Abstract
The effects of obesity and smoking in the coronavirus disease 2019 (COVID-19) pandemic remain controversial. Angiotensin converting enzyme 2 (ACE2), a component of the renin-angiotensin system (RAS), is the human cell receptor of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of COVID-19. ACE2 expression increases on lung alveolar epithelial cells and adipose tissue due to obesity, smoking and air pollution. A significant relationship exists between air pollution and SARS-CoV-2 infection, as more severe COVID-19 symptoms occur in smokers; comorbid conditions due to obesity or excess ectopic fat accumulation as underlying risk factors for severe COVID-19 strongly encourage the virus/ACE2 receptor-ligand interaction concept. Indeed, obesity, air pollution and smoking associated risk factors share underlying pathophysiologies that are related to the Renin-Angiotensin-System in SARS-CoV-2 infection. The aim of this review is to emphasize the mechanism of receptor-ligand interaction and its impact on the enhanced risk of death due to SARS-CoV-2 infection.
Keywords: COVID-19, Angiotensin II, Smoking, Air pollution, Obesity, Angiotensin-converting-enzyme inhibitors
1. Introduction
Viral infection aggression is linked to both environmental and genetic factors. Although the mortality in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection is highly age dependent, the etiology of the coronavirus disease 2019 (COVID-19)-specific mortality in these patients is largely unknown (Lyons-Weiler, 2020). SARS-CoV-2 has a size of 60−140 nm. Nasal or saliva droplet aerosols from infected individuals provide an efficient means of transport for the viral particles, as well as attachment to suspended fine particles in air (Woon Fong Leung and Sun, 2020). Positive correlations of PM2.5, PM10, nitrogen dioxide (NO2) and ozone (O3) levels with cases confirmed with new COVID-19 underlines the how air pollution is assisting in the propagation of SARS-CoV-2 infection (Zhu et al., 2020). Angiotensin-converting enzyme-2 (ACE2) protein provides the host cellular entry point for SARS-CoV-2 (Battistoni and Volpe, 2020). Thus, the relationship between ACE2 and SARS-CoV-2 is pivotal in the infection process (Ge et al., 2013; Gheblawi et al., 2020; Qiu et al., 2020). If these receptors are inhibited by the angiotensin-converting-enzyme inhibitors (ACEI) and angiotensin II type-I receptor blockers (ARBs), a concomitant fall in inflammation might occur via diminished viral invasion of tissues such as the lungs and the heart (Rico-Mesa et al., 2020). Conversely, upregulation of ACE2 or higher ACE2 gene expression may increase susceptibility to infection by SARS-CoV-2, and COVID-19 disease severity (Brake et al., 2020). Tobacco smokers have a greater predisposition (1.4 fold) to developing severe symptoms of COVID-19. This often necessitates their entry into intensive care units (ICU), alongside concomitant mechanical ventilation; moreover, their death rate is approximately 2.4 times that of non-smokers (Guan et al., 2020; Vardavas and Nikitara, 2020). Among adults aged more than 65 years approximately 89 % suffer from one or more underlying comorbidities, including obesity (48 %), cardiovascular disease (28 %), hypertension (50 %) and diabetes mellitus (28 %) as well as chronic lung disease (35 %) (Garg et al., 2020). These comorbidities show a trend towards increased disposition to COVID-19 severe disease, but no specific significant association could be shown with active smoking and obesity and severity particularly in Chinese patients (Lippi and Henry, 2020; W. Liu et al., 2020). However, among the patients admitted to ICU for SARS-CoV-2, requiring invasive mechanical ventilation (IMV), the proportion of obese patients is high. This increase in the rate of patients who need IMV is significantly linked with being male and possessing a high body mass index (BMI) (Simonnet et al., 2020).
Recently, the low mortality rate in patients with acute respiratory distress syndrome (ARDS) with obesity and morbid obesity is defined as the obesity paradox (Ball et al., 2017). To date it is unclear if this paradox is not broken by COVID-19. Whilst they can suffer less from severe COVID-19 infection, obese patients are nevertheless subject to the comorbidities associated with being overweight and they are subsequently more difficult to treat due to these factors (Jose and Manuel, 2020). It is thought that obesity or excess ectopic fat deposition may be the underlying risk factors for severe COVID-19, because of their comorbid conditions, such as cardiovascular diseases, insulin resistance, adipose tissue inflammation and detrimental effects on lung function. These risk factors are strongly associated with mortalities from COVID-19 and are more frequent among smokers. Indeed, obesity and smoking both definitely upregulate ACE2 receptor (Brake et al., 2020; Engeli et al., 2003). Interestingly, obesity, air pollution and smoking-associated risk factors share underlying pathophysiology related to the renin-angiotensin system (RAS) in SARS-CoV-2 infection.
2. Exposure to SARS-CoV-2
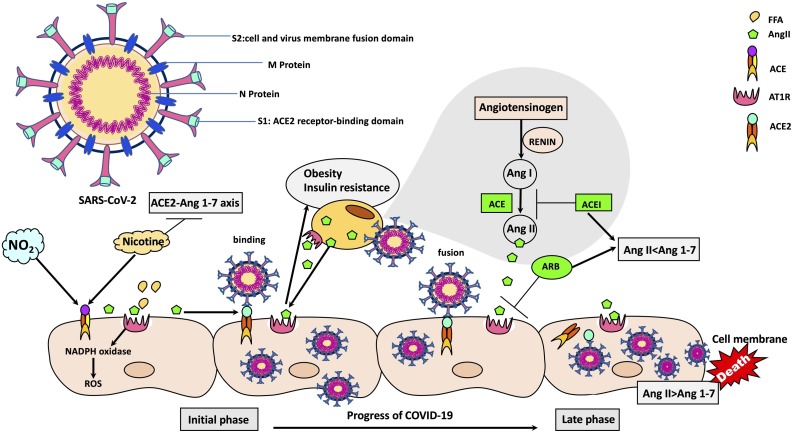
SARS-CoV-2 is single strand RNA virus which uses type 1 transmembrane spike (S) glycoprotein projections as a lock-and-key combination to breach cells. The viral glycosylated cell surface protein can be further classified as using two (S1 and S2) functional domains which manage the entry process (Fig. 1 ). Initial ACE2 receptor cell entry is operated by domain S1 (Li et al., 2005), whilst S2 sets up cell and virus membrane fusion which is necessary for full infiltration into the cell (Coutard et al., 2020). The ACE2 protein is present in abundance around the body, such as the small intestine, as well as on lung epithelia and particularly on lung type-2 pneumocytes. Thus, increase in ACE intensity effectively facilitates a most flexible and ubiquitous pathway into cells for SARS-CoV-2 (Hamming et al., 2004). After SARS-CoV-2 invasion occurs due to the interaction of its spike protein with a receptor furin-cleavage site (Hasan et al., 2020; Y. Wan et al., 2020), a transmembrane protease, serine 2 (TMPRSS2) then separates ACE2 from SARS-CoV-2; this has the effect of accelerating the progress of the infection (Heurich et al., 2014; Hoffmann et al., 2020). Indeed, the whole likelihood of the risk of contracting a severe infection, as well as progressing towards a poor clinical outcome is governed by the ACE2-COVID-19 receptor ligand interaction (Alifano et al., 2020). Affinity-modifying agents of ACE2, such as NO2 and nicotine could potentially disrupt glycosylation on the spike protein, thus interfering with the infection process. In this context, RAS can modulate infection severity (Alifano et al., 2020; Meulenbelt et al., 1992; Patel et al., 1990). As mentioned above, in countries where NO2 pollution was high, COVID-19 course was significantly severe (Alifano et al., 2020).
