Abstract
The global coronavirus disease 2019 pandemic continues to escalate at a rapid pace inundating medical facilities and creating substantial challenges globally. The risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in patients with cancer seems to be higher, especially as they are more likely to present with an immunocompromised condition, either from cancer itself or from the treatments they receive. A major consideration in the delivery of cancer care during the pandemic is to balance the risk of patient exposure and infection with the need to provide effective cancer treatment. Many aspects of the SARS-CoV-2 infection currently remain poorly characterized and even less is known about the course of infection in the context of a patient with cancer. As SARS-CoV-2 is highly contagious, the risk of infection directly affects the cancer patient being treated, other cancer patients in close proximity, and health care providers. Infection at any level for patients or providers can cause considerable disruption to even the most effective treatment plans. Lung cancer patients, especially those with reduced lung function and cardiopulmonary comorbidities are more likely to have increased risk and mortality from coronavirus disease 2019 as one of its common manifestations is as an acute respiratory illness. The purpose of this manuscript is to present a practical multidisciplinary and international overview to assist in treatment for lung cancer patients during this pandemic, with the caveat that evidence is lacking in many areas. It is expected that firmer recommendations can be developed as more evidence becomes available.
Keywords: COVID-19, Lung cancer, SARS-CoV-2, Patient care
Introduction
In December of 2019, an atypical pneumonia of unknown origin was reported in patients in Wuhan, the People’s Republic of China. The source was thought to be a wet market called the Huanan Seafood Wholesale Market. Subsequently, it was determined that the agent responsible was an enveloped RNA beta coronavirus, designated as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2, or 2019-nCoV)1 (Fig. 1 ). The condition associated with the SARS-CoV-2 virus was named Coronavirus Disease 2019 (COVID-19), and it was designated a pandemic by WHO on March 11, 2020. Genomic characterization of the virus determined that the agent was distinct from other coronaviruses like severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) and Middle East respiratory syndrome.2 SARS-CoV-2 is highly infectious. As of May 3, 2020, there were 3.6 million documented cases globally with, 248,000 deaths, resulting in a case fatality rate of 6.9%. However, these numbers are likely to be inaccurate given that asymptomatic infections occur and the rate of testing in different countries ranges from four people out of 1 million population being tested in Yemen to 146,000 tested out of 1 million population in Iceland.3 Patients with cancer are at a heightened risk for developing serious complications from COVID-19.4 , 5 As a group, they tend to be of advanced age and have an increased risk of relative immunosuppression from the underlying malignancy and anticancer treatments. Furthermore, patients with lung cancer may have additional comorbidities, including a history of smoking and preexisting lung disease. There are challenges in the management of a patient with lung cancer given the similarities in radiologic findings, respiratory symptoms, and the presence of underlying immunosuppression. In addition, immune checkpoint inhibitors are now widely used in the management of advanced lung cancer. Immune-related pneumonitis from these agents could mimic COVID-19 radiologically. In this article, we aim to provide guidance in the management of lung cancer patients during this period through a multidisciplinary perspective on the basis of clinical experience and the available data in the literature.
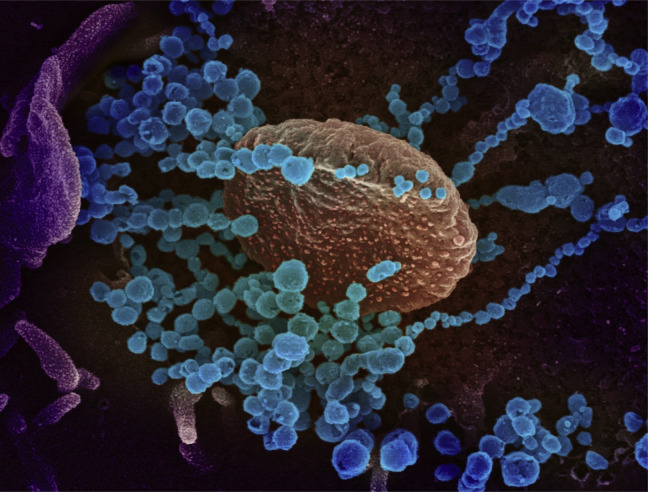
Figure 1.
This is a scanning electron microscope image, which shows severe acute respiratory syndrome coronavirus 2 (round blue objects) emerging from the surface of cells cultured in the laboratory. Severe acute respiratory syndrome coronavirus 2, also known as 2019 novel coronavirus, is the virus that causes coronavirus disease 2019. The virus exhibited here was isolated from a patient in the United States. Adapted from National Institute of Allergy and Infectious Diseases - Rocky Mountain Laboratories (NIAID-RML).
Diagnosis of COVID-19
According to WHO and Centers for Disease Control and Prevention (CDC), the preferred current diagnostic method is the detection of SARS-CoV-2 nucleic acid in patient specimens.6 , 7 SARS-CoV-2 preferentially proliferates in type II alveolar cells (AT2) and the peak of viral shedding appears 3 to 5 days after the onset of disease. Therefore, an initial negative nucleic acid test does not exclude a positive on subsequent days, as the negative predictive value is relatively low. Appropriate samples include the upper airways (pharyngeal swabs, nasal swabs, nasopharyngeal secretions), the lower airways (sputum, bronchoalveolar lavage fluid specimens), and also blood, feces, urine, and conjunctival secretions. Sputum and other lower respiratory tract specimens have a high positive rate of nucleic acids.8 When test material is scarce, the diagnosis and case definition can be made on the basis of clinical symptoms and radiologic characteristics.9 WHO has advised every country to establish and publish their case definitions appropriate for their region.
Serologic tests are currently being developed. However, because of a lack of sensitivity of a number of tests, and more importantly, the delay from the time of infection to antibody development, these tests may instead serve as a useful tool for population-based analysis for epidemiologic purposes, whereas reverse transcription–polymerase chain reaction (RT-PCR) remains the best methodology to detect acute infections.
Disease Characteristics
The main modes of SARS-CoV-2 transmission are through respiratory droplets and contact,10, 11, 12 whereas airborne transmission may be possible for situations in which aerosols are generated, such as endotracheal intubation and during bronchoscopy.13 The mean incubation period in patients is approximately 4 to 5.2 days and the mean serial interval, or time between the onset of symptoms in one individual and onset in a serial individual, is 7.5 days.11 , 14 , 15 Viral load is more similar to influenza and it does not differ between symptomatic and asymptomatic patients.16 Like SARS-CoV-1, the SARS-CoV-2 virus seems to use the angiotensin-converting enzyme 2 (ACE2) receptor to enter host cells.17 ACE2 receptors are highly expressed in cells in blood vessels, heart, kidney, and AT2 cells in the lungs. The latter is important for the synthesis, storage, and secretion of surfactant, a substance that prevents atelectasis of lung tissue by lowering the surface tension of alveoli. The destruction of AT2 cells may play a key role in the development of severe pulmonary symptoms in patients with COVID-19. It has been shown that the ACE2 receptor is markedly more expressed in chronic obstructive pulmonary disease patients, and in current smokers versus former smokers (compared with never-smokers) and shows an inverse relationship with forced expiratory volume in 1 second.18
Recent reports from the People’s Republic of China and Italy suggest that approximately 60% to 90% of patients present with fever, 55% to 70% with cough, and 33% with dyspnea.19 Other symptoms, which includes nausea, vomiting, and diarrhea, were observed in less than 5% of patients. In the United States, the CDC added other symptoms to this list—myalgia, fatigue, headache, sore throat, and new-onset loss of taste or smell. Laboratory abnormalities such as lymphopenia (83.2%), thrombocytopenia (36.2%) and leukopenia (33.7%) were observed in hospitalized patients.14 Radiologic findings will be discussed in a subsequent section.
Approximately 15% to 20% of patients will develop severe symptoms and may require hospitalization and intensive care. Severe complications may include bilateral pneumonia (75%) acute respiratory distress syndrome (17%) and multiorgan failure (11%).20, 21, 22 Emerging data indicate that vascular inflammation can result in diffuse microangiopathy with thrombosis, which contributes to multiorgan failure. In addition, pulmonary embolism, myocardial ischemia, and cerebrovascular accidents have been reported (Table 1 ).23
Table 1.
Symptoms, Signs, and Complications of Coronavirus Disease 2019
| Type | Symptom or Sign |
|---|---|
| Common symptoms or signs (2–14 d after exposure): >30% |
|
| Other symptoms: 5%–15% |
|
| Rare symptoms or signs <5% |
|
| Complications |
|
ARDS, acute respiratory distress syndrome
Those with the most severe disease on hospitalization tend to be older and have preexisting underlying diseases.14 , 24 Among the 355 patients who died from COVID-19, 70% were men, 30% had ischemic heart disease, 36% had diabetes, 25% had atrial fibrillation, and 20% had cancer.25 Only 0.3% had no preexisting diseases. Patients with a higher Sequential Organ Failure Assessment score and D-dimer greater than 1 μg/mL were found to be at higher risk for death from COVID-19.24 Any potential relationship between the smoking status of the patients and the onset or severity of the disease remains unknown.
Diagnostic Strategies for Patients With Lung Cancer
The primary aim of diagnosis in a patient with suspected lung cancer is to obtain tissue specimens for histologic diagnosis, using the least invasive method. But the risk of spreading SARS-CoV-2 infection needs to be considered. In addition, there is a risk of slow-down of diagnostic procedures as patients are afraid of going to the hospital during the current pandemic. Bronchoscopy, an aerosol-generating procedure, should be avoided whenever possible.
