Highlights
-
•
Higher inflammatory markers best select tocilizumab treatment.
-
•
The ward based tocilizumab group showed better responses and less infections than ICU tocilizumab group.
-
•
The former group may be the best for evaluating anti-cytokine therapy in COVID-19.
-
•
Poor risk factors for COVID-19 were present in the TOCI treated rather than in the good prognosis standard of care group.
Keywords: Coronavirus, COVID-19, Tocilizumab, Cytokine, Intensive care
Abstract
Objective
Approximately 5% of patients with coronavirus disease 2019 (COVID-19) develop a life-threatening pneumonia that often occurs in the setting of increased inflammation or “cytokine storm”. Anti-cytokine treatments are being evaluated but optimal patient selection remains unclear, and the aim of our study is to address this point.
Methods
Between February 29 to April 6, 2020, 111 consecutive hospitalized patients with COVID-19 pneumonia were evaluated in a single centre retrospective study. Patients were divided in two groups: 42 severe cases (TOCI) with adverse prognostic features including raised CRP and IL-6 levels, who underwent anti-cytokine treatments, mostly tocilizumab, and 69 standard of care patients (SOC).
Results
In the TOCI group, all received anti-viral therapy and 40% also received glucocorticoids. In TOCI, 62% of cases were ventilated and there were three deaths (17.8 ± 10.6 days, mean follow up) with 7/26 cases remaining on ventilators, without improvement, and 17/26 developed bacterial superinfection. One fatality occurred in the 15 TOCI cases treated on noninvasive ventilation and one serious bacterial superinfection. Of the 69 cases in SOC, there was no fatalities and no bacterial complications. The TOCI group had higher baseline CRP and IL-6 elevations (p < 0.0001 for both) and higher neutrophils and lower lymphocyte levels (p = 0.04 and p = 0.001, respectively) with the TOCI ventilated patients having higher markers than non-ventilated TOCI patients.
Conclusion
Higher inflammatory markers, more infections and worse outcomes characterized ventilated TOCI cases compared to ward based TOCI. Despite the confounding factors, this suggests that therapy time in anti-cytokine randomized trials will be key.
1. Introduction
The outbreak of novel coronavirus 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a global pandemic [1]. About twenty-five percent of patients have a seriously ill disease. A fraction of them may develop a very severe pneumonia which may progress to acute respiratory distress syndrome (ARDS) or end-organ failure that may be associated with a cytokine storm syndrome [2]. Laboratory features associated with ARDS or death included neutrophilia, coagulation dysfunction [e.g., higher lactate dehydrogenase (LDH) and D-dimer] [3]. Markedly high levels of interleukin (IL)-2R, IL-6, IL-10, and TNF-α and the absolute numbers of CD4+ and CD8+ T lymphocytes being markedly low seem to characterize the most severe cases [4]. Starting from the first preliminary experience on the apparent efficacy of tocilizumab in COVID-19 pneumonia [5], many multicenter trials are ongoing to test anti-cytokine treatments in critically ill patients.
Nevertheless, robust data to predict the outcome of COVID-19 pneumonia after the hospital admission are still lacking [6], though they are urgently needed in order to facilitate the assessment of anti-cytokine treatment efficacy in worse prognosis patient groups and not milder disease. The aim of this retrospective study was to evaluate baseline laboratory and immunological features in patients hospitalized for COVID-19 pneumonia and to explore such parameters in relationship to standard of care (SOC group) therapy versus anti-cytokine therapy, mainly tocilizumab, (TOCI group) that was mostly used either in ventilated patients in the ICU or non-invasively ventilated patients, mostly in the ward setting. Our single centre experience and approach showed that the milder hospitalized SOC group faired well as did cases with cytokine storm treated with tocilizumab outside of the ICU setting without ventilator support. Severe complications including bacterial infections complicated tocilizumab in the ICU setting but not ward-based tocilizumab therapy. Therefore, randomized trials should target non-ICU patients to prevent cytokine storm evolution.
2. Methods
This study was undertaken to identify laboratory features for more serious COVID-19 disease (i.e., to determine which cases that might theoretically benefit from anti-cytokine drugs). In this monocentric retrospective case-control study, the clinical and immunological characteristics of 111 consecutive patients with COVID-19 were analyzed. Patients were admitted to our hospital from February 29 to April 6, 2020. All but six patients presented to our hospital with six cases transferred from three other hospitals (all of whom eventually received tocilizumab).
