Highlights
-
•
Neurological manifestations has been reported in SARS-CoV2 infection recently.
-
•
SARS-CoV2 could spread to the brain hematogenously or through the cribriform plate.
-
•
Neurological symptoms can be due to systemic illness or viral invasion of the CNS.
Keywords: Coronavirus, SARS-CoV-2, COVID-19, Neurological manifestations, Encephalitis
Abstract
Background
Increasing research reports neurological manifestations of COVID-19 patients. SARS-CoV-2 shares homology with other human coronaviruses that have also had nervous system involvement.
Objective
To review the neurological aspects of SARS-cov2 and other coronavirus, including transmission pathways, mechanisms of invasion into the nervous system, and mechanisms of neurological disease.
Methods
We conducted a systematic review of articles in PubMed, SCOPUS and EMBASE data bases. Reviewed evidence is presented in sections of this manuscript which includes pathogenesis, neuro-invasion, encephalitis, Guillain-Barré, ADEM, multiple sclerosis, polyneuropathy, and cerebrovascular disease.
Results
A total 67 studies were included in the final analysis of experimental studies, case reports, series of cases, cohort studies, and systematic reviews related to neurological manifestations of SARS- CoV-2 and other human coronavirus infections.
The SARS-CoV-2 receptor is expressed in the nervous system. Common reported symptoms included hyposmia, headaches, weakness, altered consciousness. Encephalitis, demyelination, neuropathy, and stroke have been associated with COVID-19. Infection through the cribriform plate and olfactory bulb and dissemination through trans-synaptic transfer are some of the mechanisms proposed. Invasion of the medullary cardiorespiratory center by SARS-CoV-2 may contribute to the refractory respiratory failure observed in critically-ill COVID-19 patients.
Conclusion
An increasing number of reports of COVID-19 patients with neurological disorders add to emergent experimental models with neuro-invasion as a reasonable concern that SARS-CoV-2 is a new neuropathogen. How it may cause acute and chronic neurologic disorders needs to be clarified in future research.
1. Introduction
On March 11, 2020, the World Health Organization (WHO) declared the infection of coronavirus (CoV) severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) a pandemic [1]. Since being first identified in Wuhan, China [2], it has rapidly spread around the world, with more than 4,000,000 reported cases to date [3]. SARS-CoV-2 is very similar in structure and infection mechanism to other known coronaviruses, such as the SARS-CoV and Middle East respiratory syndrome (MERS) [4,5]. The respiratory system is the most commonly affected, but numerous experimental studies and case reports on these viruses have shown their potential neurotropism. According to observational studies, SARS-CoV-2 patients have presented with complaints of headache, nausea, vomiting, myalgia, dizziness [5], hypogeusia, hyposmia and impaired consciousness [6], symptoms that suggest involvement of the nervous system.
Although the exact mechanism by which SARS-CoV-2 penetrates the central nervous system (CNS) has not yet been established, two possibilities appear to offer the most likely explanations: 1) hematogenous spread of SARS-CoV-2 from systemic circulation to cerebral circulation, where the slower flow is conducive to the virus damaging the capillary endothelium and gaining access to the brain [7] and 2) dissemination through the cribriform plate and olfactory bulb [8].
Prior experimental models have shown that other coronaviruses can compromise the nervous system and the respiratory drive by directly targeting neurons located in the cardiorespiratory centers [[8], [9], [10]]. Preliminary observation of cases seen in the 2019 coronavirus disease (COVID-19) pandemic, however, suggests that the SARS-CoV-2 virus may have a higher affinity for CNS targets.
This review aims to create a systematic compilation of the neurological symptoms seen in these cases as well as reviewing possible transmission pathways of SARS-CoV-2. Finally, we will explore the mechanisms by which coronaviruses affect specific regions of the nervous system.
2. Methods
We searched PubMed, SCOPUS and EMBASE databases. We specifically screened studies that were published between January 1990 and April 2020 to ensure our results were relevant. The following research terms were used: Coronavirus, SARS, COVID-19, SARS-CoV-2, neurology, mechanism, axonal, polyneuropathy, stroke, cardiovascular disease, multiple sclerosis, neuroinvasion, acute disseminated encephalomyelitis (ADEM), myopathy, neuromuscular, Guillain–Barré syndrome (GBS), encephalitis, encephalopathy and symptoms. Restrictions were imposed to exclude studies without detailed methodological reporting. The publications that were not peer reviewed were also excluded from this study. PRISMA criteria were applied.
