For myoglobin samples, all had similar monomer content (~97%).
Figure 3:
Size exclusion chromatography for monomer percentage of BSA and myoglobin formulations. *Indicates p<0.05.
Protein Surface Coverage by XPS
XPS was used to determine the protein composition on the top 10 nm of a particle surface (Fig. 4). For excipient-free samples of both BSA and myoglobin, concentrations of nitrogen were in agreement with the bulk composition of each protein (~16% nitrogen, Fig. 4A). The addition of excipients to each of the protein formulations resulted in reduced nitrogen composition at the surface, with sucrose and trehalose leading to a significantly greater reduction in surface protein composition as determined from XPS results relative to dextran-containing formulations (Tables S1–4). For the proteins studied, a greater reduction in surface protein concentration was observed in myoglobin as compared to BSA samples.
Figure 4:
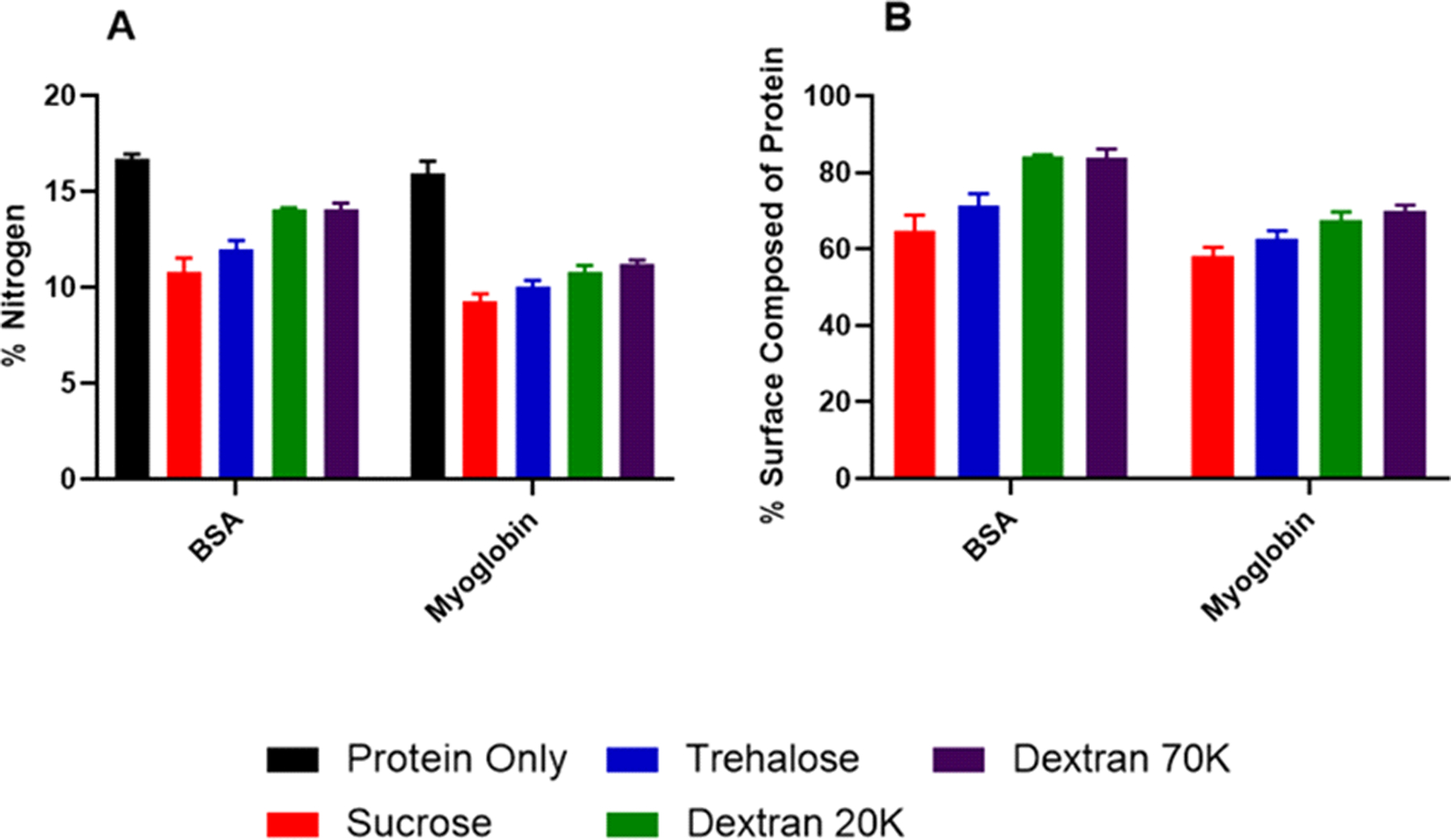
Quantification of X-ray photoelectron spectroscopy results for percentage of nitrogen on surface (A) and atomic percentage of protein found on the surface (B).
