Abstract
Background
COVID-19 predisposes patients to a prothrombotic state with demonstrated microvascular involvement. The degree of hypercoagulability appears to correlate with outcomes; however, optimal criteria to assess for the highest-risk patients for thrombotic events remain unclear; we hypothesized that deranged thromboelastography measurements of coagulation would correlate with thromboembolic events.
Study Design
Patients admitted to an ICU with COVID-19 diagnoses who had thromboelastography analyses performed were studied. Conventional coagulation assays, d-dimer levels, and viscoelastic measurements were analyzed using a receiver operating characteristic curve to predict thromboembolic outcomes and new-onset renal failure.
Results
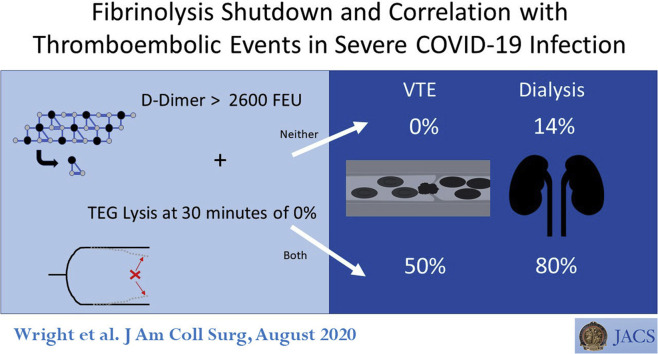
Forty-four patients with COVID-19 were included in the analysis. Derangements in coagulation laboratory values, including elevated d-dimer, fibrinogen, prothrombin time, and partial thromboplastin time, were confirmed; viscoelastic measurements showed an elevated maximum amplitude and low lysis of clot at 30 minutes. A complete lack of lysis of clot at 30 minutes was seen in 57% of patients and predicted venous thromboembolic events with an area under the receiver operating characteristic curve of 0.742 (p = 0.021). A d-dimer cutoff of 2,600 ng/mL predicted need for dialysis with an area under the receiver operating characteristic curve of 0.779 (p = 0.005). Overall, patients with no lysis of clot at 30 minutes and a d-dimer > 2,600 ng/mL had a venous thromboembolic event rate of 50% compared with 0% for patients with neither risk factor (p = 0.008), and had a hemodialysis rate of 80% compared with 14% (p = 0.004).
Conclusions
Fibrinolysis shutdown, as evidenced by elevated d-dimer and complete failure of clot lysis at 30 minutes on thromboelastography predicts thromboembolic events and need for hemodialysis in critically ill patients with COVID-19. Additional clinical trials are required to ascertain the need for early therapeutic anticoagulation or fibrinolytic therapy to address this state of fibrinolysis shutdown.
Abbreviations and Acronyms: DIC, disseminated intravascular coagulation; IQR, interquartile range; ISTH, International Society of Thrombosis and Hemostasis; LY30, lysis at 30 minutes; MA, maximum amplitude; PTT, partial thromboplastin time; TEG, thromboelastography; VTE, venous thromboembolic event
Visual Abstract
The novel coronavirus known as severe acute respiratory distress syndrome coronavirus 2, leading to COVID-19 emerged in Wuhan, China in late 2019 and has become a worldwide pandemic. More than 2.7 million cases have been confirmed worldwide, causing more than 190,000 deaths, with these numbers growing exponentially.1 A subset of patients infected with COVID-19 progress to ARDS, with 70% of critically ill patients requiring intubation and mechanical ventilation.2
Viral infection-associated inflammation clearly predisposes patients to prothrombotic states.3 In particular, infections with severe acute respiratory distress syndrome coronavirus 1 and Middle East respiratory syndrome-related coronavirus cause intra-alveolar and systemic fibrin clots in animals and humans with severe respiratory disease.4
Initial studies in Wuhan that used multivariate regression analyses suggested a higher mortality based on age, Sequential Organ Failure Assessment score, and d-dimer levels.5 In addition, previous work during the H1N1 viral pneumonia outbreak demonstrated that elevations in d-dimer levels suggest an increased thrombosis risk.6 Specific coagulation pathway product analysis demonstrated decreased survival in patients presenting with COVID-19 and elevations in the prothrombin time, d-dimer, and fibrin degradation products.7
Broad evidence exists for using rotational thromboelastometry or thromboelastography (TEG) to predict thromboembolic rates in other disease processes. Across trauma, surgical, and mixed ICU populations, hypercoagulability as demonstrated by TEG, especially an elevated maximum amplitude (MA), has reliably predicted thromboembolic events (see Discussion for references). The initial study of TEG use in 24 COVID-19 patients in Italy demonstrated that the TEG-MA and angle were elevated and the TEG R- and K-values were decreased; however, these findings were not correlated with outcomes measures, such as rate of thrombotic events.8
Based on earlier data, we hypothesized that abnormalities in TEG parameters would correlate with thromboembolic risk. The aim of our study was to develop an improved screening tool to suggest the highest risk of thromboembolic complications, including renal failure.
Methods
All patients admitted to the University of Colorado Hospital with documented COVID-19 infection were considered for inclusion beginning March 1, 2020 to April 20, 2020. Specially designed “surge” COVID-19 ICU teams in physically isolated cohort units had been established and staffed by a multidisciplinary pool of pulmonary critical care, anesthesia critical care, and surgical critical care attendings. Any patient admitted to one of these surge ICU teams who had TEG testing was included in this analysis; the first TEG drawn was used for analysis in an attempt to identify hypercoagulability early in the patients' course, thereby providing a potential window for therapeutic intervention.
IRB exemption was granted to this study by our institution (COMIRB Protocol 20-0947). Study data were collected and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at the University of Colorado Anschutz Medical Campus.
