Abstract
Objectives
Amid the increasing number of pandemic coronavirus disease 2019 (COVID-19) cases, there is a need for a quick and easy method to obtain a non-invasive sample for the detection of this novel coronavirus (severe acute respiratory syndrome coronavirus 2; SARS-CoV-2). We aimed to investigate the potential use of saliva samples as a non-invasive tool for the diagnosis of COVID-19.
Methods
From 27 March to 4 April 2020, we prospectively collected saliva samples and a standard nasopharyngeal and throat swab in persons seeking care at an acute respiratory infection clinic in a university hospital during the outbreak of COVID-19. Real-time polymerase chain reaction (RT-PCR) was performed, and the results of the two specimens were compared.
Results
Two-hundred pairs of samples were collected. Sixty-nine (34.5%) individuals were male, and the median (interquartile) age was 36 (28–48) years. Using nasopharyngeal and throat swab RT-PCR as the reference standard, the prevalence of COVID-19 diagnosed by nasopharyngeal and throat swab RT-PCR was 9.5%. The sensitivity and specificity of the saliva sample RT-PCR were 84.2% (95% CI 60.4%–96.6%), and 98.9% (95% CI 96.1%–99.9%), respectively. An analysis of the agreement between the two specimens demonstrated 97.5% observed agreement (κ coefficient 0.851, 95% CI 0.723–0.979; p < 0.001).
Conclusions
Saliva might be an alternative specimen for the diagnosis of COVID-19. The collection is non-invasive, and non-aerosol generating. This method could facilitate the diagnosis of the disease, given the simplicity of specimen collection and good diagnostic performance.
Keywords: Coronavirus disease 2019, Nasopharyngeal swab, RT-PCR, Saliva, Severe acute respiratory syndrome coronavirus 2, Throat swab
Introduction
Since December 2019, the outbreak of coronavirus disease 2019 (COVID-19), caused by the novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has emerged in the Hubei Province of China and spread to other parts of the world [1,2]. The number of cases has been increasing rapidly, with a case-fatality rate of 2.3% [3].
Detection of SARS-CoV-2 in patient specimens is the first crucial step for the guidance of treatment, effective infection control in the hospital and control of infection in the community. Screening of infection in suspected cases with a nucleic acid amplification test, such as real-time PCR (RT-PCR), in respiratory specimens, is recommended by the WHO [4]. However, the collection of nasopharyngeal and/or oropharyngeal swab specimens is a relatively invasive method and the procedure might put health-care workers at higher risk for disease transmission during patients' gag reflex, cough or sneezing.
The genome of SARS-CoV-2 is closely related to that of severe acute respiratory syndrome coronavirus (SARS-CoV), a causative agent of SARS [5]. Like SARS-CoV, SARS-CoV-2 employs the host-cell angiotensin-converting enzyme 2 as the main host receptor for cellular entry [6]. Previous experimental studies showed a higher level of angiotensin-converting enzyme 2 expression in salivary glands compared with that in the lungs [7], and the epithelial cells lining salivary gland duct were early target cells of SARS-CoV infection in rhesus macaques [8]. SARS-CoV was also detected in saliva samples [9]. This suggested that the salivary glands could be a potential target for SAR-CoV-2 infection, and hence saliva could be a potential sample for SARS-CoV-2 detection.
Recently, SARS-CoV-2 viral load was demonstrated to rise near presentation onset [10], using a saliva sample as the specimen for screening for the disease is appealing. To determine the potential of using a saliva sample for the diagnosis of COVID-19, we conducted a cross-sectional study investigating the correlation of detection of SARS-CoV-2 in saliva samples, and nasopharyngeal and throat swabs in individuals under investigation at an acute respiratory infection clinic at a university hospital in Bangkok, Thailand during the COVID-19 outbreak.
Methods
Study population
A cross-sectional study was conducted among 200 individuals under investigation who attended an acute respiratory infection clinic at Ramathibodi Hosptial, Bangkok, Thailand, between 27 March and 4 April 2020. The inclusion criteria were those who presented with a history of fever or acute respiratory symptoms together with (a) travel history from an endemic area of COVID-19 within 14 days, or (b) history of contact with an individual who was confirmed to have or suspected of having COVID-19. Individuals aged <18 years old were excluded.
Patient characteristics, symptoms at presentation and risk factors were collected. As a standard protocol, nasopharyngeal and throat swabs from individuals were collected using Copan FLOQSwabs® and a sterile tube containing Copan's Universal Transport Medium™ (UTM®; COPAN, Brescia, Italy). Before collecting the swabs, individuals were asked to provide a saliva sample, void of coughing, in a sputum collection container containing the UTM®.