Fig. 1.
The mechanism of receptor-ligand interaction and its impact on the enhanced risk of death due to SARS-CoV-2 infection.
In the renin angiotensin system, ACE cleaves Ang I to produce Ang II. Ang II action is mediated by the AT1R. SARS-CoV-2 uses the ACE2 as a receptor for entry into the cell. Coronaviruses use the surface spike (S) glycoprotein on the coronavirus envelope to attach host cells and mediate host cell membrane and viral membrane fusion during infection. The spike protein includes two regions, S1 and S2. The receptor binding domain is located in the S1 region. SARS-CoV attaches the human host cells through the binding of the receptor binding domain protein to ACE2. The expression of ACE2 and the balance of Ang II/Ang 1–7 influence the course of the disease. ACEI and ARBs cause the increase in the formation of Ang 1–7 from Ang II via increased ACE2. The loss of ACE2 function following binding by SARS-CoV-2 is driven by endocytosis and activation of proteolytic cleavage and processing. Accordingly, ACE2 may be upregulated due to the NO2 associated air pollution, and ACEIs. Nicotine has dual action by enhancing ACE and down-regulating ACE2 (Abbreviations. ACE: angiotensin-converting enzyme; ACE2: angiotensin-converting enzyme II; ACEI: angiotensin-converting enzyme inhibitors; Ang I: angiotensin I; Ang II: angiotensin II; ARB: angiotensin II type-I receptor blocker; AT1R: angiotensin II receptor type 1; NO2: Nitrogen dioxide; SARS-CoV-2: severe acute respiratory syndrome coronavirus-2).
In addition to ACE2 receptors, two different coronavirus receptor proteins, toll like receptor (TLR) and dipeptidyl peptidase-4 (DPP4) have vital roles in the pathways regulating the transduction on metabolic signals across metabolic processes, ranging from fundamental ones such as glucose homeostasis through to organ function modulation in the kidney, heart and the immune system and its inflammatory activity (Drucker, 2020). As mentioned above, SARS-CoV-2 exploits ACE2 to begin and then propagate its progression towards ARDS. Existing cardiovascular pathology and drug-mediated RAS inhibition which cause a rise in ACE2 expression may essentially increase the vulnerability of lung and heart to SARS-CoV-2 virulence. On the other hand, it has been reported that corona viruses during their infection process can cause accumulation of Angiotensin II (Ang II) through the blocking of the ACE2 receptors (Hanff et al., 2020).
Several hypotheses have been proposed regarding the effect of ACEI and ARBs on SARS-CoV-2 infection. Proteolytic cleavage processes, as well as endocytosis are all activated by viral binding and these unit to attenuate ACE2 activity. Unfortunately, SARS-CoV-2 not only effectively exploits a critical and irreplaceable system that is ACE2 to enter and multiply in the host (Gheblawi et al., 2020), but its ACE2 binding allows it to evade immune surveillance. The engulfment of ACE2 provides the virus access to the host cellular infrastructure, hence viral proliferation and immune evasion are inextricably linked with regard to successful and potentially devastating infection (Brake et al., 2020). In normal circumstances, the RAS system is antagonized by ACE2, which is protective in terms of the development of systemic damage due to hypertension, diabetes, and cardiovascular disease. SARS-CoV-2 utilizes the ACE2 receptor to invade human alveolar epithelial cells. Indeed, a bad prognosis has been shown to be linked to ACE2 receptor activity, as well as the presence of secondary ARDS and other factors such as age, and sex and multiple comorbidities (Cheng et al., 2020). Collectively, it is thought that dysfunction of the RAS is a common pathology seen in SARS-CoV-2 infected obese patients and smokers, however it has not been evaluated in detail.
3. The effect of tobacco smoke and nitrogen dioxide exposure in COVID-19
Smokers figure disproportionately highly in the numbers of severe COVID-19 victims in comparison to the non-severe patients (J.-J. Zhang et al., 2020). Their ICU admissions and subsequent IMV requirements are also considerably higher. Indeed, a fatal outcome is more likely with smokers (Guan et al., 2020; Zhou et al., 2020). Compounded by the smoking, the presence of obstructive pulmonary disease (COPD), cardiovascular pathology and diabetes is greater among severe cases (Zhou et al., 2020). In one study, (1099 COVID-19 patients), of the 19 % of patients severely affected, just under 17 % were current smokers and 5.2 % were former smokers. In another report, patients who had severe disease, where they were admitted to ICU, ventilated and/or who had fatal outcomes, about a quarter (25.5 %) smoked and 7.6 % were former smokers (Guan et al., 2020). Conversely, Liu et al. reported that among an adverse outcome group of patients, a smoking history was nine-fold more prevalent, than the group that showed improvement or stabilization. In these patients, age, history of smoking, respiratory failure, low albumin and high C-reactive protein were identified as the risk factors that lead to the acceleration of disease progress to pneumonia (Khot and Nadkar, 2020; B. Liu et al., 2020; W. Liu et al., 2020b; Y. Liu et al., 2020). In only two studies from total of five, 288 of 1399 COVID-19 patients were diagnosed with severe disease. In these patients COVID-19 progression was driven by smoking history, while the others were not thus associated (Guan et al., 2020; Huang et al., 2020; Lippi and Henry, 2020; W. Liu et al., 2020; Yang et al., 2020). The rate of ARDS development in SARS-CoV-2-infected individuals that already had pneumonia was around 50 % (Y. Liu et al., 2020). Patients who are likely to die from the infection suffer pulmonary oedema and bilateral diffuse alveolar damage which is caused by significant inflammatory infiltrates (Solaimanzadeh, 2020). Unfortunately, in these patients, obesity has serious consequences. In fact, ARDS from a number of causes, is often a major reason for significantly overweight (class III obese) patients being admitted to ICUs, where they are ventilated, which also occurs with COVID-19 patients (Marshall et al., 2016). Multiple organ failure is usually cause of mortality in both morbidly obese and COVID-19 patient groups (Bellani et al., 2016).