Citrated kaolin/kaolin-heparinase TEG (Haemonetics) in vitro point of care testing was ordered at the discretion of the intensivist to guide clinical care and was used more heavily by the anesthesia and surgical critical care group, who routinely use this tool intraoperatively and for critical care management of trauma and transplantation patient populations. TEG indices recorded included r-time, angle, MA, and lysis at 30 minutes (LY30). Additional coagulation measurements included CBC for measurements of platelets, fibrinogen level, and d-dimer. All coagulation assays were conducted by the clinical laboratory. Patients' ratio of arterial oxygen partial pressure to fractional inspired oxygen and Sequential Organ Failure Assessment score were calculated on the day of TEG assay, in addition to their International Society of Thrombosis and Hemostasis (ISTH) disseminated intravascular coagulation (DIC) score.9
The primary outcomes were venous thromboembolic events (VTE) and new-onset renal failure requiring dialysis. No routine VTE screening was performed, rather VTE were diagnosed by either ultrasound or CT imaging ordered based on clinical suspicion; empiric anticoagulation without radiographic evidence was not counted as a VTE. Only 3 CT pulmonary embolism studies were performed and they were all negative. VTE was considered a macrothrombotic event and renal failure was considered a potential microthrombotic complication based on the recent histologic reports of microthrombosis in the kidneys of COVID-19 victims.10 Arterial thrombotic events were also evaluated, which in our patient population consisted entirely of strokes. All patients received chemical VTE prophylaxis with at least enoxaparin between 40 and 60 mg/d or unfractionated heparin between 10,000 and 15,000 units/d.
SPSS Software, version 22 (IBM) was used for statistical analysis. The first set of coagulation variables from TEG were contrasted to fibrinogen and d-dimer for performance using a receiver operating characteristic curve for predicting VTEs and new-onset need for dialysis. Youden Index was used for identification of the inflection point for highest sensitivity and specificity for each significant measurement of coagulation with the specific outcomes. Patients were then dichotomized based on the Youden cut points for each associated end point. Descriptive characteristics between cohorts are displayed as the median and 25th to 75th percentile range or interquartile range (IQR). Variables contrasted between groups were tested with chi-square and Fisher exact test for categorical data, and a Mann-Whitney U test was performed for continuous variables. As both outcomes were predictors for micro- and macrothrombosis, we performed an additional analysis of patients using both coagulation variables associated with the outcomes to stratify patients from thrombotic complications.
Results
Demographic characteristics
A total of 44 patients infected with COVID-19 and in the ICU from March 22, 2020 to April 20, 2020 were eligible for the study; all patients had at least 1 TEG assessment. Twenty-eight patients (64%) were male, median age was 54 years (IQR 42 to 59 years, range 19 to 86 years), and median BMI was 30 kg/m2 (IQR 27 to 37 kg/m2, range 18.6 to 85.6 kg/m2) (Table 1 ). Median time from symptom onset to hospital admission was 5 days (IQR 4 to 7 days). Forty-one of 44 patients (93%) required mechanical ventilation, 16 patients (36%) had acute renal failure requiring dialysis, 11 patients (25%) had a VTE, and 6 patients (14%) had a thrombotic stroke. Median Sequential Organ Failure Assessment score at the time of the first TEG draw was 8 (IQR 7 to 10) with a median ratio of arterial oxygen partial pressure to fractional inspired oxygen of 163 (IQR 127 to 235), consistent with the majority of patients having moderate to severe ARDS. Using the World Health Organization's COVID-19 Ordinal Scale (8-point system) for clinical status at the nadir of illness, 36 of 44 patients (82%) scored a 7 (ventilation plus additional organ support), 5 of 44 patients (11%) scored an 8 (died during study period), 1 patient (2%) scored a 5 (high flow oxygen), and 2 patients (5%) scored a 4 (oxygen by mask). All patients with VTE had a score of 7 other than 1 patient who died; likewise, all patients with arterial thrombus had a score of 7 other than 1 patient who died.
Table 1.
Demographic Characteristics
| Variable | Data |
|---|---|
| Patients, n | 44 |
| Age, y, median (IQR) | 54 (42–59) |
| Sex, n (%) | |
| Male | 28 (63.6) |
| Female | 16 (36.6) |
| Ethnicity, n (%) | |
| Asian | 7 (15.9) |
| Caucasian | 4 (9.1) |
| African American | 10 (22.7) |
| Hispanic/Latino | 20 (45.5) |
| Other | 2 (4.5) |
| Unknown | 1 (2.3) |
| BMI, kg/m2 median (IQR) | 30 (27–37) |
| Comorbidity, n (%) | |
| Cardiac arrhythmias | 3 (6.8) |
| Hypertension | 21 (47.7) |
| Diabetes mellitus | 18 (40.9) |
| Hyperlipidemia | 11 (25) |
| COPD | 2 (4.5) |
| Asthma | 4 (9.1) |
| Smoking/vaping | 2 (4.5) |
| Ethyl alcohol/substance use disorder | 0 |
| Immunosuppression | 1 (2.3) |
| None of the above | 8 (18.2) |
IQR, interquartile range.
Coagulation measurements
The patients' conventional coagulation parameters are listed in Table 2 (ie platelet counts, prothrombin time and partial thromboplastin time [PTT], d-dimer, and fibrinogen). Pertinent abnormalities include an elevated d-dimer level, elevated fibrinogen, with normal platelet counts in the majority of patients and mildly prolonged prothrombin time and PTT with median values at or slightly above the upper limits of normal. Median ISTH DIC score was 0 (IQR 0 to 2), with no patients having a score higher that 4. TEG variables were consistent with a hypercoagulable state with an elevated MA and low LY30 (Table 2).
Table 2.
