Abstract
Since December 2019, more than 3 million cases of coronavirus disease 2019 (COVID-19) and about 200,000 deaths have been reported worldwide. The outbreak of this novel disease has become a global health emergency and continues to rapidly spread around the world. Based on the clinical data, approved cases are divided into four classes including mild, moderate, severe, and critical. About 5% of cases were considered critically ill and 14% were considered to have the severe classification of the disease. In China, the fatality rate of this infection was about 4%. This review focuses on currently available information on the etiology, clinical symptoms, diagnosis, and mechanism of action of COVID-19. Furthermore, we present an overview of diagnostic approaches and treatment of this disease according to available findings. This review paper will help the physician to diagnose and successfully treat COVID-19.
Keywords: Chloroquine, Coronavirus, Coronavirus infection, COVID-19 vaccine, Respiratory tract infections, Viral pneumonia
Highlights
-
•
C-reactive protein (CRP), lactate dehydrogenase (LDH), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin, creatinine, cardiac troponin, and cytokine levels significantly increased in COVID-19 patients.
-
•
Ground glass opacities (GGO), pure consolidation, multiple lesions, bilateral distribution, posterior part/lower lobe predilection, peripheral/subpleural distribution, and crazy-paving pattern are the main chest imaging findings in the COVID-19 patients.
-
•
Since COVID-19 quickly spread through respiratory droplets, head and neck surgeons who have close contact with the upper aerodigestive tract are principally at high risk.
-
•
Treatment with a soluble form of angiotensin-converting enzyme 2 (ACE2) probably acts as a competitive interceptor of coronavirus by inhibiting binding of the viral particle to the ACE2 receptor, consequently can slow coronavirus entry into the cells and protect the lung injury.
1. Introduction
Up to May 1, 2020, more than 3,000,000 cases with COVID-19 were diagnosed and 200,000 death cases were recorded. The coronavirus disease 2019 (COVID-19) first was reported in December 2019 in Wuhan, China. It quickly spread to other districts in the country and, a month later, to other countries across the world, impacting over 200 countries and territories. COVID-19 was reported to be a global emergency by the World Health Organization (WHO) in January 2020 and its outbreak was later declared to be a pandemic by the organization on March 2020 [1]. Like other diseases that cause pneumonia, including the Middle East respiratory syndrome (MERS) and the Severe Acute Respiratory Syndrome (SARS), COVID-19 can also cause acute respiratory distress syndrome (ARDS) [2].
COVID-19 was stated to have originated from wild bats and belongs to genera β-coronavirus. Although COVID-19 and SARS-associated Coronavirus (SARS-CoV) belong to the same β-coronavirus, the similarity at the genome level is only 70%, and the new group has been reported to display genetic differences from SARS-CoV [3].
Almost all earlier cases had a history of contact with Wuhan city, and some cases present a family clustering character. The exact source of COVID-19 is still unknown; nevertheless, it is reported that the infection may have been induced by vipers or bats [4]. This novel virus exhibits high person-to-person transmissibility and typically infects animals, including mammals and birds. Coronaviruses usually cause respiratory infections, such as those detected in the common cold, in patients. However, some new human coronavirus infections have caused lethal endemics including MERS and SARS endemics [1]. WHO, in the first report, projected about a 4% fatality rate for COVID-19. However, among hospitalized cases, the fatality rate is about 4–15% [1,5].
Most of the COVID-19 subjects are adults. Among 44,672 cases in China, 2.1% were below the age of twenty. Around 5% of patients experienced critically ill conditions and 14% experienced severe disease. Initial reports documented that disease severity was accompanied by age over 60 years and a history of a co-morbid condition. Rapid detection of COVID-19 is vital to guarantee timely treatment, and from a public health perspective, quick isolation of the patient is essential to discontinue the cascade of contamination [6].
2. Structure
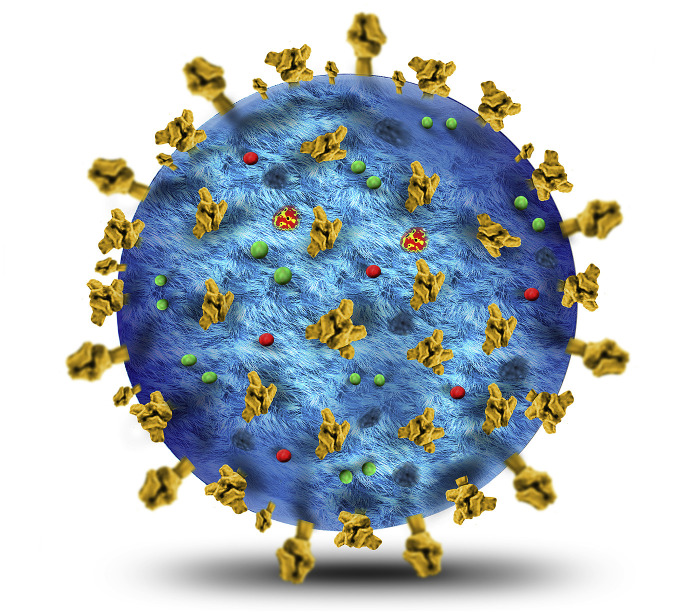
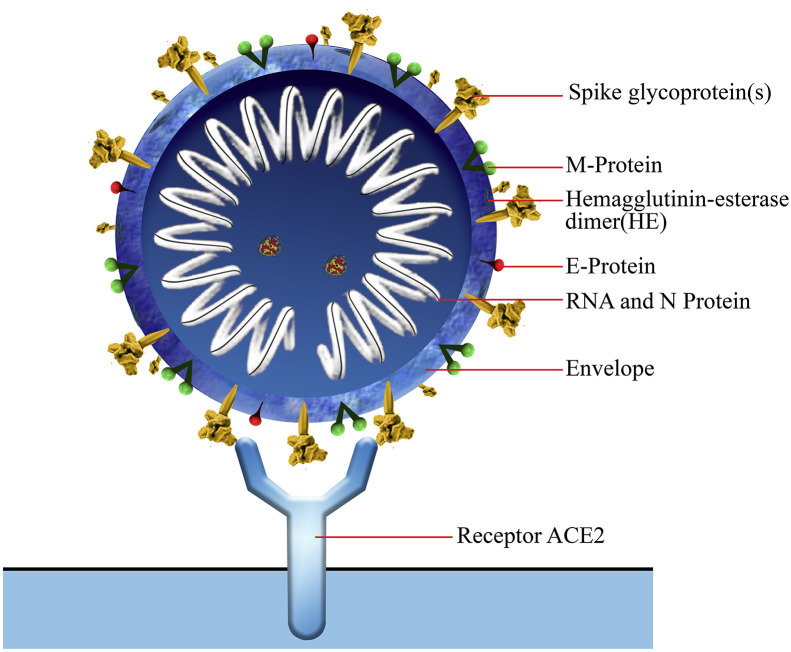
Coronaviruses (CoVs) belong to the subfamily Coronavirinae in the family of Coronaviridae of the order Nidovirales, and this subfamily includes four types named as α, β, γ, and δ coronavirus [7]. COVID-19, a β-coronavirus with positive-sense single-stranded RNA [1], is an enveloped virion that appears as oval or round with 60–140 nm diameter and is often polymorphous (Fig. 1, Fig. 2 ). COVID-19 is generally spread in humans and other mammals, and its genome is more distant from SARS-CoV and MERS-CoV [8].
