Abstract
The aim of this report is to discuss emergent repair for complex aortic diseases in patients affected by novel coronavirus pneumonia (coronavirus disease-2019 [COVID-19]), describing a case of ruptured pararenal aortic aneurysm. An eighty-year-old man with COVID-19 was admitted for ruptured aneurysm of the pararenal aorta and hemorrhagic shock. Endovascular repair was chosen, and a proximal extension of the previous abdominal endograft was performed with parallel stents in the right renal artery and the superior mesenteric artery. Endovascular treatment and early anticoagulation are the key for success for vascular emergencies in patients with COVID-19, despite the risk of late endoleak.
The novel coronavirus (NCoV) pandemic is affecting all aspects and fields of the medical profession, compelling physicians and surgeons to rethink their way of work and therapeutic choices to comply with the new challenges and the changes in their work environment.1 Although the NCoV pneumonia (coronavirus disease-2019 [COVID-19]) has spread worldwide, affecting millions of people, to our knowledge, no experience has been reported on the management of major vascular emergencies in patients with COVID-19 yet. The aim of the present case report on a ruptured pararenal abdominal aortic aneurysm (AAA) is to offer some consideration on this matter from the standpoint of a hub hospital for patients with COVID-19 in Lombardy, the Italian region that has been affected the most by the pandemic.
Materials and Methods
Anonymized publication of the patient's data was approved by the competent institutional review board.
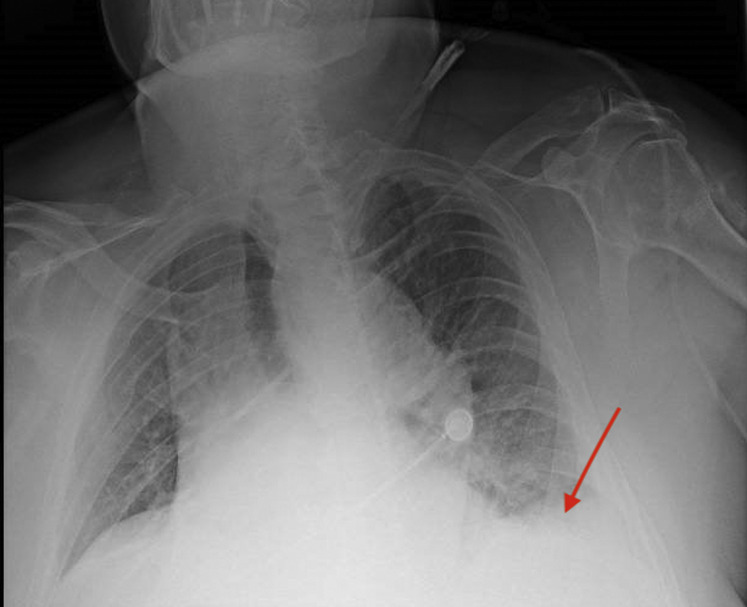
An 80-year-old man, undergone endovascular aortic repair (EVAR) in 2013 for an infrarenal AAA and lost to follow-up for 5 years, was referred to the unit of vascular surgery for severe abdominal pain, hypotension (blood pressure [BP]: 60/35 mmHg), and eventually loss of consciousness. Most significant comorbidities were previous rectal cancer, treated by Hartmann’s resection and left terminal colostomy confection, postincisional hernia of the abdominal midline, and chronic kidney failure with left kidney shrinkage. Although no respiratory symptoms such as dyspnea, cough, or fever were reported, the routine chest X-ray performed at the admittance showed a consolidation pattern spreading at the inferior lobes of the lungs (Fig. 1 ), and the nasal swab turned positive for NCoV (laboratory tests are reported in Table I ). A computed tomography angiography (CTA) was performed, showing a free rupture of the pararenal abdominal aorta, right above the previous endograft at the level of the renal arteries, and a massive intraperitoneal hematoma (Fig. 2, Fig. 3 ). Open surgical treatment was excluded, not only because of the technical difficulty caused by a hostile abdomen and the need to explant the endograft but also because a laparotomy would have necessarily worsened the respiratory impairment and probably yielded to a severe respiratory distress syndrome. Endovascular repair was therefore chosen, although the emergent setting left no time to purchase the most suitable devices, and the repair had to be carried out with the stocks available in the hospital warehouse.