The American Association for Bronchology and Interventional Pulmonology has issued a statement on the safe and effective use of bronchoscopy in patients with suspected or confirmed COVID-19.26 The following applies to suspected and confirmed patients with lung cancer:
-
•
Elective Bronchoscopy for lung mass, bronchial mass, mediastinal, or hilar lymphadenopathy, lung infiltrates, and mild-to-moderate airway stenosis should be postponed until after full recovery from COVID-19;
-
•
Bronchoscopy for urgent or emergent reasons should be considered with all precautionary measures only if it is a lifesaving intervention, e.g., massive hemoptysis, benign or malignant severe airway stenosis or suspicion of an alternative or secondary infectious cause or malignant condition with a resultant substantial endobronchial obstruction or rapidly progressing malignancy.
The Society of Interventional Radiology has categorized all procedures, such as transthoracic needle biopsies, as elective, urgent, and emergent.27 Procedures that can be delayed or rescheduled in cases of worsening local infection rates should be determined on an individual basis.
Pathologic Features
Pathologically, in the early and presymptomatic phase, the lungs exhibit exudation of proteinaceous fluid, mixed with patchy inflammatory cellular infiltrates and focal reactive hyperplasia of type II pneumocytes. Although patchy alveolar epithelial injury can be seen, hyaline membrane formation, a pivotal feature of diffuse alveolar damage, is not evident.28
In severe and fatal cases, limited gross findings from autopsy studies have shown large areas of lung consolidation and hemorrhage, with mucus plugs evident in small airways.29 Damage to alveolar epithelial cells with desquamation and mononuclear inflammatory cell infiltration in airspaces has been observed.30 , 31 Thin to quite prominent hyaline membranes, hyperplasia of type II pneumocytes, congestion of septal capillary vessels, and microthrombi are also frequently seen.30 , 32 In addition to these changes of ongoing diffuse alveolar damage, alveolar hemorrhage, and consolidation by fibroblastic proliferation with the extracellular matrix and fibrin-forming clusters in airspaces can be prominent.30 , 32 Others have observed mucous plugs in the alveoli and bronchioles and the activation of alveolar macrophages.33 In some patients, consolidation consisted of abundant intra-alveolar neutrophils, consistent with superimposed bacterial bronchopneumonia.30
Several studies have suggested the presence of fibrosis in the lungs of COVID-19 patients.32 , 33 However, it seems this mainly corresponds to microscopic findings of fibroblast proliferation with early extracellular matrix production in small airways and airspaces, with thickened alveolar walls and interstitial areas with increased stromal cells and CD4-positive lymphocytes.30, 31, 32 Whether or not true pulmonary fibrosis occurs in COVID-19 patients will depend on longitudinal follow-up of the long-term survivors, especially when symptoms appear and biopsies, when indicated, are examined.
In summary, on the basis of limited data that is currently available, the basic underlying pathology of COVID-19 pneumonia seems to be that of diffuse alveolar damage with varying degrees of organization. In addition, embolic events are frequent with vascular damage.
Imaging Features of SARS-CoV-2 Infection (COVID-19) and Implications for Patients With Lung Cancer
Typical chest radiographic features of COVID-19 patients include consolidation with limited cases of pleural effusion.34 Chest radiographs are less sensitive in the detection of COVID-19 with a sensitivity of around 30% to 70%.35 However, with the current limitations in diagnostic availability and kit performance, the total positive rate of RT-PCR from nasopharyngeal swabs has been reported to be 59% at the initial presentation.36 It is in this setting that European radiologists have used diagnostic algorithms to evaluate the use of first-line triage diagnostic radiographs.34
The Radiological Society of North America has recently published an Expert Consensus Statement on Reporting Chest Computed Tomography (CT) Findings Related to COVID-19.37 This attempts to categorize CT findings of COVID-19 pneumonia into typical, indeterminate, atypical appearances, and negative for pneumonia.37 The typical CT appearances specific for COVID-19 pneumonia is listed as peripheral, bilateral ground-glass opacities (GGOs) with or without consolidation or visible intralobular lines (crazy paving), multifocal GGO or rounded cellular structure with or without consolidation or visible intralobular lines and reverse halo sign or other findings of organizing pneumonia (seen later in the disease).
The main CT findings of COVID-19 based on the duration of symptom onset so far described are the following38, 39, 40:
-
(1)
Early stage: 0 to 4 days after onset of flulike symptoms; normal CT scans in up to 50% of patients or scans with small subpleural GGO (Fig. 2 A), mainly in the lower lobes. Typical CT findings are infrequently observed.
-
(2)
Progressive stage: 5 to 8 days after onset of symptoms; peripheral focal or multifocal GGO affecting both lungs in approximately 50% to 75% of patients, which then rapidly develop into crazy paving pattern and areas of consolidation, typically affecting both lungs (Fig. 2 B).
-
(3)
Peak stage: 9 to 13 days after onset of symptoms; as the disease progresses, crazy paving and consolidation with air bronchograms become the dominant findings (Fig. 3 A and B).
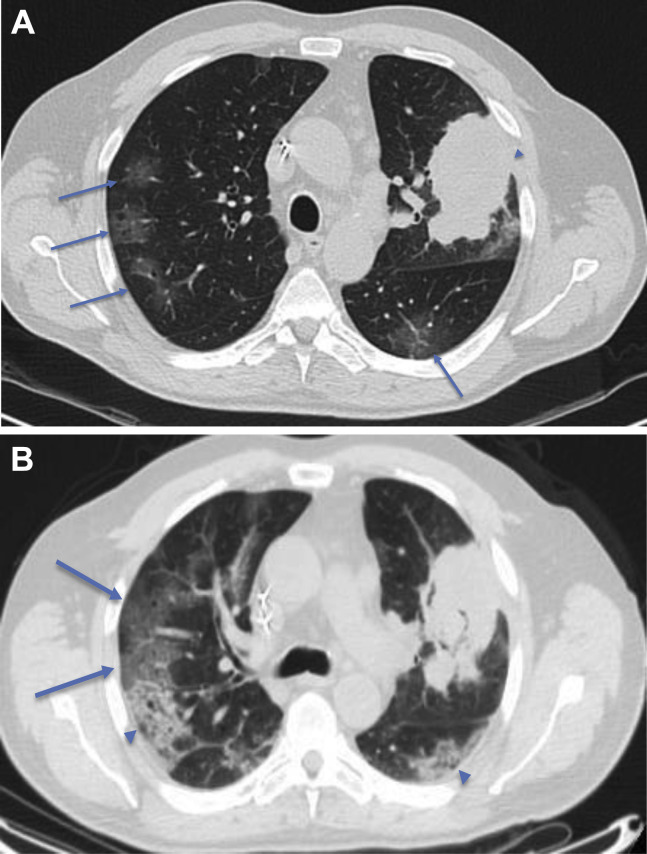
Figure 2.
(A) Early stage COVID-19 CT findings: axial CT image of the lungs of a 67-year-old Italian man presenting with hemoptysis. This CT image exhibits a left upper lobe mass (arrowhead) histologically proven to be adenocarcinoma. There are also peripheral, subpleural GGOs (arrowed) and the patient was confirmed on second throat RT-PCR swab test to also have COVID-19. (B) Progressive stage COVID-19 CT findings: reconstructed axial lung image from a CT-PET scan done for the same patient 2 days later, which exhibited progression of the GGOs into areas of crazy paving (arrows) and consolidation (arrowheads). COVID-19, coronavirus disease 2019; CT, computed tomography; GGOs, ground-glass opacities; PET, positron emission tomography; RT-PCR, reverse transcription–polymerase chain reaction.
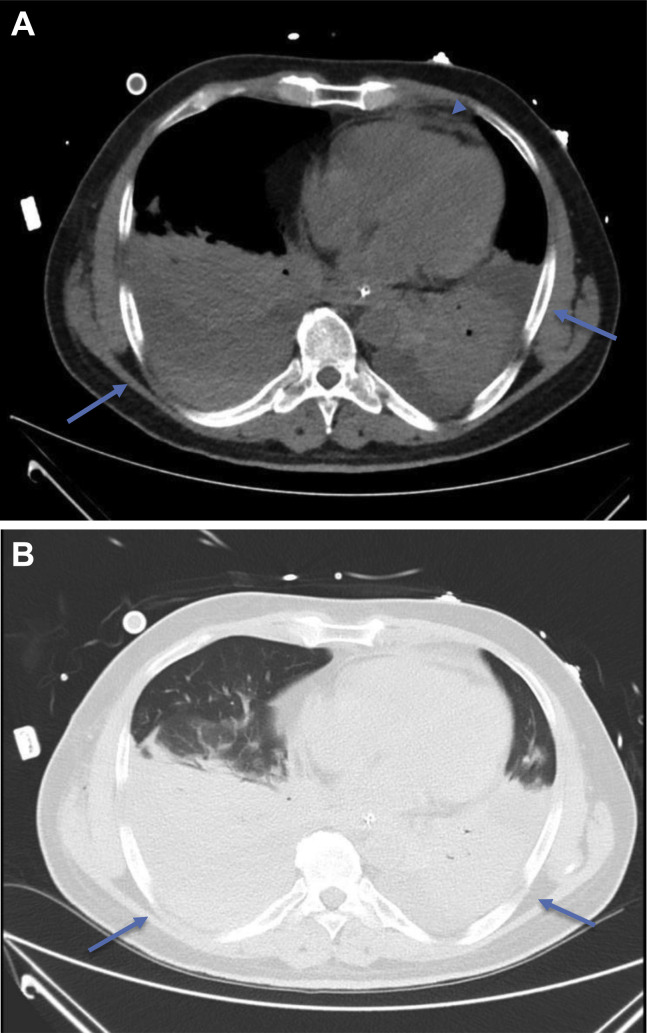
Figure 3.