Fig. 1.
A three-dimensional (3D) structure of the novel coronavirus (COVID-19).
Fig. 2.
Schematic diagram of the novel coronavirus structure (COVID-19). The spike protein on the membrane of the virus is necessary for entry into the cell. The spike protein can bind to the receptor, Angiotensin-converting enzyme 2 (ACE2) on the surface of human cells.
Coronavirus recombination rates are very high due to continuously appearing transcription errors and RNA dependent RNA polymerase (RdRP) jumps [3]. With a high mutation rate of coronaviruses, they are zoonotic pathogens that are existing in humans and other mammalians with a varied range of clinical finding from asymptomatic feature to hospitalization in the intensive care unit (ICU) leading to the infections in lungs, gastrointestinal tract, liver, and central nervous system [3].
3. Transmission and contamination with COVID-19
Aside from person-to-person close contact (less than six feet distance) and population mobility, environmental factors could influence droplet transmission and survival of viruses. Currently, subjects with COVID-19 are known as the main source of disease. Frequently, the transmission from person-to-person happens with close contact. The transmission initially occurs through the respiratory droplets formed when a patient sneezes, coughs, or even talks, just as in the spread of influenza and other respiratory viruses. Droplets can settle down in different parts of the body, such as the mouth, lungs, and eyes of subjects through inhaled air [3,9]. This disease can also be transmitted by touching a surface or an object that has the virus on it [3,10]. Moreover, transmission may occur through fomites in the environment around the COVID-19 patients. It has been reported that COVID-19 infection may lead to gastrointestinal infection and exist in stool. Hence, COVID-19 may also transmit via fecal-oral or body fluid routes. Consequently, coronavirus transmission can occur by direct route (close contact with COVID-19 patients) and indirect route (surfaces in the immediate environment or equipment used by COVID-19 patients) (Table 1, Table 2 ). Usually, as with other types of respiratory pathogens, it is accepted that the most contagious time is when subjects are most symptomatic. However, asymptomatic infections can act as a source of the disease [10]. Cases that were infected from asymptomatic subjects in the prodrome phase of COVID-19 were also documented [10]. At present, based on limited findings, there is no evidence of transmission of COVID-19 from an infected pregnant mother to baby [11].
Table 1.
COVID-19 transmission routes.
| Transmission routes | Definition |
|---|---|
| Close contact (direct or indirect) |
|
| Respiratory droplets |
|
| Airborne |
|
| Objects and surfaces |
|
Table 2.
COVID-19 duration on air and object.
| Objects and Surfaces | COVID-19 duration |
|---|---|
| Aira | up to 24 h |
| Cardboard | up to 24 h |
| Plastic | up to 2–3 days |
| Stainless Steel | up to 2–3 days |
| Cardboard | up to 1 day |
| Copper | up to 4 h |
The maximum transmission distance: up to 4 m.
COVID-19 was extensively distributed on hospital floors, sickbed handrails, trash cans, and computer keyboard and mice. The positive rate was relatively high for floor swab specimens (ICU: 70%; general COVID-19 ward: 15.4%), maybe due to air flow and gravity causing most virus droplets to float to the ground. Furthermore, as health workers walk around the ward, the virus can be transferred all over the floor, as shown by the 100% positive rate from the pharmacy floor, where there were no COVID-19 patients. Moreover, 50% of the specimens from the soles of the intensive care unit (ICU) staff shoes were positive. Hence, the soles of shoes might act as potential carriers. It has been proposed that staffs disinfect their shoe soles before walking out of wards containing infected subjects [12].
4. Gender susceptibility for COVID-19 infection
While MERS-CoV regularly infects subjects aged above 50 years, SARS-CoV infects younger individuals, and COVID-19 infects subjects at their middle age or above. Infected older men with co-morbid diseases were more likely to have lung disease because of severe alveolar injury, indicating that men are more vulnerable than women [13]. Differences in sex hormones and immunity-linked genes on the X chromosome that affect both adaptive (acquired) and innate (non-specific) immune responses may describe the high vulnerability to COVID-19 infection in males. Additionally, there may be a higher possibility of virus exposure because of occupational risk which could be another factor responsible for higher male infection rate [14].
5. Clinical manifestations of COVID-19
Understanding the clinical manifestations is vital, although these symptoms are nonspecific. Since December 2019, numerous collected subjects of “unknown viral pneumonia” have been reported, originally related to Wuhan city, China [2]. Overall, many infected subjects with COVID-19 have many clinical symptoms similar to a SARS-CoV infection [14]. It is reported that the clinical feature differs from simple lung infection findings to septic shock. Like MERS-CoV and SARS-CoV, the first symptoms of COVID-19 are usually explained as cough, myalgia or fatigue, shortness of breath, and fever [13,15]. Diarrhea was rarely documented in COVID-19 infection, while intestinal symptoms existed in approximately 20–25% of the subjects with SARS-CoV and MERS-CoV infection [3]. Some cases have complained of hemoptysis or headache while others were even relatively asymptomatic [13]. Co-morbidities such as underlying cardiovascular disease, hypertension, and diabetes were reported in nearly half of the patients [3]. The symptoms of acute respiratory infection appeared within the early stage of disease. In the next stages, septic shock, metabolic acidosis, coagulation dysfunction, and ARDS [8] can appear in severe cases [15].
The incubation period for COVID-19 on average is 5.2 days, but it is different among patients. Studies support a 14-day medical treatment period for subjects exposed to the pathogen [13].
6. Pneumonia is a significant problem
In SARS subjects, lung injury is often diagnosed in the first week from the symptom onset [16]. Then, the lesions develop into peripheral/subpleural distribution, bilateral involvement, and posterior part/lower lobe predilection mixed with ground-glass opacity (GGO) in the fourth week. Likewise, Pan et al. [2] in a cohort study showed 85.7% pneumonia progression including consolidation and GGO in early CT scans of patients with COVID-19. Notably, there is asymptomatic pneumonia among some COVID-19 patients. Therefore, care must be taken for the diagnosis of such atypical subjects as they might be sources of public transmission [16].
Other types of pneumonia induced by streptococcus, chlamydia, mycoplasma, and other coronavirus infections should be differentiated from COVID-19 pneumonia. It is very vital to separate suspected subjects with fever from the others early to reduce cross-infection. Respiratory symptoms in COVID-19, including breathlessness and lung failure, and constitutional symptoms such as fever, muscle ache, confusion, and headache accompanied by diffuse heterogeneous consolidation with GGO [13].
7. Detection of COVID-19 infection
The history of exposure or close contact with suspected or confirmed patients is critical evidence for the diagnosis. Nevertheless, for patients with an unknown history, clinical features and imaging appearances can show suspected COVID-19. After that, the real-time reverse-transcription-polymerase-chain-reaction (RT-PCR) test should be done in these cases, as a reference standard [13].