The study protocol was reviewed and approved by the Ethical Clearance Committee on Human Rights Related to Research Involving Human Subjects of the Faculty of Medicine Ramathibodi Hospital, Mahidol University.
Specimen processing
The pairs of specimens were labelled with different laboratory numbers. Technicians who performed specimen processing and RT-PCR were unaware of the names and hospital numbers of the participants. Nasopharyngeal and throat swabs in a tube and saliva samples from the collection container were treated with lysis buffer (bioMérieux, Marcy-l'Étoile, France) to inactivate the SARS-CoV-2. Viral RNA was extracted from 200 μL of the samples within 26 minutes using MagDEA® Dx reagents (Precision System Science, Chiba, Japan) and a fully automated nucleic acid extraction system, according to the manufacturer's instructions.
RT-PCR workflow
The detection of SARS-CoV-2 in the specimens was performed by RT-PCR amplification of the SARS-CoV-2 ORF1ab and N gene fragments, using a SARS-CoV-2 Nucleic Acid Diagnostic Kit (Sansure, Changsha, China), which was approved for the detection of SARS-CoV-2 by the National Medical Products Administration and certified by the China Food and Drug Administration [11]. The lower limit of detection of the test was 200 copies/sample. The detection of human RNase P gene was included in the kit as a control. RT-PCR was performed using the CFX96 Real-Time Detection System (Bio-Rad, Hercules, CA, USA). The result was considered positive if the cycle threshold (Ct) values of both target genes were ≤38, and negative when Ct values of both targets were >38. Retesting was carried out among the samples with discordancy of the Ct values; i.e. samples with one target gene with a Ct value of ≤38 and another showing a Ct value of >38. Among the retesting, the specimens with repeated discordancy were reported as negative. The turnaround time of the diagnosis was approximately 4 hours.
Statistical analysis
Data were analysed for normality and descriptive statistics were presented as a number (%) for categorical variables and mean ± standard deviation (SD) or median (interquartile range; IQR) for continuous variables. Chi-square or Fisher's exact test was used for categorical variables. Sensitivity, specificity, positive predictive value, negative predictive value and a 95% CI were calculated to assess diagnostic performance. The κ coefficient [12] was used to estimate for the agreement between the saliva RT-PCR and nasopharyngeal and throat swab RT-PCR results. All statistical analyses were performed using Stata statistical software version 15.1 (Stata, College Station, TX, USA).
Results
Two hundred sample pairs of nasopharyngeal and throat swabs and saliva samples were collected. Sixty-nine (34.5%) individuals were men. The median (IQR) age was 36 (28–48) years. Median (IQR) onset of symptoms was 3 (2–7) days. Patient characteristics are presented in Table 1 . The prevalences of COVID-19 diagnosed by nasopharyngeal and throat swab RT-PCR, and by saliva RT-PCR in this study were 9.5% and 9.0%, respectively.
Table 1.
Characteristics of patients under investigation diagnosed with COVID-19 by RT-PCR from nasopharyngeal and throat swabs
| Overall (n = 200) | COVID-191 (n = 19) | Non-COVID-19 (n = 181) | p value | |
|---|---|---|---|---|
| Age (years), median (IQR) | 36 (28–48) | 33 (26–44) | 36 (28–48) | 0.207 |
| Male, n (%) | 69 (34.5) | 9 (47.4) | 60 (33.1) | 0.217 |
| Fever (BT ≥ 37.5°C), n (%) | 18 (9.0) | 3 (15.8) | 15 (8.3) | 0.383 |
| BT (°C), mean ± SD | 36.9 ± 0.5 | 37.0 ± 0.5 | 36.9 ± 0.5 | 0.293 |
| Onset of symptoms before the test (days), median (IQR) | 3 (2-7) | 3 (2-11) | 3 (2-7) | 0.378 |
| Symptoms at presentation | ||||
| Cough, n (%) | 108 (54.0) | 11 (57.9) | 97 (53.6) | 0.813 |
| Sore throat, n (%) | 102 (51.0) | 4 (21.1) | 98 (54.1) | 0.007 |
| Runny nose, n (%) | 68 (34.0) | 5 (26.3) | 63 (34.8) | 0.613 |
| Sneezing, n (%) | 26 (13.0) | 1 (5.3) | 25 (13.8) | 0.478 |
| Dyspnoea, n (%) | 73 (36.5) | 9 (47.4) | 64 (35.4) | 0.301 |
| Risk factors | ||||
| Return from other countries, n (%) | 15 (7.5) | 3 (15.8) | 12 (6.6) | 0.162 |
| Close contact, n (%) | 170 (85.0) | 18 (94.7) | 152 (83.9) | 0.478 |
Abbreviations: BT, body temperature; COVID-19, coronavirus disease 2019; IQR, interquartile range; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation.