Tobacco smoke promotes exposure to environmental NO2, benzene, and 1,3-butadiene (Hagenbjörk-Gustafsson et al., 2014), and ambient air pollution associated with NO2 causes an increase in Ang II-binding to its receptor by upregulating ACE activity by up to 100-fold. Meanwhile, nicotine exhibits a dual effect by increasing ACE expression and causing a fall in ACE2 activity (Alifano et al., 2020; Meulenbelt et al., 1992). Nicotine upregulates activity and expression of renin, ACE and Ang II type I receptor (AT1R). Interestingly, nicotine can also switch off compensatory expression and activity of ACE2 and Ang II type 2 receptor (AT2R) (Fig. 1). Tobacco combustion products as well as nicotine administration itself promotes angiotensin I (Ang I) conversion to Ang II via promoting plasma ACE activity (Oakes et al., 2018). Tobacco smoke exposure also promotes lung inflammation, thus increasing mucosal inflammation, inflammatory cytokines and tumor necrosis factor (TNF)-alpha (TNF-α) expression, as well as increasing permeability in epithelial cells, mucus production, and impaired mucociliary clearance (Strzelak et al., 2018). Similarly, the binding of the virus to the TLR promotes pro- interleukin (IL)-1β levels, which is in turn cleaved by caspase-1. As soon as this occurs, inflammasome activation is followed by the formation of mature IL-1β; this is itself an important promotor of inflammatory processes in the lung, as well as fever and fibrosis (Conti et al., 2020). For the reasons described above, smoking increases the severity of COVID-19 associated inflammatory response. COVID-19 ICU patients suffer high systemic inflammatory responses, which are evidenced by elevated plasma levels of IL-2, IL-6, IL-7, IL-10, granulocyte colony-stimulating factor (GC-SF), interferon-gamma (IFN-γ)-inducible protein (IP10; CXCL10), monocyte chemoattractant protein 1 (MCP1), macrophage inflammatory protein 1 alpha (MIP1A), and TNF-α (Huang et al., 2020). The proportions of IFN-γ yielding CD8 + T and CD4 + T cells rise in the seriously COVID-19 patients compared with mild cases. It is likely that the so-called inflammatory ‘cytokine storm’ in seriously ill patients is promoted by CD8+ and CD4 + T cells (F. Wang et al., 2020; J. Wang et al., 2020; Z. Wang et al., 2020). It is claimed that lower counts of T lymphocyte subsets, (CD3+, CD4+, CD8+) and B-cells are associated with higher risks of in-hospital death of COVID-19 (Xu et al., 2020). Hence, the survival of the most seriously ill individuals is strongly predicated on the attenuation of the cytokine storm. The damage caused by the storm is orchestrated by IL-6, which presents a pharmacological target, for which there is already a candidate, tocilizumab (TCZ). This agent inhibits the IL-6 receptor (IL-6R), and can curtail IL-6 signal transduction (B. Liu et al., 2020; Luo et al., 2020; C. Zhang et al., 2020). In fact, TCZ was approved in 2017 by the Food and Drug Administration (FDA) for the treatment of life-threatening cytokine-release syndrome, which is commonly associated with respiratory symptoms ranging from cough and tachypnea to ARDS (Alzghari and Acuña, 2020). Interestingly, high-lactate dehydrogenase, C-reactive protein and IL-6 are observed in patients with severe COVID-19, as in cytokine release syndrome. Although at the time of writing it is yet to gain FDA approval for use in COVID-19, in preliminary trials more than half of patients with the disease exhibited a gradual reduction in IL-6 levels following TCZ administration (Luo et al., 2020; Shimabukuro-Vornhagen et al., 2018; Z. Wang et al., 2020; Yuan et al., 2020). The FDA has approved the initiation of a Phase III trial with TCZ (COVACTA) in patients with severe pneumonia as of April 2020 (Clinical Trials Hoffmann-La Roche, 2020).
In severe COVID-19, provided patients had CD4 + T cells, CD8 + T cells, IL-6, and IL-10 within normal values, their survival was more likely (S. Wan et al., 2020). Notably, pre-existing chronic obstructive pulmonary disease (COPD) and current tobacco use make a poor outcome with COVID-19 much more likely (Patwardhan, 2020; Q. Zhao et al., 2020). Tobacco smoking is a powerful driver of all infectious lung pathology. Smokers are more than a third likely to suffer from influenza compared with non-smoking (Lawrence et al., 2019). Smoking is strongly associated with COPD in the developed world, although poor local air-quality due to the increasing atmospheric pollution is also significant COPD promotors in developed and developing countries. Smoking remains the fourth leading cause of death in the world (WHO, 2017). The World Health Organization enounces that respiratory comorbidities from all sources, including tobacco, are associated with a high percentage of COVID-19 related deaths (WHO, 2017; Y.-Y. Zheng et al., 2020).
Smokers also exhibit increased ACE2 gene expression in type-2 pneumocytes, alveolar macrophages, particularly at the apical end of the small airway epithelium, which is not seen in those who do not use tobacco. COPD itself promotes ACE2 expression thus providing ample targets for SARS-CoV-2 cell entry (Brake et al., 2020; Cai et al., 2020). So significant lung damage with tobacco use promotes the risk of COVID-19 and progression to severe disease (Brake et al., 2020; J. Wang et al., 2020). Immune evasion of virus as mentioned above, may increase viral adhesion to the target host cells for relatively longer periods. This elevates the carrier status of the host due to their high local tissue viral load, which will promote the spread of the infection (Brake et al., 2020). Ang II and AT1R, are key constituents of the RAS, and they regulate mitogen-activated protein kinase (MAPK) and Janus kinase (JAK) pathways (Kemp et al., 2014). Lung injury is thought to be promoted by compromised RAS activity as this is linked with local pro-inflammatory cytokine production. Indeed, ACEI, as well as Ang II receptor antagonists are capable of ameliorating lung damage in experimental models and this suggests that RAS activity is implicated. Thus, it could be proposed that those drugs may treat diffuse parenchymal lung disease successfully (Marshall, 2003). Cigarette smoke exposure increases pulmonary ACE and ACE2 activity, however loss of ACE2 increases Ang II by inducing the ACE activity (Hung et al., 2016). As mentioned earlier, factors that promote ACE2 increase risk of severe COVID-19 (Fang et al., 2020). Any reduction in ACE2 activity restricts available virus-cell binding sites and may be protective in smokers. Unfortunately, if ACE2 function diminishes due to a high level of viral attack and binding, this would cause endocytosis, along with proteolytic cleavage; these events may lead to increase in respiratory distress (Alifano et al., 2020; Gheblawi et al., 2020).