Due to the absence of nitrogen in the excipients, the amount of nitrogen present at the surface can be used to determine the atomic percentage composed of protein (Fig. 4B; Eqn. 2). Sucrose- and trehalose-containing formulations resulted in the greatest reduction of surface protein, while dextran-containing formulations had the least, regardless of differences in molecular weight. However, the concentration of protein at the surface suggests heterogeneous distribution of molecules in the dried particle, with surface enrichment. The estimated atomic percentage of protein was calculated using the equation:
| (2) |
where At. % is the atomic percent of the molecule, Wt. % is the weight percentage of the molecule in the formulation, and MWt. is the molecular weight of the molecule. In a 1:1 w/w ratio of protein:excipient, a homogeneous matrix would yield an estimated surface composition of 0.5, 23.1 and 51.3 atomic percentage protein for sucrose, trehalose, and dextran formulations of BSA, respectively. In myoglobin, expected protein composition values would be 2.0, 54.1, and 80.5% protein for sucrose, trehalose and dextran formulations, respectively. Therefore, there is an excess concentration of protein at the surface, suggesting that the protein is not dispersed homogeneously within the matrix.
ssHDX-MS for Protein Conformational Interactions
Matrix interactions in the formulations of dried particles were monitored by measuring deuterium incorporation as a function of time using ssHDX-MS (Fig. 5). To control varying factors that can affect the rate and extent of deuterium uptake(15, 27), samples were kept at a constant temperature and relative humidity.
Figure 5:
Kinetics of hydrogen/deuterium exchange in the solid state for BSA (A) and myoglobin (B).
For both protein formulations, samples without excipient had the highest amount of deuterium incorporation. This is expected due to lack of hydrogen-bonding interactions provided by the saccharide-based excipients used in other formulations in this study. Dextran-formulated samples with two different molecular weights had similar levels of exchange, with uptake being less than the unprotected samples. The high incorporation in these samples relative to those containing sucrose or trehalose is likely due to the large size and lack of molecular flexibility in dextran. Rigidity of dextran may prevent intermolecular hydrogen-bonding interactions with the protein in specific sites due to conformational differences(17) or phase separation.(28) Samples of sucrose and trehalose had similar levels of deuterium uptake, and were the lowest of the formulations studied.
Deconvoluted mass spectra of the formulations following deuterium exposure over 10 days were examined by ssHDX-MS to identify potential differences in shape and width of mass envelopes. This can provide information about formulation differences that affect the protein populations present. For BSA without excipients (Fig. 6A), increased deuterium exposure leads to lower resolution of the isoforms present under mass spectrometric analysis. This can be attributed to variability in the protection of proteins within the matrix, so that each of the isoforms will experience varying degrees of deuteration. Similar results are observed for formulations containing dextran 20K and 70K (Fig. 6D and E). For samples dried with sucrose or trehalose (Fig. 6B and C), the deconvoluted mass envelope is better preserved during the process, with some slight broadening with increasing deuterium incorporation.
Figure 6:
Deconvoluted mass spectra of formulations prepared by spray drying BSA without excipient (A) or with sucrose (B), trehalose (C), dextran 20K (D) or dextran 70K (E).
There are fewer isoforms in myoglobin samples (Fig. 7), and a clearer resolution of mass envelopes was achieved in ssHDX-MS. As observed for the excipient-free formulation (Fig. 7A), there is a distinct broadening of the mass envelope relative to the undeuterated sample. This suggests a distribution of deuteration and/or conformational states is present in less-protected samples. Similar to BSA, the protein samples processed with sucrose or trehalose (Fig. 7B and C) showed lower levels of deuteration and mass envelopes similar to the undeuterated state. Likewise, drying with either of the dextrans (Fig. 7D and E) resulted in peak broadening and greater deuterium uptake, although less than the samples without excipient.