Detectable SARS-CoV-2 by RT-PCR from nasopharyngeal and throat swabs.
Among 19 individuals diagnosed with COVID-19 by nasopharyngeal and throat swab RT-PCR, the median age was 33 (26–44) years. Three (15.8%) individuals presented with fever, defined as temperature ≥37.5°C. The mean ± SD temperature was 37.0 ± 0.5°C and the median (IQR) onset of symptoms before the test was 3 (2–11) days. Common symptoms at presentation were cough (11; 57.9%), dyspnoea (9; 47.4%) and runny nose (5; 26.3%). When compared with 181 individuals with negative nasopharyngeal and throat swab RT-PCR, only a sore throat at presentation was significantly lower in the individuals with COVID-19. Other characteristics, symptoms at presentation and risk factors were not significantly different (Table 1).
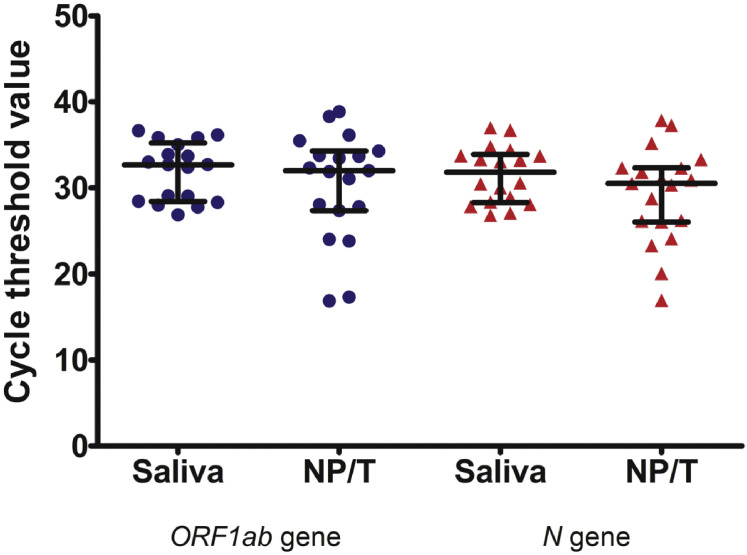
To determine the diagnostic test performance of RT-PCR of the saliva, RT-PCR results of the nasopharyngeal and throat swabs were used as the reference standard. The sensitivity and specificity of saliva samples were 84.2% (95% CI 60.4%–96.6%) and 98.9% (95% CI 96.1%–99.9%), respectively (Table 2 ). Positive predictive value and negative predictive value were 88.9% (95% CI 65.3%–98.6%), and 98.4% (95% CI 95.3%–99.7%), respectively. An analysis of the agreement between the two specimens revealed a 97.5% observed agreement (κ coefficient 0.851, 95% CI 0.723–0.979; p < 0.001). RNase P gene was detectable in all specimens. The median (IQR) Ct values of the ORF1ab and N genes were 32.7 (28.5–35.0) and 31.8 (28.4–33.7), respectively in saliva specimens, and 32.0 (27.4–34.3) and 30.5 (26.1–32.3), respectively, in nasopharyngeal and throat swabs. Ct values of the positive specimens are shown in the Supplementary material Fig. S1.
Table 2.
The comparison for the detection of SARS-CoV-2 RT-PCR between nasopharyngeal and throat swab and saliva sample
| Saliva sample | Nasopharyngeal and throat swab |
Total | |
|---|---|---|---|
| Negative | Positive | ||
| Negative | 179 | 3 | 182 |
| Positive | 2 | 16 | 18 |
| Total | 181 | 19 | 200 |
Discussion
The present study showed the value of testing a saliva sample as a non-invasive method of detection of SARS-CoV-2. The saliva RT-PCR test demonstrated high sensitivity and comparable performance to the current standard of nasopharyngeal and throat swab. The κ coefficient value showed a strong agreement of the diagnosis between the standard nasopharyngeal and throat swab and the saliva sample.
From recent findings, SARS-CoV-2 was detected from posterior oropharyngeal saliva samples, with a notable high viral load at the disease presentation [10,13]. In their protocol, an early morning saliva sample was collected after coughing up by clearing the throat. In our study, a saliva sample was self-generated by the individual, without a need for coughing up. This non-invasive procedure of saliva collection might be less aerosol-generating and might reduce the risk of infection for health-care workers working in the clinic. Although one might argue that the collected specimens were possibly mixed between saliva and sputum, the chance of patient's coughing up phlegm is rather low, as a recent study showed that dry cough was the most common symptom presented in approximately 80% of patients at the onset of the illness [14].