4. The effect of obesity on the course of COVID-19
Experience with obesity-related mortality with H1N1 influenza illustrated that much more forceful treatment of obese COVID-19 patients was required than was originally envisaged (Dietz and Santos-Burgoa, 2020). Obesity not only enhances the severity of influenza infection but also impacts viral diversity (Honce et al., 2020). More than 70 % of COVID-19 patients who require intensive care have a high rate of obesity (Malavazos et al., 2020). In patients aged less than 60 years in New York City, the progression of obesity towards morbid obesity more than doubled the risk of admission to critical care unit, compared with those with lower BMIs (<30 kg/m2) (Lighter et al., 2020). It has been established that morbid obesity doubles the risks of poor outcomes compared with those who are not clinically obese (El-Solh et al., 2001). Among the various patient comorbidities with COVID-19, the most common is hypertension (21 %), followed by diabetes (11 %) and around 7 % suffer from underlying cardiovascular problems, all of which promote the risk for hospitalization and death in COVID-19 patients (Muniyappa and Gubbi, 2020; Singh et al., 2020). In a series of COVID-19 deaths in a cohort of patients in Wuhan, China 42 % of the fatalities were diabetics (Deng and Peng, 2020). The condition of obesity related fatty liver disease increases the risk of severe COVID-19 by six-fold. This powerful association between fatty liver disease and risk of a fatal outcome with COVID-19 was valid even after adjusting for age, sex, smoking, diabetes, hypertension, and dyslipidemia (K.I. Zheng et al., 2020). Essentially, a lipotoxic state is induced by habitual overload of dietary saturated fatty acids (SFAs), which activates TLR 4 expressed on several immune cells, such as neutrophils, macrophages and dendritic cells (Engin, 2017a, 2017b; Rogero and Calder, 2018). High fat diet significantly disrupts adaptive immune response, while increasing innate immune activation. This promotes the chronic inflammation and may cause the host defense become vulnerable against viral pathogens (Butler and Barrientos, 2020). Consequently, in obese patients, viral pools can lodge in adipose tissue and promote shedding, immune activation, and chronic excessive cytokine release (Ryan and Caplice, 2020).
Mechanistically, disorders such as diabetes, cardiovascular disease and hypertension share same underlying pathophysiology related to the RAS in both COVID-19 and obesity (Hanff et al., 2020). Excessive adipose tissue in obesity secretes Ang II, which is a hormone with inflammatory properties and is generated in the RAS pathway. Obesity and insulin resistance are strongly linked with RAS activity. Furthermore, oxidative stress and inflammatory response along with mitochondrial dysfunction modulate the function of RAS (Ramalingam et al., 2017). In addition, diabetic kidney damage is promoted by the renal RAS activation through endoplasmic reticulum stress, which is in turn induced by SFAs (Li et al., 2016). Moreover, increased RAS activity in obesity initiates a series of interrelated pathological events. These include reduction of insulin secretion and sensitivity, as well as fostering a rise in arterial pressure. In all these conditions AT1R is upregulated. Contrarily, AT1R blockade improves hyperglycemia, hypertension and peripheral tissue insulin sensitivity (Rodriguez et al., 2018). Once RAS function is compromised, it causes widespread dysfunction in most tissues, through the deleterious processes described above (Ramalingam et al., 2017).
At the cellular level, renin forms angiotensin I (Ang I) from angiotensinogen. ACE cleaves Ang I forming Ang II (Lavoie and Sigmund, 2003). Ang II acts at cell surface type I G protein-coupled receptors, AT1Rs then promote a pathological cascade including insulin resistance, oxidative stress, inflammation and vasoconstriction (Luther and Brown, 2011). Reduction of the phosphoinositol-3 kinase (PI3K) activity due to elevated free fatty acids (FFAs) levels is potentiated by Ang II and consequently insulin-stimulated glucose uptake is increased by RAS inhibition. Blockade of the AT1R has been shown to stimulate the differentiation of adipocytes that store FFAs, of which leads to reduced plasma FFA levels and decreased insulin resistance (Leiter and Lewanczuk, 2005). RAS activity can also be upregulated by insulin resistance. This can in turn promote a series of linked pathophysiological issues such as inflammatory diseases, as well as the metabolic disorders associated with obesity (Muniyappa and Yavuz, 2013). In fact, the major effects of Ang II are mediated by AT1R and AT2R. Overexpression of angiotensinogen increases adiposity, which is seen through hypertrophy of adipocytes, inflammation and increased resistance to insulin. RAS inhibition can ameliorate and even reverse these obesity related alterations in adipose tissue (Pahlavani et al., 2017). The fall in glucose-mediated insulin secretion is linked with lower pancreatic insulin content and upregulated AT1R protein expression in obesity. Essentially, β-cell dysfunction is strongly associated with AT1R upregulation, which accelerates glucose intolerance and insulin resistance, through a decrease in plasma glucagon-like peptide-1 (GLP-1). In contrast, RAS blockade induces improvement in β-cell function and insulin sensitivity (Rodriguez et al., 2012; van der Zijl et al., 2012). The general progression of atherosclerotic disease is also influenced by RAS, as well as its primary mediator Ang II. The mechanisms of this process include inflammation, disruption of fibrinolytic balance and endothelial dysfunction. The inhibitors of RAS such as ACEI and angiotensin receptor blockers act either by depleting the generation of Ang II or by blocking the binding of Ang II to its receptors (Husain et al., 2015). ACE2 is expressed by epithelial cells of the lung, intestine, kidney, and blood vessels. The expression of ACE2 is substantially increased in patients with type 2 diabetes, who are treated with ACEI and ARBs (Y. Wan et al., 2020). Similarly, treatment of hypertension with ACEI and ARBs, results in an upregulation of ACE2 (Li et al., 2017). Consequently, the increased expression of ACE2 would facilitate COVID-19 due to spreading of SARS-CoV-2 (Fang et al., 2020). Since the ACE2 protein is the receptor that facilitates corona virus entry into cells, the notion that treatment with RAS blockers might increase the risk of developing a severe and fatal ARDS in COVID-19 infection has been popularized. Interestingly, the contention that the presence of ACEI or AT1R blockers promote coronavirus infection through upregulating ACE2 expression in either animals or humans is not currently supported (Danser et al., 2020). On the contrary, ACEI in addition to RAS inhibitors in COVID-19 patients with hypertension actually can improve clinical outcome (Meng et al., 2020). The use of selective AT1R antagonists in the treatment of hypertension is a useful concept. Firstly, selective AT1R blockade targets the final common pathway for all major detrimental cardiovascular actions of Ang II, and secondly; circulating Ang II levels, which increase during AT1R antagonist treatment, will be free to act only at unopposed AT2R. Also, RAS is disrupted by ACEI through blocking Ang I to Ang II conversion (Unger, 2002).
In females it is thought that adipocyte ACE2 has an ameliorating effect with regard to blood pressure response to systemic Ang II, as well as to obesity (Shoemaker et al., 2019). Compared with females, despite the males lower adiposity (Hales et al., 2017; Jackson et al., 2002), hypertension is more frequent in men compared with women until menopause, and then the situation reverses (Fryar et al., 2017). Asian men show a greater expression of ACE2 compared with women and other ethnicities, which might explain the increase in the prevalence of COVID-19 in this subgroup of patients. In this regard, the sex-balance for COVID-19 infection is in favour of men (67 %), rather than women. Considering an overview of available studies, more than half of COVID-19 patients in 552 hospitals were reported to be male, which supports the idea that a sex predisposition to COVID-19 does exist, with men being more susceptible (Guan et al., 2020; Yang et al., 2020; C. Zhang et al., 2020; J.-J. Zhang et al., 2020; Y. Zhao et al., 2020). Moreover, the sustained high levels of Ang II in these male patients strongly correlated with mortality (Huang et al., 2014). In addition, obese female subjects compared with lean females have greater adipose tissue expression of ACE2 (Gupte et al., 2012). In a mouse model, reduced ACE2 expression drives obese females toward hypertension equivalent to obese males. The Ang-(1–7) to Ang II, conversion equilibrium is controlled by ACE2. This is sex-specific and differently promotes the development of obesity related hypertension in males and females. ACE2 mRNA abundance is driven upwards by 17-β estradiol in adipocytes via the estrogen receptor alpha (ERα) (Gupte et al., 2012). Typically, adipocytes are the prevalent source of ACE2 for the development of obesity related hypertension. Unexpectedly, modulation of ACE2 by estrogen may protect against obesity related hypertension in obese females. Therefore, ACEI are not recommended for obese women. Increasing body weight and loss of vascular protection mediated by the abrupt loss of estrogen may contribute to the development of hypertension in post-menopause (Engin et al., 2019; Shoemaker et al., 2019). Systemic ACE2 activity levels are negatively correlated with BMI and blood pressure in female essential hypertension patients (Zhang et al., 2018). Since previous findings demonstrated that ACE2 activity was increased by obesity in adipose tissue of female, but not male animals (Gupte et al., 2012), then these results suggest that obesity per se may introduce sex- and tissue-specific regulation of ACE2.