Coagulation Parameters
| Conventional coagulation measurement | Median (IQR) | Reference range |
|---|---|---|
| Platelet count, 109/L | 232 (186–298) | 150–400 |
| Prothrombin time, s | 14.5 (13.5–15.9) | 12–14.5 |
| Activated partial thromboplastin time, s | 37 (31–49) | 22.6–34.1 |
| Fibrinogen, mg/dL | 656 (560–779) | 150–400 |
| d-dimer, ng/mL fibrinogen equivalent | 1,840 (935–4,085) | < 500 |
| Viscoelastic index | ||
| R-time, min | 5.8 (4.8–8.6) | 2–8 |
| Angle, degrees | 71 (66–74) | 55–78 |
| Maximum amplitude, mm | 73 (67–77) | 50–70 |
| Clot lysis at 30 min, % | 0 (0–0.4) | 0.8–3 |
IQR, interquartile range.
Coagulation association with venous thromboembolic event
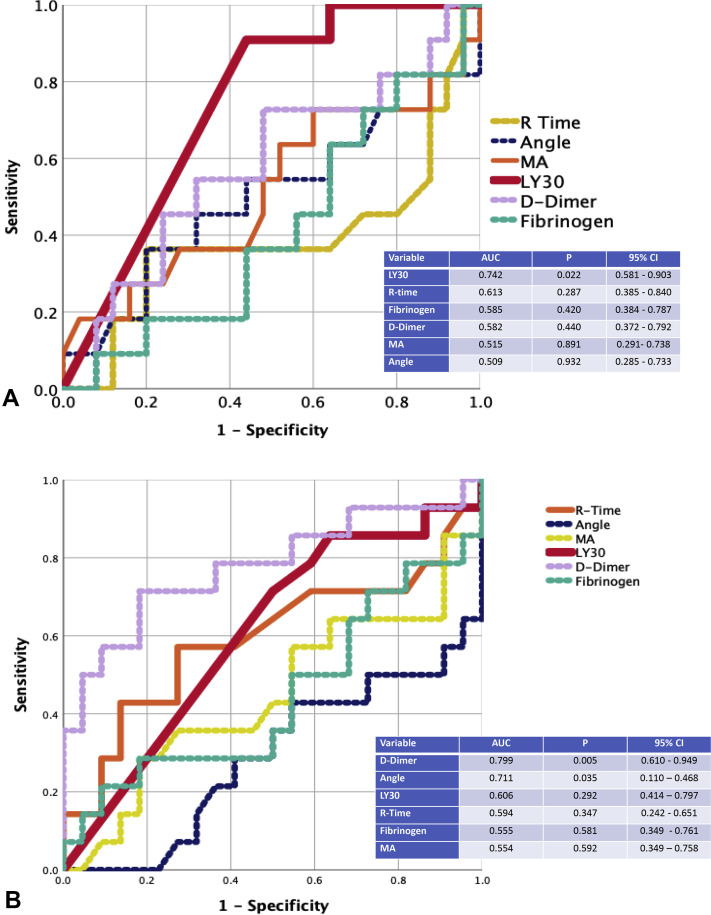
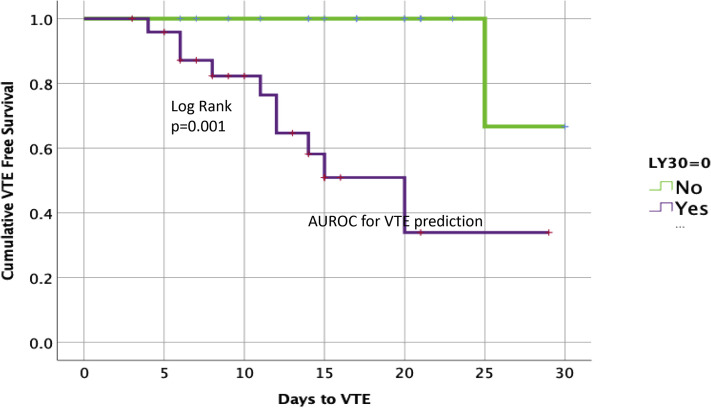
The receiver operating characteristic curve for VTE was only significant for TEG LY30 and VTE with an area under the curve of 0.742 (p = 0.021) (Fig. 1 A). The Youden Index for VTE was identified at 0. In this patient cohort, 57% had this extreme lack of fibrinolytic activity. These patients were categorized as fibrinolysis shutdown based on the presence of elevated d-dimer and low fibrinolytic activity; fibrinolysis shutdown has been described previously as LY30 < 0.8%, but the more complete shutdown seen in these patients results in an LY30 of 0% as a diagnostic cutoff based on the Youden Index for maximizing sensitivity and specificity. Descriptive variables of patient with fibrinolysis shutdown vs those without shutdown are listed in Table 3 . The only significant differences appreciated between groups were that patients with fibrinolytic shutdown had a lower incidence of history of hyperlipidemia and a lower TEG angle, even though fibrinogen levels were similar. Patients with fibrinolysis shutdown had a 40% rate of VTE compared with 5% in patients without shutdown (p = 0.013). The time to VTE was significantly shorter in patients with fibrinolysis shutdown (Fig. 2 ) (log rank p = 0.001). Overall, 24 of the 44 patients in the ICU with severe COVID-19 infection were placed on full therapeutic anticoagulation, despite only 14 of these patients having documented VTE or arterial thrombotic events; 10 additional patients received therapeutic anticoagulation empirically based on physician concern for occult thrombotic events or bedside evidence of extreme hypercoagulability, such as frequent clotting of central venous access or hemodialysis filters.
Figure 1.
(A) Area under the receiver operating characteristic curve (AUC) for venous thromboembolism prediction. (B) AUC for acute renal failure prediction. LY30, clot lysis at 30 minutes; MA, maximum amplitude.
Table 3.