Peak stage COVID-19 CT findings: axial CT images of the mediastinum (A) and lungs (B) of a 54-year-old Chinese man on day 13 of onset of symptoms exhibiting large bilateral pleural effusions with dense dependent consolidation at the lower lobes (arrows). Trivial pericardial effusion is also seen (arrowhead). Partially imaged ECMO catheter overlying the right anterior chest wall. COVID-19, coronavirus disease 2019; CT, computed tomography; ECMO, extracorporal membrane oxygenation.
These stages are then followed by a slow clearing starting approximately at (but not before) one month after onset of symptoms. The reported sensitivities of CT images for COVID-19 were 60% to 98% but had a low specificity (25% to 53%).41 CT features such as bilateral involvement, peripheral distribution, and lower zone dominance can also be assessed on chest radiograph.34
A noncontrast CT scan is recommended as intravenous contrast may mask subtle GGO.38 Axial reconstruction should be performed without a gap on 0.625 to 5 mm axial slice thickness depending on institutional logistics, data storage, and processing capabilities.
Atypical CT findings are only seen in a small minority of patients and should raise concern for superimposed bacterial pneumonia or other differential diagnoses.39 Such findings include the following: mediastinal lymphadenopathy, pleural effusions, multiple tiny pulmonary nodules, tree-in-bud opacities, and cavitation. Pneumothorax and the halo sign are also rarely seen.20 , 42
The American College of Radiology does not recommend the use of chest radiograph or CT for the screening of COVID-19 in patients without symptoms as imaging findings are not specific and may overlap with those of other infections and acute lung injury manifesting as organizing pneumonia pattern from drug toxicity, connective tissue disease, or idiopathic causes.41 , 43 However, in symptomatic patients with a high suspicion of COVID-19 but negative PCR, CT scan may make the diagnosis much more likely, especially in individuals without pulmonary comorbidities.
What Does All of This Mean for Patients With Lung Cancer?
GGO and consolidation in COVID-19 could mimic radiotherapy- or chemotherapy and immunotherapy-associated pneumonitis and viral infections, although they tend to be more peripheral. The chemotherapy and immunotherapy-associated pneumonitis seems to be more confluent and perihilar.44 In addition, CT findings suggestive of COVID-19 may be incidentally encountered in patients with lung cancer at the time of diagnosis (Fig. 2 A) or posttreatment. In such situations, the risk of infection should be evaluated by a multidisciplinary team including clinicians and radiologists along with history and the consideration of RT-PCR testing.
It is also important to highlight that CT pulmonary angiography might represent a valuable tool for detection of pulmonary thromboembolism and subsequent management in patients with COVID-19 pneumonia. In fact, elevated D-dimer and thromboembolism are thought to be common findings in these patients, especially those with severe lung damage.45
In summary, although imaging is not recommended as the first line for the screening of COVID-19 in most guidelines, it is currently used in clinical practice in most Western countries at diagnosis and can be used as an adjunct for follow-up of disease progression. The main finding of GGO and consolidation in COVID-19 may mimic treatment-induced pneumonitis (Figs. 4 A and B) or viral pneumonia in lung cancer patients.
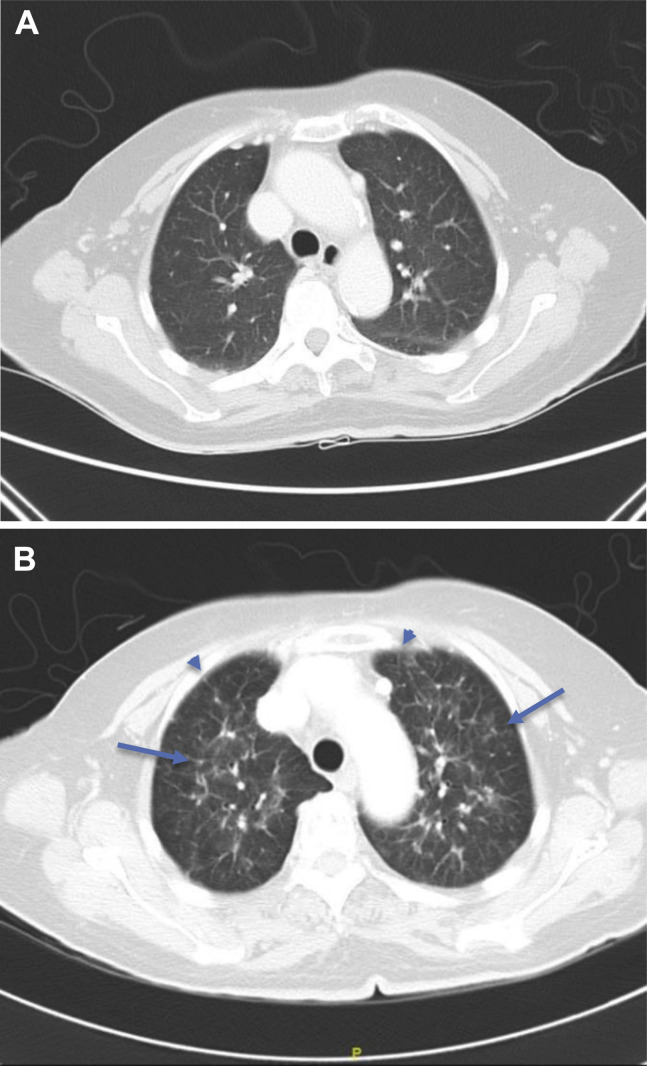
Figure 4.
(A) Axial CT lung image of a 73-year-old Chinese woman with EGFR-positive NSCLC 2 months after starting a third-generation EGFR-TKI. The upper lobes do not reveal any abnormality. (B) Axial CT lung image of the same patient 4 months after starting a third-generation EGFR-TKI. The upper lobes now reveal patchy ground-glass changes (arrows) with interstitial thickening (arrowheads) in a perihilar distribution consistent with EGFR-TKI–induced pneumonitis. CT, computed tomography; TKI, tyrosine kinase inhibitor.
Management of COVID-19
Currently, there is no specific validated treatment for COVID-19, and management comprises of supportive and symptomatic care and instituting recommended infection prevention and control measures. There are anecdotal reports and preclinical data supporting the investigation of potentially efficacious drugs.46 A number of these including chloroquine and its analogs with or without azithromycin, antivirals such as remdesivir (developed against Ebola but found to be ineffective), lopinavir and ritonavir (anti–human immunodeficiency viruses), and monoclonal antibodies against interleukin-6 (tocilizumab47) are currently being studied in clinical trials globally. Multiple studies are also evaluating the use of convalescent plasma in patients with severe COVID-19 (Table 2 ).
Table 2.
Salient Select Therapeutic Clinical Trials in the Treatment of Patients With Coronavirus Disease 2019
| Class | Agent | Mechanism of Actions | Developer | Original Use | Ongoing Trials |
|---|---|---|---|---|---|
| Treatment Of COVID-19 | |||||
| Antiviral | Remdesivir | inhibit RNA-dependent RNA polymerase | Gilead sciences | Ebola and Marburg virus infections |
NCT04252664 NCT04292730 NCT04292899 NCT04280705 NCT04321616 |
| Lopinavir-ritonavir | HIV reverse transcriptase inhibitors | AbbVie | HIV-1 infection |
NCT04255017 NCT04307693 NCT04321616 NCT04330690 NCT04321174 NCT04328285 EudraCT 2020-001113-21 |
|
| Favipiravir (fapilavir) | inhibit RNA-dependent RNA polymerase | Avigan | influenza |
NCT04346628 NCT04349241 NCT04319900 NCT04351295 NCT04310228 |
|
| Others | Hydroxychloroquine | DMARD | Multiple | Malaria, RA, SLE, Q fever, |
NCT04332991 NCT04336332 NCT04303507 NCT04341870 NCT04332094 NCT04341727 NCT04354428 NCT04325893 NCT04343092 NCT04307693 EudraCT 2020-000890-25 |
| ACE inhibitors | ACE-2 inhibitor | Multiple | Hypertension, cardiac failure |
NCT04330300 NCT04338009 NCT04355429 NCT04353596 NCT04351581 |
|
| Chloroquine sulfate | glycosylation of viral ACE-2/inhibition of quinone reductase 2 | Multiple | Malaria |
NCT04321616 NCT04303507 NCT04351191 NCT04341727 |
|
| Azithromycin | inhibit mRNA translation | Pfizer | Respiratory tract infections |
NCT04341870 NCT04341727 NCT04336332 NCT04329832 NCT04354428 |
|
| Convalescent plasma | passive immunotherapy | Multiple | NA |
NCT04355767 NCT04345523 NCT04343755 |
|
| Treatment of COVID-19–induced Cytokine Storm | |||||
| Monoclonal Ab | Tocilizumab | IL-6 receptor antagonist | Roche | RA, GCA, CRS, JIA |
NCT04306705 NCT04310228 NCT04317092 NCT04331795 NCT04332094 NCT04346355 NCT04335071 NCT04320615 NCT04332913 NCT04335305 NCT04339712 NCT04322773 NCT04345445 |
| Sarilumab | IL-6 receptor antagonist | Regeneron, Sanofi | RA |
NCT04315298 NCT04322773 NCT04327388 NCT04341870 |
|
| Lenzilumab | Antihuman GM-CSF monoclonal Ab | Humanigen | CRS | NCT04351152 | |
| Leronlimab | Anti-CCR5 receptor Ab | CytoDyn | HIV-1 infection |
NCT04343651 NCT04347239 |
|
| Eculizumab | anti-C5 antibody | Alexion | PNH, atypical HUS |
NCT04288713 NCT04355494 NCT04346797 |
|
ACE, angiotensin-converting enzyme; Ab, antibody; C5, complement C5; CCR5, chemokine receptor 5; COVID 19, coronavirus disease 2019; CRS, cytokine release syndrome; DMARD, disease-modifying antirheumatic drugs; GCA, giant cell arteritis; HUS, hemolytic uremic syndrome; HIV, human immunodeficiency virus; IL-6, interleukin-6; JIA, juvenile idiopathic arthritis; NA, not applicable; PNH, paroxysmal nocturnal hemoglobinuria; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus.