The screening of titles and abstracts was performed by the authors. The full texts were reviewed in a second screening. The papers were considered where a study was designated as a case report, cohort study, series of cases, ecological study, systematic review, metanalysis or clinical trial related to the neurological manifestations of coronavirus infections. We restricted our search to studies published in English.
3. Search results
Our literature search identified 324 abstracts, 80 of which were full text articles focused on the neurological manifestations of coronavirus infections. Among the 80 detailed full-text articles, 17 non-peer reviewed publications were excluded from the study and 6 studies were not available in full text. A total of 67 studies was included in the final analysis. Of these studies, 12 were systematic reviews, 15 were experimental model studies, 21 were series of cases, 3 were cases and controls and 16 were case reports. Some studies contributed to more than one section in this review. Detailed characteristics of the studies included are presented in Table 1, Table 2 .
Table 1.
Clinical research of non-experimental studies in human coronavirus.
| SARS | MERS | COVID-19 | HCV-229E | HCV-OC43 | |
|---|---|---|---|---|---|
| Polyneuropathy | Brynne et al.2011*. [61] Li-Kai et al. 2004**. [37] |
Kim et al. 2017** N = 4. [36] Algahtani et al. 2016*. [35] |
Ling Mao et al. 2020 **N:214. [6] Sedaghat et al.2020* [41]. Zhao et al.2020* [43]. Toscano et al.2020 **N:5 [44] Camdessanche et al 2020* [39] Alberti et al.2020* [46] Padroni et al.2020* [45] Virani et al. 2020* [47] |
||
| Demyelinating disease | Stewart et al. 1992**N = 32. [27] Arbour et al. 2000**N = 90. [25] |
Stewart et al. 1992**N = 32. [27] Murray et al. 1992**N = 22. [26] Yeh et al.2004*. [34] |
|||
| Encephalitis | Kwok-Kwong et al. 2004* [62]. | Arabi et al.2015** [63]. | Poydiadji et al. 2020*. [50] Ling Mao et al. 2020 ** N : 214. [6] |
||
| Stroke | Umapathi [52] et al.2004**N;206 | Algahtani et al. 2016* [35]. | Ling Mao et al. 2020 ** N:214. [6] Oxley et al. 2020**N: 5 [59] Avula et al. 2020**N: 4 [54] |
case report.
series of cases/observational studies.
Table 2.
Experimental and clinical studies in Alfa and Beta coronavirus with potential invasion to the nervous system.
| Viruses | Receptor | Human tissue expression | Studies suggesting neurotropism |
Associated neurological diseases |
|---|---|---|---|---|
| Alphacoronaviruses | ||||
| HCV-229E | APN | Glial cells, Neural cells, Pericytes, Astrocytes, Lung fibroblasts, Proximal renal tubule, Enterocytes, Granulocytes, Monocytes [64] | Lachane et al, 1998 * [64] | MS [25] |
| HCV-NL63 | ACE2 | Lung epithelium, Gut epithelium, Brain, Heart Kidney [15] | None | None |
| Betacoronaviruses | ||||
| HCV-OC43 | 9-O-acetylated sialic acid (9-O-Ac-Sia) | Junwei Niu et al, 2020 **. [65] Anna Nilson et al, 2020 *. [66] Mathieu Dubé et al 2018 **. [67] Jacomy H et al, 2006 ** [48]. N Arbour et al, 2000 *. [25] |
Encephalitis [66] MS [25] |
|
| SARS-CoV | ACE2 | Lung epithelium, Gut epithelium, Brain, Heart Kidney [15] | Netland et al, 2008 **. [8] Xu et al, 2005 *. [68] Gu et al, 2005 *. [69] Tsai et al, 2005*. [37] Ding et al, 2004 *. [9] |
Polyneuropathy [37,61] Myopathy [38] Stroke [52] |
| MERS-CoV | DPP4 | Pneumocytes, Renal tubular epithelial cells, Endothelial cells, T-cells, Placenta, Liver, Skeletal muscle, Heart, Brain, Pancreas [70] | Li Kai et al., 2016 **[10] Y. M. Arabi et al, 2015 *. [35] Algahatani et al, 2016 *15 |
Polyneuropathy [36] Encephalitis [63] Stroke [35] |
| SARS-CoV-2 | ACE2 | Lung epithelium, Gut epithelium, Brain, Heart Kidney [15] | Ling Mao et al., 2020 *. [6] | Polyneuropathy [6,39,41,42,43,44,45,46,47] Stroke [6,54,59] |
Observational study/case report.
Experimental studies.