Fig. 1.
Chest X-ray showing initial basal consolidation pattern (red arrow).
Table I.
Blood tests at the admittance
| Hb (g/dL) | WBC (×103/μL) | PLT (×103/μL) | aPTT (sec) | INR | d-Dimer (ug/dL) | CRP (mg/dL) | IL-6 (pg/mL) | S-Cr (mg/dL) |
|---|---|---|---|---|---|---|---|---|
| 7.5 | 10.23 | 89 | 40.70 | 1.15 | 5,334 | 18.26 | 136.08 | 1.84 |
Hb, hemoglobin; WBC, white blood cells; PLT, platelets; aPTT, activated partial thromboplastin time; INR, international normalized ratio; CRP, C-reactive protein; IL-6, interleukin-6; S-Cr, serum creatinine.
Fig. 2.
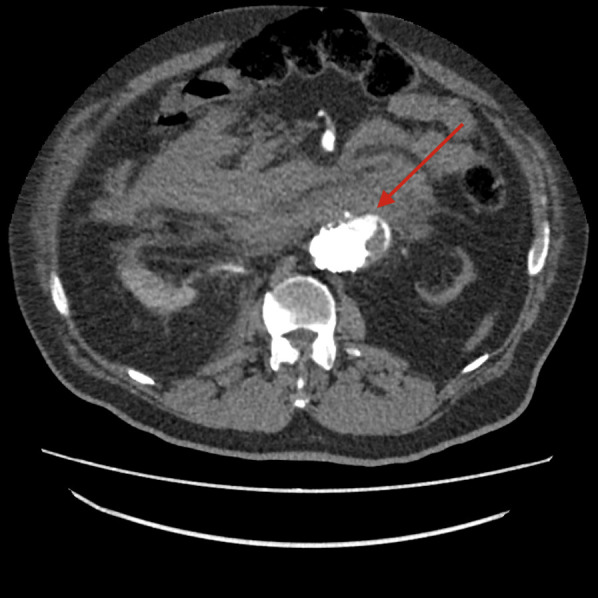
CTA scan: free ruptured AAA in the pararenal aortic tract with massive intraperitoneal hematoma (red arrow). AAA, abdominal aortic aneurysm; CTA, computed tomography angiography.
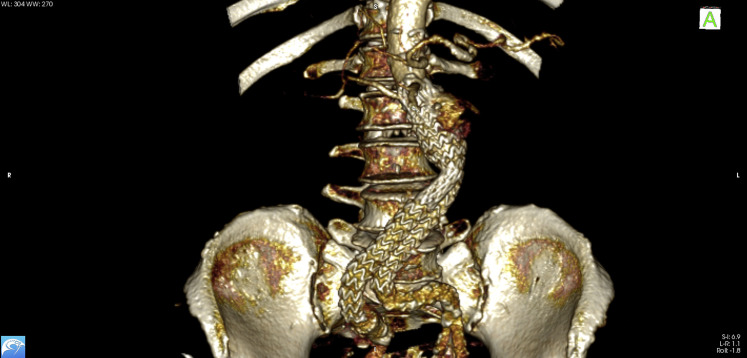
Fig. 3.
3D volume rendering of the ruptured AAA. AAA, abdominal aortic aneurysm; 3D, three-dimensional.
Under general anesthesia, a microsurgical access was obtained at the right common femoral artery (CFA), whereas the left was approached percutaneously. Introducers Cook® 8 × 45 mm and Cook 7 × 45 mm (Cook Medical Inc, Bloomington, Indiana) were placed in the left and right CFA, respectively. The right renal artery (RRA) was engaged from the left access with a 6F catheter. On the right side, the introducer was switched with a Gore® 18F introducer (Gore & Ass, Flagstaff, Arizona), and an angiogram was performed, showing aortic rupture right above the left renal artery (LRA), which was barely visible (Fig. 4 ). Owing to the shrinkage of the left kidney, it was decided that the LRA could be sacrificed and only the RRA was revascularized. On a guide wire V18, two Viabahn® 7 × 50 mm and 8 × 100 mm (Gore & Ass, Flagstaff, Arizona) were placed in the RRA. A Gore aortic cuff sized 32 × 45 mm was placed right below the origin of the superior mesenteric artery (SMA) and deployed with intentional covering of the LRA. The two Viabahn (periscope technique) were then deployed and they were postdilated simultaneously with the cuff (Cordis OPTA Pro® 7 × 100 [Cordis Corp., Milpitas, CA] was used for the stent grafts and Reliant® [Medtronic Inc, Minneapolis, Minnesota] for the cuff). At the completion angiogram, complete exclusion of the aneurysm was observed, with patency of the SMA and RRA (Fig. 5 ). The patient was extubated at the awakening and showed quick recovery of the vital parameters (BP: 105/60 mm Hg).