Results from a few studies have been reported. A study from the People’s Republic of China that randomized 237 symptomatic patients in a 2:1 ratio of remdesivir or placebo found that remdesivir use was not associated with a difference in time to clinical improvement (hazard ratio 1.23 [95% confidence interval 0.87–1.75]). Although it was not statistically significant, patients receiving remdesivir had a numerically faster time to clinical improvement than those receiving placebo among patients with symptom duration of 10 days or less (hazard ratio 1.52 [95% confidence interval 0.95–2.43]).48 Furthermore, interim results after the Data and Safety Monitoring Board mandated the unblinding of a randomized, placebo-controlled trial involving 1063 hospitalized patients with advanced COVID-19 and lung involvement revealed promising results. Patients who received remdesivir had a 31% faster time to recovery than those who received placebo (p < 0.001). Specifically, the median time to recovery was 11 days for patients treated with remdesivir compared with 15 days for those who received a placebo. Results also suggested a not statistically significant survival benefit, with a mortality rate of 8.0% for the group receiving remdesivir versus 11.6% for the placebo group (p = 0.059).49 This study conducted by the United States National Institutes of Health has not been published in the peer-reviewed literature yet, thus, the results are considered preliminary. However, U.S. Food and Drug Administration granted the emergency approval of remdesivir for the treatment of patients with severe COVID-19 on May 1, 2020.
Overall Treatment of Patients With Lung Cancer
Guiding Principles
The major goal of lung cancer management during the COVID-19 pandemic is to minimize the risk of exposing the patient and staff to infection and at the same time manage all life-threatening aspects of the disease.
This can be achieved by limiting face-to-face visits with providers and visits to the clinic or hospital, whenever possible. Patients who need to physically come to the hospital need to be screened for symptoms and tested for SARS-CoV-2 infection if there are any of the typical symptoms discussed above (also in Table 1).
Whenever possible, patients undergoing any invasive procedure or systemic chemotherapy plus immunotherapy should be tested for COVID-19 infection.
Overall clinical trial accrual, in general, has slowed down during the pandemic. New patient accrual has been put on hold at various institutions temporarily. We recommend that if adequate resources are available, clinical trial enrollment should continue with reasonable modifications. To protect trial participants, policy and procedures are being revised to manage study conduct in compliance with control of COVID-19 with appropriate protocol amendments approved by the institutional review boards and sponsors.
Specific recommendations and considerations for different stages of lung cancer are discussed below and outlined in Table 3, Table 4, Table 5 .
Table 3.
Prioritizing Treatment Options for NSCLC
| Clinical Scenario | Treatment Recommendation | Initial Delay, wk | Workup | Comments |
|---|---|---|---|---|
| Stage I, II, and resectable IIIA | ||||
| Stage I and II, untreated | Surgery SBRT for selected stage I | 2–8 | Repeat CT scan if baseline CT >8 wk | |
| Stage I and II, resected | Observation (adjuvant therapy for a subset of stage II disease) | >8 | Expand interval for CT scans up to 4– 6 mo if asymptomatic with 4 y, then annually after y 5 | Consider CT scan but perform remote follow-up |
| Stage IIIa resectable single station | Surgery followed by chemo +/- radiation | <2 | CT scan every 4 mo | |
| Stage III | ||||
| Stage III untreated | Concurrent chemotherapy and radiotherapy but may start with chemotherapy for two cycles | <2 | Same | Consider cisplatin/ pemetrexed Consider G-CSF if administering chemotherapy alone |
| Stage III completed chemoradiotherapy Immune therapy | <2 | Usual workup for immune checkpoint therapy | May delay up to 7 wk per the study, but the sooner the better | |
| Stage II completed treatment | Observation | >8 | Ct scan every 4 mo | Consider CT scan but perform remote follow-up |
| Stage IV | ||||
| Stage IV with actionable targets | ||||
| Untreated | Targeted therapy | <2 | Start on time, perform safety assessments as laboratory or ECG, but do phone clinic instead of in-person visit. Consider performing response assessment after 2 mo | |
| On treatment with disease control targeted therapy | <2 | May expand the disease assessment for 3 mo if clinically stable or longer if on treatment for a long period of time | Do virtual clinics for toxicity notation, management, and any sign of disease progression | |
| Stage IV wild-type | ||||
| Untreated | Chemotherapy alone | <2 | Standard | Consider less immune suppressive agents and use of growth factors or dose reduction as appropriate |
| Chemotherapy and immune therapy combination | <2 | Standard | Need to be very selective | |
| Immune therapy single agent | <2 | Standard | Preferred if PD-L1 score >50% consider the approved longer interval of dosing | |
| On treatment first line | Chemotherapy | |||
| Chemotherapy and immunotherapy | <2 | May do imaging every 3 cycles, if stable | Consider growth factor, aim for a lesser number of cycles (4, if disease stable), and switch to maintenance | |
| Immune therapy | <2 | May do imaging every 3 mo, if stable | Consider switching to maintenance as early as indicated, use a longer interval of administration. Skip cycles if appropriate | |
| <2 | May do imaging every 3 cycles, if stable. | Use approved longer dosing intervals and stop at 2 y. | ||
| On treatment beyond first-line | Chemotherapy | <2 or 2–8 | Extend CT scan to 3 or 4 cycles, if clinically stable | Consider chemotherapy holidays for 2–3 cycles interval. |
| Immunotherapy | <2 or 2–8 | Extend disease assessment interval | Use approved longer dosing intervals | |
| Completed treatment | ||||
| No evidence of disease | Observation | >8 | Extend interval of workup | refer to survival clinics |
| Presence of disease | Observation | 2–8 | Extend the interval of workup | per phone clinic |
CT computed tomography; ECG, electrocardiogram; G-CSF, granulocyte-colony stimulating factor; PD-L1, programmed death-ligand 1; SBRT, stereotactic body radiation therapy.
Table 4.
Prioritizing Treatment Options for SCLC
| Clinical Scenario | Treatment Recommendation | Initial Delay, wk | Workup | Comments |
|---|---|---|---|---|
| Limited Stage | ||||
| Untreated | Concurrent chemotherapy and radiotherapy | <2 | standard | if radiation therapy is not available start with chemotherapy and add XRT as early as possible |
| On treatment | Concurrent chemotherapy and radiotherapy followed by chemotherapy | <2 | standard | continue with CCRT, keep cycles of chemotherapy to 4, use growth factors away from XRT |
| Completed treatment | PCI | 2–8 | standard | |
| Observation | >8 | may delay imaging for a mo | Flow up by teleclinic | |
| Extensive Stage | ||||
| Untreated | Chemotherapy | <2 | standard | should start on time. Consider growth factors or dose reduction, consider oral etoposide for d 2 and 3 |
| Chemotherapy and immunotherapy | <2 | standard | Be selective | |
| On treatment | chemotherapy | <2 | may extend assessment for 3 cycles if stable | |
| Chemotherapy and immunotherapy | <2 | |||
| Completed treatment | Observation | 2–8 | May extend up to 2 mo | if asymptomatic by teleclinic |
CCRT, concurrent chemoradiation therapy; PCI, prophylactic cranial irradiation; XRT, radiation therapy.
Table 5.
Miscellaneous Issues Related to Lung Cancer
| Lung cancer screening | All activities should be halted for the screening of asymptomatic patients. |
| Suspected cancer cases | To be reviewed by virtual multidisciplinary team and decide case by case. |
| Smoking cessation | Impact of coronavirus disease 2019 on lung should energize tobacco control efforts. |
Early Stage Lung Cancer
For patients with stage I/II and resectable stage III NSCLC, treatment is either surgical resection or ablative radiotherapy strategies. The surgical principles of lung cancer remain the same during the COVID-19 outbreak. However, the logistics of clinical practice for early stage lung cancer may be modified. If the COVID-19 outbreak is impending, an important issue is to decide whether to delay resection or not. Guidance from CDC and most professional societies indicates that elective surgeries should be rescheduled if possible.50 The American Society of Clinical Oncology recommended that clinicians and patients need to make individual determinations on the basis of potential harms caused by delaying needed cancer-related resection.51 It has been suggested that in patients with a recent diagnosis of early stage lung cancer or those with questionable pulmonary nodules, it is advisable to reschedule the resection as undergoing surgical procedure during the incubation period of SARS-CoV-2 infection may result in a dismal outcome.52 However, the European Association of Medical Oncology recommends keeping all surgeries as a priority in the management of early NSCLC. Surgical delays should generally not be more than 6 to 8 weeks.53
The American College of Surgeons has recently published COVID-19 elective case triage guidelines for surgical care focusing on the hospital resources available depending on the phase of the COVID-19 pandemic.54 In phase I, a semiurgent setting, they recommended that surgical intervention be restricted to patients whose survivorship are likely to be compromised if the resection is not performed within the next 3 months. For lung cancer, such cases include solid or predominantly solid (>50%) lung cancer, presumed lung cancer greater than 2 cm, or node-positive lung cancers. It is also recommended that patients who finished induction therapy proceed to surgery. Predominantly ground-glass nodules, solid nodules less than 2 cm, or indolent histologic structure should be deferred. In phase II, an urgent setting, resection is restricted to patients likely to have survival compromised if a surgical intervention is not performed within the next few days, such as tumor-associated infection or surgical complications. Alternative treatment options, including transferring patients to a hospital that is in phase I, neoadjuvant therapy, or stereotactic ablative body radiotherapy are recommended.