Oral or written consent was obtained from patients. The study was conducted in accordance with the ethical principles of the Helsinki Declaration and ethical approval was given by local Ethics Committee (CEUR-2020-Os-102).
Besides clinical evaluation, the level of CRP and IL-6, when available, guided the decision towards anti-cytokine treatments. Clinical decisions for the treatment of all these patients were taken usually within the first week after the admission, and during this time, the laboratory tests were repeated. Demographic, clinical and laboratory characteristics, treatments and outcome data were collected. Identification of cases of COVID-19 virus was based on the detection of unique sequences of virus RNA by nucleic acid amplification tests (NAAT) such as RT-PCR with confirmation by nucleic acid sequencing. The following genes were investigated: E gene for screening and then RdRp and N genes of SARS-CoV-2 for confirmation [7].
Some laboratory data analysed at the admission are reported in Table 1 , including flow cytometry analysis with antibodies for the following subpopulations: CD19+ B cells, CD3+ CD4+ T cells, CD3+ CD8+ T cells, CD56+ NK cells, platelet count (cell/μL) and serum IL-6 (pg/mL), measured by CE_IVD electrochemiluminescence immunoassay (Elecsys IL6, Cobas, physiological range < 7 pg/mL) with results being available within 48 h.
Table 1.
Main comparisons between treatment groups at day 0 (hospital admission).
| TOCI (N = 42) | Number of available observations, N (%) | SOC (N = 69) | Number of available observations, N (%) | p value | |
|---|---|---|---|---|---|
| Age, mean ± SD | 62.4 ± 11.8 | 42 (100) | 56.2 ± 14.2 | 69 (100) | 0.02 |
| Gender, male (%) | 33 (78.6) | 42 (100) | 44 (63.8) | 69 (100) | 0.1 |
| Days from onset to admission, median (IQR) | 6 (3.25−7) | 42 (100) | 7 (3−9.5) | 69 (100) | 0.18 |
| Hypertension (%) | 20 (47.6) | 42 (100) | 21 (30.4) | 69 (100) | 0.11 |
| Charlson’s index ≥ 2 (%) | 5 (11.9) | 42 (100) | 12 (17.4) | 69 (100) | 0.44 |
| Antivirals* (%) | 42 (100) | 42 (100) | 54 (78.3) | 69 (100) | 0.003 |
| Antimalarials** (%) | 39 (92.9) | 42 (100) | 53 (76.8) | 69 (100) | 0.05 |
| Glucocorticoids*** (%) | 16 (38.1) | 42 (100) | 0 | 69 (100) | <0.0001 |
| Antibiotics# (%) | 12 (28.6) | 42 (100) | 9 (23.1) | 69 (100) | 0.07 |
| LMWH (%) | 31 (73.8) | 42 (100) | 15 (21.7) | 69 (100) | <0.0001 |
| WBC count (cells/μL), median (IQR) | 5540 (4270−7140) | 39 (92.9) | 5230 (3705−6305) | 69 (100) | 0.14 |
| Neutrophil count (cells/μL), median (IQR) | 4565 (3062.5−6190) | 34 (80.2) | 3670 (2285−4905) | 69 (100) | 0.04 |
| Lymphocytes (cells/μL), median (IQR) | 685 (545−1022.5) | 34 (80.2) | 940 (760−1195) | 69 (100) | 0.001 |
| Neutrophil/lymphocyte ratio, median (IQR) | 5.6 (3.5−11.8) | 34 (80.2) | 3.7 (2.2−5.4) | 69 (100) | 0.001 |
| CD4+ T cells (cells/μL), median (IQR) | 244.5 (158.75−406.25) | 24 (57.1) | 370 (269.5−497) | 25 (36.2) | 0.02 |
| CD8+ T cells (cells/μL), median (IQR) | 77 (48−195.75) | 24 (57.1) | 180 (111−366) | 25 (36.2) | 0.004 |
| CD19+ B cells (cells/μL), median (IQR) | 97 (67.5−110.5) | 17 (40.5) | 112.5 (83−174.5) | 24 (34.8) | 0.12 |
| CD56+ NK cells (cells/μL), median (IQR) | 128 (56−208.5) | 17 (40.5) | 150 (131−237) | 23 (33.3) | 0.16 |
| Platelet count (cells/μL), median (IQR) | 157,000 (125,500−195,500) | 39 (92.8) | 166,000 (136,000−216,500) | 69 (100) | 0.24 |