4. Pathogenesis
SARS-CoV-2 has a spike protein surface unit 1 that has a high binding affinity to the human receptor angiotensin-converting enzyme 2 (ACE2) [11]. The increased expression of ACE2 in the epithelial cells in the lower respiratory tract facilitates viral entry by fusion with the cell membrane [12,13]. The expression of the ACE2 receptors likely explains the involvement of medullary structures by SARS-CoV-2, but is less likely to be an explanation for the involvement of the temporo-limbic structures by the virus. ACE2 is strongly expressed in the ventrolateral medulla and the nucleus of the tractus solitarius, two areas that are closely involved in the regulation of the respiratory cycle [14,15] (Table 1). However, the affinity of the virus for the olfactory bulb is likely mediated by a different, yet unidentified mechanism. In rodents, transnasal exposure to SARS-CoV resulted in the rapid detection of the virus in the piriform and infralimbic cortex, basal ganglia and the midbrain, all of which have direct neuronal connections with the olfactory bulb [8]. Once the virus was established in the brain, there was evidence of dissemination along the neurotransmitter pathways, such as the serotonergic dorsal raphe system or hematogenously through the Virchow–Robin spaces. Prior studies have shown that both SARS-CoV and MERS [10] directly induced neuronal death in the respiratory center in the medulla through an upregulation of IL-1, IL-6 and TNF alpha cytokine response, possibly through either an inflammatory response or autophagy [8,16]. However, these observations were conducted with SARS, and further studies will be needed to determine whether they are also applicable to the novel SARS-CoV-2 virus.
5. Progression of the virus in the nervous system
A neuronal dissemination model of coronavirus invasion, in which the virus infects a peripheral neuron and relies on the machinery of active transport, synaptic terminals and retrograde transport to the neuronal cell body in remote areas of the brain, has been postulated [7]. This trans-synaptic transfer mechanism is supported by studies involving the hemagglutinating encephalomyelitis virus strain 67 N (HEV-67 N), the first CoV strain that was found to invade the porcine brain [4].
Clinical data from 214 patients with COVID-19 demonstrated neurological symptoms in 36.4 % of patients. Neurological symptoms included headache, impaired consciousness, ataxia, acute cerebrovascular disease, seizures, hyposmia, hypogeusia and neuralgias. The data suggests that patients with more severe systemic presentations were more likely to have neurologic symptoms, such as acute cerebrovascular diseases (5.7 % vs. 0.8 %), impaired consciousness (14.8 % vs. 2.4 %) and skeletal muscle injury (19.3 % vs. 4.8 %), in comparison with those with milder forms of the infection [6].
Less specific “neurological-type” symptoms that are also common with other viral infections have also been reported with COVID-19. While their presence may suggest some degree of nervous system involvement, their significance is still unclear in some cases. In an observational study of 41 patients with COVID-19, headache was found in 8% of patients and myalgia was found in 12 % [17]. In most of these cases, headaches appeared to be a non-specific symptom, without features suggestive of meningeal irritation. The occurrence of isolated headaches in the absence of other neurological-type symptoms suggests the mechanism was more likely due to the systemic illness rather than a primary invasion of the CNS by the virus. An observational report of 138 patients with COVID-19 showed that fatigue was present in 69.6 % of patients, myalgia was reported in 34.8 % and headache in 6.5 % on admission [5]. Similarly, an observational study of 99 patients with COVID-19 showed confusion in 9% of patients and headache in 8% of patients [18]. In another observational study in Wuhan, headaches were reported by 34 % of COVID-19 positive patients [19] (Table 2).
6. Multiple sclerosis and CoV
Multiple experimental models have used human coronavirus (HCV) to explore the environmental component triggering the autoimmune changes seen in multiple sclerosis (MS) [[20], [21], [22], [23]]. Toll-like receptors (TLRs), which are involved in host defense and in the recognition of invading pathogens, play a role in MS pathophysiology. It has been postulated that viral particles are recognized by these receptors and contribute to the modification of the immune response of patients developing MS. This interaction shows a potential link between viral infections, including coronavirus, and the development of demyelinating diseases [24].
An observational study targeted the detection of HCV RNA in a large panel of human brain autopsy samples. Reverse transcription-PCR for 229E and OC43 coronavirus strains was performed on samples from 39 patients with MS and 51 patients with other neurological diseases or normal controls. A statistically significant higher prevalence of OC43 was observed in MS patients (35.9 %; 14 of 39) as compared to controls (13.7 %; 7 of 51) [25]. Another observational study showed that 4 out of 21 specimens from a white matter plaque, normal-appearing white matter, gray matter and cervical cord tissue of MS patients tested for HCV-229E nucleic acid, consistently gave positive results while none of the 11 control brain specimens did so. A similar study reported the detection of murine like coronaviruses in 12 of 22 MS brains [26]. This association is also supported by the observation of coronavirus-like particles in the perivascular cuffing of an MS plaque intrathecal synthesis of antibodies to HCV-229E and HCV-OC43 in a proportion of MS patients, in addition to the isolation of HCV from MS patients’ brains [27].