If a SARS-CoV-2 test is positive, surgical resection should be delayed for 2 to 3 weeks, if possible. If the patient’s condition is urgent, it is recommended that the resection proceeds within a specialized negative-pressure operating room with full personal protection equipment and postoperative care in a negative-pressure isolation room. All patients should be retested for SARS-CoV-2 when delayed resection is rescheduled.
As a specific example, in the setting of a widespread outbreak throughout the whole region, as experienced in Lombardy, Italy,25 an approach based on the stage of the disease and other oncologic clinical evaluations was implemented. Lung cancer patients were categorized into two groups: (1) red code, or patients with stage IC, II or III diseases with a real risk of progression and patients who already received induction chemo- or chemoradiation treatment, for which resection should be guaranteed in 4 weeks; and (2) yellow code, or those patients with stage I tumor (<2 cm) or indolent malignancies that can be postponed for 1 or 2 months. The Lombardy region identified and selected several hospital hubs that should theoretically be “COVID-free” for oncology cases. Surgical red code cases from other hospitals were diverted to these hub hospitals.
In patients with resectable locally advanced disease with a single positive mediastinal station (resectable nonbulky IIIA) or T3N1 tumors, for which surgical treatment is scheduled after induction therapy,55 the timing of resection could be planned such that adjuvant chemotherapy starts at a later date. This approach is based on two main reasons: (1) to avoid exposing the patient to the risk of infection during the frequent trips to and from the hospital for chemotherapy cycles at the apex of the COVID-19 emergency period; and (2) to reduce chemotherapy-induced immunosuppression, which can expose the patient to an increased risk of COVID-19 and, in case of infection, to serious pulmonary complications with a delay of potential curative surgical resection. Neoadjuvant therapy is recommended for appropriate patients to mitigate any deleterious effects from postponing surgical intervention for situations in which surgical services are overwhelmed. In general, measures that allow home management of cancer patients are encouraged, including telemedicine and phone calls replacing physical visits.56
Stereotactic body radiotherapy (SBRT) or stereotactic ablative radiotherapy is a well-established noninvasive method of treating early stage (<5 cm) node-negative NSCLC. Treatment with SBRT results in highly effective cure and local control rates with minimal risk. The delivery of SBRT can involve treatment with 50 Gy to 70 Gy in as many as 5 to 10 fractions for central tumors but can be delivered in a single fraction of 24 Gy to 34 Gy for peripheral tumors of less than 2 cm.57 For patients whom SBRT is appropriate, careful consideration should be given to whether treatment should be delivered immediately or delayed for small slow-growing tumors. Whenever possible, SBRT fractionation schemes during the COVID-19 pandemic should be shortened as much as possible with maximal use of single fraction treatment.
Brachytherapy is another modality for radiotherapy primarily of early stage, recurrent, or small endobronchial obstructive lesions involving the insertion of a radioactive source to treat small areas with less deposition of radiation dose to surrounding tissues. However, brachytherapy requires multidisciplinary coordination in a protected operating room or brachytherapy suite, patient sedation, bronchoscopy, and planning that increases the risk of exposure to patients and providers. During the COVID-19 pandemic, it is suggested to consider avoiding all brachytherapy procedures if there are any external beam radiotherapy or alternative options.
Adjuvant therapy is not recommended for stage I NSCLC patients. For cases in which local conditions render systemic chemotherapy hazardous resulting in the inability to start adjuvant cytotoxic chemotherapy, adjuvant EGFR tyrosine kinase inhibitor (TKI) therapy could be considered for resected EGFR mutation–positive NSCLC.58 , 59 If patients are clinically stable after adjuvant therapy, follow-up imaging can be delayed for 3 to 4 months.
Locally Advanced Lung Cancer
The treatment of locally advanced lung cancer could involve resection, radiotherapy, and systemic therapy; but most patients with stage III NSCLC will be treated with combined concurrent chemoradiotherapy typically consisting of platinum-based chemotherapy with radiotherapy delivered as 60 Gy in 30 fractions60 followed by consolidation durvalumab.61 As the aim of treatment is curative, the decision for treatment will need to take into consideration factors including the risk of developing COVID-19, the risk of developing treatment-related toxicities, and the availability of resources to administer treatment safely. At this time, the relationship between SARS-CoV-2 infection and severity with chemotherapy, radiotherapy, or immunotherapy has not been clearly defined, but it has been reported that anticancer therapy within 14 days of COVID-19 diagnosis was associated with an increased risk of developing severe complications.20 However, this was not confirmed in the most recent large-series reports.62, 63, 64 Careful consideration should be given by the institution performing adjuvant therapy, particularly in frail patients. The start of treatment after resection should be delayed for as long as possible consistent with the adjuvant chemotherapy data (up to 12 weeks after resection).
Systemic therapies associated with a reduced risk of myelosuppression, shorter treatment time, and lower frequency of treatment visits are recommended. A three-weekly schedule such as cisplatin plus pemetrexed65 may be reasonable, although one limitation is its longer infusion time. In contrast, therapies with frequent visits such as daily66 or weekly schedules should be avoided. Paclitaxel should also be avoided if possible, given the need for relatively high doses of steroids as a premedication and the longer infusion time. Because of shorter infusion time and steroid-sparing properties, nanoparticle albumin-bound paclitaxel may be a preferred alternative to paclitaxel, if it is available. The use of granulocyte-colony-stimulating factor (G-CSF) should be strongly encouraged as prophylaxis for early secondary prevention of neutropenia, as appropriate.
Though alternative chemoradiotherapy treatment schemes exist,57 themes for treatment remain largely consistent: patients must come to the clinic once a day, 5 days per week, for several weeks. This requires daily contact with other patients, treating staff, and transportation to the clinic, which all represent contact modes for infection over a prolonged treatment period
An alternative approach is the use of hypofractionation to decrease the number of radiotherapy fractions. If clinical resources are strained or if exposure risk is high, radiotherapy could be delayed but at the risk of increased mortality,67 so risks and benefits need to be discussed among the treatment team and the patient. The contemporary use of alternative fractionation schemes combined with chemotherapy and immunotherapy in the curative setting has not been tested.68 Alternative fractionations could include 55 Gy in 20 fractions with reasonable toxicity profiles.69, 70 Sequential chemotherapy followed by radiotherapy could be considered but would expose patients to a prolonged course of cancer treatment during an ongoing pandemic.
As discussed previously, SARS-CoV-2 infection may induce radiologic abnormalities similar to radiation-induced pneumonitis or immunotherapy induced pneumonitis. In a patient treated with chemoradiation or an immune checkpoint inhibitor, presentation with dyspnea and radiologic evidence of pneumonitis can often provide a diagnostic challenge. In this situation, after appropriate investigations, corticosteroid treatment may be considered for patients who have been tested negative for COVID-19.
However, the development of new infiltrates during radiotherapy was exhibited in a case report to precede COVID-19 symptoms and confirmed infection by 3 days.71 Radiation oncologists can review daily radiotherapy imaging to ascertain if any new infiltrates develop and this may prove to be useful for early detection.