In contrast, food restriction by between 20 % and 40 % improves metabolic profile, ameliorates inflammatory status and down-regulates the RAS (Pinheiro et al., 2017). Interestingly, the available data does not support a deleterious effect of RAS blockers in COVID-19. Therefore, there is currently no reason to discontinue RAS blockers in stable patients facing the COVID-19 pandemic (Kreutz et al., 2020). Combining ACEI and ARBs to provide more extensive RAS inhibition may provide greater efficacy for end-organ protection (Weir, 2007).
5. Conclusion
COVID-19 patients have increased Ang II compared to healthy people. Obesity, air pollution and smoking are associated risk factors which share underlying pathophysiology related to the RAS in SARS-CoV-2 infection. Air pollution via the interference of NO2 increases the ACE activity. Meanwhile, nicotine exhibits dual effect on RAS. Obese adipose tissue synthesizes excess Ang II, thus the combination of an AT1R antagonist with an ACEI for more complete blockade of the RAS may be a rationally viable therapeutic strategy in obese COVID-19 patients. In this context, using ACEI or ARBs potentially contribute to the improvement of clinical outcomes of COVID-19 patients.
Declaration of Competing Interest
The authors have no conflict of interest.
References
- Alifano M., Alifano P., Forgez P., Iannelli A. Renin-angiotensin system at the heart of COVID-19 pandemic. Biochimie. 2020;174:30–33. doi: 10.1016/j.biochi.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzghari S.K., Acuña V.S. Supportive treatment with Tocilizumab for COVID-19: a systematic review. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2020;127:104380. doi: 10.1016/j.jcv.2020.104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2020:102452. doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball L., Serpa Neto A., Pelosi P. Obesity and survival in critically ill patients with acute respiratory distress syndrome: a paradox within the paradox. Crit. Care Lond. Engl. 2017;21:114. doi: 10.1186/s13054-017-1682-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistoni A., Volpe M. Might renin-angiotensin system blockers play a role in the COVID-19 pandemic? Eur. Heart J. Cardiovasc. Pharmacother. 2020 doi: 10.1093/ehjcvp/pvaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellani G., Laffey J.G., Pham T., Fan E., Brochard L., Esteban A., Gattinoni L., van Haren F., Larsson A., McAuley D.F., Ranieri M., Rubenfeld G., Thompson B.T., Wrigge H., Slutsky A.S., Pesenti A., LUNG SAFE Investigators, ESICM Trials Group Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- Brake S.J., Barnsley K., Lu W., McAlinden K.D., Eapen M.S., Sohal S.S. Smoking upregulates angiotensin-converting Enzyme-2 receptor: a potential adhesion site for novel coronavirus SARS-CoV-2 (Covid-19) J. Clin. Med. 2020;9 doi: 10.3390/jcm9030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler M.J., Barrientos R.M. The impact of nutrition on COVID-19 susceptibility and long-term consequences. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Wu Z., Li J.-W., Zhao H., Wang G.-Q. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105954. 105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai G., Bossé Y., Xiao F., Kheradmand F., Amos C.I. Tobacco smoking increases the lung gene expression of ACE2, the receptor of SARS-CoV-2. Am. J. Respir. Crit. Care Med. 2020 doi: 10.1164/rccm.202003-0693LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Wang Y., Wang G.-Q. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J. Med. Virol. 2020 doi: 10.1002/jmv.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical Trials Hoffmann-La Roche . Hoffmann-La Roche; USA: 2020. A Study to Evaluate the Safety and Efficacy of Tocilizumab in Patients With Severe COVID-19 Pneumonia (COVACTA) (No. NCT04320615) [Google Scholar]
- Conti P., Ronconi G., Caraffa A., Gallenga C., Ross R., Frydas I., Kritas S. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020;34 doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- Coutard B., Valle C., de Lamballerie X., Canard B., Seidah N.G., Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. 2020;176:104742. doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danser A.H.J., Epstein M., Batlle D. Renin-angiotensin system blockers and the COVID-19 pandemic: At present there is No evidence to abandon renin-angiotensin system blockers. Hypertens. 2020 doi: 10.1161/HYPERTENSIONAHA.120.15082. Dallas Tex 1979 HYPERTENSIONAHA12015082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng S.-Q., Peng H.-J. Characteristics of and public health responses to the coronavirus disease 2019 outbreak in China. J. Clin. Med. 2020;9 doi: 10.3390/jcm9020575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz W., Santos-Burgoa C. Obesity and its implications for COVID-19 mortality. Obes. Silver Spring Md. 2020 doi: 10.1002/oby.22818. [DOI] [PubMed] [Google Scholar]
- Drucker D.J. Coronavirus infections and type 2 diabetes-shared pathways with therapeutic implications. Endocr. Rev. 2020 doi: 10.1210/endrev/bnaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Solh A., Sikka P., Bozkanat E., Jaafar W., Davies J. Morbid obesity in the medical ICU. Chest. 2001;120:1989–1997. doi: 10.1378/chest.120.6.1989. [DOI] [PubMed] [Google Scholar]
- Engeli S., Schling P., Gorzelniak K., Boschmann M., Janke J., Ailhaud G., Teboul M., Massiéra F., Sharma A.M. The adipose-tissue renin-angiotensin-aldosterone system: role in the metabolic syndrome? Int. J. Biochem. Cell Biol. 2003;35:807–825. doi: 10.1016/s1357-2725(02)00311-4. [DOI] [PubMed] [Google Scholar]
- Engin A.B. Adipocyte-macrophage cross-talk in obesity. Adv. Exp. Med. Biol. 2017;960:327–343. doi: 10.1007/978-3-319-48382-5_14. [DOI] [PubMed] [Google Scholar]
- Engin A.B. What is lipotoxicity? Adv. Exp. Med. Biol. 2017;960:197–220. doi: 10.1007/978-3-319-48382-5_8. [DOI] [PubMed] [Google Scholar]
- Engin A.B., Engin A., Gonul I.I. The effect of adipocyte-macrophage crosstalk in obesity-related breast cancer. J. Mol. Endocrinol. 2019;62:R201–R222. doi: 10.1530/JME-18-0252. [DOI] [PubMed] [Google Scholar]
- Wang F., Hou H., Luo Y., Tang G., Wu S., Huang M., Liu W., Zhu Y., Lin Q., Mao L., Fang M., Zhang H., Sun Z. The laboratory tests and host immunity of COVID-19 patients with different severity of illness. JCI Insight. 2020 doi: 10.1172/jci.insight.137799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryar C.D., Ostchega Y., Hales C.M., Zhang G., Kruszon-Moran D. 2017. Hypertension Prevalence and Control Among Adults: United States, 2015-2016. NCHS Data Brief 1–8. [PubMed] [Google Scholar]