Figure 7:
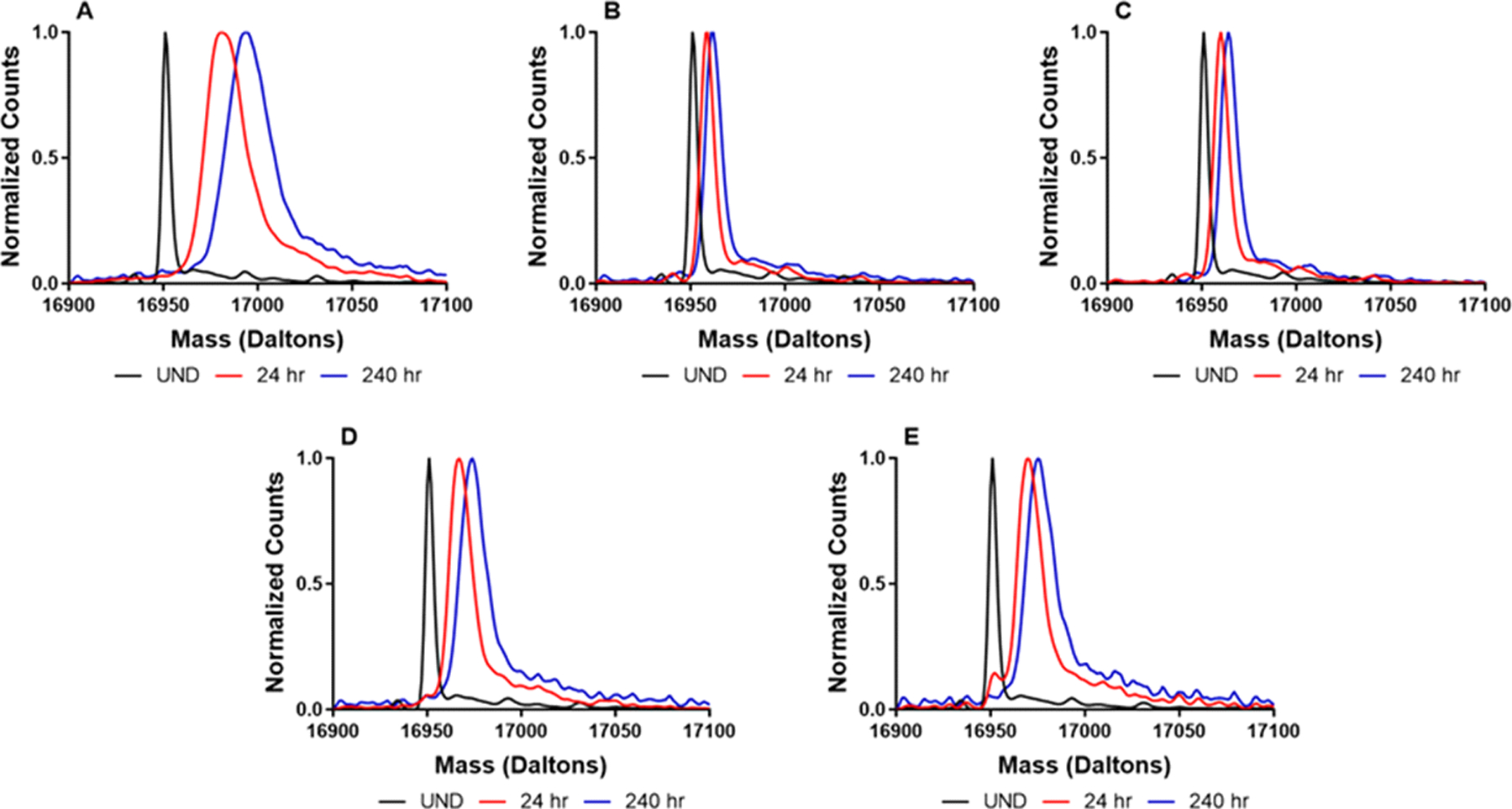
Deconvoluted mass spectra of formulations prepared by spray drying myoglobin without excipient (A) or with sucrose (B), trehalose (C), dextran 20K (D) or dextran 70K (E).