According to the National Health Commission (NHC) of China, the diagnostic criteria are: 1. history of exposure to cases with respiratory symptoms from Wuhan or infected cities within two weeks before the onset of disease, 2. clinical findings (fever, normal or decreased WBC count or reduced lymphocyte count, and/or imaging features of pneumonia), and 3. Real-time PCR showing positive results for COVID-19. The confirmed COVID-19 pneumonia case should be hospitalized and isolated for therapy [2]. Based on the WHO recommendations for SARS and MERS, the NHC of China proposed criteria for diagnosis and treatment of COVID-19 pneumonia [13]. A person with two clinical conditions and one contact history is regarded as a suspected patient. Without any clear contact history, suspected cases should have three clinical features [13]. The discharge criteria were: 1. afebrile for more than 3 days, 2. significant improvement of respiratory symptoms, and 3. improvement in the imaging abnormalities of the chest. The definitive etiology diagnosis of infection is needed, which can be made stronger by PCR assay using blood or lung specimen or by viral gene sequencing. Based on the clinical findings, approved subjects are divided into different classes, including critical, severe, moderate, and mild [13].
8. Laboratory tests
About nearly half of the patients infected with COVID-19 had a reduced WBC count and lymphopenia (reduced lymphocytes), or thrombocytopenia (low blood platelet count) with increased activated thromboplastin time. C-reactive protein (CRP) levels were increased in most patients, but procalcitonin (PCT) concentrations were normal [8]. Elevated serum ferritin levels reported in some patients [14]. All these alterations further explained that COVID-19 may exert a potential effect on lymphocytes, especially T cells. COVID-19 outbreaks and invades via lung mucosa stimulates inflammation cascades and motivates a cytokine storm, resulting in alteration in immune response such as peripheral blood leukocytes and lymphocytes. Hence, intravenous immunoglobulin was applied in most subjects with reduced WBC and lymphocyte count [8]. Augmented serum pro-inflammatory cytokine levels have also been documented and are associated with the severity of the disease. Nevertheless, elevated levels of interleukin-10, which is known as an anti-inflammatory cytokine, indicate a diverse pattern from that of SARS-CoV infection [14]. Some patients showed increased liver enzymes and glucose levels, and few patients exhibited abnormal muscle enzymes. Liver test abnormality of COVID-19 may not be induced by liver cell damage but by bile duct cell dysfunction and other reasons [8]. In summary, blood routine test, CRP, PCT, coagulation function, arterial blood gas, and tissue function tests (e.g. transaminases, bilirubin, myocardial enzyme, creatinine, urea nitrogen, urine volume, etc.) as tabulated in Table 3 should be monitored in patients [17]. Furthermore, subjects having upper respiratory tract symptoms and fever with leukopenia or lymphopenia should be suspected, particularly for cases with close contact or exposure history [18].
Table 3.
Laboratory findings in Covid-19 patients.
| Increased in most patients | Increased in few patients | Decreased in most patients | Normal in most patients |
|---|---|---|---|
| CRP | D-dimera | Lymphocyte count | Procalcitonin |
| LDH | Procalcitonin | Albumin | |
| ALT | Urea | WBCb | |
| AST | Blood glucose | ||
| Total bilirubin | Myohemoglobin | ||
| Creatinine | CK | ||
| Cardiac troponin | Ferritin | ||
| PT | |||
| ESR Cytokines (IL-6, IL-10, IL-2, IL-7) |
CRP (C-reactive protein), LDH (Lactate dehydrogenase), ALT (Alanine aminotransferase), AST(Aspartate aminotransferase), PT (Prothrombin time), ESR (Erythrocyte sedimentation rate), IL- (Interleukin-), Creatinine Kinase (CK), WBC (White blood cell).
Increased in severe cases.
Patients have normal or reduced levels.
9. Imaging
Radiological evaluations, particularly thin slice chest computed tomography (CT) scan, have a critical role in diagnosis, management, and follow-up of COVID-19 infections [18]. Radiologists play the main role in the outbreak of COVID-19. Early diagnosis of the imaging abnormality could offer suspect pneumonia in cases at risk. Although the final detection of COVID-19 is based on RT-PCR, the findings of imaging are vital for pneumonia detection [16]. CT scans are proposed in cases with suspicious lung abnormalities. Appropriate recognition of COVID-19 pneumonia could provide quick management and follow-ups. Lung imaging shows the severity of COVID-19, therefore physicians should be informed about radiological reports. Clinical findings from COVID-19 cases with high abnormality shown in the CT scan who were admitted to the ICU [16] show that, on admission, these patients frequently showed subsegmental consolidation and bilateral multiple lobular, whereas CT reports from non-ICU patients showed subsegmental consolidation and bilateral ground-glass opacity (GGO) [15]. In severe infection, imaging can confirm heterogeneous consolidation with GGOs in bilateral lungs and bronchiectasis, indicating as “white lung” when most lobes of the lung are affected [19]. Furthermore, COVID-19 cases might show intralobular septal thickening and bilateral pleura along with little pleural effusion [13]. CT scanning enables recognition of the initial phase of respiratory infection and provides opportunity for a quick public health care response [20]. Slice chest CT has been shown as the main evidence for approved findings. Since chest radiography is not sensitive for the diagnosis of GGO and might show normal results in the initial stage of disease [20], it is not considered as the first-line imaging method for COVID-19. Nevertheless, bilateral multifocal consolidation can be observed in severe cases, partially fused into high consolidation with minor pleural effusions and even showing with “white lung” [13]. In this respect, the thin slice chest CT test is more useful for the early diagnosis of COVID-19 pneumonia [18,20]. High-resolution computed tomography (HRCT) can detect GGOs more easily in the early stage [4]. A study with a large sample size (3665 confirmed cases of the disease) has reported that 95.5% of cases were detected as pneumonia. Pan et al. [2] conducted an experiment with 21 approved COVID-19 cases who underwent repeated CT with about 4-day intervals and observed negative results in four cases in the initial stages (0–4 days after onset of the early symptom), but repeated CT displayed abnormalities in the lung in all of these four patients.
The usual imaging features of COVID-19 pneumonia include interstitial inflammation, extensive consolidation with multifocal bilateral GGOs, bilateral involvement, noticeable peripheral or subpleural distribution, posterior part or lower lobe predilection, and multiple lesions [13]. Nevertheless, certain cases with COVID-19 pneumonia regularly revealed no respiratory distress or hypoxemia during the course of hospitalization [2]. Furthermore, since the CT scan results of COVID-19 overlap with other viral pneumonia, RT-PCR assay is strongly recommended for rapid detection and treatment [4].
