Highlights
-
•
Zhejiang was the third worst-affected province of COVID-19 in China
-
•
This study monitored transmissibility of COVID-19, accounting transmission from imported cases
-
•
An interruption of local transmission was observed in early/mid-February 2020
-
•
Early response followed by large-scale measures was effective for the interruption
Keywords: COVID-19, Zhejiang, Reproduction number, Serial interval, Travel restriction
Abstract
Owing to the frequent travel connections between Wuhan and Zhejiang, Zhejiang was the third worst-affected province in China with 1,205 cases confirmed before 26 February 2020. The transmissibility of the 2019 novel coronavirus disease was monitored in Zhejiang, accounting for the transmissions from imported cases. Even though Zhejiang was one of the worst-affected provinces, an interruption of disease transmission (i.e. instantaneous reproduction numbers <1) was observed in early/mid-February after a comprehensive set of interventions combating the outbreak.
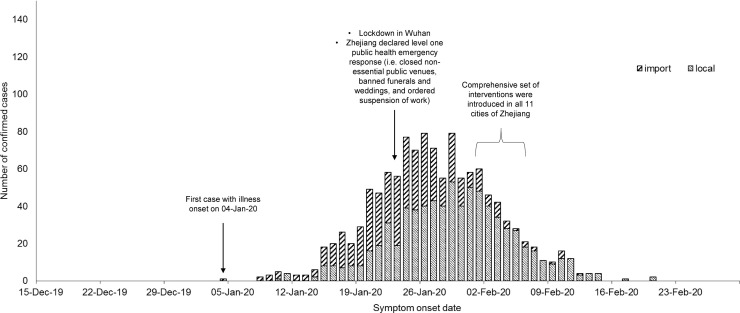
In December 2019, a novel strain of coronavirus emerged and caused an outbreak in Wuhan, Hubei province, China (WHO, 2020). The disease responsible for the outbreak has been officially named by the World Health Organization (WHO) as COVID-19. To halt the spread of COVID-19, the Chinese government announced a complete lockdown in Wuhan on 23 January 2020. A set of draconian countermeasures were issued to other cities in Hubei province since 26 January 2020, aiming to quarantine the epicentre of the outbreak (Amy and Wuhan, 2020). Owing to the frequent travel connections between Wuhan and Zhejiang (an eastern province with 57 million residents), Zhejiang was the third worst-affected province with 1,205 cases confirmed before 26 February 2020, and the first province to declare the highest provincial level public health emergency in response to the outbreak (23 January 2020). As of 27 January 2020, Zhejiang released many travel-related measures by suspending all inter-province land and water passenger transportation. Starting from 1 February to 6 February 2020, all 11 cities in Zhejiang imposed a comprehensive set of interventions–including personal protective measures, environmental measures, social distancing measures, and city-level travel restrictions–to mitigate the risk and impact of the COVID-19 epidemic. In 10 of the 11 Zhejiang cities, arrivals with a travel history to other provinces within the last 14 days were quarantined; individuals were requested to stay at home for at least 2 weeks and immediately report any fever or cough cases to their neighbourhood committee (cunweihui and juweihui); enforced temperature check and face mask wearing were applied in public places to protect the people; gatherings, including weddings, were suspended to keep social distance; and it was suggested that only one person per household leave home once every 2 days for supplies (Hangzhou Municipal Health Commission, 2020). In addition to these measures, more restrictive interventions were applied for Wenzhou city, which was most affected by the epidemic. All factories and schools were shut down; inter-city and intra-city public transportation were mostly suspended; transportation hubs were frequently sterilized; and no inter-community movement was allowed without necessary reasons (Wenzhou Government Information, 2020). This type of comprehensive intervention was widely applied in many provinces in China, while Zhejiang was one of the very first to apply these measures outside Hubei.