To better examine the effects of formulation and spray drying on protein populations, the peak areas of the normalized deconvoluted mass spectra as a percentage of an experimentally fully-deuterated sample were examined as a function of deuterium incorporation (Fig. 8). As in previous work, this ratio is thought to reflect differences in the distribution of protein conformational states and matrix interactions in the sample, and may be correlated with long-term physical stability.(13, 14). When formulated with an excipient, both BSA (Fig. 8A) and myoglobin (Fig. 8B) show increasing peak area with increasing deuterium incorporation. At a given level of deuterium incorporation, formulations containing dextran showed greater peak area than those formulated with sucrose or trehalose, consistent with greater population heterogeneity. This suggests that loss of the hydrogen-bonding interactions provided by the saccharide-based excipients leads to a greater number of conformational states and/or matrix interactions after drying.
Figure 8:

Peak areas of the deconvoluted mass envelope as a function of deuterium incorporation for BSA (A) and myoglobin (B). Peak areas are measured as a percentage of the area of the fully deuterated (FD) sample.
SEM for Particle Morphology
The morphology of the spray-dried particles was examined using scanning electron microscopy (Fig. 9). Pure spray-dried BSA (Fig. 9A) exhibited highly wrinkled particles, which is consistent with literature reports.(29) Particles spray-dried with this protein retain these morphological characteristics despite the addition of excipients.
Figure 9:
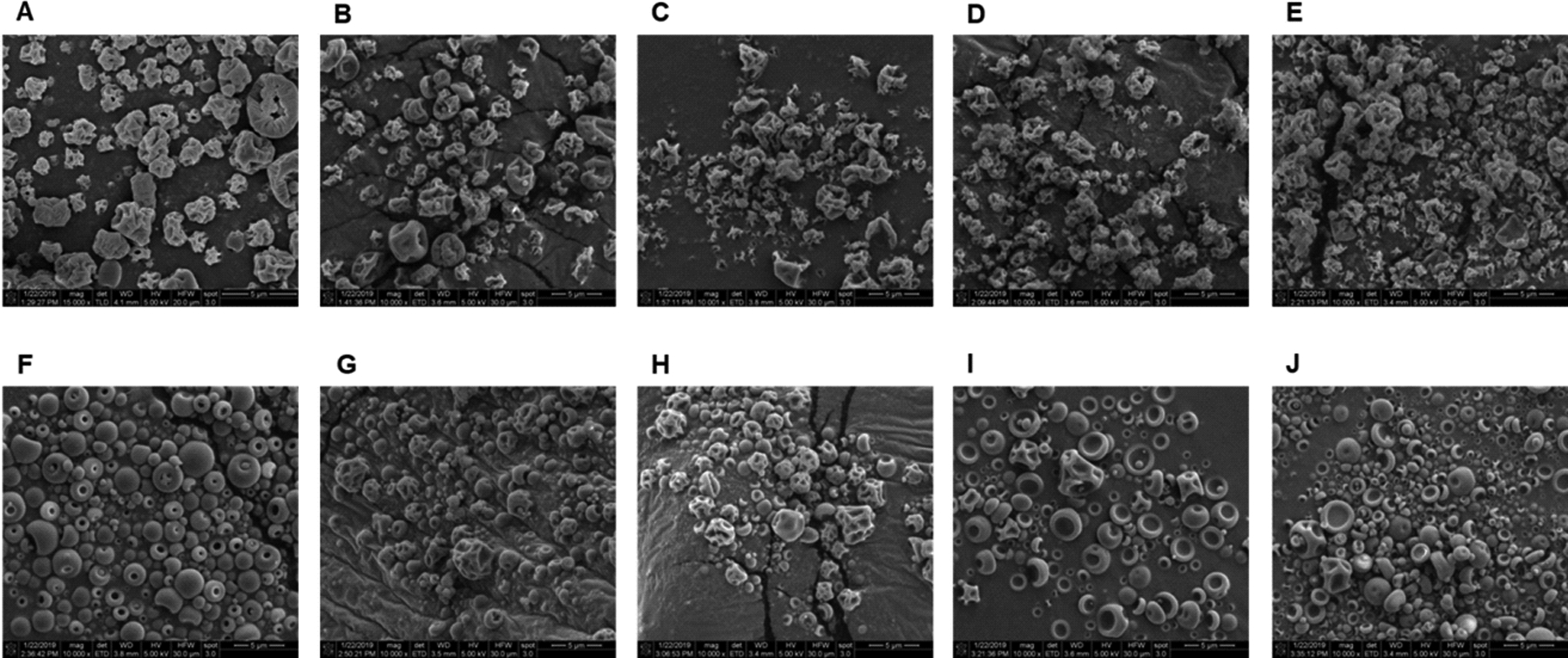
Scanning electron microscopy images of spray-dried particles. BSA formulations were processed without excipient (A) or with sucrose (B), trehalose (C), dextran 20K (D) or dextran 70K (E). Samples of myoglobin were formulated without excipient (F) or with sucrose (G), trehalose (H), dextran 20K (I) or dextran 70K (J).
