To the Editor:
Decontamination and reuse of personal protective equipment such as N95 respirators is not recommended but may be considered in crisis situations such as shortages encountered during the coronavirus disease 2019 (COVID-19) pandemic.1 A variety of decontamination technologies are under investigation and some vaporous hydrogen peroxide technologies have received emergency use authorization for respirator decontamination from the Food and Drug Administration.1 , 2 For many technologies, relatively long-treatment cycles are required and respirators must be transferred to a central in-house or off-site processing area. Thus, it is often not feasible to decontaminate respirators after each use. Rather, potentially contaminated N95 respirators may be reused multiple times with once daily or even less frequent decontamination.
To minimize the risks associated with reuse of respirators, it would be beneficial to provide rapid decontamination at the point-of-care between each reuse. Short cycles of ultraviolet-C light could be used, but efficacy may be limited against organisms associated with irregular, soft surfaces such as respirators.3 Steam treatment also has the potential to rapidly reduce non–spore-forming organisms.1 , 4 We previously reported that a 13-15-minute steam treatment was effective for decontamination of face masks and N95 respirators.4 Here, we investigated the efficacy of shorter steam treatments that could potentially allow decontamination between each use.
We studied 3M 1860 N95 respirators (3M; Saint Paul, MN) and medical procedure face masks (Precept; Arden, NC). The test organisms included methicillin-resistant Staphylococcus aureus (MRSA), Geobacillus stearothermophilus spores, and the nonenveloped, single-stranded RNA virus bacteriophage MS2.3 , 4 Ten-μL aliquots containing ∼106 colony-forming units (CFU) or plaque-forming units of the test organisms suspended in 8% simulated mucus were inoculated onto 1-cm2 areas on both the outer or inner surfaces of the respirators and face masks.3 , 4 The inoculated masks and respirators were subjected to 100°C steam treatments of 2, 10, or 30 seconds by placing them inside a steamer (Aroma; San Diego, CA) for the specified time during the steam cycle. After treatment, the inoculated sections were cut out and processed to quantify viable organisms.3 , 4 All tests were performed in triplicate. Log10 reductions were calculated in comparison to untreated controls. A reduction of 3-log10 or greater was considered effective for decontamination.3, 4, 5 To assess the impact on respirator performance, qualitative and quantitative (Portacount Respirator Fit Tester, TSI Incorporated, Shoreview, MN) fit testing was performed before and after N95 respirators were subjected to 20-30-second steam treatments.
To assess the real-world efficacy of rapid steam treatment, we collected used medical procedure masks from personnel. Two-cm2 sections of mask material were cut out before and after a 30-second steam treatment, processed as described previously, and plated on nonselective blood agar plates to quantify total bacterial counts. For plates with CFU too numerous to count, the CFU count was designated as 1,000 CFU.
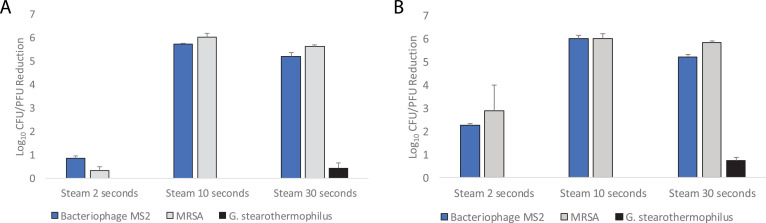
As shown in Figure 1 , the 10- and 30-second steam treatments met criteria for decontamination of bacteriophage MS2 and MRSA on N95 respirators, whereas the 2-second treatment did not. The steam treatments did not substantially reduce G. stearothermophilus spores. Similar results were obtained with inoculated medical procedure masks (data not shown). N95 respirators passed fit testing after 20-30-second steam treatments. After steam treatment, the respirators were slightly damp to touch, but this resolved within 5 minutes at room temperature or within 2 minutes when placed in a dry oven at 70°C.
Fig 1.