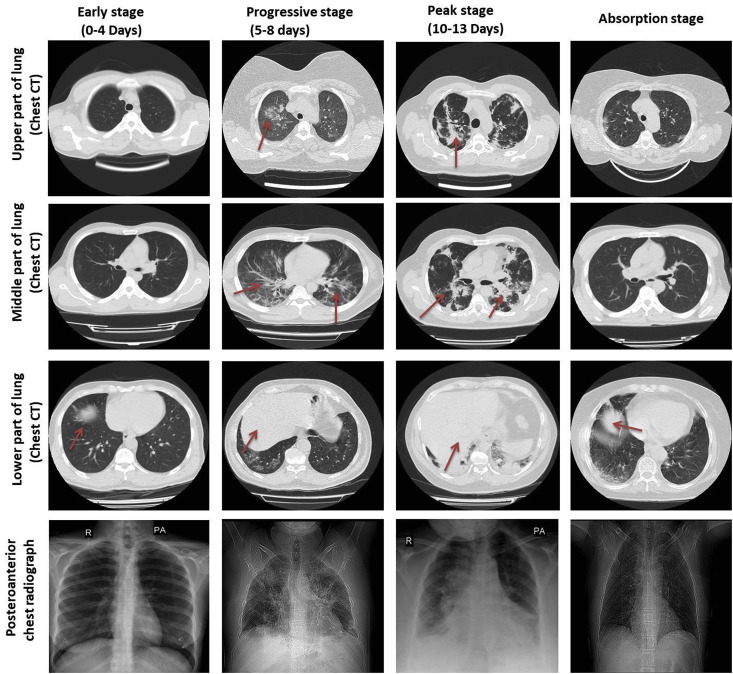
According to the Pan et al. report, the cases who recovered from this pneumonia due to COVID-19 experienced four stages based on their CT results: (1) early stage (0–4 days) that shows small GGO distributed subpleurally in the lower part, (2) progressive stage (5–8 days) with infection quickly extended to a bilateral multi-lobe with diffused GGO, consolidation, and crazy-paving pattern, (3) peak stage (10–13 days) that shows slowly expanding of the involved part to the peak involvement, including diffused GGO, crazy-paving pattern, residual parenchymal bands, and consolidation, and finally (4) absorption stage which occurs two weeks after the onset of first symptoms and shows that the disease is managed and the consolidation is slowly absorbed (Fig. 3 and Table 4 ). No crazy-paving signs exist anymore. Nevertheless, in this step, widespread GGO can be observed as an indication of the consolidation absorption. According to the CT scores, the absorption step extended beyond 26 days after the onset of the first symptom [2].
Fig. 3.
Chest X-rays and computerized tomography (CT) images of COVID-19 patients. Four stages in COVID-19 patients; 1: early stage (0–4 days), 2: progressive stage (5–8 days), 3: peak stage (10–13 days), and 4: absorption stage (more detail in the text).
Table 4.
Chest CT findings of COVID-19 pneumonia.
| Chest CT imaging features | Definition | Severity grades |
|---|---|---|
| Ground glass opacities (GGO) +/− consolidation | An area of hazy elevated lung opacity | +++++ |
| Multiple lesions | Damage or abnormal changes in a different area | ++++ |
| Bilateral distribution | Two sides distribution of GGO | ++++ |
| Posterior part/lower lobe predilection | Dorsal part/lower lobe predilection | ++++ |
| Pure consolidation | Replacement of air in the alveoli by different matters (e.g., cells, blood, and pus) | ++++ |
| Peripheral/subpleural distribution | Peripherally and subpleural distributed multifocal GGOs | +++ |
| Crazy-paving pattern | A linear pattern superimposed on a background of GGO | +++ |
| Reticular pattern | Presence of countless lines, either due to fibrosis or thickening of the interlobular septa | ++ |
| Pleural thickening | Extensive scarring thickens the pleura | ++ |
| Bronchial wall thickening | Damage of the bronchial wall | ++ |
| Bronchiectasis | Lungs become abnormally enlarged | ++ |
| Nodules | Irregular or rounded opacity (any space-occupying lesion in single or multiple forms) | + |
| Pleural effusion | Fluid on the pleural cavity | + |
| Subpleural curvilinear line | A thin curvilinear opacity (1–3 mm), located in the subpleural area and having a parallel distribution over the pleural surface | + |
| Fibrosis | The alveoli become stiff and scarred | + |
| Mediastinal lymphadenopathy | Mediastinal lymph node enlargement | Rare |
| Pericardial effusion | An abnormal levels of fluid in the pericardial space | Rare |
| Halo sign | Pulmonary nodules surrounded by ground glass | Very rare |
| Cavitation | A gas-filled spaces | Absent |
| Calcification | Deposition of calcium salt | Absent |
10. RT-PCR test
The most common detection assay is RT-PCR based on the RNA isolated from respiratory specimens such as oropharyngeal swabs, sputum, nasopharyngeal aspirate, bronchoalveolar lavage, or deep tracheal aspirate [3].
At the moment, the RT-PCR method is used in clinics to confirm the COVID-19 infection. While this assay remains the reference standard to the detection of COVID-19, the high false-negative RT-PCR results [21] and inapplicability of RT-PCR in the initial phase of the disease limited the rapid diagnosis of infected subjects [13]. It has been reported that the sensitivity of chest CT was superior to that of RT-PCR (98% vs. 71%, respectively). The causes for the low effectiveness of viral nucleic acid measurement might include low viral load, inappropriate sample, variation in the diagnosis rate among different kits, and undeveloped technology for detection of the nucleic acid [22]. Lower respiratory tract sample (bronchoalveolar lavage fluid, deep tracheal aspirates) and induced sputum has the highest genome fraction and viral load compared to upper respiratory tract samples, which are optimal for improving detection accuracy [3]. Nevertheless, with sample collection and transportation and kit quality, the total positive rate of RT-PCR for throat swab specimens was about 60% at initial presentation in different studies (Table 5 ) [23,24].
Table 5.
COVID-19 detection in various clinical samples (positive rate).
| Clinical specimen | Wang et al. study [24] |
Wölfel et al. study [52] |
|---|---|---|
| Total cases: 1070 samples from 205 patients | Total cases: 13 samples from 4 patients | |
| BAL fluid | 93% | NT |
| Sputum | 72% | 83.33% |
| Nasal swabs | 63% | 16.66% |
| Brush biopsy | 46% | NT |
| Pharyngeal swabs | 32% | NT |
| Stool | 29% | Test was not successful |
| Blood | 1% | NT |
| Urine | 0% | NT |
Samples were prepared during the first week of symptoms. BAL (bronchoalveolar lavage), NT (not tested).
The positive rate of chest CT imaging and RT-PCR assay in a cohort study by Ai et al. [23] was 88%, and 59% for the detection of suspected subjects with COVID-19, respectively. They showed that chest CT imaging had higher sensitivity for the diagnosis of the COVID-19 infection as compared with initial RT-PCR from swab samples. They also reported that 42% of infected subjects exhibited improvement of follow-up chest CT scans before the RT-PCR finding turning negative [23].
Xie et al. [25], evaluating 167 infected cases, showed that 3% (5/167) of patients had negative RT-PCR while they had positive chest CT. PCR procedure for COVID-19 may be falsely negative because of a laboratory error, inappropriate sample, or inadequate viral load in the sample. The chest CT gives fast results, is easy to do, and enables quick diagnoses of initial COVID-19 pneumonia [13].
11. Mechanism of action
The COVID-19 spreads and invades via the lung, stimulates inflammation, and induces a cytokine storm, leading to alteration in immune cells such as WBCs and lymphocytes. Accordingly, administration of intravenous immunoglobulins is being used as the therapeutic strategy for most subjects with decreased levels of WBCs and lymphocytes [8].