Differences in particle morphology were observed for myoglobin formulations. Without excipient, spray-dried myoglobin (Fig. 9F) exhibits spherical particles with a dimpled surface, which is attributed to buckling due to pressure differences during drying. In sucrose- and trehalose-containing samples (Fig. 9G and H), highly wrinkled particles were formed. For both samples processed with dextran (Fig. 9I and J), the particles collapsed with a mushroom-cap shape.
Discussion
With the growing exploration of alternative drying approaches for biopharmaceuticals, understanding the effects of process and formulation on protein conformation, protein surface coverage, and population heterogeneity are important in developing robust products. Previously, our group has shown that drying method and formulation affect protein populations as measured by ssHDX-MS, which correlates well with protein aggregation on storage.(14) In the present study, ssHDX-MS was used together with XPS and other characterization techniques to explore the effects of formulation on population heterogeneity in protein formulations processed by spray drying.
The amide I region of ssFTIR spectra (Fig. 2) indicated secondary structural differences of protein formulations with and without excipients. For both BSA and myoglobin, the excipient-free formulations showed greater structural disorder than excipient-containing samples, which was expected due to lack of a stabilizing excipient. Inclusion of sucrose or trehalose resulted in better preservation of native structure, which is consistent with previous reports.(24, 25) In formulations containing dextran 20K or 70K, some structural perturbations were noted. As these dextran excipients are significantly larger than sucrose or trehalose, they may be less able to interact with the protein to stabilize the secondary structure.(17) In addition, dextran has been shown to undergo phase separation in some protein formulations after lyophilization.(28, 30) For this study, only a single glass transition temperature was observed for each formulation (Table I), which suggests a single phase, although there are challenges in identifying multiple phases based on assessment of calorimetric Tg.
Surface coverage of the proteins was measured by XPS in order to examine the effects of excipients on protein surface distribution following drying. For surface-sensitive proteins, reducing the concentration of protein at the air-liquid interface during drying can lead to reduced aggregation and help retain efficacy.(20) In the present study, higher molecular weight excipients (i.e., dextrans) resulted in greater protein concentrations on the surface when compared to formulations with lower molecular weight excipients (Fig. 4). As previously mentioned, this is probably due to the lack of molecular flexibility in the dextrans, which may be incapable of providing site-specific protection of the protein. This steric hindrance reduces the degree of interaction with the protein(17), whereby the interface adsorption of the protein can lead to a higher protein concentration at the surface and decreased physical stability.(10) For structurally smaller excipients such as sucrose and trehalose, increased protein interaction as compared to dextran can occur, which reduces the amount of protein that may adsorb to the droplet surface prior to drying.
All of the excipient-containing formulations had higher protein surface concentrations than the theoretical concentrations for a 1:1 w/w ratio of protein:excipient (Fig. 4B). The higher surface concentration of the biomolecules suggests slow diffusion of proteins during the drying process. This surface enrichment has been demonstrated to depend on the Peclet number, Pe, given by the equation:
| (3) |
where k is the evaporation rate of solvent and Di is the diffusivity of a component in the solvent system(31), in this instance a droplet. Under spray drying conditions, the rapid evaporation of the solvent and the differences in diffusion rates of the components in the formulation can lead to a heterogeneous distribution of materials in the dried particle. Proteins, which are surface active molecules with high molecular weight, have a propensity to concentrate at the air-liquid interface, producing a surface concentration greater than that in the bulk. This results in a final product with more unprotected protein on the surface exposed to the environment, which can result in reduced long-term stability.