Comparison Between Patients With and Without Complete Fibrinolysis Shutdown
| Variable | No fibrinolysis shutdown | Fibrinolysis shutdown | p Value |
|---|---|---|---|
| Age, y, median (IQR) | 56 (46–58) | 53 (45–62) | 0.859 |
| BMI, kg/m2, median (IQR) | 30 (27–38) | 30 (28–33) | 0.760 |
| Cardiac arrhythmias, % | 10 | 4 | 0.570 |
| Hypertension, % | 53 | 44 | 0.761 |
| Diabetes mellitus, % | 37 | 44 | 0.760 |
| Hyperlipidemia, % | 42 | 12 | 0.035∗ |
| COPD, % | 5 | 4 | 0.999 |
| Asthma, % | 0 | 16 | 0.122 |
| Smoking/vaping, % | 0 | 8 | 0.498 |
| Alcohol or substance use disorder, % | 0 | 0 | 0.999 |
| Immunosuppression, % | 0 | 4 | 0.999 |
| None of the above, % | 43 | 57 | 0.999 |
| Platelet count, 109/L, median (IQR) | 243 (198–341) | 226 (184–280) | 0.343 |
| Prothrombin time, s, median (IQR) | 14 (13–15) | 16 (14–16) | 0.117 |
| Activated partial thromboplastin time, s, median (IQR) | 31 (30–40) | 37 (34–49) | 0.146 |
| Fibrinogen, mg/dL, median (IQR) | 728 (563–944) | 649 (556–773) | 0.360 |
| d-dimer, ng/mL fibrinogen equivalent, median (IQR) | 2,685 (1,850–8,740) | 2,720 (1,595–17,270) | 0.355 |
| C-reactive protein, mg, median (IQR) | 181 (73–271) | 127 (87–250) | 0.485 |
| R-time, min, median (IQR) | 7.1 (4.5–7.8) | 6 (4.5–10.2) | 0.537 |
| Angle, degrees, median (IQR) | 74 (72–75) | 67(63–72) | 0.019∗ |
| Maximum amplitude, mm, median (IQR) | 77 (72–78) | 73 (66–78) | 0.999 |
IQR, interquartile range.
Statistically significant.
Figure 2.
Clot lysis at 30 minutes (LY30) of any value > 0% predicts fewer venous thromboembolic events (VTEs). AUROC, area under the receiver operating characteristic curve.
Coagulation association with need for dialysis
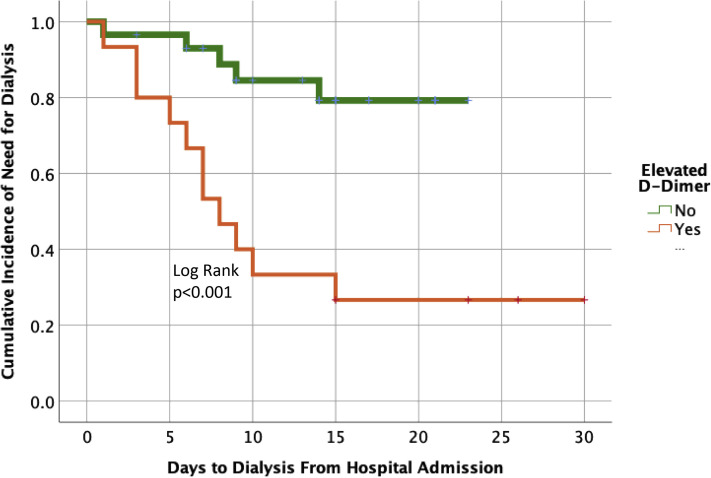
The receiver operating characteristic curve for new-onset need for dialysis was only significant for d-dimer and TEG angle. The area under the curve for d-dimer was 0.779 (p = 0.005) and TEG angle was 0.771 (p = 0.035). The remaining coagulation variables were not significant (Fig. 1B). Due to the higher performance with d-dimer, this coagulation measurement was used to find the inflection point for need for dialysis. The Youden Index was found to be 2,600 ng/mL. An elevated d-dimer above this threshold was appreciated in 34% of the study population. Variables contrasting patients with high d-dimers vs lower are listed in Table 4 . An elevated d-dimer was associated with a 73% new-onset need for dialysis vs 17% for lower d-dimer levels (p = 0.001) (Fig. 3 ). Need for dialysis occurred at a median of day 7 of hospitalization (IQR 4.5 to 9 days), with acute kidney injury occurring on day 3 (IQR 1 to 5 days), and VTE and thrombotic events did not occur until 12 days (IQR 7 to 14.5 days) and 11.5 days (IQR 9.5 to 21 days) from hospital admission, respectively; all VTE or thrombotic events were noted after acute kidney injury or dialysis need had been established. In patients with VTE, all were either directly admitted to the ICU from the emergency department (n = 9) or upgraded from ward status within 48 hours of hospital admission (n = 2).
Table 4.