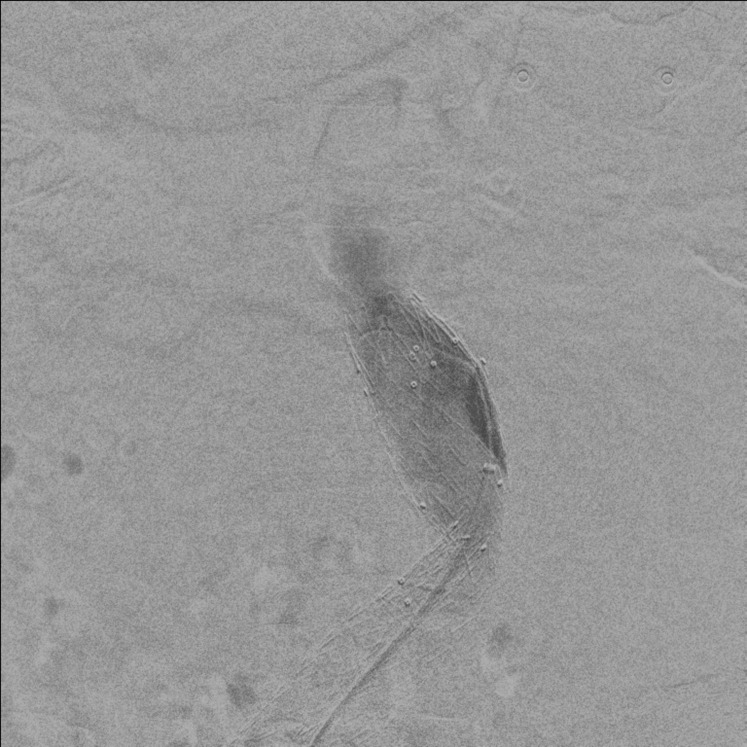
Fig. 4.
Preoperative angiogram.
Fig. 5.
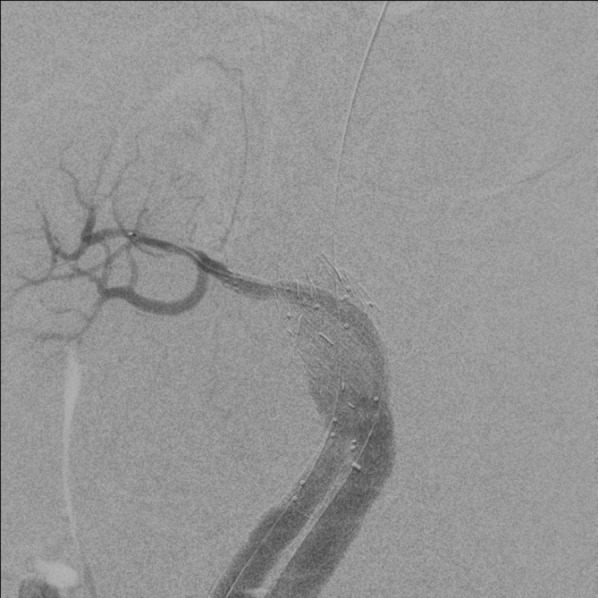
Completion angiogram after the first intervention showing patency of the RRA. RRA, right renal artery.
Results
On postoperative day 2, he performed a CTA that showed a type IA endoleak because of slight caudad migration of the aortic cuff, with reduction of the retroperitoneal hematoma (Fig. 6, Fig. 7 ). Reintervention was carried out, with surgical access at the right CFA and the left brachial artery. From the brachial access, a Gore introducer 9 × 70 was placed at the origin of the SMA, which was engaged from above, and a covered stent (VBX 7 × 59 mm) (Gore &Ass, Flagstaff, Arizona) was inserted. From the CFA access, a Gore endograft aortic cuff 32 × 45 was placed right below the celiac trunk (CT) origin and deployed along with the SMA VBX after safety engagement of the right renal Viabahn. Completion angiography showed absence of leak and patency of the CT, SMA, and RRA (Fig. 8 ).