| CRP (mg/L), median (IQR) | 79.05 (47.77−186.22) | 40 (95.2) | 24.1 (7.35−72.6) | 69 (100) | <0.0001 |
| D-dimer (ng/mL), median (IQR) | 835 (602−1163) | 14 (33.3) | 660 (270.5−846.5) | 25 (36.2) | 0.1 |
| LDH (IU/L), median (IQR) | 625 (482−829) | 35 (83.3) | 442 (375−577) | 67 (97.1) | 0.001 |
| CK (IU/L), median (IQR) | 134 (84.5−365.5) | 29 (69.0) | 93 (57−146) | 65 (94.2) | 0.007 |
| IL-6 (pg/mL), median (IQR) | 63.5 (37.2−135.5) | 34 (80.9) | 18.5 (10.25−33) | 56 (81.1) | <0.0001 |
Legend: SD, standard deviation; WBC, white blood cells; CRP, C-reactive protein; LDH, lactate dehydrogenase; CK, creatine kinase; LMWH, low molecular weight heparin; TOCI, tocilizumab treatment group; SOC standard of care group.
As prophylactic treatment, before tocilizumab therapy.
Lopinavir/Ritonavir (L/R) in 56 patients (all as first-line antiviral treatment); Darunavir/Cobicistat (D/C) in 57 patients (as first-line antiviral treatment in 40, as second-line in 17); Remdesivir in 3 patients, all as second- or third-line treatment. Seventeen patients switched from L/R to D/C due to side effects.
Hydroxychloroquine in 87 patients; chloroquine in 5 patients.
Glucocorticoids were always administered intravenously at the dose of 1 mg/kg of methylprednisolone in the first two days, then steroids were tapered and finally suspended in 7 days.
Variables were reported as mean and standard deviation or median and interquartile range (IQR), as appropriate, or frequency rates and percentages if categorical; consequently, comparisons between TOCI and SOC groups were made by parametric tests (t-test for two independent samples) or no parametric tests (Mann--Whitney test) for continuous variables. Proportions were compared by χ2 test, or Fisher exact test. Bivariate correlation was made by two tailed Pearson or Spearman tests. All statistical analyses were performed using SPSS version 15.0 software (SPSS Inc.). For unadjusted comparisons, a 2-sided α of less than 0.05 was considered statistically significant. No corrections were made for multiple comparisons due to the explorative nature of the study.
When the laboratory parameters were available, the patients were classified into two groups: the first group comprised 42 cases who developed a serious COVID-19 disease that were deemed suitable for tocilizumab 8 mg/kg intravenously as a single infusion. In TOCI failures, two patients were then treated with anakinra 200 mg/day subcutaneously for three consecutive days. A second group of 69 cases who received supportive therapy [standard of care group (SOC)] comprised those initially admitted to the hospital for COVID-19, and who were treated with SOC based on clinical and laboratory features (Table 1).
3. Results
3.1. Patients’ characteristics and outcome
Table 1 reports the main demographic and clinical features of the two groups. Patients were predominantly male (77/111, 69.4%) with a mean age of 58.5 ± 13.6 years. Patients in TOCI were slightly older than SOC (p = 0.02) (Table 1). Globally, at the hospital admission, resting oxygen saturation equal or below 93% was available for 45 patients (40.5%).
Antiviral treatments were employed in 100% of TOCI group and 80% of SOC group (Table 1). Notably, nearly 40% of TOCI group received glucocorticoids but none of the SOC group did (Table 1). There was no difference between groups regarding the time of reaching a negative swab test (supplemental file).