COVID-19 patients are susceptible to liver failure, since COVID-19 directly binds to angiotensin-converting enzyme-2 (ACE2) positive bile duct cells [26]. It has been shown that ACE2 is protective against several lung diseases, including ARDS, asthma, acute lung injury (ALI), chronic obstructive pulmonary disease (COPD), and pulmonary hypertension [27]. ACE2 has also been demonstrated to be the receptor for both the SARS-CoV and the human respiratory coronavirus NL63. The previous experiment established the positive correlation of ACE2 expression and the infection of SARS-CoV in vitro [28]. SARS-CoV significantly decreased ACE2 protein expression after infecting the host [28]. Consequently, the ACE2 expression in different organs might be vital for the vulnerability, signs, and outcome of COVID-19. An analysis of single-cell RNA-sequencing (RNA-seq) showed that Asian males might have higher ACE2 expression levels [28].
ACE2 is one of the components of the renin angiotensin system (RAS) which regulates blood pressure, systemic vascular resistance, and electrolyte balance. In the respiratory system, local lung RAS activation can influence the pathogenesis of lung damage through numerous mechanisms, including elevation of vascular permeability and changes in alveolar epithelial cells. In this cascade, renin increases angiotensinogen to produce angiotensin I (Ang I, a decapeptide hormone) [29]. The ACE hydrolyzes Ang I to angiotensin II. The angiotensin II binds to its receptors and induces vasoactive effects. ACE2 catalyzes Ang I and Ang II to generate angiotensin-(1–9) and Ang-(1–7), respectively, and antagonizes several effects of Ang II. ACE2, by reducing local Ang II levels, acts as a counter-regulatory enzyme [29,30]. ACE2 deficiency and the consequent high Ang II concentration, lead to increased vascular permeability, neutrophil accumulation, pulmonary oedema, disruption of gas exchange, and exacerbation in lung function. On the other hand, active recombinant ACE2 protein alleviates ALI symptoms in ACE2 knockout animals [31]. In the lungs, RAS activity is basically high, and the activity of the ACE2 is also highly increased to control the balance of Ang II/Ang-(1–7) concentration [29,30]. It has been shown that ACE2 participates in the severe ALI and failure that is induced by SARS, influenza A H5N1 virus, acid aspiration, sepsis, and lethal avian. Currently, ACE2 is proposed as a potential therapeutic target for the treatment of ALI in humans [32].
In animal models of ARDS, ACE2 knockout animals showed more severe symptoms, whereas the upregulation of the ACE2 has protective effects. In animals infected by SARS-CoV, both the viral spike protein and replication protein alone can decrease ACE2 but not ACE expression. Furthermore, SARS-CoV also motivates quick ACE2 downregulation from the cell surface. These findings suggest that the SARS-CoV interrupts the physiological balance between ACE/ACE2 and Ang II/Ang-(1–7) [29]. Consequently, high Ang II concentration in the lung tissue aggravates acid-induced acute lung injury and causes severe lung failure. Likewise, the spike protein of COVID-19 interacts with ACE2, and the pathogenic mechanism might probably be shared between SARS-CoV and COVID-19 [29].
12. Surgery in COVID-19 patients
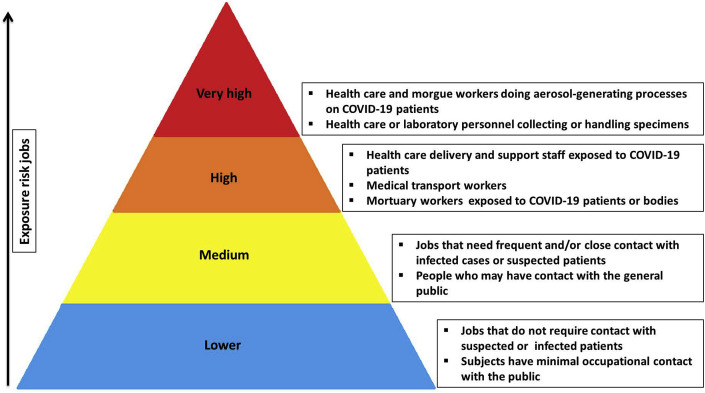
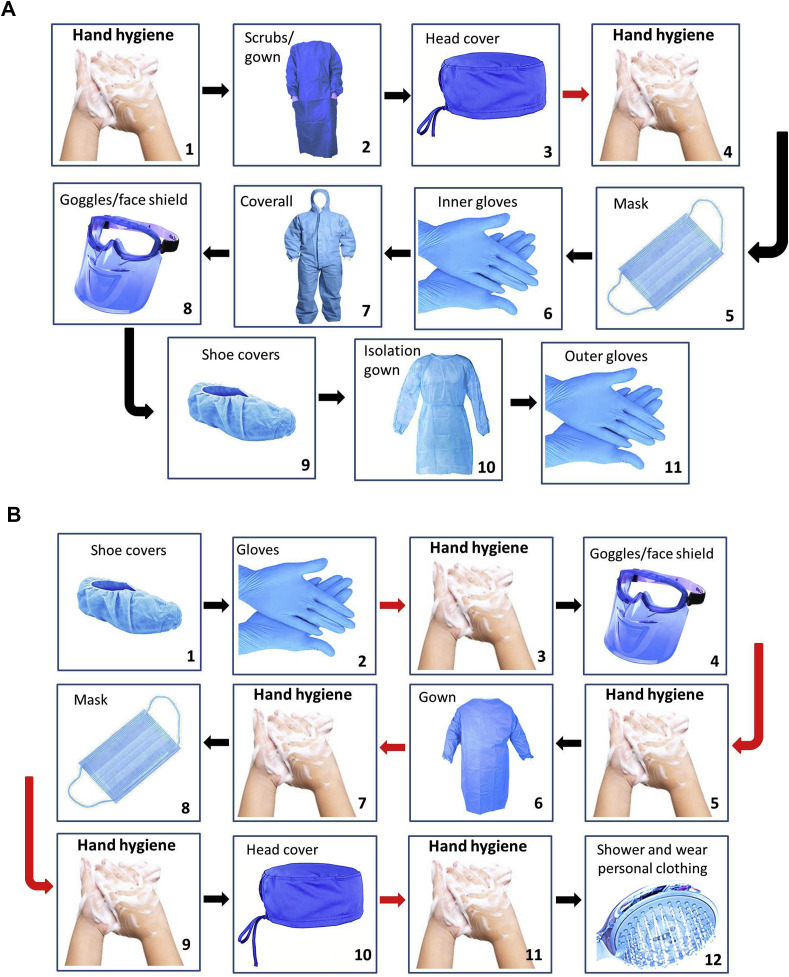
Healthcare workers are on the front lines of battling COVID-19 which puts them at high risk of COVID-19 infection. Occupational Safety and Health Administration (OSHA) has separated job tasks into four risk exposure levels, as presented in Fig. 4 . Since Covid-19 spreads quickly through respiratory droplets, head and neck surgeons who have close contact with the upper aerodigestive tract are principally at high risk. Given the high number of surgeries done worldwide, it is essential for the surgeons and surgical team to be adequately protected from coronavirus transmission. In the COVID–19 patients who need surgery, risks versus benefits of the procedure for the patient should be cautiously evaluated. The surgeon may temporarily postpone an emergency or urgent surgery on cases which show coronavirus symptoms (e.g., cough, sore throat, fever). For all suspected cases that are undergoing operation, chest CT and blood tests need to be checked before admission. The surgical team can also order an in-house RT-PCR assay within 24 h. If the subject's condition does not allow for a 24 h wait, the patient is assumed to be COVID-19-positive. For suspected or confirmed COVID-19 cases, non-operative management is preferred. If surgery is essential in these subjects, suitable personal protective equipment (PPE) should be used (Fig. 5 ). Furthermore they should remove their PPE and place the PPE in a labeled waste bag in an anteroom. There are various levels of emergency related to COVID-19 patient needs, and assessment is required to distinguish between them. Table 6 summarizes the key recommendations for the surgeon and surgical team in different stages [[33], [34], [35]].