Comparison Between Patients With and Without d-Dimer Elevation
| Variable | d-dimer < 2,600 ng/mL | d-dimer > 2,600 ng/mL | p Value |
|---|---|---|---|
| Age, y, median (IQR) | 48 (37–56) | 58 (53–59) | 0.147 |
| BMI, kg/m2, median (IQR) | 30 (28–32) | 30 (28–33) | 0.516 |
| Cardiac arrhythmias, % | 6 | 7 | 0.999 |
| Hypertension, % | 55 | 53 | 0.752 |
| Diabetes mellitus, % | 41 | 40 | 0.999 |
| Hyperlipidemia, % | 24 | 27 | 0.999 |
| COPD, % | 7 | 0 | 0.540 |
| Asthma, % | 7 | 13 | 0.596 |
| Smoking/vaping, % | 0 | 13 | 0.111 |
| Alcohol or substance abuse disorder, % | 0 | 0 | 0.999 |
| Immunosuppression, % | 3 | 0 | 0.999 |
| None of the above, % | 45 | 52 | 0.999 |
| Platelet count, 109/L, median (IQR) | 262 (167–409) | 229 (198–254) | 0.820 |
| Prothrombin time, s, median (IQR) | 14 (13–16) | 15 (14–16) | 0.278 |
| Activated partial thromboplastin time, s, median (IQR) | 38 (30–42) | 35 (30–50) | 0.820 |
| Fibrinogen, mg/dL, median (IQR) | 675 (544–759) | 686 (568–862) | 0.455 |
| C-reactive protein, mg, median (IQR) | 112 (67–218) | 186 (120–271) | 0.240 |
| R-time, min, median (IQR) | 6.3 (4.8–7.5) | 7.6 (4.1–10.6) | 0.577 |
| Angle, degrees, median (IQR) | 71 (64–76) | 68 (64–75) | 0.528 |
| Maximum amplitude, mm, median (IQR) | 75 (68–77) | 75 (68–77) | 0.999 |
| Clot lysis at 30 min, %, median (IQR) | 0 (0–0.6) | 0 (0–0.2) | 0.366 |
IQR, interquartile range.
Figure 3.
d-dimer levels and timing of dialysis.
Combination score
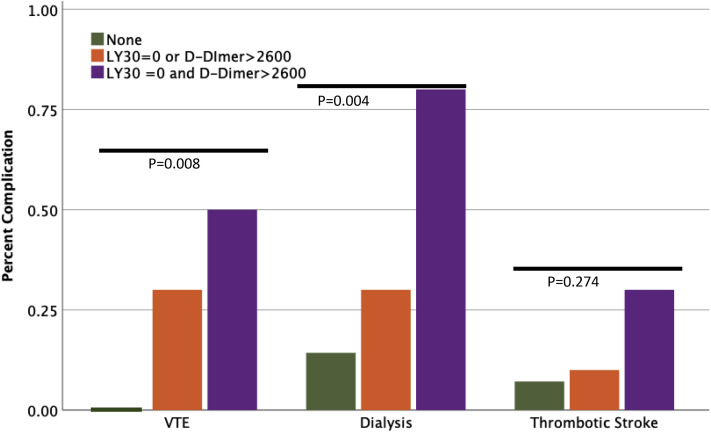
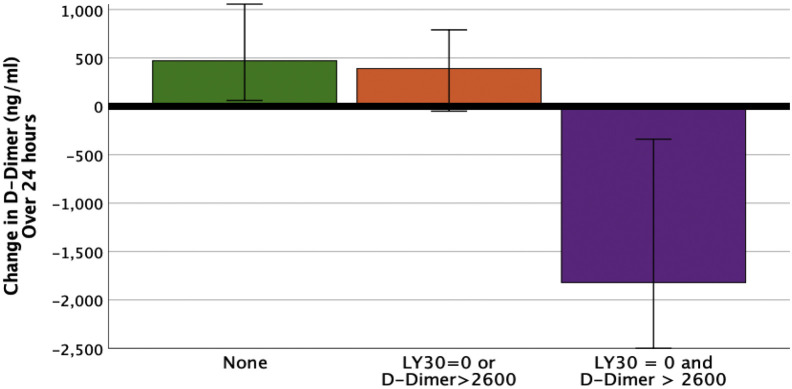
Patients were grouped into coagulation cohorts using both LY30 and d-dimer inflection points. Neither risk factor for hypercoagulability was present in 32% of the patient population, 1 coagulation risk factor represented 45% of the patient population and 23% of patients had both. The number of patients with VTE increased from 0 to 50% for patients with 0 to 2 coagulation risk factors, respectively (p = 0.008) (Fig. 4 ). Similarly, the need for dialysis increased from 14% to 80% (p = 0.004) (Fig. 4). Although not significant, thrombotic stroke rate was also increased from 7% to 30% (p = 0.274) (Fig. 4). Patients with an LY30 of 0% and d-dimer > 2,600 ng/mL had a significant decrease in d-dimer levels over 24 hours compared with other cohorts (eFig.1) (p = 0.009).
Figure 4.
Combination score predicts venous thromboembolic event (VTE) and dialysis risk. LY30, clot lysis at 30 minutes.
Discussion
Patients with COVID-19 in the ICU undergoing viscoelastic testing have a uniquely hypercoagulable profile. This patient cohort had a high rate of renal failure that preceded VTE diagnosis. An elevated d-dimer was associated with the need for dialysis, and a low LY30 was associated with VTE. Nearly one-quarter of these critically ill patients had an LY30 of 0% and d-dimer > 2,600 ng/mL and 80% of these patients required dialysis, 50% had a VTE, and 30% had a thrombotic stroke. These results demonstrate that fibrinolysis shutdown correlates with thrombotic complications. Of note, this cohort of critically ill COVID-19 patients was clearly hypercoagulable, despite high normal or frankly elevated prothrombin time and PTT levels, demonstrating the importance of using whole blood coagulation assays (which more closely approximate in vivo conditions, including the presence of cells and platelets), such as the TEG for improved risk stratification. As a rapid test to demonstrate complete fibrinolysis shutdown, an LY30 of 0% in conjunction with d-dimer levels > 2,600 ng/mL can serve as a sensitive marker for the patients most at risk for VTE and other thrombotic complications.