Direct inoculation of the blood-brain-barrier endothelial cells and persistence of infection in a leukocytic cell line allows CoV to not only infect the macrophages and dendritic cells, but to also modulate the innate immunity system as a whole [28]. Experimental models performed in susceptible strains of mice inoculated with the coronavirus JHMV strain resulted in an acute encephalomyelitis followed by a chronic demyelinating disease [29]. In addition, multiple models of murine coronavirus induced demyelinating diseases in rodents [21], either through a persistent infection of oligodendrocytes or astrocytes or by autoimmune stimulation against myelin basic protein that contributed to the postulated molecular mimicry [30]. Despite these findings associating some alfa and beta coronaviruses with demyelinating disease, a connection between SARS-CoV-2 and MS has not been demonstrated, however an eventual association can be expected.
7. Acute disseminated encephalomyelitis (ADEM) and CoV
Multiple experimental studies inoculating CoV in mice showed myelin loss in the spinal cords of mice in the acute phase of infection [31] and myelin destruction 2–3 weeks post infection [32,33]. There was a case of a 15 year old child with initial upper respiratory symptoms and subsequent ADEM, in which both the cerebrospinal fluid (CSF) and nasopharyngeal specimens were reported as positive for HCV-OC43 [34]. Another report showed a case of a 71 years old positive for MERS-CoV infection who developed new lesions in the periventricular deep white matter, corpus callosum, bilateral pons, midbrain, left cerebellum and upper cervical cord on the 24th day of the disease [35]. Despite evidence showing the persistence CoV RNA in the nervous system, even after the acute phase of infection has occurred and has caused neuronal loss, more clinical research is required to support a relationship between ADEM and CoV.
8. Peripheral nervous disease and CoV
Multiple reports have documented acute polyneuropathy in patients infected with SARS-CoV, MERS-CoV and SARS-CoV-2 [6,[35], [36], [37], [38]]. Acute autoimmune polyneuropathy triggered by coronavirus infection has been postulated [36]. As found in case report publications, most of these patients were observed in a critically ill context. Critically ill polyneuropathy (CIP), prolonged neuromuscular blockage, vitamin deficiencies and electrolyte disturbances, and drug related neuromuscular disorders were usually included in the differential diagnosis list as pointed in their discussions [6,[35], [36], [37], [38]].
From a case report including 4 patients with acute neuromuscular disorders and SARS infection, 3 developed weakness, sensorimotor peripheral nerve symptoms and decreased deep tendon reflexes (DTRs) within 21–25 days after the onset of SARS. Nerve conduction studies (NCSs) indicated temporarily reduced compound muscle action potential (CMAP) amplitudes, and there was no evident slowing of nerve conduction velocity, prolonged distal motor latency, conduction block or temporal dispersion. Electromyography (EMG) showed acute denervation with increased polyphasia. These patients received intensive care for multiple organ failure from their systemic inflammatory response syndrome and, thereby, CIP likely played a significant role [37].
In an observational study of 214 patients with a confirmed diagnosis of COVID-19, 8.9 % presented with peripheral nervous system (PNS) symptoms including hypogeusia, hyposmia, hypoplasia and neuralgia. The most common complaints were hypogeusia and hyposmia. These symptoms could be evidence of a coronavirus neurotropic invasion pathway shown in previous research [19].
Another report studying acute polyneuropathy in CoV included 4 patients with Guillain-Barré syndrome (GBS) attributed to the MERS-CoV infection. One of those was diagnosed with Bickerstaff's brainstem encephalitis (BBE), a variant of GBS, after showing hypersomnolence, ophthalmoplegia and weakness in all four limbs with cerebrospinal fluid (CSF) studies being positive for antiganglioside antibodies and albumin cytologic dissociation in the CSF after a severe MERS-CoV infection. The other two of these patients were diagnosed with acute sensory neuropathy in the context of treatment with ribavirin and lopinavir/ritonavir. Findings in the fourth patient were attributed to a CIP neuropathy [36].
A single case report showed a 28-year-old male with a MERS-CoV severe respiratory infection that gradually improved. He was found to have weakness in both legs and an inability to walk with numbness and tingling in stocking distribution. Electroneurography showed axonal polyneuropathy. A CIP was assumed as the final diagnosis [35].