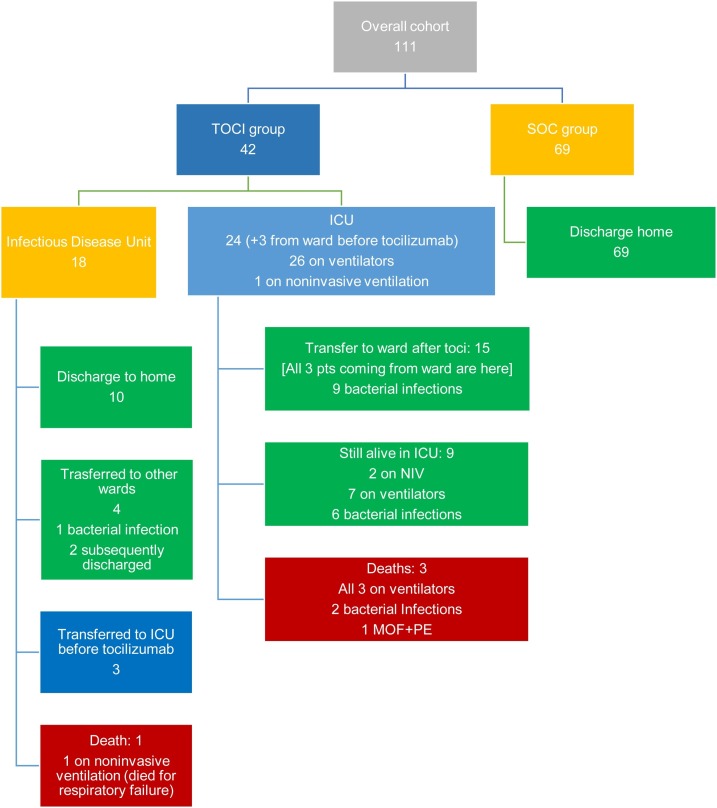
Among TOCI group, 18 (43%) patients were originally referred to the Infectious Disease Unit with three being subsequently transferred to ICU before tocilizumab administration (Fig. 1 ) with 24/42 patients (57%) ICU transfers within 24 h of hospital admission. The majority of patients received tocilizumab in the ICU (27/42, 64.3%) with the remaining 15 cases receiving TOCI on the ward. Tocilizumab was administered after a mean time of 8.4 ± 3.7 days from disease onset as add-on treatment. Of the 27 patients that were transferred to ICU, 26 (96.3%) were intubated with subsequent tracheostomies in 8 (7.2%), while only one was on noninvasive ventilation.
Fig. 1.
The chart illustrates the outcomes of the two treatment groups.
Legend: TOCI, tocilizumab treatment group; SOF, standard of care group; MOF, multi-organ failure; ICU, intensive care unit; NIV, non-invasive ventilation; PE, pulmonary embolism.
There were no fatalities in the SOC group (Fig. 1). Overall, at April 18, 2020, 4/42 TOCI patients had died (9.5%). Of the TOCI ventilated patients 15/26 (57.7%) had a good outcome. When combined with fatality rate, 11/26 (42.3%) patients in the TOCI ventilated group can be deemed as non-responders. By contrast, 15/16 (93.7%) TOCI non-ventilated patients can be deemed as responders with a single fatality (Fig. 1). Importantly, at the hospital admission, TOCI patients who required invasive ventilation showed higher levels of inflammation markers, higher LDH and, notably, lower lymphocyte count than non-ventilated TOCI patients (Table 2 ).
Table 2.
Laboratory marker comparison between the two Tocilizumab treated subgroups.
| TOCI on NIV/O2 (N = 16) | TOCI on ventilators (N = 26) | p value | |
|---|---|---|---|
| CRP, mg/L | 59.3 (21.6−112.7) | 114.6 (5.25−210) | 0.04 |
| IL-6, pg/mL | 58 (28.45−78.5) | 78.8 (46−161) | 0.06 |
| WBC, cell/μL | 4425 (3210−6115) | 6180 (5230−8130) | 0.009 |
| Neutrophil, cell/μL | 3130 (2310−4885) | 7235 (5430−9072) | 0.01 |
| Lymphocyte, cell/μL | 1020 (635−1165) | 650 (445−775) | 0.01 |
| NLR | 3.5 (2.5−5.1) | 8.2 (4.7−15.7) | 0.001 |
| LDH, IU/L | 494 (246.5−599) | 744 (580.75−1057) | 0.001 |
| CK, IU/L | 101 (78−179) | 197 (104.5−382.75) | 0.13 |
Legend: TOCI, tocilizumab treatment group; NIV, non-invasive ventilation; CRP, C-reactive protein; IL, interleukin; LDH, lactate dehydrogenase; CK, creatine kinase; WBC, white, blood cell count; NLR, neutrophil to lymphocyte ratio. Data are presented as median (IQR).