- Garg S., Kim L., Whitaker M., O’Halloran A., Cummings C., Holstein R., Prill M., Chai S.J., Kirley P.D., Alden N.B., Kawasaki B., Yousey-Hindes K., Niccolai L., Anderson E.J., Openo K.P., Weigel A., Monroe M.L., Ryan P., Henderson J., Kim S., Como-Sabetti K., Lynfield R., Sosin D., Torres S., Muse A., Bennett N.M., Billing L., Sutton M., West N., Schaffner W., Talbot H.K., Aquino C., George A., Budd A., Brammer L., Langley G., Hall A.J., Fry A. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 states, March 1-30, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X.-Y., Li J.-L., Yang X.-L., Chmura A.A., Zhu G., Epstein J.H., Mazet J.K., Hu B., Zhang W., Peng C., Zhang Y.-J., Luo C.-M., Tan B., Wang N., Zhu Y., Crameri G., Zhang S.-Y., Wang L.-F., Daszak P., Shi Z.-L. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503:535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.-C., Turner A.J., Raizada M.K., Grant M.B., Oudit G.Y. Angiotensin converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system. Circ. Res. 2020 doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X., Liu L., Shan H., Lei C.-L., Hui D.S.C., Du B., Li L.-J., Zeng G., Yuen K.-Y., Chen R.-C., Tang C.-L., Wang T., Chen P.-Y., Xiang J., Li S.-Y., Wang J.-L., Liang Z.-J., Peng Y.-X., Wei L., Liu Y., Hu Y.-H., Peng P., Wang J.-M., Liu J.-Y., Chen Z., Li G., Zheng Z.-J., Qiu S.-Q., Luo J., Ye C.-J., Zhu S.-Y., Zhong N.-S., China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte M., Thatcher S.E., Boustany-Kari C.M., Shoemaker R., Yiannikouris F., Zhang X., Karounos M., Cassis L.A. Angiotensin converting enzyme 2 contributes to sex differences in the development of obesity hypertension in C57BL/6 mice. Arterioscler. Thromb. Vasc. Biol. 2012;32:1392–1399. doi: 10.1161/ATVBAHA.112.248559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbjörk-Gustafsson A., Tornevi A., Andersson E.M., Johannesson S., Bellander T., Merritt A.-S., Tinnerberg H., Westberg H., Forsberg B., Sallsten G. Determinants of personal exposure to some carcinogenic substances and nitrogen dioxide among the general population in five Swedish cities. J. Expo. Sci. Environ. Epidemiol. 2014;24:437–443. doi: 10.1038/jes.2013.57. [DOI] [PubMed] [Google Scholar]
- Hales C.M., Carroll M.D., Fryar C.D., Ogden C.L. 2017. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. NCHS Data Brief; pp. 1–8. [PubMed] [Google Scholar]
- Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanff T.C., Harhay M.O., Brown T.S., Cohen J.B., Mohareb A.M. Is there an association between COVID-19 mortality and the renin-angiotensin System-a call for epidemiologic investigations. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020 doi: 10.1093/cid/ciaa329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan A., Paray B.A., Hussain A., Qadir F.A., Attar F., Aziz F.M., Sharifi M., Derakhshankhah H., Rasti B., Mehrabi M., Shahpasand K., Saboury A.A., Falahati M. A review on the cleavage priming of the spike protein on coronavirus by angiotensin-converting enzyme-2 and furin. J. Biomol. Struct. Dyn. 2020:1–9. doi: 10.1080/07391102.2020.1754293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurich A., Hofmann-Winkler H., Gierer S., Liepold T., Jahn O., Pöhlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J. Virol. 2014;88:1293–1307. doi: 10.1128/JVI.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honce R., Karlsson E.A., Wohlgemuth N., Estrada L.D., Meliopoulos V.A., Yao J., Schultz-Cherry S. Obesity-related microenvironment promotes emergence of virulent influenza virus strains. mBio. 2020;11 doi: 10.1128/mBio.03341-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F., Guo J., Zou Z., Liu J., Cao B., Zhang S., Li H., Wang W., Sheng M., Liu S., Pan J., Bao C., Zeng M., Xiao H., Qian G., Hu X., Chen Yuanting, Chen Yu, Zhao Y., Liu Q., Zhou H., Zhu J., Gao H., Yang S., Liu X., Zheng S., Yang J., Diao H., Cao H., Wu Y., Zhao M., Tan S., Guo D., Zhao X., Ye Y., Wu W., Xu Y., Penninger J.M., Li D., Gao G.F., Jiang C., Li L. Angiotensin II plasma levels are linked to disease severity and predict fatal outcomes in H7N9-infected patients. Nat. Commun. 2014;5:3595. doi: 10.1038/ncomms4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond. Engl. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung Y.-H., Hsieh W.-Y., Hsieh J.-S., Liu F.-C., Tsai C.-H., Lu L.-C., Huang C.-Y., Wu C.-L., Lin C.-S. Alternative roles of STAT3 and MAPK signaling pathways in the MMPs activation and progression of lung injury induced by cigarette smoke exposure in ACE2 knockout mice. Int. J. Biol. Sci. 2016;12:454–465. doi: 10.7150/ijbs.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain K., Hernandez W., Ansari R.A., Ferder L. Inflammation, oxidative stress and renin angiotensin system in atherosclerosis. World J. Biol. Chem. 2015;6:209–217. doi: 10.4331/wjbc.v6.i3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Luo Q., Chen R., Chen T., Li J. 2020. Susceptibility Analysis of COVID-19 in Smokers Based on ACE2. [DOI] [Google Scholar]
- Zhang J.-J., Dong X., Cao Y.-Y., Yuan Y.-D., Yang Y.-B., Yan Y.-Q., Akdis C.A., Gao Y.-D. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020 doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- Jackson A.S., Stanforth P.R., Gagnon J., Rankinen T., Leon A.S., Rao D.C., Skinner J.S., Bouchard C., Wilmore J.H. The effect of sex, age and race on estimating percentage body fat from body mass index: the Heritage Family Study. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2002;26:789–796. doi: 10.1038/sj.ijo.0802006. [DOI] [PubMed] [Google Scholar]
- Jose R.J., Manuel A. Does COVID-19 disprove the obesity paradox in ARDS? Obes. Silver Spring Md. 2020 doi: 10.1002/oby.22835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng K.I., Gao F., Wang X.-B., Sun Q.-F., Pan K.-H., Wang T.-Y., Ma H.-L., Liu W.-Y., George J., Zheng M.-H. Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020 doi: 10.1016/j.metabol.2020.154244. 154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp J.R., Unal H., Desnoyer R., Yue H., Bhatnagar A., Karnik S.S. Angiotensin II-regulated microRNA 483-3p directly targets multiple components of the renin-angiotensin system. J. Mol. Cell. Cardiol. 2014;75:25–39. doi: 10.1016/j.yjmcc.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutz R., Algharably E.A.E.-H., Azizi M., Dobrowolski P., Guzik T., Januszewicz A., Persu A., Prejbisz A., Riemer T.G., Wang J.-G., Burnier M. Hypertension, the renin-angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID-19. Cardiovasc. Res. 2020 doi: 10.1093/cvr/cvaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie J.L., Sigmund C.D. Minireview: overview of the renin-angiotensin system--an endocrine and paracrine system. Endocrinology. 2003;144:2179–2183. doi: 10.1210/en.2003-0150. [DOI] [PubMed] [Google Scholar]