Fig. 6.
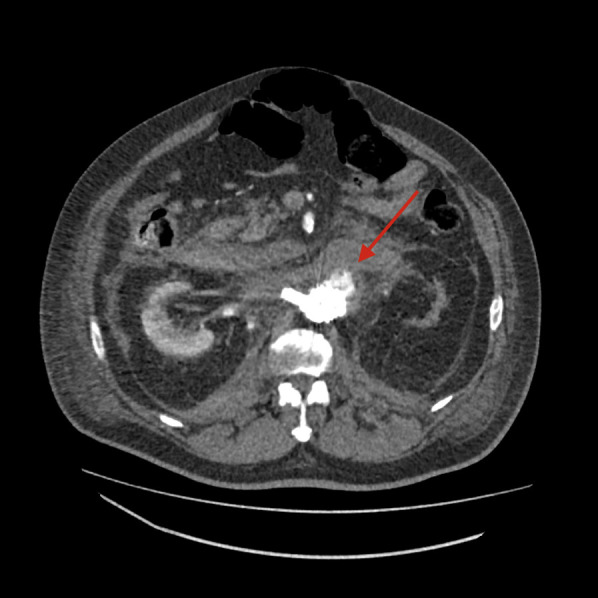
CTA on postoperative day 2, showing a type IA endoleak (red arrow) and reduced intraperitoneal hematoma. CTA, computed tomography angiography.
Fig. 7.
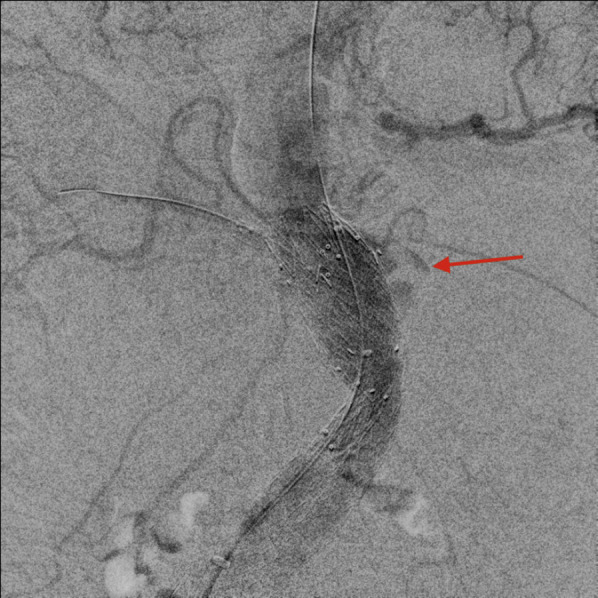
Angiogram showing contrast blushing at the level of the left renal artery (red arrow).
Fig. 8.
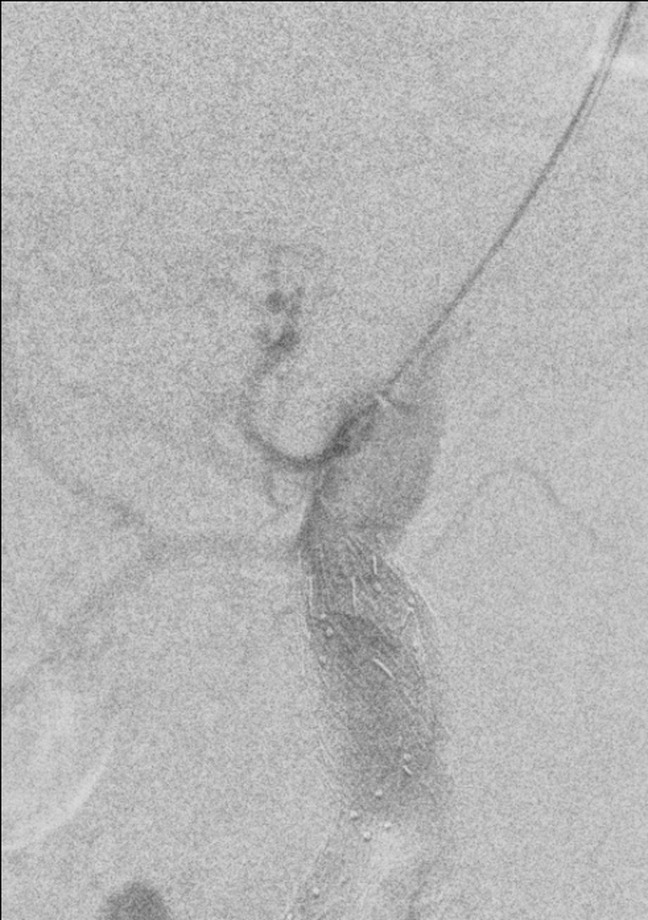
Completion angiogram after the second intervention, showing aneurysm exclusion and patency of the SMA and the RRA. RRA, right renal artery; SMA, superior mesenteric artery.