Similarly, a 3 pat [39]ient case report showed 2 women experiencing motor-predominant peripheral nerve disorders and another woman experiencing neuropathy and myopathy 3 weeks after the onset of SARS-CoV. Clinical and electrophysiologic improvement was evident during follow-up examinations, with a good prognosis [38]. GBS in a child with another beta coronavirus (HCV-OC43) infection, presenting with unilateral peripheral palsy and bulbar palsy has also been reported [40].
Based on the current literature twelve cases of GBS have been reported in patients with COVID-19 infection. Some of them required mechanical ventilation. The interval between the onset of viral illness and the development of GBS symptoms was approximately ten days. Most patients presented with paresthesia and progressive, flaccid quadriparesis and showed Albumin-cytologic dissociation in CSF study. Acute Inflammatory Demyelinating Polyneuropathy subtype was most commonly observed and Immunoglobulin was the treatment of choice in all these reports. [39,[41], [42], [43], [44], [45], [46], [47]]
Developing axonal polyneuropathies in the context of a viral infection suggests that the virus can cause a neural inflammatory reaction through immune mimicry, or present as part of an inflammatory response syndrome [36,38]. However, those mechanism of SARS-CoV-2 related neuropathy needs to be clarified.
9. Acute encephalitis and CoV
The notion that an infectious respiratory pathogen can infiltrate the CNS and lead to brain inflammation was tested using HCV-OC43. In this study, infection led to the degeneration of neurons and ultimately apoptosis. Susceptible mice samples after inoculation of HCV-OC43 developed acute encephalitis with viral RNA present for several months causing neuronal degeneration [48].
Full-length HCV-OC43 RNA was recovered from the brain of an 11-month-old boy, with severe combined immunodeficiency (SCID), who had symptoms of viral encephalitis after undergoing a cord-blood transplantation. The patient died 1.5 months post-transplantation. RNA sequencing of a brain biopsy sample obtained 2 months after the onset of symptoms showed HCV-OC43, which was subsequently confirmed on real-time PCR and brain immunohistochemical analysis [49].
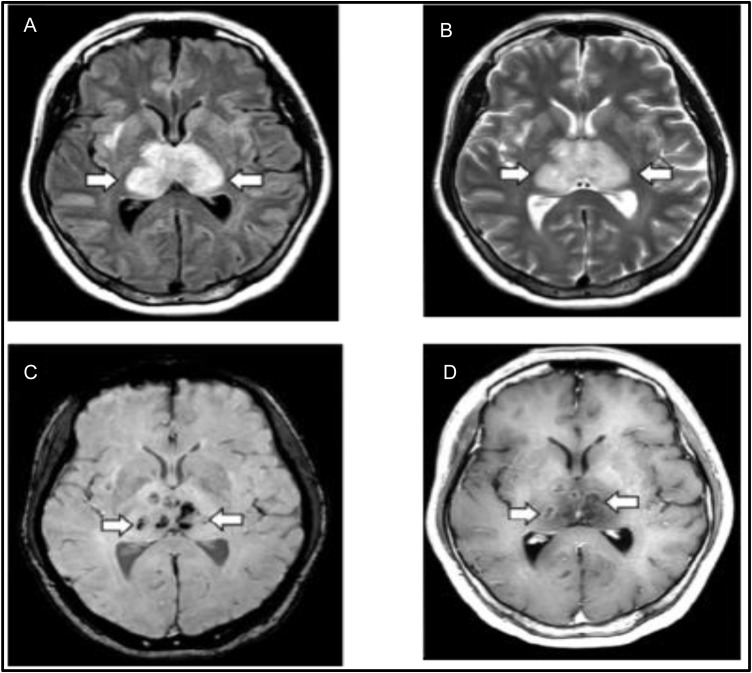
A middle-aged female with COVID-19 was diagnosed with necrotizing hemorrhagic encephalitis after presenting with a 3-day history of cough, fever and altered mental status. Non-contrast head CT images demonstrated symmetric hypoattenuation within the bilateral medial thalami and MRI demonstrated hemorrhagic lesions within the bilateral thalami, medial temporal lobes and sub insular regions (Fig. 1 ) [50]. In another case report, a 24-year-old man, after experiencing headaches, generalized fatigue and fever, presented with generalized seizures and altered mental status that progressed to impaired consciousness. Clinical and laboratory evidence was suggestive of a viral meningoencephalitis, SARS-CoV-2 was detected in an RT-PCR analysis of the CSF. A brain MRI revealed changes in the right wall of the lateral ventricle, the right mesial temporal lobe and hippocampus, which probably correlates with a SARS-CoV-2 meningitis. Interestingly, the nasopharyngeal swab specimen for RT-PCR was found to be negative for SARS-CoV-2, raising an awareness of COVID-19 possible independent mechanisms of neuropathogenesis [51]. Despite postulated mechanisms of neuronal colonization and clinical reports, stronger evidence for the association between COVID-19 and encephalitis is needed.