Fig. 4.
Occupational risk pyramid for COVID-19 infection based on the Occupational Safety and Health Administration (OSHA) report.
Fig. 5.
Sequence for putting on (A) and removing of (B) personal protective equipment (PPE) (designed according to Chen et al. [35] paper).
Table 6.
| A. Preoperative recommendation |
|
| B. Operative recommendation |
Operating room
|
| C. Postoperative recommendation |
|
13. Treatment of COVID-19
Currently, there is no effective medicine for the treatment for COVID-19 patients, which can, in some patients, lead to lethal lung failure. Furthermore, there is no specific antiviral medicine or vaccine for this virus [8]. Pathogenesis of coronavirus is an extremely complex process, and much of the required details of host-pathogen interaction remain unknown. Discovering the useful medicine options for the treatment of the COVID-19 outbreak is vital [36,37] (Table 7 ). There are various possible treatment approaches [29] including: (i) raising of ACE2 expression by injection of soluble recombinant ACE2 protein or using therapeutic vectors expressing high levels of ACE2 which may be applicable in the future [38], (ii) using specific ACE inhibitors such as lisinopril, and (iii) inhibiting Ang II receptors. In particular, the type I Ang II receptor has been reported to promote disease by motivating edemas and disturbing lung function [38]. Hence, a type I Ang II receptor blocker (e.g., losartan) has been successively examined for improving COVID-19 pneumonia [29]. Treatment with a soluble form of ACE2 probably acts as a competitive interceptor of coronavirus by inhibiting binding of the viral particle to the ACE2 receptor, which can consequently slow coronavirus entry into the cells and protect against lung injury. Remdesivir (adenosine analogue), which is used for the treatment of the Ebola disease, is incorporated in the nascent chains of viral RNA, causing premature termination. This drug is currently used for the treatment of COVID-19 infection. Randomized trial studies have reported the safety and efficacy of interferon-α (5 million U per time, twice a day) and lopinavir-ritonavir (two capsules each time, twice a day) in COVID-19 patients [39]. Wang et al. determined the efficacy of some FDA-approved drugs such as penciclovir, ribavirin, nitazoxanide, chloroquine, nafamostat, and favipiravir (T-705) for treatment of COVID-19 infection in vitro [40]. They found that chloroquine and remdesivir are potentially effective for the control of COVID-19 infection in vitro [40]. Chloroquine is a well-known drug that has currently been documented as a strong broad-spectrum antiviral agent for the treatment of malarial and autoimmune disease. This drug interferes with glycosylation of the cellular receptor, elevates endosomal pH needed for virus/cell fusion, and consequently reduces virus infection [41]. The use of traditional medicine to recover the physical signs of cases has also been recommended by some Chinese researchers [8]. Luo et al. showed that some Chinese medicines such as Lonicerae Japonicae Flos (Jinyinhua), Radix saposhnikoviae (Fangfeng), Radix glycyrrhizae (Gancao), Fructus forsythia (Lianqiao), and Rhizoma Atractylodis Macrocephalae (Baizhu) are useful in the prevention of COVID-19 [42]. Some other herbal plants, through their antioxidant and anti-inflammatory activity might be considered as useful agents in the treatment of COVID-19 infection [[43], [44], [45], [46]]. Furthermore, zinc nanoparticles, due to their potential antioxidant, anti-inflammatory [47], and antiviral effects, have been found to inhibit influenza viral load and COVID-19 replication in an in vitro experiment [48].
Table 7.
Treatment options for COVID-19.
| Drug | Proposed dose for COVID-19 | Mechanism of action | Target diseases | Route of administration | Safety concerns and toxicities |
|---|---|---|---|---|---|
| Ritonavir + Lopinavir (Kaletra) (Repurposed agent) | 500 mg once, twice a day, 2 weeks | Protease inhibitors Inhibits coronavirus replication |
HIV infection | Oral | Elevated risk of cardiac arrhythmias, pancreatitis, cardiac conduction abnormalities, and hepatotoxicity Caution in cases with liver disease, hemophilia, cardiovascular disease, and pancreatitis Potential drug interactions Common side effects: diarrhea, gastrointestinal intolerance, nausea, vomiting, |
| Ribavirin (Repurposed agent) | 500 mg each time, 2 to 3 times/day in combination with IFN-α or lopinavir/ritonavir | Nucleoside inhibitor (Interfering with the synthesis of viral mRNA) | Hepatitis C, SARS, MERS | Oral or intravenous infusion | Elevated risk of anemia Is a contraindicated and teratogen in pregnancy Leads to severe dose-dependent hematologic toxicity |
| Chloroquine phosphate, chloroquine (Repurposed agent) | 500 mg each time, 2 times/day for 5–10 days (300 mg for chloroquine) | Increasing endosomal pH Autophagy inhibitors Inhibits viral RNA polymerase Immunomodulating Probably inhibit ACE2 cellular receptor |
Antimalarial agent, autoimmune disease | Oral | Elevated risk of cardiac arrhythmias, hypoglycemia, retinal damage, particularly with long time use Caution in cases with G6PD deficiency and diabetes Potential drug interactions Common side effects: Abdominal cramps, anorexia, vomiting, nausea, diarrhea |
| Hydroxychloroquine sulphate (Repurposed agent) | 400 mg each time, 2 times/day in first day, then 200 mg 2 times/day for 4 days (Alternative dose: 400 mg daily for 5 days or 200 mg 3 times/day for 10 days) | Has same mechanism as Chloroquine | Antimalarial agent, autoimmune disease | Oral | Side effects are similar to chloroquine but less common |
| Arbidol (umifenovir) (Repurposed agent) | 200 mg each time, 3 times/day | S protein/ACE2, membrane fusion inhibitor Inhibits the replication of coronavirus in vitro |
Influenza infection | Oral | Safety and efficacy not established Common side effects: allergic reaction, gastrointestinal intolerance, increased liver enzymes |
| Favipiravir (T-705) (Investigational agent) | 1600 mg*2/first day followed by 600 mg*2/day | Nucleoside analogue (RNA polymerase inhibitor) | Influenza A (H1N1), Ebola | Oral | Increased risk for embryotoxicity and teratogenicity Common side effects: diarrhea, increased liver enzymes, hyperuricemia, decreased neutrophil count |
| Remdesivir (GS-5734) (Investigational agent) | 200 mg on day 1, then 100 mg on days 2–10 | Nucleoside analogue (terminates RNA synthesis) Interfering with virus post-entry |
SARS, Ebola, and MERS | Intravenous infusion | Safety and efficacy not established Common side effects: increased liver enzymes (reversible), kidney injury |
| Interferon alpha (IFN-α) (Adjunctive/Supportive therapy) | 5 million U, 2 times/day | Increase cellular immunity, Inhibits viral replication |
Broad-spectrum antiviral | Oral or injectable | Failed to suppress viral replication and had some side effects when prescribe later |
| Tocilizumab (Actemra) (Adjunctive/Supportive therapy) | 400 mg IV or 8 mg/kg × 1–2 doses Next dose 8–12 h after the first dose if insufficient response |
Inhibits IL-6-mediated signaling (also reduce cytokine storm) | Rheumatoid arthritis | Intravenous infusion | Caution in patients with neutropenia a (<500 cells/μL) or thrombocytopenia (<50,000/μL) Safety in pregnancy is unknown and may cause harm to the fetus Increased risk of URTI, hepatotoxicity, hypersensitivity reactions, infections, nasopharyngitis, hematologic effects, gastrointestinal problem Common side effects: hypertension, headache, increased AST level |
Note: Most of these drugs should not be used for more than 10 days.