After awakening and extubation, the patient was stable and in good clinical conditions and was transferred to the internal medicine ward for an observation period owing to the COVID-19 pneumonia, which showed a stepwise but constant recovery during the postoperative course (improvement of the lung translucency at the chest X-ray on postoperative day 12 and drop of C-reactive protein to 3.6 mg/dL).
Discussion
Endovascular repair of complex AAAs involving the renal arteries, whether with fenestrated, branched endografts or parallel grafting, has been largely validated in the latest years, and it has proven safe and effective, provided that adequate surgical expertise and suitable endovascular materials are available.2 Those conditions still limit the feasibility of EVAR of pararenal aneurysms in emergent settings, especially in case of free-rupture and hemodynamic instability, with little time for accurate planning and sizing and often lack of the optimal endograft size.3 In those situations, open repair is still considered the treatment of choice, despite the heavy burden of possible complications in a high-risk patient, as in this case: in fact, open repair would have required delayed conversion of the previous EVAR with an endograft explant, and the presence of a left colostomy, midline laparocele, and intraperitoneal hematoma would have made the access to the aorta extremely difficult and time-consuming, with a considerable damage of the respiratory mechanism and a prolonged condition of respiratory distress. The patient was actually asymptomatic for respiratory infection, but the imaging and laboratory tests showed clear signs of a moderate-to-severe involvement of the pulmonary area and initial signs of systemic involvement. Although no specific indication has been given yet about the management of patients with COVID-19 warranting urgent aortic surgery, the consequences of laparotomy on the respiratory system are well known, especially in already compromised patients, and so is the great advantage of EVAR over open aortic surgery in terms of pulmonary involvement4; therefore, the advanced endovascular treatment was the most reasonable choice, even in absence of a wide selection of endovascular materials covering all possible sizes. Parallel grafting has already been described in semiurgent, urgent, and even emergent settings, with satisfactory outcomes, including lower mortality and lower complication rate and shorter intensive care unit (ICU) stay, and its role can result even more precious now, to avoid open surgery in patients with COVID-19.3 In this respect, the importance of having a wide variety of endovascular devices stored in the hospital warehouse is crucial because it offers the possibility to treat safely many and different types of aortic diseases and anatomic configurations, not only in urgency but also in elective settings because supplies could be delayed owing to the lockdown and other measures to contain the viral spread.