Fig. 1.
MRI sequence of images from a patient with suspected COVID-19 associated acute necrotizing hemorrhagic encephalopathy. A. T2 FLAIR image with bilateral thalamic hyperintensity and surrounding edema. B. T2 image revealing bilateral thalamic hyperintensity. C. SWI reveals evidence of bilateral thalamic hemorrhage. D. T2 image with post contrast ring enhancement lesions in bilateral thalami. Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19–associated Acute Hemorrhagic Necrotizing Encephalopathy: CT and MRI Features. Radiology 2020:201187. doi:https://doi.org/10.1148/radiol.2020201187. Copyright Radiological Society of North America 2020.
10. Cerebrovascular disease (CVD) and CoV
Strokes are uncommon complication of viral CNS infections. Large vessel strokes were reported in 5 of 206 SARS-CoV patients in Singapore. Four of those were critically ill and 3 died. Two patients had cardiac dysfunction and disseminated intravascular coagulation (DIC), and significant hypotension was present just before the onset of stroke [52]. An elevated number of venous thromboembolisms was observed in critically ill patients with SARS-CoV in another observational report [53].
Case series from Wuhan reported 14 strokes out of 214 patients with COVID-19. Data showed that patients with severe systemic presentation and cardiovascular risk factors were more likely to have acute cerebrovascular diseases [6]. In a retrospective study, 4 elderly patients with multiple cardiovascular risk factors diagnosed with COVID-19 developed stroke. Large vessel disease was documented as the mechanism of vascular damage in all of them [54]. Similarly, 5 cases of stroke in the context of COVID-19 were reported more recently. Three of these younger patients, had vascular risk factors including diabetes, dyslipidemia and hypertension. Evidence of occlusion of large vessels, treated with endovascular therapy was documented in all of them.
This apparent association of COVID-19 and stroke, however, is likely due to the fact that both conditions share similar risk factors. There is ample evidence that the severity of COVID-19 infection in humans is directly related to the presence of cardiovascular co-morbidities, such as hypertension (HTN), diabetes mellitus (DM) and elderly status predisposing to large vessel disease [55]. A recent meta-analysis of 8 studies from China, including 46,248 infected patients, showed the most prevalent comorbidities were HTN (17 %) and DM (8%), followed by cardiovascular diseases (5%) [57,58]. In those few reported cases, of patients without vascular risk factors, the SARS-CoV-2 induced hypercoagulability may be the most important mechanism of the cerebrovascular disease [54,56].
In the setting of a COVID-19 infection, patients with a previous history of vascular risk factors [55,57,58] may have an increased risk of stroke by facing complications such as hypotension, shock, arrhythmogenic cardiomyopathy, heart failure and DIC that can potentially contribute to hypoperfusion, embolic mechanisms of stroke and large vessel occlusion [5,18,54,58,59]. In an observational study of 138 hospitalized patients with COVID-19, shock was observed in 8.7 % of infected patients, acute cardiac injury was present in 7.2 %, and arrhythmia in 16.7 % [5]. Recently, another observational study of 191 patients in China showed that 23 % were complicated with heart failure, 20 % with septic shock, 19 % with coagulopathy, and 17 % with acute cardiac injury [58]. All of which are factors potentially predisposing patients to stroke [60]. The presence of specific viral factors directly causing hypercoagulability, arteritis, and endothelial dysfunction, which can lead to ischemic stroke or brain bleeding, need to be clarified in further research.
11. Conclusion
The COVID-19 pandemic has become a challenging world issue after its emergence in December 2019. Despite its most characteristic symptom of respiratory distress, patients with COVID-19 have also shown neurologic manifestations. An increasing number of reports of COVID-19 patients with neurological issues, in addition to emergent experimental models evidencing neuroinvasion, brings a reasonable concern of SARS-CoV-2 being a new neuropathogenic that remains underdiagnosed. How it may cause acute and chronic neurologic issues and whether possible targeting of the medullary cardiorespiratory center is contributing to the poor outcomes observed remains unclear. Exploring the neurologic manifestations of COVID-19 is a step towards better understanding the virus, preventing further spread and treating patients affected by this pandemic.