Eighteen out 111 patients (16.2%) experienced bacterial superinfection that were almost exclusively in the TOCI group (Fig. 1). Three out of four deaths and 17/18 bacterial complications occurred in ICU (all three deaths as well as all the bacterial complications occurred in patients on ventilators or in the non-ventilated TOCI group (Fig. 1).
While all the patients in the SOC group recovered, in the TOCI group, 9/42 (21.4%) patients completely recovered, and 21/42 (50%) patients showed a clear and rapid improvement after tocilizumab. A rapid improvement on anakinra after tocilizumab occurred in one case. In the 21 recovered TOCI treated group complicating infections arose in 11 (52.4%). In the remaining 12 non-responder patients, four of them died, including one treated with anakinra after tocilizumab failure, and almost all showed co-morbidities including hypertension, obesity, ischemic heart disease or diabetes, or experienced superinfections, which substantially complicated the course. At the last available follow-up on May 13th, i.e. after two months from the first TOCI patient and more than one month from the last TOCI patient, 7/42 TOCI patients had died (16.7%) (another 3 patients from the TOCI-ventilated group), 30/42 (71.4%) were discharged home, whilst 5/42 (11.9%) remained hospitalised, only one of whom in ICU.
3.2. Retrospective laboratory marker comparison between treatment groups
At hospital admission, TOCI group showed a significantly higher level of systemic inflammation as resulted by the significant difference of CRP levels [mg/L, median (IQR)] [79.05 (47.8–186.22) vs 24.1 (7.3–72.6) p < 0.0001)], and IL-6 levels [pg/mL, median (IQR)] [63.5 (37.25–135.5) vs 18.5 (10.25−33), p < 0.0001]. Also, some other laboratory features mirrored a higher level of systemic disease and organ damage in TOCI group, such as LDH [IU/L, median (IQR)] [625 (482−829) vs 442 (375−577), p = 0.001] and CK [IU/L, median (IQR)] [134 (84.5−365.5) vs 93 (57−146), p = 0.007].
The TOCI group showed a significantly higher neutrophil count (cells/μL) [4565 (3062.5−6190) vs 3670 (2285−4905), p = 0.04], lower lymphocyte count [cell/μL, median (IQR)] [685 (545−1022.5) vs 940 (760−1195), p = 0.001], CD4+ T cell [244.5 (158.75−406.25) vs 370 (269.5−497), p = 0.02], CD8+ T cell subpopulation [77 (48−195.75) vs 180 (111−366), p = 0.004]. Also, neutrophil to lymphocyte ratio (NLR) was significantly higher in TOCI group than in SOC group [5.6 (3.5−11.8) vs 3.6 (2.2−5.4), p = 0.001]. The TOCI group also showed basal higher levels of LDH (p = 0.001) and CK (p = 0.007), possibly indicating cardiac injury that is a known bad prognostic sign.
Table 3 A reports the correlations between CRP levels and the levels of the other biomarkers in the whole population (TOCI + SOC) and table 3B resports the same results after excluding those patients with the worst clinical presentation at the admission (N = 24). A moderate to high correlation (>0.5) was found between CRP and the following variables: D-dimer, LDH, neutrophil count and NLR (Table 3 A) .
Table 3.
(A) Bivariate correlations between CRP levels and other biomarkers. (B) Bivariate correlations between CRP levels and other biomarkers by excluding those patients transferred to ICU within 24 h from the admission (N = 24).
| Variable | Spearman’s rho correlation coefficient | p value | Number of observations, N (%) |
|---|---|---|---|
| IL-6 | 0.46 | <0.0001 | 90 (81.1) |
| D-dimer | 0.63 | <0.0001 | 39 (35.1) |
| LDH | 0.62 | <0.0001 | 102 (91.9) |
| CK | 0.23 | 0.03 | 94 (84.7) |
| Total WBC count | 0.49 | <0.0001 | 108 (97.3) |
| Neutrophil count | 0.60 | <0.0001 | 103 (92.8) |
| Lymphocyte count | −0.26 | 0.002 | 103 (92.8) |
| NLR | 0.57 | <0.0001 | 103 (92.8) |
| B | |||
|---|---|---|---|
| Variable | Spearman’s rho correlation coefficient | p value | Number of observations, N (%) |
| IL-6 | 0.42 | 0.0002 | 71 (63.4) |
| D-dimer | 0.62 | 0.0002 | 31 (27.9) |
| LDH | 0.48 | <0.0001 | 82 (73.9) |
| CK | 0.12 | 0.29 | 78 (70.3) |
| Total WBC count | 0.43 | <0.0001 | 87 (78.4) |
| Neutrophil count | 0.50 | <0.0001 | 83 (74.8) |
| Lymphocyte count | −0.1 | 0.38 | 83 (74.8) |
| NLR | 0.44 | <0.0001 | 83 (74.8) |
Legend: IL, interleukin; LDH, lactate dehydrogenase; CK, creatine kinase; WBC, white blood cell count; NLR, neutrophil to lymphocyte ratio.