Critically ill patients in ICU settings experience VTE rates of 13% to 30% without chemoprophylaxis and 5% to 15% with routine chemoprophylaxis.11 An early series of 184 COVID-19 ICU status patients had a VTE rate of 27% and arterial thrombosis rate of 3.7%, supporting a unique predisposition to hypercoagulation in this new patient population.12 In addition, an autopsy series in COVID-19 patients in the US has suggested a possible role for thrombotic microangiopathy in the lungs.13 Low-molecular-weight heparin or unfractionated heparin given at prophylactic doses in COVID-19 patients with elevated sepsis-induced coagulopathy scores showed a mortality benefit in a retrospective study.14 Interestingly, when comparing COVID-19 patients with a cohort with non-COVID-19 pneumonia, the non-COVID-19 patients had similar d-dimer elevations but did not have a survival benefit with prophylactic low-molecular-weight heparin/unfractionated heparin.15
In the trauma population, hypercoagulable TEG measurements predict VTE 2.4- to 6.7-fold higher based on higher MA parameters, despite appropriate prophylactic anticoagulation.16, 17, 18, 19 In a broad surgical patient population, patients with a TEG MA > 68 mm were 6 times more likely to have thrombotic events including VTE, MI, and CVA.20 In a mixed medical-surgical ICU population with baseline abnormal coagulation parameters, a TEG-MA of > 72 mm predicted thromboembolic events more effectively than conventional coagulation parameters, such as international normalized ratio, PTT, fibrinogen, or platelet count.21 In a more recent study, a low LY30 suggestive of fibrinolysis shutdown was associated with an increased risk of thrombotic complications as early as 12 hours from injury.18 Medical inhibition of fibrinolysis with tranexamic acid has also been associated with an increased risk of thrombotic complications in trauma patients.22 TEG MA in this COVID-19 population was universally elevated regardless of whether thrombotic complications developed or not, but LY30 strongly differentiated those patients with and without VTE. The first publication to report use of TEG in the ICU with COVID-19 patients drew similar conclusions that viscoelastic testing demonstrated hypercoagulability in this patient population,8 however, this study of 24 patients from Italy was descriptive and did not include any outcomes associated with TEG indices. Our results suggest that TEG LY30 serves as a prognostic marker that this patient population is at risk for thrombotic complications and can have better performance identifying these at-risk patients than other coagulation measurements.
Elevated d-dimer levels were also associated with potential microthrombotic disease leading to renal failure. These results align with previous reports that patients with elevated d-dimer levels have poor outcomes in the setting of COVID-19 infection.7 Elevated d-dimer and low LY30 have previously been defined as fibrinolysis shutdown in trauma.23 This fibrinolytic phenotype has been associated with poor outcomes after trauma.24, 25, 26, 27, 28 The cause of elevated d-dimer levels in COVID-19 patients remains unclear but could be reflective of an excessive amount of intravascular polymerized fibrin. Fibrin acts as co-factor for its own destruction,29 therefore, a high burden of fibrin will result in elevated d-dimer levels with minimal systemic fibrinolytic activity. Specifically, there is more reagent that is readily broken down because fibrin increases the binding opportunities for plasminogen activators and plasminogen to co-localize. Alternatively, it is possible that a high d-dimer level could be representative of a viral activator of the fibrinolytic system with subsequent shutdown. Patients meeting this fibrinolysis shutdown laboratory definitions of low systemic fibrinolysis and elevated d-dimers had marked drops in d-dimer during 24-hour periods compared with the other patient cohorts that had mild increases in d-dimer during this time frame. This rise and fall of fibrinolytic activity has been demonstrated in animal models of endotoxemia in which plasminogen activation occurs within 2 hours of IV infusion but is followed by an abrupt inhibition of fibrinolysis.30 In a study evaluating the addition of recombinant tissue plasminogen activator to viscoelastic blood analysis of septic patients without evidence of DIC, even low doses of recombinant tissue plasminogen activator did not correct the hypofibrinolytic state.31
Recently, acute fibrinolysis shutdown has been documented in early sepsis and found to correlate to increased morbidity and mortality.32 Fibrinolysis shutdown and organ failure has historically been associated with DIC,33 however, the ISTH score of 4 or higher to define overt DIC9 was not present in a single patient in our study, despite the majority of patients having respiratory and renal failure with laboratory evidence of a hypercoagulable state. COVID-19 autopsies have shown diffuse microthrombi, including the kidney and lungs, and are believed to be the cause of organ failure in this patient population.10 , 13 Microthrombi have been demonstrated in animal models of shock34 and these can be reversed with pretreatment using heparin35 or post-treatment with a fibrinolytic agent.36 Despite evidence of a DIC-type picture in lungs and kidneys supported by COVID-19 autopsy studies, platelet counts remain relatively normal and fibrinogen levels are elevated in these patients, suggesting that the conventional ISTH DIC score fails to capture the prothrombotic coagulopathy seen in this patient population.
Surprisingly, in our patient population of COVID-19-infected patients, marked d-dimer elevation and TEG LY30 levels of 0% were seen in patient samples drawn more than 2 weeks into their ICU course and the ISTH score for DIC remained low. These findings suggest the possibility of a prolonged imbalance between native tissue plasminogen activator and plasminogen activator inhibitor 1, a strong inhibitor of the fibrinolytic system. The use of fibrinolytic agents in phase I clinical trials to treat ARDS in critically ill patients has also demonstrated promising results in improving oxygenation.37 , 38 The use of fibrinolytics to treat ARDS in COVID-19 has been proposed,39 used as salvage therapy in New York City,40 and projected to have a large impact on patient outcomes,41 if efficacy is as promising as early use of fibrinolytic therapy to treat ARDS in earlier phase I trials.
The need for more aggressive anticoagulation or fibrinolytic therapy remains unclear, and scant data exist to guide more aggressive therapies to mitigate the hypercoagulable state. Microvascular thrombosis can contribute to ARDS, acute renal failure, and liver dysfunction frequently observed in these patients. It is unclear but biologically plausible that treatment of the hyperthrombotic state of these patients with COVID-19 could prevent or decrease the extent of renal injury seen.