The instantaneous reproduction number (R t) of COVID-19 in Zhejiang was estimated to monitor the impact of control measures on disease transmissibility over time. R t was defined as the average number of secondary cases generated by a primary case at time t in a population and is a time-varying measure of disease transmissibility when intervention measures are in place for outbreak control (De Serres et al., 2000). If R t is below unity, disease transmission is unlikely to be sustained and the outbreak should be under control. The data of confirmed cases, including both local and imported cases from 26 December 2019 to 25 February 2020, from all cities in Zhejiang were analysed. Cases with travel history to other provinces (with ≥1 case reported) 14 days prior to symptom onset were defined as imported cases. To ensure a robustness of estimation, two different methods were used to estimate R t: 1) Wallinga and Teunis approach (M1) (Wallinga and Teunis, 2004, Cori et al., 2013) and 2) susceptible-exposed-infectious-recovered (SEIR) model-based approach (M2). In M1, it was assumed that the serial interval followed a lognormal distribution with a mean of 7.5 days and standard deviation of 3.4 days (Li et al., 2020), whereas in M2, the mean latent duration and mean infectious duration were assumed to be 5.2 (Li et al., 2020) and 2.3 days (i.e. difference between mean serial interval and mean latent duration), respectively. Using the time series data of local and imported cases, the likelihoods were formulated and the model parameter R t was solved (additional details in Supplementary File).
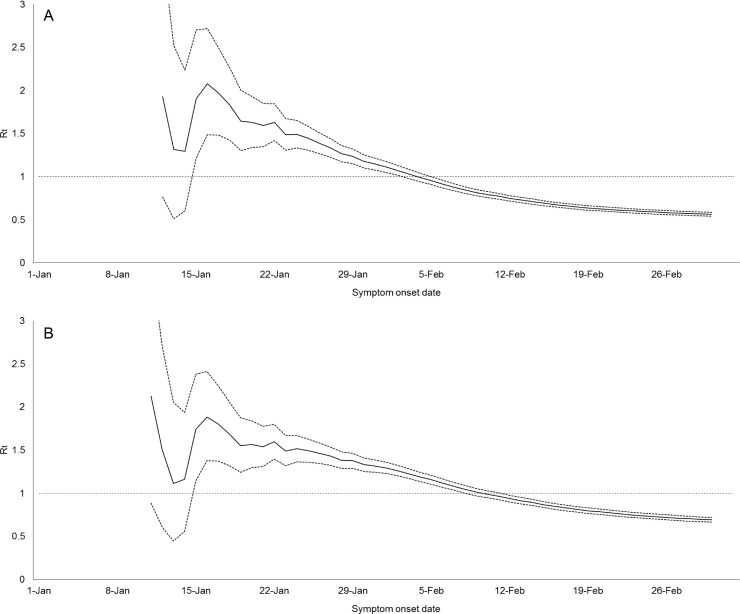
Over the study period, R t attained peak values of 2.08 (95% CI 1.49 to 1.72) and 1.88 (95% CI 1.38 to 2.41) on 16 January 2020 for method M1 and method M2, respectively (Fig. 1, Fig. 2 ). The R t remained stable and kept above 1.40 until 28 January 2020, 5 days after the declaration of the highest-level public health emergency. Although the number of local cases had gradually surpassed that of imported cases, the R t decreased over time. After the lockdown of cities, the upper bounds of CI of R t for M1 and M2 were below the threshold of unity on 6 February 2020 and 12 February 2020, respectively: M1 0.91 (95% CI 0.87 to 0.96) and M2 0.94 (95% CI 0.90 to 0.98). The R t was to be 0.63 (95% CI 0.60 to 0.65) for M1 and 0.78 (95% CI 0.75 to 0.82) for M2 on 20 February 2020, the day the last cases were reported to date. The trends of R t were generally not sensitive when the mean infectious duration varied but with a shorter infectious duration, a later of time for R t being below unity (Figure S1 in Supplementary File).
Fig. 1.
Number of imported cases and local cases of COVID-19 in Zhejiang against symptom-onset date.
Fig. 2.
Estimated instantaneous reproduction number (Rt) (solid line) and 95% confidence intervals (dotted lines) using: (A) Wallinga and Teunis approach (M1); and (B) susceptible-exposed-infectious-recovered (SEIR) model-based approach.