By excluding those patients admitted to the ICU within 24 h (i.e., the most serious) (Table 3B),correlations between CRP and IL-6, total white blood cell count, neutrophil count, NLR, LDH were still significant (Table 3B).
Furthermore, the same analysis in the whole cohort by splitting the two group (N = 42 for TOCI and N = 69 for SOC), showed that baseline CRP value correlated with IL-6, D-dimer, LDH, WBC, neutrophil count and NLR only in the SOC group, while in the TOCI group, baseline CRP correlated only with LDH, WBC, neutrophil and NLR (data not shown).
4. Discussion
Our retrospective study was designed to evaluate which baseline standardized laboratory features in hospitalized COVID-19 pneumonia may facilitate optimal employment of experimental anti-cytokine therapy [8,9]. Some case reports and one case series on the treatment with tocilizumab have been reported in the literature, suggesting some benefits in seriously ill patients [[10], [11], [12], [13], [14], [15], [16]]. More clearly, our data suggested that tocilizumab treatment in patients with cytokine storm features may be more effective outside of the ICU setting in non-ventilated patients. However, there were differences in the degree of inflammation between non-ventilated and ventilated patients treated with tocilizumab, so it cannot be inferred that use of tocilizumab prior to ICU admission is superior, given the generally milder inflammation in the former group. Also, serious superimposed bacterial infections were largely confined to the ICU. However, looking at long-term follow-up (i.e. at least 30 days after tocilizumab), around 70% of TOCI patients recovered with a fatality rate of 17%.
Our findings confirmed that the milder patient group receiving standard of care therapy without the utilization of tocilizumab all made full recoveries. Our findings do point towards trials focused on the earlier use of such therapeutic strategies. Notably, our SOC and TOCI groups were different in terms of co-treatments, which could have affected the overall outcome, and all of the TOCI cases also received antiviral therapy. These findings are preliminary and the results of ongoing randomized controlled trials will definitely clarify anti-cytokine use.
In our study, neutrophilia, lymphopenia, in particular low CD8 + T cell count rather than CD4+ T cell, higher CRP, higher LDH and higher CK showed the highest significance to distinguish the two patient groups at initial hospital admission. Also, serum IL-6 was significantly higher in the TOCI group, thus reflecting the very high inflammatory state of those patients at baseline. Very recently, IL-6 serum levels were also closely correlated with viral load in critically ill patients and it is important to point out that all our patients belonging to TOCI group received anti-viral agents [17]. Notably, baseline CRP and IL-6 continued to distinguish the two groups (TOCI versus SOC) even after excluding the most seriously ill patients from analyses. Thus, these biomarkers could useful for decision making. Notably, a higher NLR, as well as a higher monocyte to lymphocyte ratio, have been associated with mortality and imaging progression in hospitalized patients for COVID-19 [[18], [19], [20]]. It is well known that NLR is a biomarker for poor outcome even in various cancers [21]. An interesting feature of our TOCI treated cases was that the lymphocyte counts were substantially higher on ward treated subjects suggesting that the COVID-19 pneumonia was not being treated at an earlier time point but that an intrinsically milder group of COVID-19 was being targeted in that setting.
Lymphocyte biology probably plays a great role in the pathogenesis of COVID-19 disease [4,[22], [23], [24], [25]].
Since CD4+ T cells and CD8+ T cells are a crucial arm against infections [26], our findings also indicated that the lymphopenia in the TOCI group may be relevant for secondary infections. Given that, treatment with tocilizumab might favor the persistence of the virus and iatrogenic infections. Anakinra might be safer and more flexible than repeating tocilizumab infusion in seriously ill patients.