There are inherent limitations to this retrospective descriptive study. First, there was variability in the laboratory testing patterns based on intensivist preference. In addition, the TEG and other coagulation parameters were drawn at variable times of the patient disease processes. Studies done to evaluate for thromboembolic events were performed for clinical suspicion rather than routine screening and, therefore, some VTE or arterial emboli were likely not captured or had delays in diagnosis. Clotting of central lines and intermittent hemodialysis/continuous renal replacement therapy circuits was commonly noted in these critically ill patients with COVID-19, but was not reliably captured in our data set, again potentially leading to an underestimation of hypercoagulable outcomes. Due to the sudden influx of patients with a COVID-19 diagnosis, outcomes data are inherently limited because many patients still remain hospitalized and there are logistical hurdles to effective diagnosis of VTE. In addition, less common outcomes, such as stroke and mortality, would require a large cohort of patients to obtain adequate power to determine whether the coagulation measurements identified that were associated with VTE and renal failure are also associated with these adverse events.
Conclusions
COVID-19 causes not only hypercoagulability, but also fibrinolysis shutdown, which is associated with VTE, stroke, and renal failure. Defining the optimum predictive test for micro- or macrothromboses requires additional study; however, the TEG in conjunction with the d-dimer appears to be a sensitive marker for severity of disease, which will need to be studied in a prospective fashion. A TEG LY30 of 0% and a d-dimer of > 2,600 ng/mL together suggest complete fibrinolysis shutdown and markedly elevated risk of renal failure, VTE, and thrombotic events. The optimum medical therapy to address this hypercoagulable and hypofibrinolytic state is still unknown; however, these results suggest the need to consider therapeutic anticoagulation and potentially tissue plasminogen activator therapy to directly address the failure in the coagulation cascade of this high-risk group of patients.
Author Contributions
Study conception and design: Wright, EE Moore, HB Moore, PK Moore, McIntyre
Acquisition of data: Vogler, PK Moore
Analysis and interpretation of data: Wright, EE Moore, HB Moore, McIntyre
Drafting of manuscript: Wright, Vogler, HB Moore, Wohlauer, Urban
Critical revision: EE Moore, Nydam, McIntyre
Footnotes
Disclosure Information: Drs EE Moore and HB Moore receive researchsupportfrom Haemonetics and Instrumentation Laboratory.
Disclosures outside the scope of this work: Drs EE Moore and HB Moore hold stock options with Thrombo Therapeutics.
Support: REDCap is provided through the Colorado Clinical & Translational Sciences Institute (CCTSI) with the Development and Informatics Service Center grant support (NIH/National Center for Research Resources CCSTI grant UL1 RR025780).
Appendix
eFigure 1.
d-dimer decreases in fibrinolysis shutdown. LY30, clot lysis at 30 minutes.
References
- 1.Johns Hopkins University Johns Hopkins University Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html Available at:
- 2.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subramaniam S., Scharrer I. Procoagulant activity during viral infections. Front Biosci (Landmark Ed) 2018;23:1060–1081. doi: 10.2741/4633. [DOI] [PubMed] [Google Scholar]
- 4.Giannis D., Ziogas I.A., Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127:104362. doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z.F., Su F., Lin X.J. Serum D-dimer changes and prognostic implication in 2009 novel influenza A (H1N1) Thromb Res. 2011;127:198–201. doi: 10.1016/j.thromres.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 7.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panigada M., Bottino N., Tagliabue P. Hypercoagulability of COVID-19 patients in intensive care unit. A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020 Apr 17 doi: 10.1111/jth.14850. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bakhtiari K., Meijers J.C., de Jonge E., Levi M. Prospective validation of the International Society of Thrombosis and Haemostasis scoring system for disseminated intravascular coagulation. Crit Care Med. 2004;32:2416–2421. doi: 10.1097/01.ccm.0000147769.07699.e3. [DOI] [PubMed] [Google Scholar]
- 10.Yao X.H., Li T.Y., He Z.C. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:411–417. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 11.Boonyawat K., Crowther M.A. Venous thromboembolism prophylaxis in critically ill patients. Semin Thromb Hemost. 2015;41:68–74. doi: 10.1055/s-0034-1398386. [DOI] [PubMed] [Google Scholar]
- 12.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020 Apr 10 doi: 10.1016/j.thromres.2020.04.013. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox SE, Akmatbekov A, Harbert J, et al. Pulmonary and cardiac pathology in Covid-19: the first autopsy series from New Orleans. Preprint. Posted online April 20, 2020. bioRxiv. 10.1101/2020.04.06.20050575. [DOI]
- 14.Tang N., Bai H., Chen X. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin S., Huang M., Li D., Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J Thromb Thrombolysis. 2020 Apr 3 doi: 10.1007/s11239-020-02105-8. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brill J.B., Badiee J., Zander A.L. The rate of deep vein thrombosis doubles in trauma patients with hypercoagulable thromboelastography. J Trauma Acute Care Surg. 2017;83:413–419. doi: 10.1097/TA.0000000000001618. [DOI] [PubMed] [Google Scholar]
- 17.Coleman J.R., Kay A.B., Moore E.E. It's sooner than you think: blunt solid organ injury patients are already hypercoagulable upon hospital admission—results of a bi-institutional, prospective study. Am J Surg. 2019;218:1065–1073. doi: 10.1016/j.amjsurg.2019.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cotton B.A., Minei K.M., Radwan Z.A. Admission rapid thrombelastography predicts development of pulmonary embolism in trauma patients. J Trauma Acute Care Surg. 2012;72:1470–1475. doi: 10.1097/TA.0b013e31824d56ad. [DOI] [PubMed] [Google Scholar]