- Lawrence H., Hunter A., Murray R., Lim W.S., McKeever T. Cigarette smoking and the occurrence of influenza - Systematic review. J. Infect. 2019;79:401–406. doi: 10.1016/j.jinf.2019.08.014. [DOI] [PubMed] [Google Scholar]
- Leiter L.A., Lewanczuk R.Z. Of the renin-angiotensin system and reactive oxygen species Type 2 diabetes and angiotensin II inhibition. Am. J. Hypertens. 2005;18:121–128. doi: 10.1016/j.amjhyper.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Li F., Li W., Farzan M., Harrison S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309:1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- Li C., Lin Y., Luo R., Chen S., Wang F., Zheng P., Levi M., Yang T., Wang W. Intrarenal renin-angiotensin system mediates fatty acid-induced ER stress in the kidney. Am. J. Physiol. Renal Physiol. 2016;310:F351–363. doi: 10.1152/ajprenal.00223.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.C., Zhang J., Zhuo J.L. The vasoprotective axes of the renin-angiotensin system: Physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases. Pharmacol. Res. 2017;125:21–38. doi: 10.1016/j.phrs.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighter J., Phillips M., Hochman S., Sterling S., Johnson D., Francois F., Stachel A. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020 doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G., Henry B.M. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19) Eur. J. Intern. Med. 2020;75:107–108. doi: 10.1016/j.ejim.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J. Med. Virol. 2020 doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther J.M., Brown N.J. The renin-angiotensin-aldosterone system and glucose homeostasis. Trends Pharmacol. Sci. 2011;32:734–739. doi: 10.1016/j.tips.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons-Weiler J. Pathogenic priming likely contributes to serious and critical illness and mortality in COVID-19 via autoimmunity. J. Transl. Autoimmun. 2020:100051. doi: 10.1016/j.jtauto.2020.100051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malavazos A.E., Corsi Romanelli M.M., Bandera F., Iacobellis G. Targeting the adipose tissue in COVID-19. Obes. Silver Spring Md. 2020 doi: 10.1002/oby.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall R.P. The pulmonary renin-angiotensin system. Curr. Pharm. Des. 2003;9:715–722. doi: 10.2174/1381612033455431. [DOI] [PubMed] [Google Scholar]
- Marshall R.V., Haas P.J., Schweinfurth J.M., Replogle W.H. Tracheotomy outcomes in super obese patients. JAMA Otolaryngol. - Head Neck Surg. 2016;142:772–776. doi: 10.1001/jamaoto.2016.1089. [DOI] [PubMed] [Google Scholar]
- Meng J., Xiao G., Zhang J., He X., Ou M., Bi J., Yang R., Di W., Wang Z., Li Z., Gao H., Liu L., Zhang G. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg. Microbes Infect. 2020;9:757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulenbelt J., van Bree L., Dormans J.A., Boink A.B., Sangster B. Biochemical and histological alterations in rats after acute nitrogen dioxide intoxication. Hum. Exp. Toxicol. 1992;11:189–200. doi: 10.1177/096032719201100307. [DOI] [PubMed] [Google Scholar]
- Muniyappa R., Gubbi S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am. J. Physiol. Endocrinol. Metab. 2020;318:E736–E741. doi: 10.1152/ajpendo.00124.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniyappa R., Yavuz S. Metabolic actions of angiotensin II and insulin: a microvascular endothelial balancing act. Mol. Cell. Endocrinol. 2013;378:59–69. doi: 10.1016/j.mce.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes J.M., Fuchs R.M., Gardner J.D., Lazartigues E., Yue X. Nicotine and the renin-angiotensin system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018;315:R895–R906. doi: 10.1152/ajpregu.00099.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahlavani M., Kalupahana N.S., Ramalingam L., Moustaid-Moussa N. Regulation and functions of the renin-angiotensin system in white and brown adipose tissue. Compr. Physiol. 2017;7:1137–1150. doi: 10.1002/cphy.c160031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J.M., Sekharam K.M., Block E.R. Oxidant injury increases cell surface receptor binding of angiotensin II to pulmonary artery endothelial cells. J. Biochem. Toxicol. 1990;5:253–258. doi: 10.1002/jbt.2570050408. [DOI] [PubMed] [Google Scholar]
- Patwardhan P. COVID-19: risk of increase in smoking rates among England’s 6 million smokers and relapse among England’s 11 million ex-smokers. Bjgp Open. 2020 doi: 10.3399/bjgpopen20X101067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro Thalesde Almeida, Barcala-Jorge A.S., Andrade J.M.O., Pinheiro Thaisade Almeida, Ferreira E.C.N., Crespo T.S., Batista-Jorge G.C., Vieira C.A., Lelis D., de F., Paraíso A.F., Pinheiro U.B., Bertagnolli M., Albuquerque C.J.B., Guimarães A.L.S., de Paula A.M.B., Caldeira A.P., Santos S.H.S. Obesity and malnutrition similarly alter the renin-angiotensin system and inflammation in mice and human adipose. J. Nutr. Biochem. 2017;48:74–82. doi: 10.1016/j.jnutbio.2017.06.008. [DOI] [PubMed] [Google Scholar]
- Zhao Q., Meng M., Kumar R., Wu Y., Huang J., Lian N., Deng Y., Lin S. The impact of COPD and smoking history on the severity of Covid-19: a systemic review and meta-analysis. J. Med. Virol. 2020 doi: 10.1002/jmv.25889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y., Zhao Y.-B., Wang Q., Li J.-Y., Zhou Z.-J., Liao C.-H., Ge X.-Y. Predicting the angiotensin converting enzyme 2 (ACE2) utilizing capability as the receptor of SARS-CoV-2. Microbes Infect. 2020 doi: 10.1016/j.micinf.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalingam L., Menikdiwela K., LeMieux M., Dufour J.M., Kaur G., Kalupahana N., Moustaid-Moussa N. The renin angiotensin system, oxidative stress and mitochondrial function in obesity and insulin resistance. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863:1106–1114. doi: 10.1016/j.bbadis.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Rico-Mesa J.S., White A., Anderson A.S. Outcomes in patients with COVID-19 infection taking ACEI/ARB. Curr. Cardiol. Rep. 2020;22:31. doi: 10.1007/s11886-020-01291-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R., Viscarra J.A., Minas J.N., Nakano D., Nishiyama A., Ortiz R.M. Angiotensin receptor blockade increases pancreatic insulin secretion and decreases glucose intolerance during glucose supplementation in a model of metabolic syndrome. Endocrinology. 