Taking transmission arising from imported cases into account, disease transmissibility of COVID-19 reached its peak on 16 January 2020, before Zhejiang declared the highest-level public health emergency and the government imposed a lockdown in Wuhan. A fast growth in disease transmission during the initial phase of this epidemic can thus be expected, especially in areas that have tight travel connections with the source region (Zhao et al., 2020). With the successive imported cases from Wuhan, the local infections were likely to be generated by them and this resulted in sustained transmission initially in Zhejiang, as indicated by R t >1. Because of this, it is advised that early and strict screening and tracing measures be implemented before a large number of imported cases arises in a community, for example, mandatory quarantine for travellers to the region. In addition, this study showed that the transmission of COVID-19 reduced and remained at a low level, not only after the lockdown of Wuhan, after the implementation of a comprehensive set of interventions in Zhejiang. As a result, disease transmission in the population was interrupted (R t <1) within several weeks alongside a drop in local cases. The decline in transmissibility might be attributed to the early declaration of a public health emergency, followed by implementation of large-scale measures, which seemed to interrupt and slow down the transmission of COVID-19 in the population. These findings could thus inform high-risk regions on disease control planning in a timely manner.
Funding source
The work is supported by National Natural Science Foundation of China (31871340, 71974165). The funder has no role in the study design, in the collection, analysis and interpretation of data.
Conflict of interest
No conflict of interest to declare.
Ethical approval
As this investigation was a part of the surveillance of emerging infectious diseases of Zhejiang Provincial Center for Disease Control and Prevention (CDC), ethics approval and informed consent were exempted by CDC. The data were analysed anonymously.
Acknowledgments
We thank the physicians and staffs at Hangzhou, Huzhou, Jiaxing Wenzhou, Shaoxing, Ningbo, Quzhou, Jinhua, Zhoushan, Lishui, Taizhou Municipal Center for Disease Control and Prevention for their support and assistance with this investigation.
Footnotes
Preprint of this article is published at https://doi.org/10.1101/2020.03.02.20028704.
Supplementary material related to this article can be found, in the online version, at https://doi.org/10.1016/j.ijid.2020.04.036.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- World Health Organization. Novel coronavirus–China. Jan 12, 2020. [cited 2020 Feb 22]. Available from: https://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/.
- Amy Q., Wuhan Vivian W. Center of Coronavirus Outbreak. Is Being Cut Off by Chinese Authorities; 2020 Jan 22 [cited 2020 Feb 22]. In: The New York Times. Available from: https://www.nytimes.com/2020/01/22/world/asia/china-coronavirus-travel.html; 2020. [Google Scholar]
- Hangzhou Municipal Health Commission. Prevention and control of pneumonia in a new coronavirus infection. Notice of the Hangzhou Municipal People's Government on the Implementation of the Ten Measures for the Prevention and Control of Epidemic. 2020 Feb 4 [cited 2020 Feb 22]. Available from: http://wsjkw.hangzhou.gov.cn/art/2020/2/4/art_1228996568_41882478.html [in Chinese].
- Wenzhou Government Information Release. Wenzhou's epidemic prevention and control: 25 emergency measures are closely related to life. 2020 Feb 1 [cited 2020 April 9]. Available from: http://www.wenzhou.gov.cn/art/2020/2/1/art_1217832_41865281.html [in Chinese].
- De Serres G., Gay N.J., Farrington C.P. Epidemiology of transmissible diseases after elimination. Am J Epidemiol. 2000;151(11):1039–1048. doi: 10.1093/oxfordjournals.aje.a010145. [DOI] [PubMed] [Google Scholar]
- Wallinga J., Teunis P. Different epidemic curves for severe acute respiratory syndrome reveal similar impacts of control measures. Am J Epidemiol. 2004;160(6):509–516. doi: 10.1093/aje/kwh255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cori A., Ferguson N.M., Fraser C., Cauchemez S. A new framework and software to estimate time-varying reproduction numbers during epidemics. Am J Epidemiol. 2013;178(9):1505–1512. doi: 10.1093/aje/kwt133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001316. Jan 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Zhuang Z., Ran J., Lin J., Yang G., Yang L. The association between domestic train transportation and novel coronavirus (2019-nCoV) outbreak in China from 2019 to 2020: A data-driven correlational report. Travel Med Infect Dis. 2020;33:101568. doi: 10.1016/j.tmaid.2020.101568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.