In the present case, a longer proximal neck could have been obtained by performing a chimney graft on the SMA from the beginning, which would have spared the patients the second intervention, but the emergent conditions of the patients (hemorrhagic shock with hemodynamic instability) made it preferable to try for a quicker and less invasive treatment. That was in fact successful in excluding the aneurysm and stop the intraperitoneal hemorrhage, as demonstrated by the hemodynamic stabilization and the CTA, and made it possible to perform the reintervention on a stable patient, in non-emergent setting. Although EVAR is at high risk for late failure and complications, and despite being technically demanding due to the parallel grafting, endovascular repair appears in those cases the treatment of choice, because it represents the safest and quickest way to stop the hemorrhage preserving the respiratory function. In fact, not only did the patient regain his consciousness and hemodynamic stability shortly after the end of the procedure, but he was also extubated and has had no further need for ventilatory support so far. Moreover, endovascular repair avoided postoperative ICU stay, which would have been necessary in case of open repair, in a moment of dramatic shortage of beds owing to the critically ill patients with COVID-19. The outbreak of COVID-19 in Northern Italy, in fact, has imposed the conversion of many hospitals, including ours, into COVID-19–dedicated centers, with only little resources available for the management of other emergencies. Theoretically, non-COVID emergencies should be referred to other centers, but often these patients cannot be safely transferred, so it is of the utmost importance to have a protocol for all those cases requiring nondeferrable treatment. The one followed in our center consists of early screening for COVID-19 through nasal swab and chest X-ray for all patients accessing to the emergency room and separate routes for inhospital admission for patients with and without COVID-19. The operatory theaters and the hospital wards are also divided between patients with and without COVID-19. If COVID-19 positivity cannot be determined before admission and surgery, the patients is managed through the “clean” route but with all precaution of staff individual protection and room sanification that are used for patients with COVID. In the present case, the result of the nasal swab was only available after the first intervention began, but the patient was considered COVID-19 positive since the beginning based on the chest X-ray. All these considerations are crucial to contain the viral spread among inhospital patients and were extremely helpful in choosing the most suitable approach of treatment.
As acknowledged previously, the risk of failure of emergent parallel grafting in ruptured AAAs is high, ranging between 10% and 26%, but the most common complications of EVAR, namely endoleak, can be addressed by endovascular treatment, which makes the risk of failure less worrisome than the perspective of an open conversion.5
The last suggestion regarding the management of patients with COVID-19 needing immediate aortic surgery regards anticoagulation: in fact, although the incidence of thrombotic events in these patients is still unknown, an altered coagulation profile and an increased tendency to develop microthromboses and macrothromboses in both arterial and venous system have been confirmed by several authors, so early anticoagulation with systemic heparin administration should be started in all these patients before starting endovascular procedures.6 , 7
Conclusions
Despite being a single case report, this is to our knowledge the first experience regarding emergent complex aortic surgery in a patient with COVID-19. The main suggestions that can be drawn from this regard are the benefits of endovascular surgery, even in absence of adequate materials, the importance of keeping a well-supplied warehouse, and early systemic anticoagulation before surgery. Minimal invasiveness and prevention of the complications, in fact, are the key to manage the vascular urgency and avoid progression of the infection that can evolve from pneumonia to multiorgan failure.
Acknowledgments
None.
Footnotes
Conflict of interest: None.
No funding was received regarding the publication of this article.
References
- 1.Hu Y., Sun J., Dai Z. Prevalence and severity of corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. J Clin Virol. 2020;127:104371. doi: 10.1016/j.jcv.2020.104371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katsargyris A., Oikonomou K., Klonaris C. Comparison of outcomes with open, fenestrated, and chimney graft repair of juxtarenal aneurysms: are we ready for a paradigm shift? J Endovasc Ther. 2013;20:159–169. doi: 10.1583/1545-1550-20.2.159. [DOI] [PubMed] [Google Scholar]
- 3.Bin Jabr A., Lindblad B., Kristmundsson T. Outcome of visceral chimney grafts after urgent endovascular repair of complex aortic lesions. J Vasc Surg. 2016;63:625–633. doi: 10.1016/j.jvs.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 4.IMPROVE Trial Investigators Endovascular strategy or open repair for ruptured abdominal aortic aneurysm: one-year outcomes from the IMPROVE randomized trial. Eur Heart J. 2015;36:2061–2069. doi: 10.1093/eurheartj/ehv125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson A., Zhou A., Bachoo A. Systematic review of chimney and periscope grafts for endovascular aneurysm repair. Br J Surg. 2013;100:1557. doi: 10.1002/bjs.9274. [DOI] [PubMed] [Google Scholar]
- 6.Marone E.M., Rinaldi L.F. Upsurge of deep venous thrombosis in patients affected by COVID-19: preliminary data and possible explanations. J Vasc Surg Venous Lymphat Disord. 2020 doi: 10.1016/j.jvsv.2020.04.004. [Epub ahed of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhai Z., Li C., Chen Y. Prevention and treatment of venous thromboembolism associated with coronavirus disease 2019 infection: a consensus statement before guidelines. Thromb Haemost. 2020 doi: 10.1055/s-0040-1710019. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]