2012;153:1684–1695. doi: 10.1210/en.2011-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez R., Minas J.N., Vazquez-Medina J.P., Nakano D., Parkes D.G., Nishiyama A., Ortiz R.M. Chronic AT1 blockade improves glucose homeostasis in obese OLETF rats. J. Endocrinol. 2018;237:271–284. doi: 10.1530/JOE-17-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogero M.M., Calder P.C. Obesity, inflammation, toll-like receptor 4 and fatty acids. Nutrients. 2018;10 doi: 10.3390/nu10040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan P.M., Caplice N.M. Is adipose tissue a reservoir for viral spread, immune activation and cytokine amplification in COVID-19. Obes. Silver Spring Md. 2020 doi: 10.1002/oby.22843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S., Yi Q., Fan S., Lv J., Zhang X., Guo L., Lang C., Xiao Q., Xiao K., Yi Z., Qiang M., Xiang J., Zhang B., Chen Y., Gao C. Relationships among lymphocyte subsets, cytokines, and the pulmonary inflammation index in coronavirus (COVID-19) infected patients. Br. J. Haematol. 2020 doi: 10.1111/bjh.16659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimabukuro-Vornhagen A., Gödel P., Subklewe M., Stemmler H.J., Schlößer H.A., Schlaak M., Kochanek M., Böll B., von Bergwelt-Baildon M.S. Cytokine release syndrome. J. Immunother. Cancer. 2018;6:56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker R., Tannock L.R., Su W., Gong M., Gurley S.B., Thatcher S.E., Yiannikouris F., Ensor C.M., Cassis L.A. Adipocyte deficiency of ACE2 increases systolic blood pressures of obese female C57BL/6 mice. Biol. Sex Differ. 2019;10:45. doi: 10.1186/s13293-019-0260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonnet A., Chetboun M., Poissy J., Raverdy V., Noulette J., Duhamel A., Labreuche J., Mathieu D., Pattou F., Jourdain M., Lille Intensive Care COVID-19 and Obesity study group High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obes. Silver Spring Md. 2020 doi: 10.1002/oby.22831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A.K., Gupta R., Misra A. Comorbidities in COVID-19: outcomes in hypertensive cohort and controversies with renin angiotensin system blockers. Diabetes Metab. Syndr. 2020;14:283–287. doi: 10.1016/j.dsx.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaimanzadeh I. Acetazolamide, nifedipine and phosphodiesterase inhibitors: rationale for their utilization as adjunctive countermeasures in the treatment of coronavirus disease 2019 (COVID-19) Cureus. 2020;12:e7343. doi: 10.7759/cureus.7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strzelak A., Ratajczak A., Adamiec A., Feleszko W. Tobacco smoke induces and alters immune responses in the lung triggering inflammation, allergy, asthma and other lung diseases: a mechanistic review. Int. J. Environ. Res. Public Health. 2018;15 doi: 10.3390/ijerph15051033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger T. The role of the renin-angiotensin system in the development of cardiovascular disease. Am. J. Cardiol. 2002;89:3A–9A. doi: 10.1016/s0002-9149(01)02321-9. discussion 10A. [DOI] [PubMed] [Google Scholar]
- van der Zijl N.J., Moors C.C.M., Goossens G.H., Blaak E.E., Diamant M. Does interference with the renin-angiotensin system protect against diabetes? Evidence and mechanisms. Diabetes Obes. Metab. 2012;14:586–595. doi: 10.1111/j.1463-1326.2012.01559.x. [DOI] [PubMed] [Google Scholar]
- Vardavas C.I., Nikitara K. COVID-19 and smoking: a systematic review of the evidence. Tob. Induc. Dis. 2020;18:20. doi: 10.18332/tid/119324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Tao Z.-W., Lei W., Ming-Li Y., Kui L., Ling Z., Shuang W., Yan D., Jing L., Liu H.-G., Ming Y., Yi H. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin. Med. J. (Engl.) 2020 doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir M.R. Effects of renin-angiotensin system inhibition on end-organ protection: can we do better? Clin. Ther. 2007;29:1803–1824. doi: 10.1016/j.clinthera.2007.09.019. [DOI] [PubMed] [Google Scholar]
- WHO . 2017. Chronic Obstructive Pulmonary Disease (COPD) [WWW Document]. URL.https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd) (accessed 4.29.20) [Google Scholar]
- Woon Fong Leung W., Sun Q. Electrostatic charged nanofiber filter for filtering airborne novel coronavirus (COVID-19) and nano-aerosols. Sep. Purif. Technol. 2020 doi: 10.1016/j.seppur.2020.116886. 116886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B., Fan C.-Y., Wang A.-L., Zou Y.-L., Yu Y.-H., He C., Xia W.-G., Zhang J.-X., Miao Q. Suppressed T cell-mediated immunity in patients with COVID-19: a clinical retrospective study in Wuhan, China. J. Infect. 2020 doi: 10.1016/j.jinf.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., Wang Z., Li Jinxiu, Li Jianming, Feng C., Zhang Z., Wang L., Peng L., Chen L., Qin Y., Zhao D., Tan S., Yin L., Xu J., Zhou C., Jiang C., Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94 doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Zhao Z., Wang Y., Zhou Y., Ma Y., Zuo W. Single-cell RNA expression profiling of ACE2, the putative receptor of Wuhan 2019-nCov. bioRxiv. 2020 doi: 10.1101/2020.01.26.919985. 2020.01.26.919985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.-Y., Ma Y.-T., Zhang J.-Y., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., Zou R., Zeng L., Kou S., Lan J., Li X., Liang Y., Ding X., Tan G., Tan G.S., Liu L., Liu Y., Pan Y., Wang Z. The correlation between viral clearance and biochemical outcomes of 94 COVID-19 infected discharged patients. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. Al. 2020;69:599–606. doi: 10.1007/s00011-020-01342-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020 doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Cong M., Wang N., Li X., Zhang H., Zhang K., Jin M., Wu N., Qiu C., Li J. Association of angiotensin-converting enzyme 2 gene polymorphism and enzymatic activity with essential hypertension in different gender: a case-control study. Medicine (Baltimore) 2018;97 doi: 10.1097/MD.0000000000012917. e12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet Lond. Engl. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Xie J., Huang F., Cao L. Association between short-term exposure to air pollution and COVID-19 infection: evidence from China. Sci. Total Environ. 2020;727 doi: 10.1016/j.scitotenv.2020.138704. 138704. [DOI] [PMC free article] [PubMed] [Google Scholar]