- 19.Gary J.L., Schneider P.S., Galpin M. Can thromboelastography predict venous thromboembolic events in patients with severe extremity trauma? J Orthop Trauma. 2016;30:294–298. doi: 10.1097/BOT.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 20.McCrath D.J., Cerboni E., Frumento R.J. Thromboelastography maximum amplitude predicts postoperative thrombotic complications including myocardial infarction. Anesth Analg. 2005;100:1576–1583. doi: 10.1213/01.ANE.0000155290.86795.12. [DOI] [PubMed] [Google Scholar]
- 21.Harahsheh Y., Duff O.C., Ho K.M. Thromboelastography predicts thromboembolism in critically ill coagulopathic patients. Crit Care Med. 2019;47:826–832. doi: 10.1097/CCM.0000000000003730. [DOI] [PubMed] [Google Scholar]
- 22.Myers S.P., Kutcher M.E., Rosengart M.R. Tranexamic acid administration is associated with an increased risk of posttraumatic venous thromboembolism. J Trauma Acute Care Surg. 2019;86:20–27. doi: 10.1097/TA.0000000000002061. [DOI] [PubMed] [Google Scholar]
- 23.Moore H.B., Moore E.E., Neal M.D. Fibrinolysis shutdown in trauma: historical review and clinical implications. Anesth Analg. 2019;129:762–773. doi: 10.1213/ANE.0000000000004234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moore H.B., Moore E.E., Gonzalez E. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: the spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J Trauma Acute Care Surg. 2014;77:811–817. doi: 10.1097/TA.0000000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore H.B., Moore E.E., Liras I.N. Acute fibrinolysis shutdown after injury occurs frequently and increases mortality: a multicenter evaluation of 2,540 severely injured patients. J Am Coll Surg. 2016;222:347–355. doi: 10.1016/j.jamcollsurg.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leeper C.M., Neal M.D., McKenna C.J., Gaines B.A. Trending fibrinolytic dysregulation: fibrinolysis shutdown in the days after injury is associated with poor outcome in severely injured children. Ann Surg. 2017;266:508–515. doi: 10.1097/SLA.0000000000002355. [DOI] [PubMed] [Google Scholar]
- 27.Meizoso J.P., Karcutskie C.A., Ray J.J. Persistent fibrinolysis shutdown is associated with increased mortality in severely injured trauma patients. J Am Coll Surg. 2017;224:575–582. doi: 10.1016/j.jamcollsurg.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 28.Roberts D.J., Kalkwarf K.J., Moore H.B. Time course and outcomes associated with transient versus persistent fibrinolytic phenotypes after injury: a nested, prospective, multicenter cohort study. J Trauma Acute Care Surg. 2019;86:206–213. doi: 10.1097/TA.0000000000002099. [DOI] [PubMed] [Google Scholar]
- 29.Collen D. Molecular mechanisms of fibrinolysis and their application to fibrin-specific thrombolytic therapy. J Cell Biochem. 1987;33:77–86. doi: 10.1002/jcb.240330202. [DOI] [PubMed] [Google Scholar]
- 30.Biemond B.J., Levi M., Ten Cate H. Plasminogen activator and plasminogen activator inhibitor 1 release during experimental endotoxaemia in chimpanzees: effect of interventions in the cytokine and coagulation cascades. Clin Sci (Lond) 1995;88:587–594. doi: 10.1042/cs0880587. [DOI] [PubMed] [Google Scholar]
- 31.Kuiper G.J., Kleinegris M.C., van Oerle R. Validation of a modified thromboelastometry approach to detect changes in fibrinolytic activity. Thromb J. 2016;14:1. doi: 10.1186/s12959-016-0076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitt F.C.F., Manolov V., Morgenstern J. Acute fibrinolysis shutdown occurs early in septic shock and is associated with increased morbidity and mortality: results of an observational pilot study. Ann Intensive Care. 2019;9(1):19. doi: 10.1186/s13613-019-0499-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hardaway R.M., Johnson D.G. Influence of fibrinolysin on shock. JAMA. 1963;183:597–599. doi: 10.1001/jama.1963.63700070034020a. [DOI] [PubMed] [Google Scholar]
- 34.Hardaway R.M. Disseminated intravascular coagulation with special reference to shock and its treatment. Mil Med. 1965;130:451–460. [PubMed] [Google Scholar]
- 35.Hardaway R.M., Brune W., Geever E. Studies on the role of intravascular coagulation in irreversible hemorrhagic shock. Ann Surg. 1962;155:241–250. doi: 10.1097/00000658-196200000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hardaway R.M., Burns J.W. Mechanism of action of fibrinolysin in the prevention of irreversible hemorrhagic shock. Ann Surg. 1963;157:305–309. doi: 10.1097/00000658-196302000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greene R., Lind S., Jantsch H. Pulmonary vascular obstruction in severe ARDS: angiographic alterations after i.v. fibrinolytic therapy. AJR Am J Roentgenol. 1987;148:501–508. doi: 10.2214/ajr.148.3.501. [DOI] [PubMed] [Google Scholar]
- 38.Hardaway R.M.H.H., Tyroch A.H. Treatment of severe acute respiratory distress syndrome: a final report on a phase I study. Am Surg. 2001;67:377–382. [PubMed] [Google Scholar]
- 39.Moore H.B., Barrett C.D., Moore E.E. Is there a role for tissue plasminogen activator (tPA) as a novel treatment for refractory COVID-19 associated acute respiratory distress syndrome (ARDS)? J Trauma Acute Care Surg. 2020 March 20 doi: 10.1097/TA.0000000000002694. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J., Hajizadeh N., Moore E.E. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): a case series. J Thromb Haemost. 2020 Apr 9 doi: 10.1111/jth.14828. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choudhury R., Barrett C.D., Moore H.B. Salvage use of tissue plasminogen activator (tPA) in the setting of acute respiratory distress syndrome (ARDS) due to COVID-19 in the USA: a Markov decision analysis. World J Emerg Surg. 2020;15:29. doi: 10.1186/s13017-020-00305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]