ACE2 (angiotensin-converting enzyme 2), AST (aspartate aminotransferase), G6PD (glucose-6-phosphate dehydrogenase), HIV (human immunodeficiency viruses), IL-6 (interleukin 6), IV (intravenous therapy), MERS (middle east respiratory syndrome), SARS (severe acute respiratory syndrome), URTI (upper respiratory tract infection).
Isolation of infected cases and supportive cares such as fluid management, oxygen therapy, and antimicrobials agents for the treatment of secondary bacterial infections and prevention of end-organ dysfunction are suggested by the WHO for patients needing hospital admission [8,10]. Interferon-α is a wide spectrum antivirus medicine that can be used for the treatment of hepatitis B virus (HBV). Lopinavir is a protease inhibitor used for the treatment of human immunodeficiency viruses (HIV) with ritonavir as a booster that showed potential anti-coronavirus activity. Patients with SARS treated with lopinavir/ritonavir and ribavirin had a lower risk of ARDS or death as compared with ribavirin alone [49]. Beck et al. used some antiviral drugs, including arbidol, lopinavir/ritonavir, and Shufeng Jiedu Capsule (SFJDC, herbal medicine) for the treatment of four cases with mild or severe COVID-19 pneumonia. After the drug therapy, the cases showed noticeable improvement and were discharged from the hospital [39].
Some COVID-19 infected patients might have co-infections with fungi and bacteria. Chen et al. detected some bacteria and fungi as secondary infections, including Klebsiella pneumonia, Acinetobacter baumannii, Aspergillus flavus, Candida albicans, and Candida glabrata. They showed that Acinetobacter baumannii has a high drug resistance rate, causing the possibility of septic shock [50]. The host immune system is one of the main factors in secondary infections. Other factors involved which may increase mortality in COVID-19 patients are obesity, old age, diabetics, HIV infection, autoimmune disease, and pregnancy in women [50]. Therefore, early diagnosis and timely treatment of these critical patients to prevent secondary infection are necessary. Immunoglobulin injection is recommended to increase the anti-infection drug ability for severe cases [50]. Furthermore, paracetamol (400 mg per time, every 8 h when required) is recommended in patients with high temperature [9,37].
In summary, at present, there are no vaccines and specific antiviral medicine for the treatment of COVID-19. All of the recommended drugs come from the knowledge gained by treating MERS, SARS, or another family of coronavirus. Further researches are required to provide evidences of the effectiveness of these drugs.
Ethical approval
No ethical approval required.
Sources of funding
No funding received.
Author contribution
EAO and FM wrote the manuscript with support from FF, HT and IK. EAO designed the experiments, revised the manuscript. FF prepared surgery section and revised the manuscript. HT contributed to data collections and revised the manuscript. All authors read and approved the final.
Unique Identifying number (UIN)
Name of the registry:
Unique Identifying number or registration ID:
Hyperlink to your specific registration (must be publicly accessible and will be checked):
Guarantor
Ebrahim Aabbasi-Oshaghi accepts full responsibility for this review manuscript.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Data statement
Data statement don't required for this review article.
Declaration of competing interest
The author declared no interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijsu.2020.05.018.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ahmed S.F., Quadeer A.A., McKay M.R. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 2020;12:254. doi: 10.3390/v12030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan F., Ye T., Sun P., Gui S., Liang B., Li L. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020:200370. doi: 10.1148/radiol.2020200370. ([Epub ahead of print]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sahin A.R., Erdogan A., Agaoglu P.M., Dineri Y., Cakirci A.Y., Senel M.E. Novel Coronavirus (COVID-19) outbreak: a review of the current literature. Eur. J. Med. Oncol. 2019;4:1–7. doi: 10.14744/ejmo.2020.12220. 2020. [DOI] [Google Scholar]
- 4.Wei J., Xu H., Xiong J., Shen Q., Fan B., Ye C. Novel Coronavirus (COVID-19) pneumonia: serial computed tomography findings. Korean J. Radiol. 2019;21(4):501–550. doi: 10.3348/kjr.2020.0112. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K. Covid-19 in critically ill patients in the seattle region-case series. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2004500. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team . vol. 2. CCDCW; 2020. pp. 113–122. (The Epidemiological Characteristics of Anoutbreak of 2019 Novel Coronavirus Diseases (COVID-19) – China, 2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu J., Liu J., Zhao X., Liu C., Wang W., Wang D. Clinical characteristics of imported cases of covid-19 in jiangsu province: a multicenter descriptive study. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa199. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sohrabi C., Alsafi Z., O'Neill N., Khan M., Kerwan A., Al-Jabir A. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int. J. Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin Y.H., Cai L., Cheng Z.S., Cheng H., Deng T., Fan Y.P. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil. Med. Res. 2020;7:4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karimi-Zarchi M., Neamatzadeh H., Dastgheib S.A., Abbasi H., Mirjalili S.R., Behforouz A. Vertical transmission of coronavirus disease 19 (COVID-19) from infected pregnant mothers to neonates: a review. Fetal Pediatr. Pathol. 2020:1–5. doi: 10.1080/15513815.2020.1747120. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo Z.D., Wang Z.Y., Zhang S.F., Li X., Li L., Li C. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China. Emerg. Infect. Dis. 2020;26(2020) doi: 10.3201/eid2607.200885. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zu Z.Y., Jiang M.D., Xu P.P., Chen W., Ni Q.Q., Lu G.M. Coronavirus disease 2019 (COVID-19): a perspective from China. Radiology. 2020:200490. doi: 10.1148/radiol.2020200490. ([Epub ahead of print]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Habibzadeh P., Stoneman E.K. The novel coronavirus: a bird's eye View. Int. J. Occup. Environ. Med. 2020;11:65. doi: 10.15171/ijoem.2020.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim H. Outbreak of novel coronavirus (COVID-19): what is the role of radiologists? Eur. Radiol. 2020 doi: 10.1007/s00330-020-06748-2. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mardani R., Ahmadi Vasmehjani A., Zali F., Gholami A., Mousavi Nasab S.D., Kaghazian H. Laboratory Parameters in detection of COVID-19 patients with positive RT-PCR; a diagnostic accuracy study. Arch. Acad. Emerg. Med. 2020;8:e43. doi: 10.22037/aaem.v8i1.632.g775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Worth Health Organization . 28 January 2020. Clinical Management of Severe Acute Respiratory Infection when Novel Coronavirus ( 2019-nCoV) Infection Is Suspected: Interim Guidance. [Google Scholar]