References
- 1.Cucinotta D., Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Velavan T.P., Meyer C.G. The COVID-19 epidemic. Trop. Med. Int. Health. 2020;25(3):278–280. doi: 10.1111/tmi.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020 doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao L. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baig A.M. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 8.Netland J. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 2008;82(15):7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding Y. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J. Pathol. 2004;203(2):622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li K. Middle east respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J. Infect. Dis. 2016;213(5):712–722. doi: 10.1093/infdis/jiv499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walls A.C. Structure, function, and Antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020 doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan Y. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94(7):e00127–20. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doobay M.F. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292(1):R373–81. doi: 10.1152/ajpregu.00292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palasca O. 2018. TISSUES 2.0: An Integrative Web Resource on Mammalian Tissue Expression. Database (Oxford) 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCray P.B., Jr. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J. Virol. 2007;81(2):813–821. doi: 10.1128/JVI.02012-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang C. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen N. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu X.W. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun N. Activation of astrocytes in the spinal cord of mice chronically infected with a neurotropic coronavirus. Virology. 1995;213(2):482–493. doi: 10.1006/viro.1995.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Houtman J.J., Hinze H.C., Fleming J.O. Demyelination induced by murine coronavirus JHM infection of congenitally immunodeficient mice. In: Talbot P.J., Levy G.A., editors. Corona- and Related Viruses: Current Concepts in Molecular Biology and Pathogenesis. Springer US; Boston, MA: 1995. pp. 159–163. [DOI] [PubMed] [Google Scholar]
- 22.Wege H. Immunopathological aspects of coronavirus infections. Semin. Immunopathol. 1995;17(2–3):133–148. doi: 10.1007/BF00196162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arbour N. Persistent infection of human oligodendrocytic and neuroglial cell lines by human coronavirus 229E. J. Virol. 1999;73(4):3326–3337. doi: 10.1128/jvi.73.4.3326-3337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duffy L., O’Reilly S.C. Toll-like receptors in the pathogenesis of autoimmune diseases: recent and emerging translational developments. Immunotargets Ther. 2016;5:69–80. doi: 10.2147/ITT.S89795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arbour N. Neuroinvasion by human respiratory coronaviruses. J. Virol. 2000;74(19):8913–8921. doi: 10.1128/jvi.74.19.8913-8921.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray R.S. Detection of coronavirus RNA and antigen in multiple sclerosis brain. Ann. Neurol. 1992;31(5):525–533. doi: 10.1002/ana.410310511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart J.N., Mounir S., Talbot P.J. Human coronavirus gene expression in the brains of multiple sclerosis patients. Virology. 1992;191(1):502–505. doi: 10.1016/0042-6822(92)90220-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerna G. Human respiratory coronavirus HKU1 versus other coronavirus infections in Italian hospitalised patients. J. Clin. Virol. 2007;38(3):244–250. doi: 10.1016/j.jcv.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Libbey J.E., Lane T.E., Fujinami R.S. Axonal pathology and demyelination in viral models of multiple sclerosis. Discov. Med. 2014;18(97):79–89. [PMC free article] [PubMed] [Google Scholar]
- 30.Boucher A. Long-term human coronavirus-myelin cross-reactive T-cell clones derived from multiple sclerosis patients. Clin. Immunol. 2007;123(3):258–267. doi: 10.1016/j.clim.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu G.F., Perlman S. Macrophage infiltration, but not apoptosis, is correlated with immune-mediated demyelination following murine infection with a neurotropic coronavirus. J. Virol. 1999;73(10):8771–8780. doi: 10.1128/jvi.73.10.8771-8780.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Savarin C. Memory CD4+ T-cell-mediated protection from lethal coronavirus encephalomyelitis. J. Virol. 2008;82(24):12432–12440. doi: 10.1128/JVI.01267-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol. Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeh E.A. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics. 2004;113(1 Pt 1):e73–6. doi: 10.1542/peds.113.1.e73. [DOI] [PubMed] [Google Scholar]
- 35.Algahtani H., Subahi A., Shirah B. Neurological complications of middle east respiratory syndrome coronavirus: a report of two cases and review of the literature. Case Rep. Neurol. Med. 2016;2016:3502683. doi: 10.1155/2016/3502683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim J.E. Neurological complications during treatment of middle east respiratory syndrome. J. Clin. Neurol. 2017;13(3):227–233. doi: 10.3988/jcn.2017.13.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsai L.K., Hsieh S.T., Chang Y.C. Neurological manifestations in severe acute respiratory syndrome. Acta Neurol. Taiwan. 2005;14(3):113–119. [PubMed] [Google Scholar]