A role for anticoagulation is increasingly recognized in severe COVID-19 [27,28]. In our study, a significant correlation between CRP and D-dimer, as well as with LDH and neutrophil count (and NLR) was shown. Very recent data showed that low molecular weight heparin or unfractionated heparin at prophylactic doses are associated with a reduced short-term mortality in more severe COVID-19 patients [28], and most of our patients, particularly, in ICU, were administered heparin which may have impacted on the overall outcome. Moreover, inflammatory diseases carry a higher risk of thrombosis, as seen in chronic autoimmune diseases [29]. It remains to be seen whether the possible efficacy of anti-cytokine therapy may be even to mitigate against immunothrombosis. Increased levels of LDH and CK may also reflect the level of the organ damage in a systemic disease, as occurs in the macrophage activation syndrome [30], where a hypercoagulable state often complicates the course, and it may be the case for COVID-19. Thus, it is not surprisingly that LDH has been already noticed as biomarker of severity as long as neutrophils, in COVID-19 [3,31].
This study has several limitations. It is a retrospective study, with some missing data due to the emergency context in which it has been realized. No conclusions on the efficacy and safety of treatment approach employed can be provided. Six patients were transferred from other hospitals so original baseline values from the first admission were unavailable. About 50% of the TOCI group were admitted to the ICU within 24 h from admission, thus they already presented a more serious disease at the time of admission. The follow-up started from admission to our hospital, however, only 6 patients were transferred from other hospitals. Finally, measurement of viral load was not available, while viral clearance was finally assessed by repeating swab test in almost all the patients (see supplemental material). Nevertheless, the cohort is monocentric and it showed similar characteristics to those reported by Wang et al. [2], thus supporting the results, though preliminary.
To conclude, our study showed that TOCI treated patients COVID-19 pneumonia were at the highest risk of cytokine storm [32]. However, long-term data are reassuring, overall fatality rate being around 17% in TOCI group. Tocilizumab use prior to ventilation in ICU may be optimal since 50% of such cases died, remain ventilated and show serious superinfection. Whether the use of tocilizumab prior to ventilation will be vindicated in randomized trials is of major interest. Our findings also showed that cases receiving tocilizumab on ventilation generally had higher levels of inflammation and lower lymphocyte count than non-ventilated TOCI treated subjects, possibly suggesting that the latter group has an intrinsically milder disease with a better prognosis. Timing of anti-cytokine therapy appears to be a key issue.
5. Contributions
LQ designed data collection tools, monitored data collection for the whole study, wrote the statistical analysis plan, cleaned and analysed the data, drafted and revised the paper. He is guarantor. AS collected the data, analysed the data, and revised the paper. FC, MF, TB, ADM, FB, MP, DP collected the data, analysed the data, and revised the paper. SDV, DM analysed the data, drafted and revised the paper. CT designed data collection tools, analysed the data, revised the paper.
6. Ethical approval information
The study was conducted in accordance with the ethical principles of the Helsinki Declaration. Patients’ consents for using data for research purpose were obtained at the time of hospital admission.
Ethical approval for the present retrospective observational study was given by “Comitato Etico Unico Regionale (CEUR)”, with the following registration number: CEUR-2020-Os-102.
Patients treated with tocilizumab were then enrolled into the observational part of the TOCIVID-19 Italian study (EudraCT: 2020-−001110-38), a single arm, open-label trial on the efficacy and safety of tocilizumab in COVID-19 pneumonia.
Data sharing statement
The data that support the findings of this study are available on request from the corresponding author, [LQ].