- 19.Pan Y., Guan H. Imaging changes in patients with 2019-nCov. Eur. Radiol. 2020:2026. doi: 10.1007/s00330-020-06713-z. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng M.Y., Lee E.Y., Yang J., Yang F., Li X., Wang H. Imaging profile of the COVID-19 infection: radiologic findings and literature review. Radiol. Cardiothorac. Imaging. 2020;2 doi: 10.1148/ryct.2020200034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan J.F.W., Yuan S., Kok K.-H., To K.K.-W., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang Y., Zhang H., Xie J., Lin M., Ying L., Pang P. Sensitivity of Chest CT for COVID-19: Comparison to RT-PCR. Radiology. 2020:200432. doi: 10.1148/radiol.2020200432. ([Epub ahead of print]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ai T., Yang Z., Hou H., Zhan C., Chen C., Lv W. Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020:200642. doi: 10.1148/radiol.2020200642. ([Epub ahead of print]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W., Xu Y., Gao R., Lu R., Han K., Wu G. Detection of SARS-CoV-2 in different types of clinical specimens. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.3786. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie X., Zhong Z., Zhao W., Zheng C., Wang F., Liu J. Chest CT for Typical 2019-nCoV Pneumonia: Relationship to Negative RT-PCR Testing. Radiology. 2020:200343. doi: 10.1148/radiol.2020200343. ([Epub ahead of print]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS. J. Virol. 2020;94 doi: 10.1128/JVI.00127-20. e00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jia H. Pulmonary angiotensin-converting enzyme 2 (ACE2) and inflammatory lung disease, Shock. J. Virol. 2016;46:239–248. doi: 10.1128/JVI.00127-20. [DOI] [PubMed] [Google Scholar]
- 28.Cao Y., Li L., Feng Z., Wan S., Huang P., Sun X. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020;6:1–4. doi: 10.1038/s41421-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Y. Compensation of ACE2 function for possible clinical management of 2019-ncov-induced acute lung injury. Virol. Sin. 2020:1–3. doi: 10.1007/s12250-020-00205-6. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiao J. What are the risks of COVID-19 infection in pregnant women? Lancet. 2020;395:760–762. doi: 10.1016/S0140-6736(20)30365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu X., Lin Q., Qin X., Ruan Z., Zhou J., Lin Z. ACE2 antagonizes VEGFa to reduce vascular permeability during acute lung injury. Cell. Physiol. Biochem. 2016;38:1055–1062. doi: 10.1159/000443056. [DOI] [PubMed] [Google Scholar]
- 32.Yang P., Gu H., Zhao Z., Wang W., Cao B., Lai C. Angiotensin-converting enzyme 2 (ACE2) mediates influenza H7N9 virus-induced acute lung injury. Sci. Rep. 2014;4:7027. doi: 10.1038/srep07027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coccolini F., Perrone G., Chiarugi M., Di Marzo F., Ansaloni L., Scandroglio I. Surgery in COVID-19 patients: operational directives, World. J. Emerg. Surg. 2020;15:25. doi: 10.1186/s13017-020-00307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Givi B., Schiff B.A., Chinn S.B., Clayburgh D., Iyer N.G., Jalisi S. Safety recommendations for evaluation and surgery of the head and neck during the COVID-19 pandemic. JAMA Otolaryngol. Head Neck Surg. 2020 doi: 10.1001/jamaoto.2020.0780. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Chen X., Liu Y., Gong Y., Guo X., Zuo M., Li J. Anesthesiology; 2020. Perioperative Management of Patients Infected with the Novel Coronavirus: Recommendation from the Joint Task Force of the Chinese Society of Anesthesiology and the Chinese Association of Anesthesiologists. ([Epub ahead of print]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov. Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 37.Smith T., Prosser T. Clinical Drug Information| Clinical Solutions. Elsevier; 2020. COVID-19 Drug Therapy–potential options.https://www.elsevier.com/__data/assets/pdf_file/0007/988648/COVID-19-Drug-Therapy_Mar-2020.pdf [Google Scholar]
- 38.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beck B.R., Shin B., Choi Y., Park S., Kang K. bioRxiv; 2020. Predicting Commercially Available Antiviral Drugs that May Act on the Novel Coronavirus (2019-nCoV), Wuhan, China through a Drug-Target Interaction Deep Learning Model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2:69. doi: 10.1186/1743-422x-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo H., Tang Q.-l., Shang Y.-x., Liang S.-b., Yang M., Robinson N. Can Chinese Medicine be used for prevention of corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chin. J. Integr. Med. 2020:1–8. doi: 10.1007/s11655-020-3192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mehrdad Kassaee S., Goodarzi M., Oshaghi E. Antioxidant, antiglycation and anti-hyperlipidemic effects of Trigonella foenum and Cinnamon in type 2 diabetic rats. Jundishapur J. Nat. Pharm. Prod. 2018;13 doi: 10.8512/jjnpp.38414. [DOI] [Google Scholar]
- 44.Abbasi Oshaghi E., Goodarzi M.T., Higgins V., Adeli K. Role of resveratrol in the management of insulin resistance and related conditions: mechanism of action. Crit. Rev. Clin. Lab Sci. 2017;54:267–293. doi: 10.1080/10408363.2017.1343274. [DOI] [PubMed] [Google Scholar]
- 45.Ravan A.P., Bahmani M., Ghasemi Basir H.R., Salehi I., Oshaghi E.A. Hepatoprotective effects of Vaccinium arctostaphylos against CCl4-induced acute liver injury in rats. J. Basic Clin. Physiol. Pharmacol. 2017;28:463–471. doi: 10.1515/jbcpp-2016-0181. [DOI] [PubMed] [Google Scholar]
- 46.Lin L.T., Hsu W.C., Lin C.C. Antiviral natural products and herbal medicines. J. Tradit. Compl. Med. 2014;4:24–35. doi: 10.4103/2225-4110.124335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abbasi E., Mirzaei F., Mirzaei A. Effects of ZnO nanoparticles on intestinal function and structure in normal/high fat diet-fed rats and Caco-2 cells. Nanomedicine. 2018;13:2791–2816. doi: 10.2217/nnm-2018-0202. [DOI] [PubMed] [Google Scholar]
- 48.Ghaffari H., Tavakoli A., Moradi A., Tabarraei A., Bokharaei-Salim F., Zahmatkeshan M. Inhibition of H1N1 influenza virus infection by zinc oxide nanoparticles: another emerging application of nanomedicine. J. Biomed. Sci. 2019;26:70. doi: 10.1186/s12929-019-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil. Med. Res. 2020;7:11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ye Z., Zhang Y., Wang Y., Huang Z., Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur. Radiol. 2020 doi: 10.1007/s00330-020-06801-0. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wolfel R., Corman V.M., Guggemos W., Seilmaier M., Zange S., Muller M.A. Virological Assessment of Hospitalized Patients with COVID-2019. Nature. 2020 doi: 10.1038/s41586-020-2196-x. ([Epub ahead of print]) [DOI] [PubMed] [Google Scholar]
- 53.Liu Z., Zhang Y., Wang X., Zhang D., Diao D., Chandramohan K. Recommendations for surgery during the novel coronavirus (COVID-19) Epidemic. Indian J. Surg. 2020;3:133–135. doi: 10.3779/j.issn.1009-3419.2020.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.