- 38.Tsai L.K. Neuromuscular disorders in severe acute respiratory syndrome. Arch. Neurol. 2004;61(11):1669–1673. doi: 10.1001/archneur.61.11.1669. [DOI] [PubMed] [Google Scholar]
- 39.Camdessanche J.P. COVID-19 may induce Guillain-Barre syndrome. Rev Neurol (Paris) 2020 doi: 10.1016/j.neurol.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turgay C. A rare cause of acute flaccid paralysis: human coronaviruses. J. Pediatr. Neurosci. 2015;10(3):280–281. doi: 10.4103/1817-1745.165716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sedaghat Z., Karimi N. Guillain Barre syndrome associated with COVID-19 infection: a case report. J. Clin. Neurosci. 2020 doi: 10.1016/j.jocn.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El Otmani H. Covid-19 and Guillain-Barre syndrome: more than a coincidence! Rev Neurol (Paris) 2020 doi: 10.1016/j.neurol.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao H. Guillain-Barre syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19(5):383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toscano G. Guillain-barre syndrome associated with SARS-CoV-2. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Padroni M. Guillain-Barre syndrome following COVID-19: new infection, old complication? J. Neurol. 2020 doi: 10.1007/s00415-020-09849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alberti P. Guillain-Barre syndrome related to COVID-19 infection. Neurol. Neuroimmunol. Neuroinflamm. 2020;7(4) doi: 10.1212/NXI.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Virani A. Guillain-Barre Syndrome associated with SARS-CoV-2 infection. IDCases. 2020:e00771. doi: 10.1016/j.idcr.2020.e00771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacomy H. Human coronavirus OC43 infection induces chronic encephalitis leading to disabilities in BALB/C mice. Virology. 2006;349(2):335–346. doi: 10.1016/j.virol.2006.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morfopoulou S. Human coronavirus OC43 associated with fatal encephalitis. N. Engl. J. Med. 2016;375(5):497–498. doi: 10.1056/NEJMc1509458. [DOI] [PubMed] [Google Scholar]
- 50.Poyiadji N. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020:201187. doi: 10.1148/radiol.2020201187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moriguchi T. A first case of Meningitis/Encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Umapathi T. Large artery ischaemic stroke in severe acute respiratory syndrome (SARS) J. Neurol. 2004;251(10):1227–1231. doi: 10.1007/s00415-004-0519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lew T.W. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290(3):374–380. doi: 10.1001/jama.290.3.374. [DOI] [PubMed] [Google Scholar]
- 54.Avula A. COVID-19 presenting as stroke. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo J. Coronavirus disease 2019 (COVID-19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin-converting enzyme Inhibitors/Angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J. Am. Heart Assoc. 2020;9(7):e016219. doi: 10.1161/JAHA.120.016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Panigada M. Hypercoagulability of COVID-19 patients in intensive care unit. A report of thromboelastography findings and other parameters of hemostasis. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang J. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020 [Google Scholar]
- 58.Zhou F. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oxley T.J. Large-vessel stroke as a presenting feature of Covid-19 in the young. N. Engl. J. Med. 2020:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Powers W.J. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–e418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 61.Stainsby B., Howitt S., Porr J. Neuromusculoskeletal disorders following SARS: a case series. J. Can. Chiropr. Assoc. 2011;55(1):32–39. [PMC free article] [PubMed] [Google Scholar]
- 62.Lau K.K. Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis. 2004;10(2):342–344. doi: 10.3201/eid1002.030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arabi Y.M. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV) Infection. 2015;43(4):495–501. doi: 10.1007/s15010-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lachance C. Involvement of aminopeptidase N (CD13) in infection of human neural cells by human coronavirus 229E. J. Virol. 1998;72(8):6511–6519. doi: 10.1128/jvi.72.8.6511-6519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Niu J. Non-invasive bioluminescence imaging of HCoV-OC43 infection and therapy in the central nervous system of live mice. Antiviral Res. 2020;173:104646. doi: 10.1016/j.antiviral.2019.104646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nilsson A. Fatal encephalitis associated with coronavirus OC43 in an immunocompromised child. Infect Dis (Lond) 2020:1–4. doi: 10.1080/23744235.2020.1729403. [DOI] [PubMed] [Google Scholar]
- 67.Dube M. Axonal transport enables neuron-to-neuron propagation of human coronavirus OC43. J. Virol. 2018;92(17) doi: 10.1128/JVI.00404-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu J. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin. Infect. Dis. 2005;41(8):1089–1096. doi: 10.1086/444461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gu J. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005;202(3):415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alsaad K.O. Histopathology of Middle East respiratory syndrome coronovirus (MERS-CoV) infection - clinicopathological and ultrastructural study. Histopathology. 2018;72(3):516–524. doi: 10.1111/his.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]