Funding info
This research received no external funding.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgement
We thank the following colleagues for their valued contribution to this work: Ginevra De Marchi, MD, Miriam Isola, prof, BS, Sara Zandonella, MD, Ivan Giovannini, MD, Elena Treppo, MD, Donatella Colatutto, MD, Marco Binutti, MD, Giulia Del Frate, MD, Roberto Agarinis, MD, Valeria Manfrè, MD, Daniela Cesselli, prof, MD, Roberta Giacomello, BS, Federica D’Aurizio, MD, Michele Zuliani, MD, Corrado Marescalco, MD.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jcv.2020.104444.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;(395):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen G., Wu D., Guo W. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020 doi: 10.1172/JCI137244. pii: 137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J. Med. Virol. 2020 doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wynants L., Van Calster B., Bonten M.M.J. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. BMJ. 2020;369 doi: 10.1136/bmj.m1328. m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Udugama B., Kadhiresan P., Kozlowski H.N. 2020. Diagnosing COVID-19: The Disease and Tools for Detection ACS Nano. acsnano.0c02624. Published online 2020 Mar 30. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W., Zhao Y., Zhang F. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the Perspectives of clinical immunologists from China. Clin. Immunol. 2020;25 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGonagle D., Sharif K., O’Regan A., Bridgewood C. The role of cytokines including Interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 2020 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu X.L., Han M.F., Li T.T. Effective treatment of severe COVID-19 patients with tocilizumab. ChinaXiv. 2020 doi: 10.1073/pnas.2005615117. 202003(00026):V1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michot J.M., Albiges L., Chaput N. Tocilizumab, an anti-IL6 receptor antibody, to treat Covid-19-related respiratory failure: a case report. Ann. Oncol. 2020 doi: 10.1016/j.annonc.2020.03.300. pii: S0923-7534(20)36387-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Luna G., Habibi A., Deux J.F. Rapid and severe Covid-19 pneumonia with severe acute chest syndrome in a sickle cell patient successfully treated with tocilizumab. Am. J. Hematol. 2020 doi: 10.1002/ajh.25833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cellina M., Orsi M., Bombaci F., Sala M., Marino P., Oliva G. Favorable changes of CT findings in a patient with COVID-19 pneumonia after treatment with tocilizumab. Diagn. Interv. Imaging. 2020 doi: 10.1016/j.diii.2020.03.010. pii: S2211-5684(20)30087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Giambenedetto S., Ciccullo A., Borghetti A. ; GEMELLI AGAINST COVID-19 group (Members are listed in the Acknowledgments section). Off-label use of Tocilizumab in patients with SARS-CoV-2 infection. J. Med. Virol. 2020 doi: 10.1002/jmv.25897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu B., Xu X., Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J. Transl. Med. 2020;18(1) doi: 10.1186/s12967-020-02339-3.1. 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J. Med. Virol. 2020 doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X., Zhao B., Qu Y. Detectable serum SARS-CoV-2viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa449. ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y., Du X., Chen J. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. pii: ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin C., Zhou L., Hu Z. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. J. Infect. 2020 doi: 10.1016/j.jinf.2020.04.002. pii: S0163-4453(20)30208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Z., Shi J., He Z. Predictors for imaging progression on chest CT from coronavirus disease 2019 (COVID-19) patients. Aging (Albany NY) 2020:12. doi: 10.18632/aging.102999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Templeton A.J., McNamara M.G., Šeruga B. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J. Natl. Cancer Inst. 2014;106(6) doi: 10.1093/jnci/dju124. dju124. [DOI] [PubMed] [Google Scholar]
- 22.Ji D., Zhang D., Xu J. Prediction for progression risk in patients with COVID-19 pneumonia: the CALL score. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa414. pii: ciaa414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 Pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. pii: S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiong Y., Liu Y., Cao L. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microbes Infect. 2020;9:761–770. doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao J., Zhao J., Mangalam A.K. Airway memory CD4(+) T cells mediate protective immunity against emerging respiratory coronaviruses. Immunity. 2016;44:1379–1391. doi: 10.1016/j.immuni.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciceri F., Beretta L., Scandroglio A.M. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit. Care Resusc. 2020 doi: 10.51893/2020.2.pov2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang N., Bai H., Chen X. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quartuccio L. Risk of thrombosis in Sjögren syndrome: the open question of endothelial function immune-mediated dysregulation. J. Rheumatol. 2017;44:1106–1108. doi: 10.3899/jrheum.170462. [DOI] [PubMed] [Google Scholar]
- 30.Li X., Xu S., Yu M. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 2020 doi: 10.1016/j.jaci.2020.04.006. pii: S0091-6749(20)30495-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henderson L.A., Canna S.W., Schulert G.S. On the alert for cytokine storm: immunopathology in COVID-19 . 2020 doi: 10.1002/art.41285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quartuccio L., Semerano L., Benucci M., Boissier M.C., De Vita S. Urgent avenues in the treatment of COVID-19: Targeting downstream inflammation to prevent catastrophic syndrome. Joint Bone Spine. 2020;87:191–193. doi: 10.1016/j.jbspin.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.