COVID-19 and Immunotherapy
Programmed cell death protein 1 (PD-1) plays a role in both central and peripheral immune tolerance. Its ligation by programmed death-ligand 1 (PD-L1) or programmed death-ligand 2 leads to inhibition of an ongoing or starting immune response. PD-1 determines the threshold, the strength, and duration of an immune response and is sometimes called the immune “rheostat.” Blocking PD-1 by monoclonal antibodies has resulted in anticancer efficacy. Anti–PD-1 or PD-L1 monoclonal antibodies are currently approved for many cancer types as a new standard of care, including first-line and second-line treatment of NSCLC and first-line treatment of SCLC.72
During an acute viral infection, CD8 T-cells up-regulate cell-surface PD-1. Blockade of PD-1 at this stage results in accelerated viral clearance.73, 74 Depending on the type of virus, this may be accompanied by more severe inflammation of the infected tissue. After viral clearance, the expanded virus-specific T-cell population contracts and T-cell memory is formed. One type of T-cell memory cells, the so-called tissue-resident memory T-cells, permanently populate the infected tissue, such as lung tissue, during virus infections of the lower airways.75 At this stage, expression of PD-1 and its ligands PD-L1/2 may prevent further tissue damage, whereas blockade of the PD-1/PD-L1 axis could result in immunopathology. Also, PD-L1 expression especially may be differentially regulated during an acute viral infection. PD-L1 is much more widely expressed compared with PD-1. Apart from cells belonging to the hematopoietic lineages, endothelial and parenchymal cells can also up-regulate PD-L1.76 With an acute viral infection, in addition to PD-1 expression by CD8 and CD4 T-cells, PD-L1 is also up-regulated by cytokines, especially interferon type 1 and interferon-γ, and by pathogen recognition receptors, such as TLR and others, depending on the type of virus. Expression of PD-L1 by virus-infected cells may inhibit T-cells from efficiently eliminating these cells. In other models of acute viral infection, the PD-L1 expression occurring later during acute infection could limit tissue damage by controlling PD-1 expressing virus-specific T-cells.76 Hence, ideally, an immune response proceeds in such a way that viral clearance is optimal with as little tissue damage as possible (as reviewed by Schonrich et al.).77 What would be the effect of blockade of the PD-1/PD-L1 axis during acute viral infection, such as COVID-19? Could this lead to a better or worse outcome, to even more tissue damage? Whether or not this occurs is probably virus-dependent, and so far, very little is known about COVID-19. On the basis of current scarce data, it is hard to predict how checkpoint blockade will influence SARS-CoV-2 infection. There is an urgent need to collect data from patients with COVID-19 who are on checkpoint inhibitor treatment. Recently, a worldwide initiative, the Thoracic Cancers International COVID-19 Collaboration (TERAVOLT Registry) has been instituted to collect these data.62 , 78 The first analysis, which was done on 200 patients with thoracic cancers, revealed that the overall mortality rate in thoracic malignancies is 34.6%. However, many patients were not admitted to intensive care units. With the present analysis, it seems that immunotherapy has no detrimental effect on the outcome of COVID-19 compared with other treatments. In addition, the multivariable analysis failed to reveal that comorbidities were associated with an increased risk of death. For this reason, it is impossible to identify a category of patients with thoracic cancer who were at higher risk to have a severe course of COVID-19. Therefore, prevention remains the only safeguard for these patients.
Advanced Stage NSCLC
The use of molecular-targeted therapy, immunotherapy, and chemoimmunotherapy in advanced NSCLC has resulted in long-term survival in a proportion of patients. Thus, the decision to initiate or interrupt treatment poses a challenge for both the patient and their physicians. As lung cancer–related symptoms are similar to COVID-19, a careful history and examination are essential before treatment in order not to miss COVID-19 infection. All patients and health care providers should follow the general measures described in previous sections to minimize exposure and to reduce side effects. Response evaluation can be deferred from every two cycles to three or four cycles to reduce the frequency of hospital visits, provided that patients are clinically stable. Radiologic findings of SARS-CoV-2 infection are difficult to differentiate from drug-induced pneumonitis or immune-related pneumonitis, in which a GGO pattern is dominant. Thus, every patient with radiological findings suggestive of SARS-CoV-2 must be evaluated corresponding diagnostic tests.
Treatment-Naive Patients
Many patients at initial diagnosis may require immediate therapy and should be treated according to institutional guidelines. However, whenever possible, particularly in high-risk patients (such as those who are frail, elderly, or with comorbidities), treatment should be delayed if the tumor burden is low. All decisions should be discussed with the patient and family. Regimens with low myelosuppressive potential are preferred, and the use of G-CSF should be used as needed, notwithstanding the standard guidelines.
For nonsquamous carcinoma with high PD-L1 expression, single-agent pembrolizumab is preferred to a chemotherapy and PD1 or PD-L1 combination to reduce the incidence of hematologic or other adverse effects. Given the concerns about the interaction of checkpoint inhibitors with COVID-19 and the lack of data as guidance, in specific cases, it is reasonable if the use of pembrolizumab is deferred and systemic chemotherapy alone is administered. For pemetrexed treatment, doses of dexamethasone can be reduced to minimize immunosuppression.
Similar to nonsquamous carcinoma, for squamous carcinoma with high expression of PD-L1, pembrolizumab is preferred to a chemotherapy and PD1 or PD-L1 combination to reduce the incidence of hematologic or other side effects. If a chemotherapy combination is used, an effort should be made to use the least myelosuppressive regimen.
Patients on Treatment With Single-Agent Immunotherapy
For patients on single-agent immunotherapy, a number of approaches have been proposed to minimize the risk of infection. One recommendation is to continue treatment for patients in the early induction phase or short-term maintenance phase of therapy. In these patients, every attempt should be made to limit the number of visits, such as lengthening the duration of cycles. The pharmacology of most of the immune checkpoint inhibitors used in lung cancer lends itself to much less frequent dosing than currently used.79 The plasma half-lives of atezolizumab, nivolumab, pembrolizumab, and durvalumab are 27, 26.7, 26, and 12 days respectively. Currently, nivolumab can be given at a dose of 480 mg every 4 weeks. Atezolizumab can be given at a 1680 mg flat-dose, and durvalumab can be given at a dose of 1500 mg every 4 weeks as maintenance for SCLC. These regimens can be adopted for NSCLC. Pembrolizumab at a dose of 400 mg every 6 weeks for all approved indications just received regulatory approval by the U.S. Food and Drug Administration and should be the schedule of choice in the current COVID-19 pandemic.
In patients who have been on therapy for over a year, consideration could be given to deferring treatments for even longer periods.
Oncogene-Driven NSCLC
For patients with oncogene-driven NSCLC who are treated with a TKI, treatment can continue as prescribed. Follow-up evaluation through telemedicine is encouraged when possible. Response evaluation visits can be delayed, and CT scans are only advised in patients who are suspected of symptomatic progression. Whenever possible, medications can be mailed to patients to reduce the need for frequent visits. For situations in which this is not possible, the patient’s healthy family member can pick up the medication from the hospital or clinic.
Although it is quite rare, TKI-induced pneumonitis might be difficult to distinguish from COVID-19 pneumonia. Extensive evaluation and monitoring are required. Steroids should be avoided as much as possible. Preliminary results of the TERAVOLT registry suggest that these patients have a risk of long hospitalization compared with those who had other treatments.
SCLC
SCLC is a highly aggressive disease and is characterized by a rapid response to chemotherapy. Postponing first-line treatment will therefore rarely be possible. For patients with limited disease SCLC, the standard treatment is concurrent chemoradiotherapy, in which radiotherapy is given twice daily for three weeks or once daily for 6 weeks with comparable disease control and toxicity outcomes.80 A shortened treatment time would facilitate optimal care with a decreased total time of SARS-CoV-2 exposure risk. The Concurrent ONce -daily VERses Twice-daily chemotherapy in patients with limited-stage small-cell lung cancer (CONVERT) trial revealed that with modern radiotherapy techniques, severe radiotherapy-induced toxicity is limited; however, more than 70% of patients experience grade 3 to 4 neutropenia.80 Dose reductions should be considered especially in patients expected to be high risk for both neutropenia and COVID-19 (i.e., frail, elderly, have hypertension, or undergoing sequential chemoradiotherapy). Given the relatively modest benefit of prophylactic cranial irradiation and consolidative radiotherapy, it has been suggested that both can be removed from care patterns.81 It is also suggested that oral etoposide could be considered an option for SCLC patients during COVID-19 to reduce the frequency of hospital or clinic visits.
For decades the standard treatment of patients with metastatic SCLC was etoposide-platinum. Recently, improved progression-free survival and overall survival have been shown when atezolizumab was added to chemotherapy.82 However, the improvement in outcome is modest and no predictive biomarker is available to the few patients who will benefit. It is, therefore, reasonable to omit atezolizumab in patients at high risk of COVID-19 mortality, as described previously. When used, a less frequent schedule with every 4 weeks of atezolizumab should be considered. The use of G-CSF or dose reductions of the chemotherapy regimen in patients at a high risk of neutropenia should be considered. Second- and further-line treatment should be postponed after a full discussion with patients and families was done on the basis of the risk/benefit ratio.
Conclusion
The rapid onset of the COVID-19 pandemic requires careful consideration of urgent decisions to treat lung cancer by oncologists. Treatment decisions balancing the risk of exposure with effective care require close multidisciplinary discussions and thorough deliberation between caregivers and patients. The duration and severity of the COVID-19 pandemic are unclear, and treatment delay alone will be insufficient to provide optimal treatment to cancer patients. In combination with determining a treatment path for lung cancer, physicians should educate patients to help them prevent further spread of COVID-19 according to WHO and CDC guidelines. Patients who commit to treatment should further commit to self-isolation and safe practices for themselves, other patients, and providers.
COVID-19 will eventually be controlled. However, outbreaks are likely to recur. To be prepared, a number of international COVID study groups have been organized and active participation is encouraged.
Acknowledgments
The authors thank the expert secretarial, administrative, and editorial support provided by Vun-Sin Lim, PhD.