Although testing of saliva samples might provide an advantage as a simple procedure, a comparison of diagnostic studies between saliva sample and confirmed-case bronchoalveolar lavage fluid or convalescence serum titre has not been available. A recent study that detected the virus from multiple sites showed a lower test positivity rate from the nasal swab (63%) compared with bronchoalveolar lavage fluid (93%) [15]. Therefore, a lower detection rate of SARS-CoV-2 from saliva, compared with bronchoalveolar lavage fluid, among individuals with severe disease might be possible. Of interest, two specimens had detectable SARS-CoV-2 from saliva samples, but not from nasopharyngeal and throat swabs. Of these two samples, the Ct values of the ORF1ab and N genes were 33.9 and 34.8, respectively, in one specimen, and 36.2 and 33.7, respectively, in another specimen. These two individuals later reported having anosmia. Therefore, the results of these tests could represent a true infection. The yield of the saliva specimen as a complementary diagnostic test for COVID-19 needs further study.
The study has several strengths. We prospectively collected data on consecutive individuals who were at high risk of COVID-19, including those with acute respiratory symptoms and risk factors, so minimizing potential spectrum effect. In addition, all enrolled patients were verified with the reference standard. As for the limitations, our study only focused on saliva testing among symptomatic individuals under investigation but the spectrum of the disease ranges from asymptomatic, through upper respiratory tract symptoms and pneumonia, to acute respiratory distress syndrome [16,17]. Therefore, the performance of the saliva test for the detection of SARS-CoV-2 among asymptomatic individuals remains unknown. In addition, the number of COVID-19 cases in our study was limited by the decline in the number of cases in Bangkok since April 2020.
With the current situation, involving a shortage of personal protective equipment during the pandemic and moderate risk of infection among health-care workers, a saliva sample is an alternative specimen to collect for the diagnosis of COVID-19, especially in resource-limited settings.
Transparency declaration
All authors declare that there are no conflicts of interests.
Funding
This study was supported by a grant from the Faculty of Medicine Ramathibodi Hospital, Mahidol University.
Contribution of authors
EP, SPW and AP designed the study and wrote the manuscript. PJ, GW and WS performed the study. KB and AP analysed the data. KB and SS edited the manuscript.
Acknowledgements
We are grateful to the physicians and nurses at the acute respiratory infection clinic at Ramathibodi Hospital for their help in collecting the samples.
Editor: F. Allerberger
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.05.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Figs1.
Cycle threshold values of ORF1ab and N genes in the SARS-CoV-2 detectable saliva samples, and nasopharyngeal and throat swab specimens1
References
- 1.Chan J.F., Yuan S., Kok K.H., To K.K., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . 2020. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases.https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspected-human-cases-20200117 Available from: [Google Scholar]
- 5.Xu X., Chen P., Wang J., Feng J., Zhou H., Li X. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63:457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu J., Li Y., Gan F., Du Y., Yao Y. Salivary glands: potential reservoirs for COVID-19 asymptomatic infection. J Dent Res. 2020 doi: 10.1177/0022034520918518. 22034520918518 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 8.Liu L., Wei Q., Alvarez X., Wang H., Du Y., Zhu H. Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in the upper respiratory tracts of rhesus macaques. J Virol. 2011;85:4025–4030. doi: 10.1128/JVI.02292-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang W.K., Chen S.Y., Liu I.J., Chen Y.C., Chen H.L., Yang C.F. Detection of SARS-associated coronavirus in throat wash and saliva in early diagnosis. Emerg Infect Dis. 2004;10:1213–1219. doi: 10.3201/eid1007.031113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.To K.K., Tsang O.T., Leung W.S., Tam A.R., Wu T.C., Lung D.C. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30196-1. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loeffelholz M.J., Tang Y.W. Laboratory diagnosis of emerging human coronavirus infections—the state of the art. Emerg Microbe. Infect. 2020;9:747–756. doi: 10.1080/22221751.2020.1745095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McHugh M.L. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 2012;22:276–282. [PMC free article] [PubMed] [Google Scholar]
- 13.To K.K., Tsang O.T., Chik-Yan Yip C., Chan K.H., Wu T.C., Chan J.M.C. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa149. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Q., Zheng Z., Zhang C., Zhang X., Wu H., Wang J. Clinical characteristics of 145 patients with corona virus disease 2019 (COVID-19) in Taizhou, Zhejiang, China. Infection. 2020 doi: 10.1007/s15010-020-01432-5. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020 doi: 10.1001/jama.2020.3786. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bai Y., Yao L., Wei T., Tian F., Jin D.Y., Chen L. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.2565. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]