The kinetics of deuterium incorporation by ssHDX-MS was fitted to the mono-exponential model (Equation 1) to quantify any differences in the rate of exchange in the various formulations (Table II). For BSA, there were no differences in rate constant, k, for deuterium incorporation among samples. However, BSA formulations containing dextran had higher Dmax values than those dried with sucrose or trehalose. This suggests the excipient used had a significant effect on the extent of deuterium uptake that can occur over time. In myoglobin samples, k was also similar across all formulations. As with BSA, Dmax values for myoglobin formulations were similar for sucrose and trehalose, which were lower than that of the dextran formulations.
Table II:
Deuterium exchange kinetics for protein formulations fitted to the mono-exponential model in Equation 1. (mean ± SD, n=3)
| Formulation | Dmax | k (h−1) |
|---|---|---|
| Myo Only | 34.2 ± 1.6 | 0.067 ± 0.015 |
| Myo Suc | 8.8 ± 0.4 | 0.043 ± 0.008 |
| Myo Tre | 9.6 ± 0.5 | 0.058 ± 0.013 |
| Myo Dex 20K | 19.6 ± 1.0 | 0.049 ± 0.012 |
| Myo Dex 70K | 20.6 ± 0.9 | 0.045 ± 0.009 |
| BSA Only | 197 ± 6.5 | 0.028 ± 0.005 |
| BSA Suc | 78.7 ± 5.1 | 0.011 ± 0.002 |
| BSA Tre | 85.7 ± 4.3 | 0.016 ± 0.002 |
| BSA Dex 20K | 139.3 ± 2.4 | 0.019 ± 0.003 |
| BSA Dex 70K | 155.8 ± 5.1 | 0.019 ± 0.003 |
Differences in ssHDX-MS peak areas suggest differences in the heterogeneity of the protein conformation and/or matrix interactions in the samples (Fig. 8). As with the structural perturbations observed in ssFTIR, formulations with higher molecular weight excipients showed increased heterogeneity by ssHDX-MS than those with lower molecular weight excipients. In formulations without any excipient, BSA showed less ssHDX-MS peak broadening consistent with a narrower distribution of states than samples spray-dried with excipients, albeit with higher deuterium incorporation. This inconsistency cannot be attributed to aggregation, as the monomer content was the same for samples analyzed by SEC (Fig. 3). The more homogeneous distribution of states for the excipient-free formulation may be due to consistent unfolding or partial unfolding of the protein throughout the matrix, or to other types of protein-protein intermolecular interactions, which may produce similar conformations while not providing increased hydrogen-bonding interactions.
To examine the relationship between protein surface concentration and population heterogeneity, the ssHDX-MS peak area was plotted as a function of the concentration at the interface determined by XPS (Fig. 10). Due to the differences in interactions that may be found in protein-only formulations, these samples were treated as outliers and excluded from the correlation. In formulations containing saccharide-based excipients, a clear linear trend exists between surface coverage and peak area, with higher surface concentrations corresponding to increased population heterogeneity. This may be due to the unprotected protein at the surface, which can unfold, leading to loss of conformation and an increase in observed heterogeneity. Interestingly, this correlation was found to be consistent across not only different formulations, but with the two different proteins as well. Together, the results suggest that lower molecular weight excipients capable of hydrogen-bonding to proteins in the solid state, also reduce surface enrichment of proteins, producing a more homogeneous distribution of protein states and better physical stability.
Figure 10:

Correlation of percentage surface composition of protein to peak area of the deconvoluted mass envelope relative to an experimentally fully-deuterated sample.
Conclusion
The effects of spray drying and sugar-containing excipients on protein structure, surface coverage, and population heterogeneity were examined by ssFTIR, XPS, and ssHDX-MS. For all techniques, distinct differences were observed in secondary structure, surface coverage, and conformation when processed without excipient and with excipients of different molecular weights. The concentration of protein at the surface of the dried particle showed a linear correlation with protein population heterogeneity (as indicated by ssHDX-MS peak area) when formulated with a sugar-containing excipient. The use of low molecular weight, hydrogen bond-replacing excipients during spray drying thus can reduce both the destabilizing stress at the surface and formulation heterogeneity, leading to improved physical stability and more homogeneous biopharmaceutical products.