Footnotes
Disclosure: Dr. Dingemans reports receiving personal fees from Roche, Pharma Mar, Boehringer Ingelheim, Eli Lilly, and Merck Sharp and Dohme; and grants from Bristol-Myers Squibb outside of the submitted work. Dr. Soo reports receiving grants and personal fees from AstraZeneca and Boehringer Ingelheim; and personal fees from Bristol-Myers Squibb, Eli Lilly, Merck, Novartis, Pfizer, Roch0065, Taiho, Takeda, Yuhan, and Amgen outside of the submitted work. Dr. Jazieh reports receiving grants from Merck Sharp and Dohme and other fees from Bristol-Myers Squibb outside of the submitted work. Dr. Aerts reports receiving personal fees and nonfinancial support from Merck Sharp and Dohme; received personal fees from Bristol-Myers Squibb, Boehringer Ingelheim, Amphera, Eli Lilly, Takeda, Bayer, Roche, and AstraZeneca outside of the submitted work; has a patent allogenic tumor cell lysate licensed to Amphera; has a patent combination immunotherapy in cancer pending; and has a patent biomarker for immunotherapy pending. Dr. Yoon reports receiving research grants from GE Healthcare outside of the submitted work. Dr. Veronesi reports receiving grants from Associazione Italiana per la Ricerca sul Cancro, the Ministry of Health, and Istituto Nazionale Assicurazione Infortuni sul Lavoro outside of the submitted work; and received honoraria from Ab Medica SpA, Medtronic, and Verb Medical. Dr. Ramalingam reports receiving grants and other fees from Amgen, AstraZeneca, and Bristol-Myers Squibb; received other fees from Abbvie, Genentech, and Roche; and received grants from Merck, Takeda, Tesaro, and Advaxis outside of the submitted work. Dr. Garassino reports receiving personal fees from Bayer Incyte, Sanofi-Aventis, Inivata Takeda, Boehringer Ingelheim, Otsuka Pharma, Seattle Genetics, and Daiichi Sankyo; personal fees and other fees from AstraZeneca, Eli Lilly, Novartis, Bristol-Myers Squibb, Roche, Pfizer, and Celgene; and other fees from Tiziana Sciences, Clovis GlaxoSmithKline, Spectrum Pharmaceuticals, Blueprint Medicine, Bayer Healthcare Pharmaceuticals, Janssen, and GlaxoSmithKline outside of the submitted work. Dr. Haanen reports receiving grants from Bristol-Myers Squibb, Merck, Sharp and Dohme, Novartis, and Neon Therapeutics outside of the submitted work; and advisory roles for AIMM, Amgen, Achilles Tx, AstraZeneca, Bristol-Myers Squibb, Bayer, Celsius Tx, GSK, Gadeta, Immunocore, MSD, Merck Serono, Neon Tx, Neogene Tx, Novartis, Pfizer, Roche/Genentech, Sanofi, Seattle Genetics, Third Rock Ventures, and Vaximm. Dr. Peters reports receiving personal fees from Abbvie, Amgen, AstraZeneca, Bayer, Biocartis, Boehringer Ingelheim, and Bristol-Myers Squibb; personal fees from Clovis, Daiichi Sankyo, Debiopharm, Eli Lilly, F. Hoffmann-La Roche, Foundation Medicine, Illumina, Janssen, Merck Sharp, and Dohme, Merck Serono, Merrimack, Novartis, Pharma Mar, Pfizer, Regeneron, Sanofi, Seattle Genetics, Takeda, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, and Eli Lilly; nonfinancial support from Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Clovis, F. Hoffmann-La Roche, Illumina, Merck Sharp, and Dohme, Merck Serono, Novartis, Pfizer, and Sanofi; and fees for the institution from Bioinvent outside of the submitted work. Dr. Scagliotti reports receiving personal fees from AstraZeneca, Roche, Merck Sharp, and Dohme, Takeda, and Eli Lilly outside of the submitted work. Dr. Belani reports receiving personal fees from Genentech outside of the submitted work. The remaining authors declare no conflict of interest.
References
- 1.Lu R., Zhao X., Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Worldometer. https://www.worldometers.info/
- 4.Zhang L., Zhu F., Xie L. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China [e-pub ahead of print]. Ann Oncol. https://doi.org/10.1016/j.annonc.2020.03.296 accessed May 5, 2020. [DOI] [PMC free article] [PubMed]
- 5.Liang W., Guan W., Chen R. Cancer patients in SARS-Co.-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO 2020 coronavirus disease (COIID-19) technical guidance: laboratory testing for 2019-now in humans. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/laboratory-guidance Accessed May 5,2020.
- 7.Centers for Disease Control and Prevention 2020 testing for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/testing.html Accessed May 5, 2020. [PubMed]
- 8.China: First Affiliated Hospital, Zhejiang University School of Medicine; Zhejiang; P.R: 2020. Handbook of COVID-19 Prevention and Treatment. [Google Scholar]
- 9.WHO Global surveillance for COVID-19 caused by human infection with COVID-19 virus: interim guidance, 20 March 2020. https://www.who.int/docs/default-source/coronaviruse/2020-03-20-surveillance.pdf?sfvrsn=e6be6ef1_2 Accessed May 5, 2020.
- 10.Yu P., Zhu J., Zhang Z., Han Y., Huang L. A familial cluster of infection associated with the 2019 novel coronavirus indicating potential person-to-person transmission during the incubation period. J Infect Dis. 2020;221:1757–1761. doi: 10.1093/infdis/jiaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan J.F., Yuan S., Kok K.H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pung R., Chiew C.J., Young B.E. Investigation of three clusters of COVID-19 in Singapore: implications for surveillance and response measures. Lancet. 2020;395:1039–1046. doi: 10.1016/S0140-6736(20)30528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holshue M.L., DeBolt C., Lindquist S. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung J.M., Yang C.X., Tam A. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19 [e-pub ahead of print]. Eur Respir J. https://doi.org/10.1183/13993003.00688-2020 accessed May 5, 2020. [DOI] [PMC free article] [PubMed]
- 19.Caruso D., Zerunian M., Polici M. Chest CT features of COVID-19 in Rome, Italy [e-pub ahead of print]. Radiolo. https://doi.org/10.1148/radiol.2020201237 accessed May 5, 2020. [DOI] [PMC free article] [PubMed]
- 20.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J.J., Dong X., Cao Y.Y. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China [e-pub ahead of print]. Allergy. https://doi.org/10.1111/all.14238 accessed May 5, 2020. [DOI] [PubMed]
- 23.Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy [e-pCChead of print]. JAMA. https://doi.org/10.1001/jama.2020.4683 accessed May 5, 2020. [DOI] [PubMed]
- 26.American Association for Bronchology and Interventional Pulmonology 2020 AABIP Statement on COVID-19 Infections; March 19th Updates. https://aabronchology.org/2020/03/12/2020-aabip-statement-on-bronchoscopy-covid-19-infection [DOI] [PMC free article] [PubMed]
- 27.Society of Interventional Radiology A COVID−19 Toolkit for Interventional Radiologists. https://www.sirweb.org/globalassets/aasociety-of-interventional-radiology-home-page/practice-resources/covid-19-toolkit.pdf
- 28.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.Y. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15:700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Q., Wang R.S., Qu G.Q. Gross examination report of a COVID-19 death autopsy. Fa Yi Xue Za Zhi. 2020;36:21–23. doi: 10.12116/j.issn.1004-5619.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Tian S., Xiong Y., Liu H. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies [e-pub ahead of print]. Mod Pathol. https://doi.org/10.1038/s41379-020-0536-x accessed May 5, 2020. [DOI] [PMC free article] [PubMed]
- 31.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao X.H., Li T.Y., He Z.C. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi. 2020;49:E009. doi: 10.3760/cma.j.cn112151-20200312-00193. [DOI] [PubMed] [Google Scholar]
- 33.Wang C., Xie J., Zhao L. Aveolar macrophage activation and cytokine Storm in the pathogenesis of severe COVID-19. Res Square Prepr. 2020 doi: 10.1016/j.ebiom.2020.102833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong H.Y.F., Lam H.Y.S., Fong A.H. Frequency and distribution of chest radiographic findings in COVID-19 positive patients [e-pub ahead of print]. Radiology. https://doi.org/10.1148/radiol.2020201160 accessed May 5, 2020. [DOI] [PMC free article] [PubMed]
- 35.Yoon S.H., Lee K.H., Kim J.Y. Chest radiographic and CT findings of the 2019 novel coronavirus disease (COVID-19): analysis of nine patients treated in Korea. Korean J Radiol. 2020;21:494–500. doi: 10.3348/kjr.2020.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ai T., Yang Z., Hou H. Correlation of chest CT and RT-PCR testing in coronavirus Disease 2019 (COVID-19) in China: a report of 1014 cases [e-pub ahead of print]. Radiology. https://doi.org/10.1148/radiol.2020200642 accessed May 5, 2020. [DOI] [PMC free article] [PubMed]
- 37.Simpson S., Kay F.U., Abbara S. Radiological Society of North America expert consensus statement on reporting chest CT findings related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA. Rad Cardiothorac Imaging. 2020;2 doi: 10.1148/ryct.2020200152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodrigues J.C.L., Hare S.S., Edey A. An update on COVID-19 for the radiologist - A British society of Thoracic Imaging statement. Clin Radiol. 2020;75:323–325. doi: 10.1016/j.crad.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanne J.P., Little B.P., Chung J.H., Elicker B.M., Ketai L.H. Essentials for radiologists on COVID-19: an update-Radiology Scientific Expert Panel [e-pub ahead of print]. Radiology. https://doi.org/10.1148/radiol.2020200527 accessed May 5, 2020. [DOI] [PMC free article] [PubMed]
- 40.Zu Z.Y., Jiang M.D., Xu P.P. Coronavirus Disease 2019 (COVID-19): A perspective from China [e-pub ahead of print]. Radiology. https://doi.org/10.1016/j.sapharm.2020.04.020 accessed May 5, 2020. [DOI] [PMC free article] [PubMed]
- 41.Song F., Shi N., Shan F. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology. 2020;295:210–217. doi: 10.1148/radiol.2020200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li X., Zeng X., Liu B., Yu Y. COVID-19 infection presenting with CT halo sign. Rad Cardiothorac Imaging. 2020;2 doi: 10.1148/ryct.2020200026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.American College of Radiology ACR recommendations for the use of chest radiography and computed tomography (CT) for suspected COVID-19 infection. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection Accessed May 5, 2020.