Efficacy of 100°C steam treatment for decontamination of methicillin-resistant Staphylococcus aureus (MRSA), bacteriophage MS2, and Geobacillus stearothermophilus spores inoculated on the outside surface (A) and inside surface (B) of 3M 1860 N95 respirators. Error bars indicate standard error.
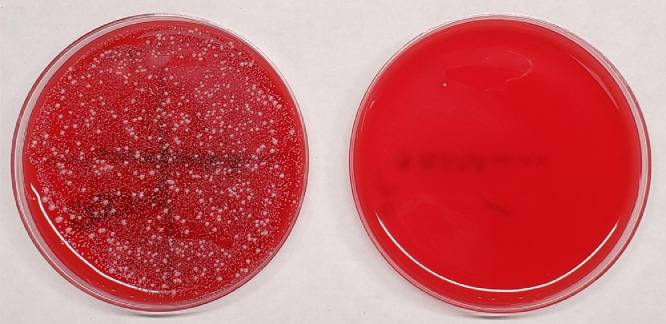
All 30 used medical procedure masks cultured were contaminated with bacteria with an average of 2.4 log10 CFU recovered, predominantly Streptococcus species and coagulase-negative staphylococci. Staphylococcus aureus was recovered from 3 (10%) masks. The 30-second steam treatment eliminated all bacteria from 29 of 30 (97%) masks. Figure 2 shows pictures before and after treatment for the one mask that had a positive culture after treatment with one colony of coagulase-negative staphylococci recovered.
Fig 2.
Pictures of blood agar culture plates showing organisms recovered before and after a 30-second 100°C steam treatment for the one mask of 30 tested that had a positive culture after treatment. Pretreatment the total colony-forming units (CFU) of bacteria were too numerous to count and Staphylococcus aureus was recovered. Post-treatment one colony of coagulase-negative staphylococci was recovered.
In summary, steam treatment resulted in rapid decontamination of bacteriophage MS2 and MRSA on N95 respirators and medical procedure masks. The reductions in bacteriophage MS2 met the current Food and Drug Administration Enforcement Policy for Face Masks and Respirators of a >3 log10 reduction of viruses, but the requirement for a >6 log10 inactivation of bacterial spores was not met.5 Nevertheless, steam treatment deserves further investigation because the short-treatment cycles and ease of use could allow for rapid decontamination of respirators or face masks at the point-of-care between each use. Twenty cycles of steam treatment did not adversely affect fit testing performance, consistent with previous reports that short cycles of steam treatment may have minimal effect on N95 filtration and fit performance.1 , 4 Further work is needed to assess the impact of short cycles of steam treatment on filtration efficiency and to develop technologies that could provide steam treatments for respirators and face masks in health care settings.
Footnotes
Conflicts of interest: C.J.D has received research grants from Pfizer, Clorox, and PDI. All other authors report no conflicts of interest relevant to this article.
This work was supported by the Department of Veterans Affairs.
References
- 1.Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html
- 2.3M Personal Safety Division. Disinfection of filtering facepiece respirators technical bulletin. 2020. Available at: https://multimedia.3m.com/mws/media/1824869O/decontamination-methods-for-3m-n95-respirators-technical-bulletin.pdf. Accessed May 7, 2020.
- 3.Cadnum JL, Li D, Redmond SN, John AR, Pearlmutter B, Donskey CJ. Effectiveness of ultraviolet-C light and a high-level disinfection cabinet for decontamination of N95 respirators. Pathog Immun. 2020;5:52–67. doi: 10.20411/pai.v5i1.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li DF, Cadnum JL, Redmond SN, Jones LD, Donskey CJ. It's not the heat, it's the humidity: effectiveness of a rice cooker-steamer for decontamination of cloth and surgical face masks and N95 respirators. Am J Infect Control. 2020;48:854–855. doi: 10.1016/j.ajic.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Food & Drug Administration. Enforcement policy for face masks and respirators during the Coronavirus Disease (COVID-19) public health emergency (Revised). Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents. Revised April 2020. Accessed May 4, 2020.