Supplementary Material
Acknowledgements
This study was partially supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R01AI132681 and R01AI146160. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Carpenter JF, Chang BS, Garzon-Rodriguez W, Randolph TW. Rational Design of Stable Lyophilized Protein Formulations: Theory and Practice In: Carpenter JF, Manning MC, editors. Rational Design of Stable Protein Formulations: Theory and Practice. Boston, MA: Springer US; 2002. p. 109–133. [DOI] [PubMed] [Google Scholar]
- 2.Langford A, Bhatnagar B, Walters R, Tchessalov S, Ohtake S. Drying technologies for biopharmaceutical applications: Recent developments and future direction. Drying Technology. 2018;36(6):677–684. [Google Scholar]
- 3.Gaiani C, Mullet M, Arab-Tehrany E, Jacquot M, Perroud C, Renard A, Scher J. Milk proteins differentiation and competitive adsorption during spray-drying. Food Hydrocolloids. 2011;25(5):983–990. [Google Scholar]
- 4.Kim EHJ, Chen XD, Pearce D. Surface composition of industrial spray-dried milk powders. 2. Effects of spray drying conditions on the surface composition. Journal of Food Engineering. 2009;94(2):169–181. [Google Scholar]
- 5.Maa Y-F, Prestrelski SJ. Biopharmaceutical Powders Particle Formation and Formulation Considerations. Current Pharmaceutical Biotechnology. 2000;1(3):283–302. [DOI] [PubMed] [Google Scholar]
- 6.Lee G Spray-Drying of Proteins In: Carpenter JF, Manning MC, editors. Rational Design of Stable Protein Formulations: Theory and Practice. Boston, MA: Springer US; 2002. p. 135–158. [Google Scholar]
- 7.White S, Bennett DB, Cheu S, Conley PW, Guzek DB, Gray S, Howard J, Malcolmson R, Parker JM, Roberts P, Sadrzadeh N, Schumacher JD, Seshadri S, Sluggett GW, Stevenson CL, Harper NJ. EXUBERA®: Pharmaceutical Development of a Novel Product for Pulmonary Delivery of Insulin. Diabetes Technology & Therapeutics. 2005;7(6):896–906. [DOI] [PubMed] [Google Scholar]
- 8.Abdul-Fattah AM, Kalonia DS, Pikal MJ. The challenge of drying method selection for protein pharmaceuticals: Product quality implications. Journal of Pharmaceutical Sciences. 2007;96(8):1886–1916. [DOI] [PubMed] [Google Scholar]
- 9.Manning MC, Chou DK, Murphy BM, Payne RW, Katayama DS. Stability of Protein Pharmaceuticals: An Update. Pharmaceutical Research. 2010;27(4):544–575. [DOI] [PubMed] [Google Scholar]
- 10.Webb SD, Golledge SL, Cleland JL, Carpenter JF, Randolph TW. Surface adsorption of recombinant human interferon-γ in lyophilized and spray-lyophilized formulations. Journal of Pharmaceutical Sciences. 2002;91(6):1474–1487. [DOI] [PubMed] [Google Scholar]
- 11.Ameri M, Maa Y-F. Spray Drying of Biopharmaceuticals: Stability and Process Considerations. Drying Technology. 2006;24(6):763–768. [Google Scholar]
- 12.Patapoff TW, Esue O. Polysorbate 20 prevents the precipitation of a monoclonal antibody during shear. Pharmaceutical Development and Technology. 2009;14(6):659–664. [DOI] [PubMed] [Google Scholar]
- 13.Moussa EM, Wilson NE, Zhou QT, Singh SK, Nema S, Topp EM. Effects of Drying Process on an IgG1 Monoclonal Antibody Using Solid-State Hydrogen Deuterium Exchange with Mass Spectrometric Analysis (ssHDX-MS). Pharmaceutical Research. 2018;35(1):12. [DOI] [PubMed] [Google Scholar]
- 14.Wilson NE, Topp EM, Zhou QT. Effects of drying method and excipient on structure and stability of protein solids using solid-state hydrogen/deuterium exchange mass spectrometry (ssHDX-MS). International Journal of Pharmaceutics. 2019;567:118470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moorthy BS, Iyer LK, Topp EM. Mass spectrometric approaches to study protein structure and interactions in lyophilized powders. Journal of Visualized Experiments. 2015(98):52503–52503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lueckel B, Helk B, Bodmer D, Leuenberger H. Effects of Formulation and Process Variables on the Aggregation of Freeze-Dried Interleukin-6 (IL-6) After Lyophilization and on Storage. Pharmaceutical Development and Technology. 1998;3(3):337–346. [DOI] [PubMed] [Google Scholar]