- 44.Calandri M., Solitro F., Angelino V., Moretti F., Veltri A. The role of radiology in the evaluation of the immunotherapy efficacy. J Thorac Dis. 2018;10(suppl 13):S1438–S1446. doi: 10.21037/jtd.2018.05.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie Y., Wang X., Yang P., Zhang S. COVID-19 complicated by acute pulmonary embolism. Rad Cardiothorac Imaging. 2020;2 doi: 10.1148/ryct.2020200067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scavone C., Brusco S., Bertini M. Current pharmacological treatments for COVID-19: what’s next [e-pub ahead of print]? Br J Pharmacol. https://doi.org/10.1111/bph.15072 accessed May 5, 2020. [DOI] [PMC free article] [PubMed]
- 47.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. The cytokine release syndrome (CRS) of severe COVID-19 and interleukin-6 receptor (IL-6R) antagonist tocilizumab may be the key to reduce the mortality [e-pub ahead of print]. Int J Antimicrob Agents. https://doi.org/10.1016/j.ijantimicag.2020.105954 accessed May 5, 2020. [DOI] [PMC free article] [PubMed]
- 48.Wang Y., Zhang D., Du G. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial [e-pub ahead of print]. Lancet. accessed May 5, 2020. [DOI] [PMC free article] [PubMed]
- 49.National Institutes of Health 2020 NIH clinical trial shows Remdesivir accelerates recovery from advanced COVID-19. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-shows-remdesivir-accelerates-recovery-advanced-covid-19
- 50.Centers for Disease Control and Prevention Interim guidance for healthcare facilities: preparing for community transmission of COVID-19 in the United States. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-hcf.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fhealthcare-facilities%2Fguidance-hcf.html Accessed May 5, 2020.
- 51.American Society of Oncology COVID-19 Patient Care information. https://www.asco.org/asco-coronavirus-information/care-individuals-cancer-during-covid-19 Accessed May 5, 2020.
- 52.Lei S., Jiang F., Su W. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection [e-pub ahead of print]. EClinicalmedicine. https://doi.org/10.1016/j.eclinm.2020.100331 accessed May 5, 2020. [DOI] [PMC free article] [PubMed]
- 53.ESMO ESMO management and treatment adapted recommendations in the COVID-19 era: lung cancer. https://www.esmo.org/guidelines/lung-and-chest-tumours/lung-cancer-in-the-covid-19-era Accessed May 5, 2020. [DOI] [PMC free article] [PubMed]
- 54.American Society of Surgeons 2020 COVID-19 guidelines for triage of thoracic patients. https://www.facs.org/covid-19/clinical-guidance/elective-case/thoracic-cancer Accessed May 5, 2020.
- 55.Eberhardt W.E., De Ruysscher D., Weder W. 2nd ESMO Consensus Conference in lung cancer: locally advanced stage III non-small-cell lung cancer. Ann Oncol. 2015;26:1573–1588. doi: 10.1093/annonc/mdv187. [DOI] [PubMed] [Google Scholar]
- 56.You B., Ravaud A., Canivet A. The official French guidelines to protect patients with cancer against SARS-CoV-2 infection. Lancet Oncol. 2020;21:619–621. doi: 10.1016/S1470-2045(20)30204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.National Comprehensive Cancer Network Guidelines in Oncology Non-Small Cell Lung Cancer. https://www.nccn.org/professionals/physician_gls/Default.aspx Accessed May 5, 2020.
- 58.Yue D., Xu S., Wang Q. Erlotinib versus vinorelbine plus cisplatin as adjuvant therapy in Chinese patients with stage IIIA EGFR mutation-positive non-small-cell lung cancer (EVAN): a randomised, open-label, phase 2 trial. Lancet Respir Med. 2018;6:863–873. doi: 10.1016/S2213-2600(18)30277-7. [DOI] [PubMed] [Google Scholar]
- 59.Zhong W.Z., Wang Q., Mao W.M. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA. Lancet Oncol. 2018;19:139–148. doi: 10.1016/S1470-2045(17)30729-5. [DOI] [PubMed] [Google Scholar]
- 60.Bradley J.D., Hu C., Komaki R.R. Long-term results of NRG oncology RTOG 0617: standard- versus high-dose chemoradiotherapy with or without cetuximab for unresectable Stage III non-small-cell lung cancer. J Clin Oncol. 2020;38:706–714. doi: 10.1200/JCO.19.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Antonia S.J., Villegas A., Daniel D. Overall survival with Durvalumab after chemoradiotherapy in Stage III NSCLC. N Engl J Med. 2018;379:2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 62.Garassino MC. Session VCTPL09 - COVID-19 and Cancer - TERAVOLT (Thoracic cancERs international coVid 19 cOLlaboraTion): first results of a global collaboration to address the impact of COVID-19 in patients with thoracic malignancies. AACR Annual Meeting. April 24–29, 2020; San Diego, CA.
- 63.Rubinstein S., Steinharter J.A., Warner J., Rini B.I., Peters S., Choueiri T.K. The COVID-19 & Cancer Consortium (CCC19): a collaborative effort to understand the effects of COVID-19 on patients with cancer [e-pub ahead of print]. Cancer Cell. accessed May 5, 2020. [DOI] [PMC free article] [PubMed]
- 64.Miyashita H., Mikami T., Chopra N. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City [e-pub ahead of print]. Ann Oncol. https://doi.org/10.1016/j.annonc.2020.04.006 accessed May 5, 2020. [DOI] [PMC free article] [PubMed]
- 65.Senan S., Brade A., Wang L.H. PROCLAIM: randomized Phase III trial of pemetrexed-cisplatin or etoposide-cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2016;34:953–962. doi: 10.1200/JCO.2015.64.8824. [DOI] [PubMed] [Google Scholar]
- 66.Hanna N., Neubauer M., Yiannoutsos C. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: the Hoosier Oncology Group and U.S. Oncology. J Clin Oncol. 2008;26:5755–5760. doi: 10.1200/JCO.2008.17.7840. [DOI] [PubMed] [Google Scholar]
- 67.Vinod S.K., Chandra A., Berthelsen A., Descallar J. Does timeliness of care in Non-Small Cell Lung Cancer impact on survival? Lung Cancer. 2017;112:16–24. doi: 10.1016/j.lungcan.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 68.O’Rourke N., Roque I.F.M., Farre Bernado N., Macbeth F. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst Rev. 2010;6 doi: 10.1002/14651858.CD002140.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iqbal M.S., Vashisht G., McMenemin R. Hypofractionated concomitant chemoradiation in inoperable locally advanced non-small cell lung cancer: A report on 100 patients and a systematic review. Clin Oncol R Coll Radiol. 2019;31:e1–e10. doi: 10.1016/j.clon.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 70.Din O.S., Harden S.V., Hudson E. Accelerated hypo-fractionated radiotherapy for non small cell lung cancer: results from 4 UK centres. Radiother Oncol. 2013;109:8–12. doi: 10.1016/j.radonc.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 71.Suppli M.H., De Blanck S.R., Elgaard T., Josipovic M., Pøhl M. Early appearance of COVID-19 associated pulmonary infiltrates during daily radiotherapy imaging for lung cancer. J Thorac Oncol. 2020 doi: 10.1016/j.jtho.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Remon J., Passiglia F., Ahn M.J. Immune checkpoint inhibitors in thoracic malignancies: review of the existing evidence by an IASLC Expert Panel and Recommendations [e-pub ahead of print]. J Thorac Oncol. https://doi.org/10.1016/j.jtho.2020.03.006 accessed May 5, 2020. [DOI] [PubMed]
- 73.Ahn E., Araki K., Hashimoto M. Role of PD-1 during effector CD8 T cell differentiation. Proc Natl Acad Sci U S A. 2018;115:4749–4754. doi: 10.1073/pnas.1718217115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.David P., Megger D.A., Kaiser T. The PD-1/PD-L1 pathway affects the expansion and function of cytotoxic CD8(+) T cells during an acute retroviral infection. Front Immunol. 2019;10:54. doi: 10.3389/fimmu.2019.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shin H. Formation and function of tissue-resident memory T cells during viral infection. Curr Opin Virol. 2018;28:61–67. doi: 10.1016/j.coviro.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 76.Keir M.E., Liang S.C., Guleria I. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schonrich G., Raftery M.J. The PD-1/PD-L1 axis and virus infections: a delicate balance. Front Cell Infect Microbiol. 2019;9:207. doi: 10.3389/fcimb.2019.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.TERAVOLT Thoracic cancERs international coVid 19 cOLlaboraTion. http://teravolt-consortium.org Accessed May 5, 2020. [DOI] [PMC free article] [PubMed]
- 79.Hurkmans D.P., Basak E.A., van Dijk T. A prospective cohort study on the pharmacokinetics of nivolumab in metastatic non-small cell lung cancer, melanoma, and renal cell cancer patients. J Immunother Cancer. 2019;7:192. doi: 10.1186/s40425-019-0669-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Faivre-Finn C., Snee M., Ashcroft L. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017;18:1116–1125. doi: 10.1016/S1470-2045(17)30318-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Simcock R., Thomas T.V., Estes C. COVID-19: global radiation oncology’s targeted response for pandemic preparedness. Clin Transl Radiat Oncol. 2020;22:55–68. doi: 10.1016/j.ctro.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Horn L., Mansfield A.S., Szczesna A. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]