- 17.Tonnis WF, Mensink MA, de Jager A, van der Voort Maarschalk K, Frijlink HW, Hinrichs WLJ. Size and Molecular Flexibility of Sugars Determine the Storage Stability of Freeze-Dried Proteins. Molecular Pharmaceutics. 2015;12(3):684–694. [DOI] [PubMed] [Google Scholar]
- 18.Bhujbal SV, Zemlyanov DY, Cavallaro A, Mangal S, Taylor LS, Zhou QT. Qualitative and Quantitative Characterization of Composition Heterogeneity on the Surface of Spray Dried Amorphous Solid Dispersion Particles by an Advanced Surface Analysis Platform with High Surface Sensitivity and Superior Spatial Resolution. Molecular pharmaceutics. 2018;15(5):2045–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adler M, Lee G. Stability and surface activity of lactate dehydrogenase in spray-dried trehalose. Journal of Pharmaceutical Sciences. 1999;88(2):199–208. [DOI] [PubMed] [Google Scholar]
- 20.Lechuga-Ballesteros D, Charan C, Stults CLM, Stevenson CL, Miller DP, Vehring R, Tep V, Kuo MC. Trileucine Improves Aerosol Performance and Stability of Spray-Dried Powders for Inhalation. Journal of Pharmaceutical Sciences. 2008;97(1):287–302. [DOI] [PubMed] [Google Scholar]
- 21.Simperler A, Kornherr A, Chopra R, Bonnet PA, Jones W, Motherwell WDS, Zifferer G. Glass Transition Temperature of Glucose, Sucrose, and Trehalose: An Experimental and in Silico Study. The Journal of Physical Chemistry B. 2006;110(39):19678–19684. [DOI] [PubMed] [Google Scholar]
- 22.Larsen BS, Skytte J, Svagan AJ, Meng-Lund H, Grohganz H, Löbmann K. Using dextran of different molecular weights to achieve faster freeze-drying and improved storage stability of lactate dehydrogenase. Pharmaceutical Development and Technology. 2019;24(3):323–328. [DOI] [PubMed] [Google Scholar]
- 23.Towns JK. Moisture content in proteins: its effects and measurement. Journal of Chromatography A. 1995;705(1):115–127. [DOI] [PubMed] [Google Scholar]
- 24.Fu K, Griebenow K, Hsieh L, Klibanov AM, Robert L. FTIR characterization of the secondary structure of proteins encapsulated within PLGA microspheres. Journal of Controlled Release. 1999;58(3):357–366. [DOI] [PubMed] [Google Scholar]
- 25.Sinha S, Li Y, Williams TD, Topp EM. Protein Conformation in Amorphous Solids by FTIR and by Hydrogen/Deuterium Exchange with Mass Spectrometry. Biophysical Journal. 2008;95(12):5951–5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barbosa LRS, Ortore MG, Spinozzi F, Mariani P, Bernstorff S, Itri R. The importance of protein-protein interactions on the pH-induced conformational changes of bovine serum albumin: a small-angle X-ray scattering study. Biophysical journal. 2010;98(1):147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sophocleous AM, Zhang J, Topp EM. Localized hydration in lyophilized myoglobin by hydrogen-deuterium exchange mass spectrometry. 1. Exchange mapping. Molecular pharmaceutics. 2012;9(4):718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heller MC, Carpenter JF, Randolph TW. Manipulation of Lyophilization-Induced Phase Separation: Implications For Pharmaceutical Proteins. Biotechnology Progress. 1997;13(5):590–596. [DOI] [PubMed] [Google Scholar]
- 29.Maa Y-F, Costantino HR, Nguyen P-A, Hsu CC. The Effect of Operating and Formulation Variables on the Morphology of Spray-Dried Protein Particles. Pharmaceutical Development and Technology. 1997;2(3):213–223. [DOI] [PubMed] [Google Scholar]
- 30.Mensink MA, Nethercott MJ, Hinrichs WLJ, van der Voort Maarschalk K, Frijlink HW, Munson EJ, Pikal MJ. Influence of Miscibility of Protein-Sugar Lyophilizates on Their Storage Stability. The AAPS Journal. 2016;18(5):1225–1232. [DOI] [PubMed] [Google Scholar]
- 31.Vehring R Pharmaceutical particle engineering via spray drying. Pharmaceutical research. 2008;25(5):999–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


